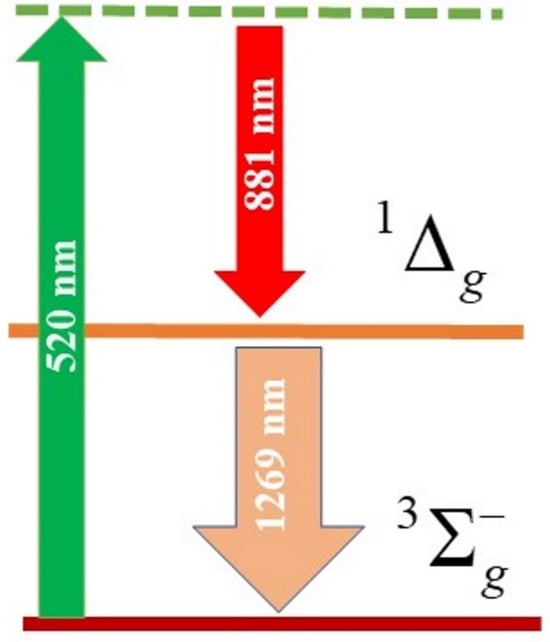
Near-Infrared Phosphorescence of Raman Photogenerated Singlet Oxygen
Abstract
1. Introduction
2. Materials and Methods
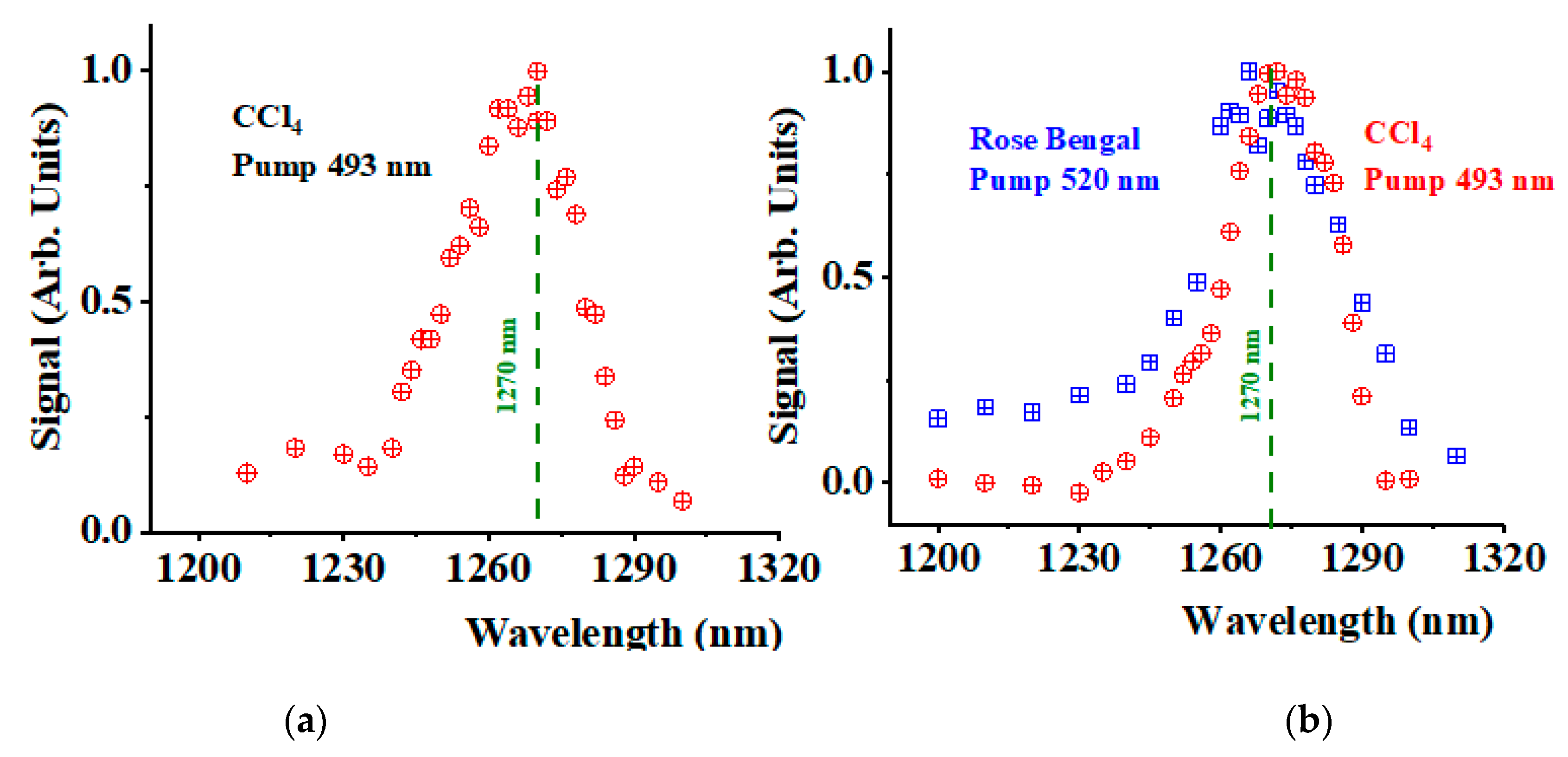
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dahl, T.A.; Midden, W.R.; Hartman, P.E. Comparison of killing of gram-negative and gran-positive bacteria by pure singlet oxygen. J. Bacteriol. 1989, 171, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Skupin-Mrugalska, P.; Mielcarek, J.; Goslinski, T.; Balzarini, J. Photosensitizers Mediated Photodynamic Inactivation against Virus Particles. Mini Rev. Med. Chem. 2015, 15, 503–521. [Google Scholar] [CrossRef]
- Preuß, A.; Saltsman, I.; Mahammed, A.; Pfitzner, M.; Goldberg, I.; Gross, Z.; Röder, B. Photodynamic inactivation of mold fungi spores by newly developed charged corroles. J. Photochem. Photobiol. B Bio. 2014, 113, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. JNCI J. Nat. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Beermann, A.E.; Jay, D.G. Chromophore-Assisted Laser Inactivation of Cellular Proteins. Methods Cell Biol. 1994, 44, 715–732. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; He, S.; Wu, S.; Yang, C. Singlet Oxygen: Properties, generation, detection, and environmental applications. J. Hazardous Mater. 2024, 461, 132538. [Google Scholar] [CrossRef]
- Singlet Oxygen: Applications in Biosciences and Nanosciences—Comprehensive Series in Photochemical & Photobiological Sciences, 1st ed.; Nonell, S., Flors, C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; Volume 14. [Google Scholar]
- Marcano Olaizola, A.; Kuis, R.; Johnson, A.; Kingsley, D. Stimulated Raman Generation of Aqueous Singlet Oxygen without Photosensitizers. J. Photochem. Photobio. B Biol. 2022, 235, 112562. [Google Scholar] [CrossRef]
- Marcano Olaizola, A.; Zerrad, A.; Jenneto, F.; Kingsley, D. Confirming the Stimulated Raman Origin of Singlet-Oxygen Photogeneration. J. Raman Spectros. 2024, 55, 58–64. [Google Scholar] [CrossRef]
- Krasnovskiĭ, A.A. Fotosensibilizirovannaia liuminestsentsiia singletnogo kisloroda v rastvore [Photosensitized luminescence of singlet oxygen in solution]. Biofizika 1976, 21, 748–749. (In Russian) [Google Scholar] [PubMed]
- Khan, A.U. Direct spectroscopic observation of 1.27 μm and 1.58 μm emission of singlet (1Δg) molecular oxygen in chemically generated and dye-photosensitized liquid solutions at room temperature. Chem. Phys. Lett. 1980, 72, 112–114. [Google Scholar] [CrossRef]
- Adam, W.; Kazakov, D.V.; Kazakov, V.P. Singlet oxygen chemiluminescence in peroxide reactions. Chem. Rev. 2005, 105, 3371–3387. [Google Scholar] [CrossRef] [PubMed]
- Ossola, R.; Jönsson, O.M.; Moor, K.; McNeill, K. Singlet Oxygen quantum yields in environmental waters. Chem. Rev. 2021, 121, 4100–4146. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.A.; McNeill, K.; Janssen, E.M.I. Non-singlet oxygen kinetic solvent isotope effects in aquatic photochemistry. Environ. Sci. Technol. 2018, 52, 9908–9916. [Google Scholar] [CrossRef]
- Moskalensky, A.E.; Karagodina, T.Y.; Vorobev, A.Y.; Sokolovski, S.G. Singlet oxygen luminescence detector on low-cost InGaAs avalanche photodiode. Hardware X 2021, 10, e00224. [Google Scholar] [CrossRef]
- Sivery, A.; Anquez, F.; Perlot, C.; Aubry, J.M.; Courtade, E. Singlet oxygen (1O2) generation upon 1270 nm laser irradiation of ground statet oxygen (3O2) dissolved in organic solvents: Simultaneous and independent determination of 1O2 production rate and reactivity with chemical traps. Chem. Phys. Lett. 2013, 555, 252–257. [Google Scholar] [CrossRef]
- Anquez, A.F.; Suret, P.; Randoux, S.; Courtade, E.; Belkoura, E.Y. Cell death induced by direct laser activation of singlet oxygen at 1270 nm. Laser Phys. 2013, 23, 025601. [Google Scholar] [CrossRef]
- Bregnhøj, M.; Blázquez-Castro, A.; Westberg, M.; Breitenbach, T. Direct optical excitation of molecular oxygen in solution and in simple mammalian cells. J. Phys. Chem. B 2015, 119, 5422–5429. [Google Scholar] [CrossRef]
- Sokolovski, S.G.; Zolotovskaya, S.A.; Goltsov, A.; Pourreyron, C.; South, A.P.; Rafailov, E.U. Infrared laser pulse triggers increased singlet oxygen production in tumour cells. Sci. Rep. 2013, 3, 3484. [Google Scholar] [CrossRef]
- Krasnovsky, A.A., Jr.; Kozlov, A.S.; Roumbal, Y.V. Photochemical investigation of the IR absorption bands of molecular oxygen in organic and aqueous environment. Photochem. Photobiol. Sci. 2012, 11, 988–997. [Google Scholar] [CrossRef]
- Krasnovsky, A.A., Jr.; Kozlov, A.S. Photonics of dissolved oxygen molecules. Comparison of the rates of direct and photosensitized excitation of oxygen and revaluation of the oxygen absorption coefficients. J. Photochem. Photobio. A Chem. 2016, 329, 167–174. [Google Scholar] [CrossRef]
- Blázquez-Castro, A. Direct 1O2 optical excitation: A tool for redox biology. Redox Biol. 2017, 13, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Bregnhøj, M.; Ogilby, P. Two-photon excitation of neat aerated solvents with visible light produces singlet oxygen. J. Phys. Chem. A. 2019, 123, 7567–7575. [Google Scholar] [CrossRef]
- Bagrov, I.V.; Kiselev, V.M.; Kislyakov, L.M.; Sosnov, E.N. Direct optical excitation of singlet oxygen in organic solvents. Opt. Spectrosc. 2014, 116, 567–574. [Google Scholar] [CrossRef]
- Schweitzer, C.; Schimdt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef]
- Diaz, A.M.; Bonifácio, R.R.; Marrucho, I.M.; Padua, A.A.H.; Costa Gomes, M.F. Solubility of oxygen in a n-hexane and in n-perfluorohexane. Experimental determination and prediction by molecular simulation. Phys. Chem. Phys. 2003, 5, 543–549. [Google Scholar] [CrossRef]
- Sato, T.; Hamada, Y.; Sumikawa, M.; Araki, S.; Yamamoto, H. Solubility of oxygen in organic solvents and calculation of the Hansen solubility parameters of oxygen. Ind. Eng. Chem. Res. 2014, 53, 19331–19337. [Google Scholar] [CrossRef]
- Quaranta, M.; Mukovic, M.; Klimant, I. A new method to measure oxygen solubility in organic solvents through optical oxygen sensing. Analyst 2013, 138, 6243–6245. [Google Scholar] [CrossRef]
- Krasnovsky, A.A., Jr. Singlet molecular oxygen in photobiochemical systems: IR phosphorescence studies. Membr. Cell Biol. 1998, 12, 665–690. [Google Scholar] [PubMed]
- Wessels, J.M.; Rodgers, A.J. Effects of solvent polarizability on the forbidden 1Δg→3Σg- transition in molecular oxygen: A Fourier transform near-infrared luminescence study. J. Phys. Chem. 1995, 99, 17585–17592. [Google Scholar] [CrossRef]
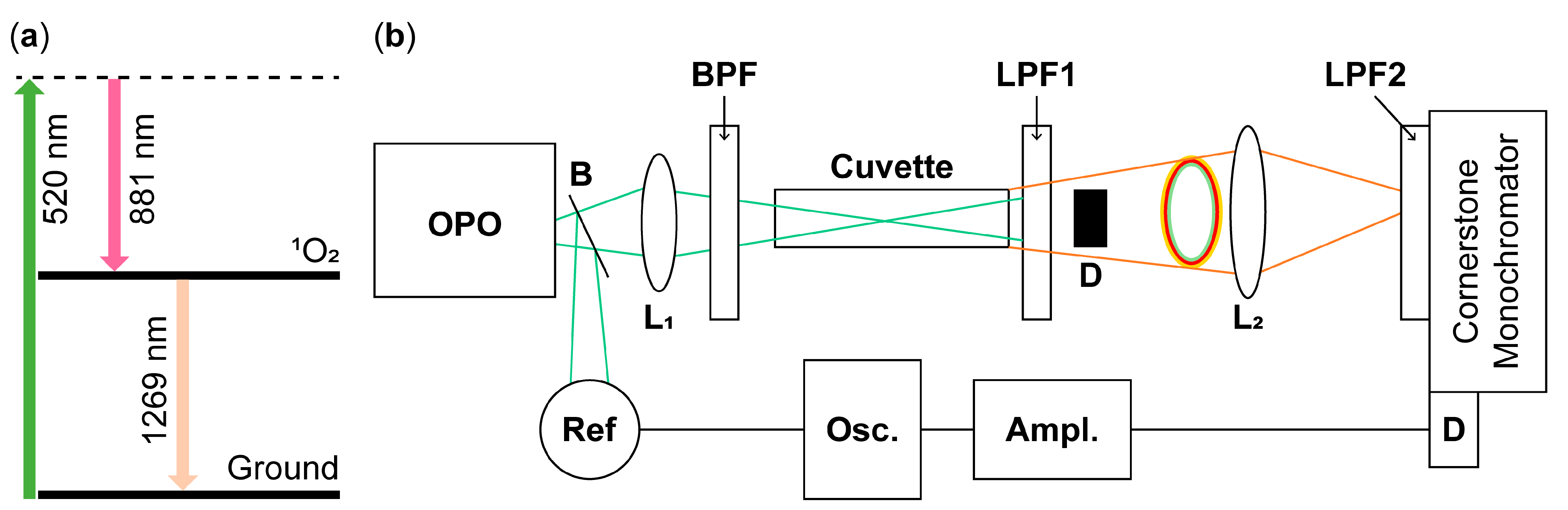
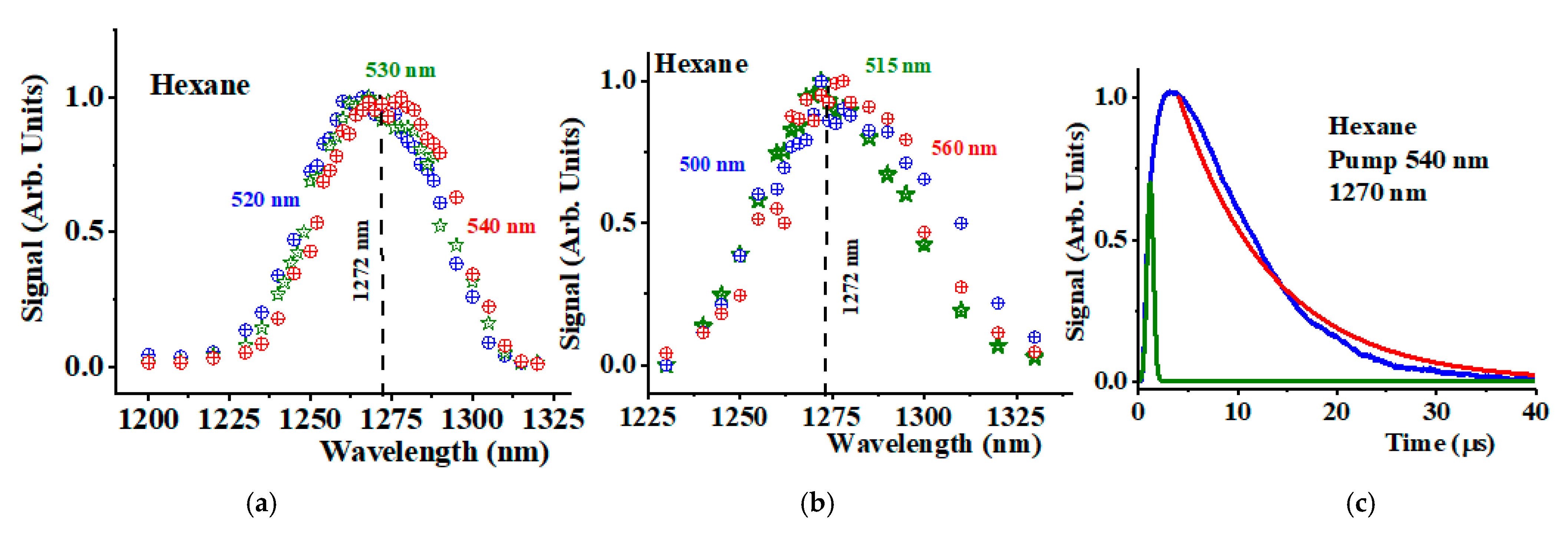
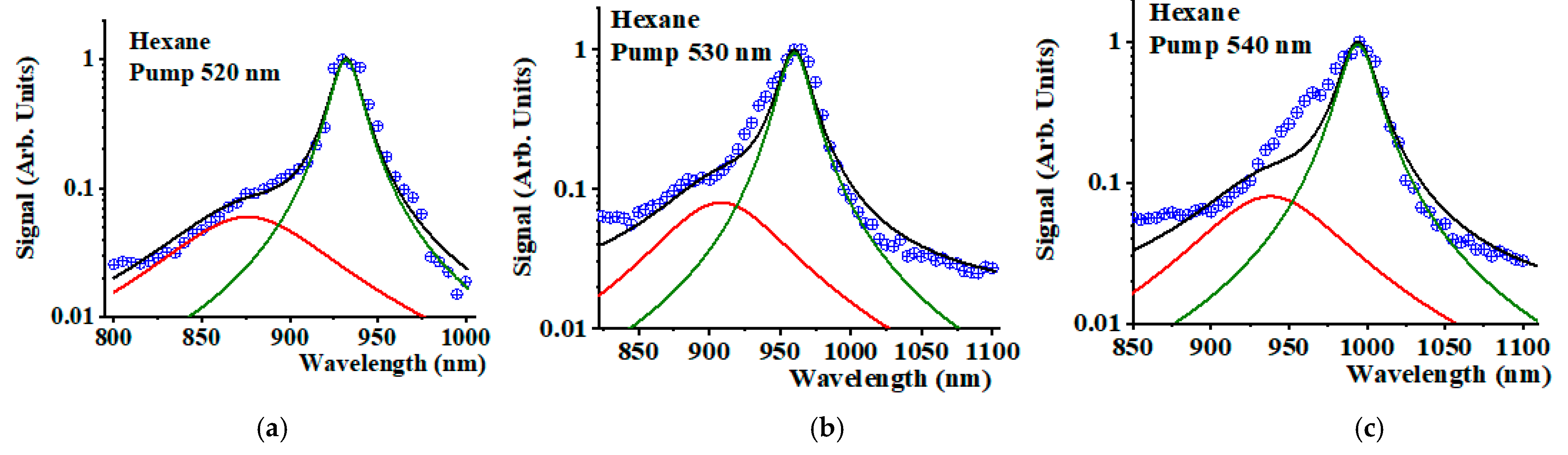
| Pump Wavelength (nm) | 2nd Overtone (nm) | 1O2 Stokes (nm) | 1O2 Phosphorescence (nm) |
|---|---|---|---|
| 520 | 932 | 879 | 1272 |
| 530 | 964 | 908 | 1272 |
| 540 | 998 | 938 | 1272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcano Olaizola, A. Near-Infrared Phosphorescence of Raman Photogenerated Singlet Oxygen. Photochem 2025, 5, 7. https://doi.org/10.3390/photochem5010007
Marcano Olaizola A. Near-Infrared Phosphorescence of Raman Photogenerated Singlet Oxygen. Photochem. 2025; 5(1):7. https://doi.org/10.3390/photochem5010007
Chicago/Turabian StyleMarcano Olaizola, Aristides. 2025. "Near-Infrared Phosphorescence of Raman Photogenerated Singlet Oxygen" Photochem 5, no. 1: 7. https://doi.org/10.3390/photochem5010007
APA StyleMarcano Olaizola, A. (2025). Near-Infrared Phosphorescence of Raman Photogenerated Singlet Oxygen. Photochem, 5(1), 7. https://doi.org/10.3390/photochem5010007