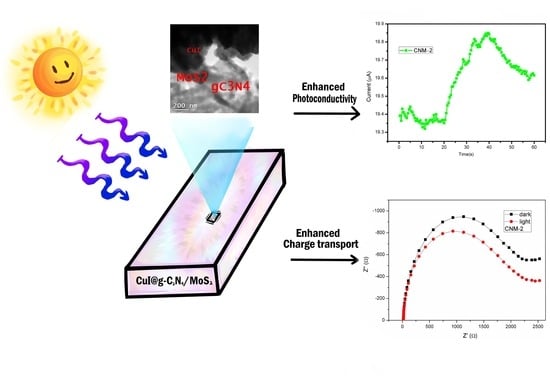
Hybrid CuI@g-C3N4/MoS2 Thin Films for Energy Conversion Applications: A Photoelectrochemical Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CuI@g-C3N4/MoS2 Thin Films
2.1.1. Chemicals
2.1.2. Synthesis of CuI Thin Films
2.1.3. Synthesis of g-C3N4/MoS2 Composite
2.1.4. Synthesis of CuI@g-C3N4/MoS2 Hybrid Thin Films
2.2. Characterization
3. Results
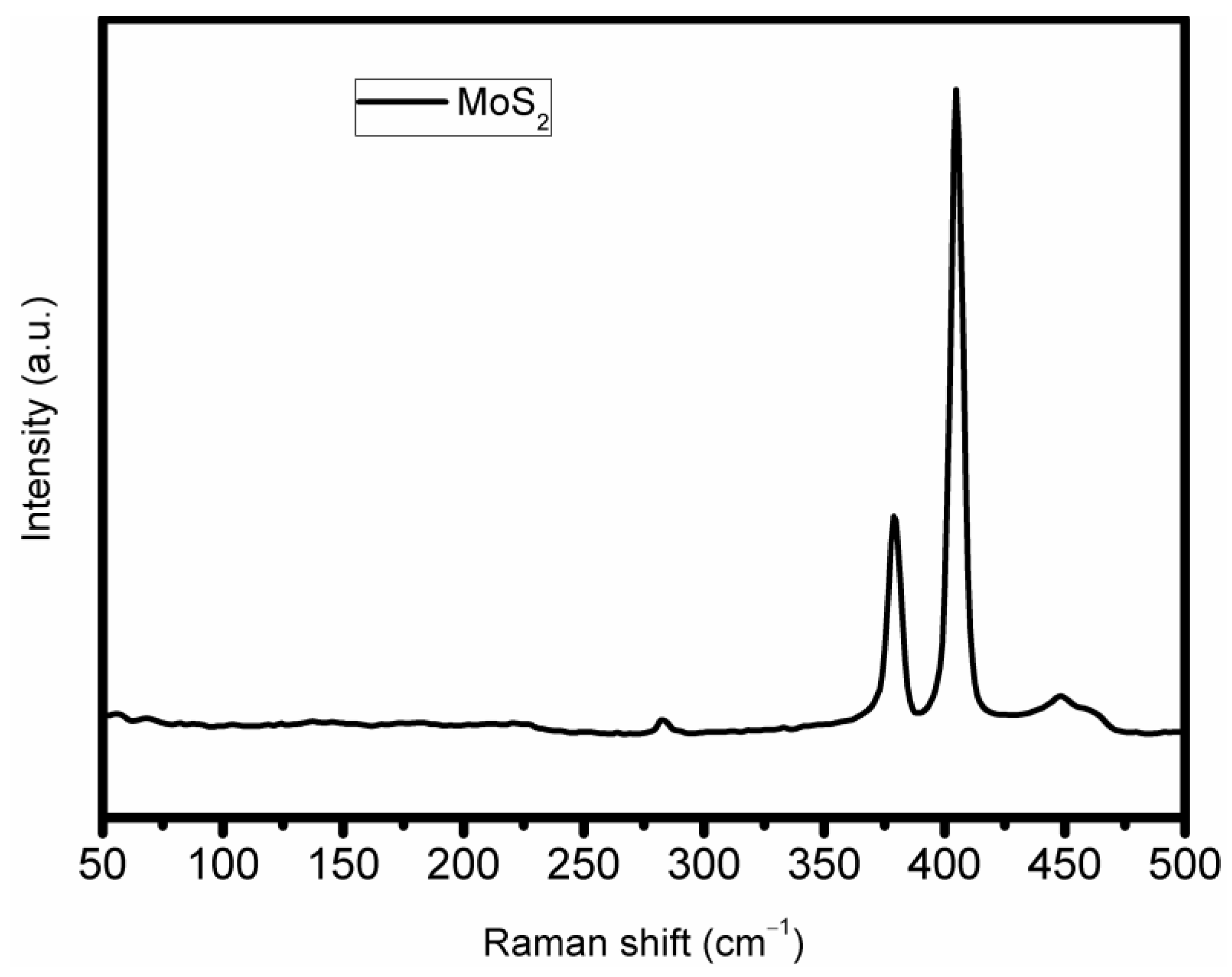
3.1. Structural Characterization
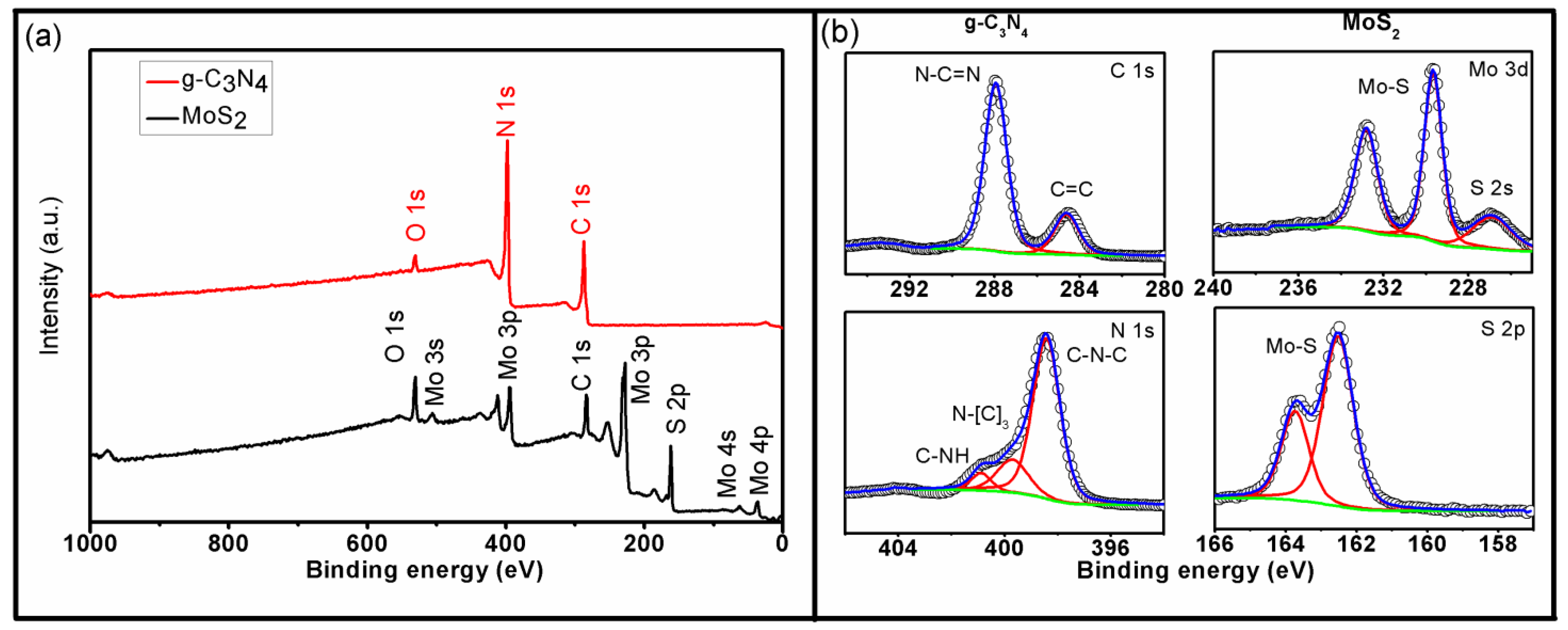
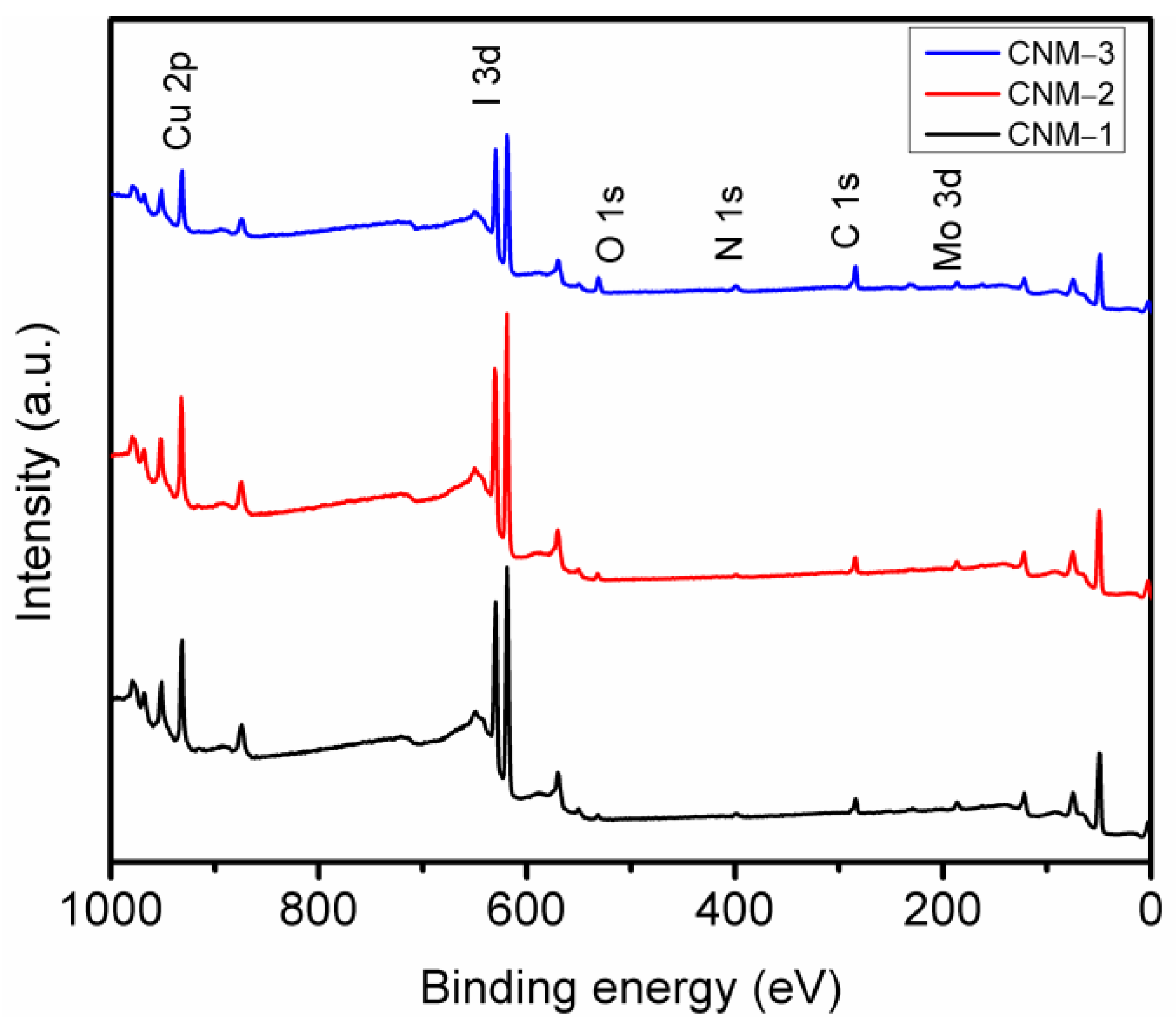
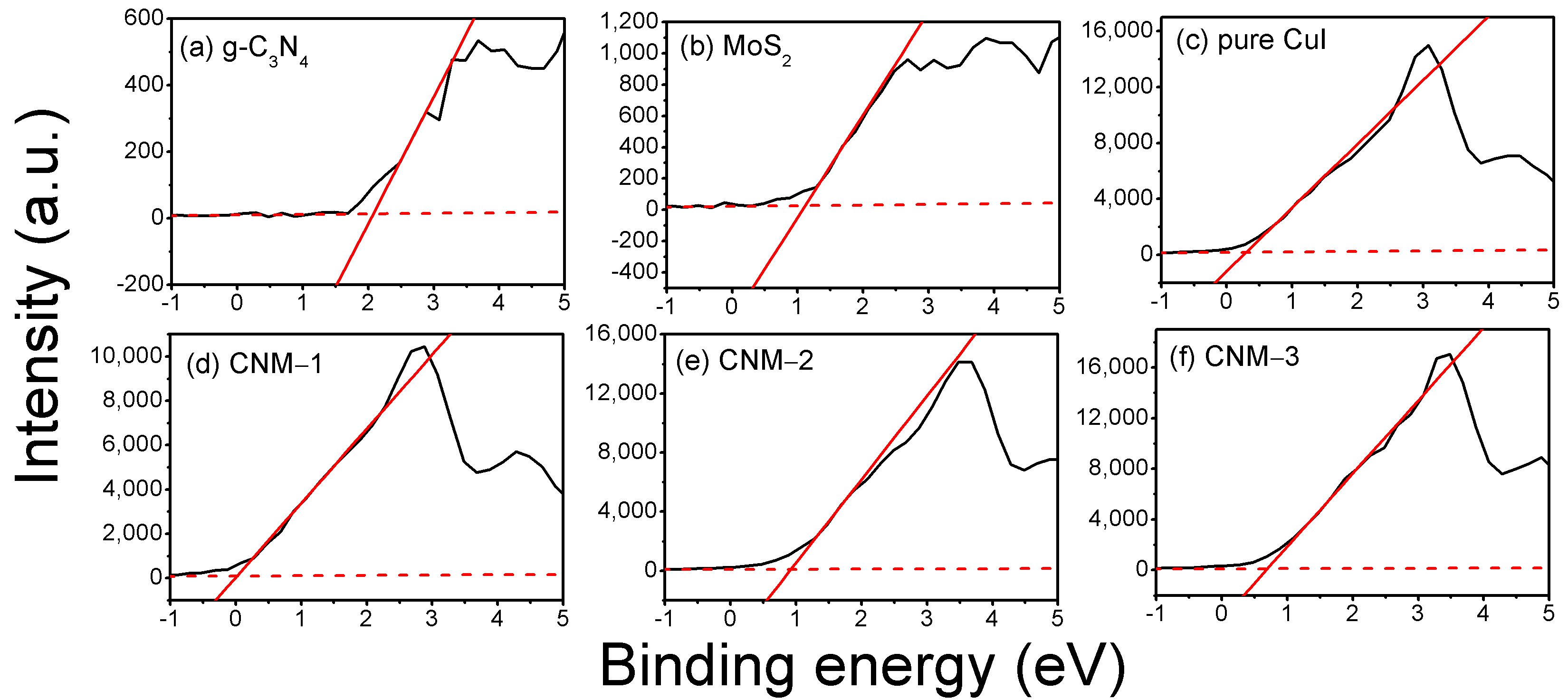
3.2. Chemical Composition
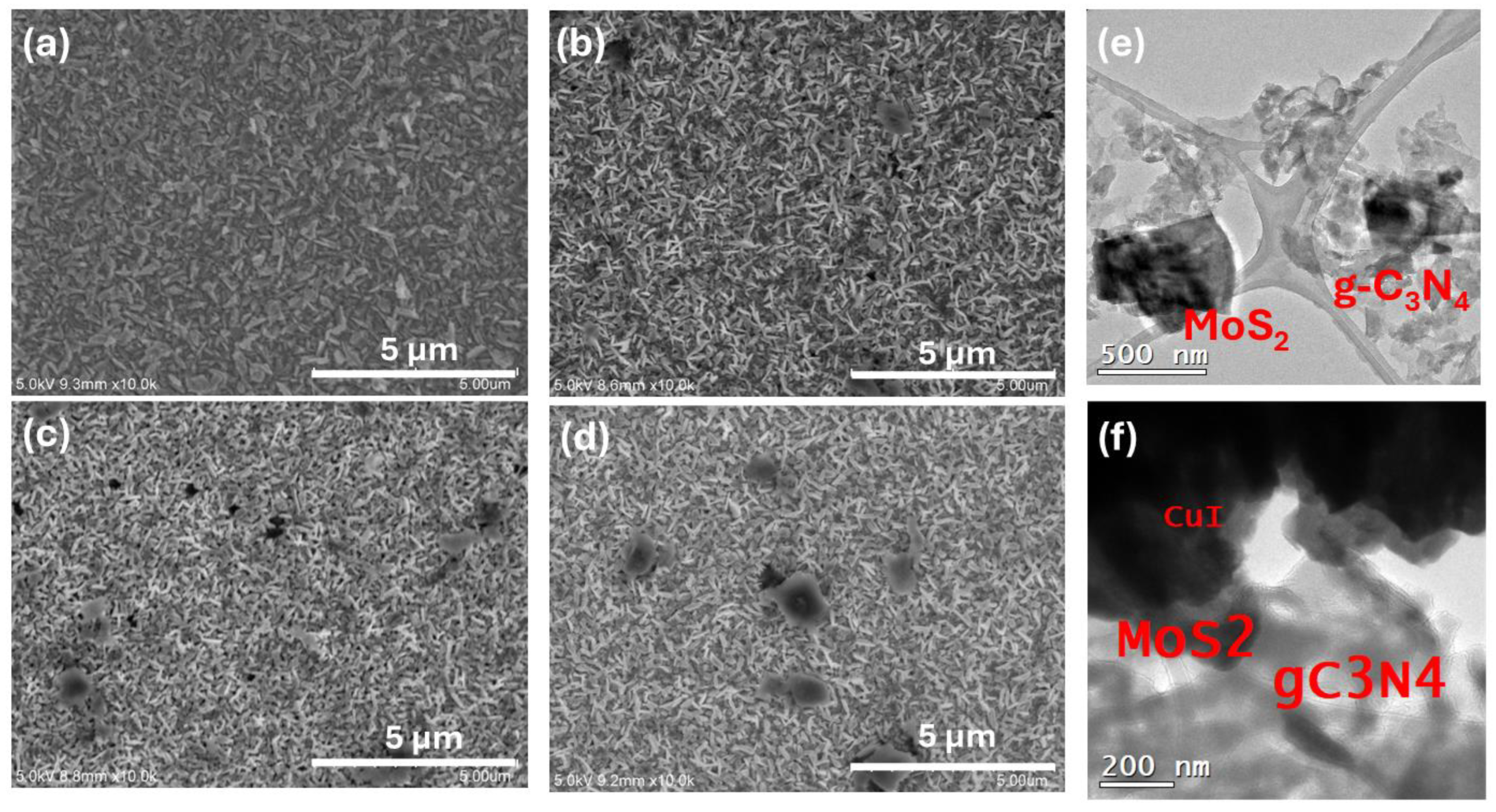
3.3. Morphology
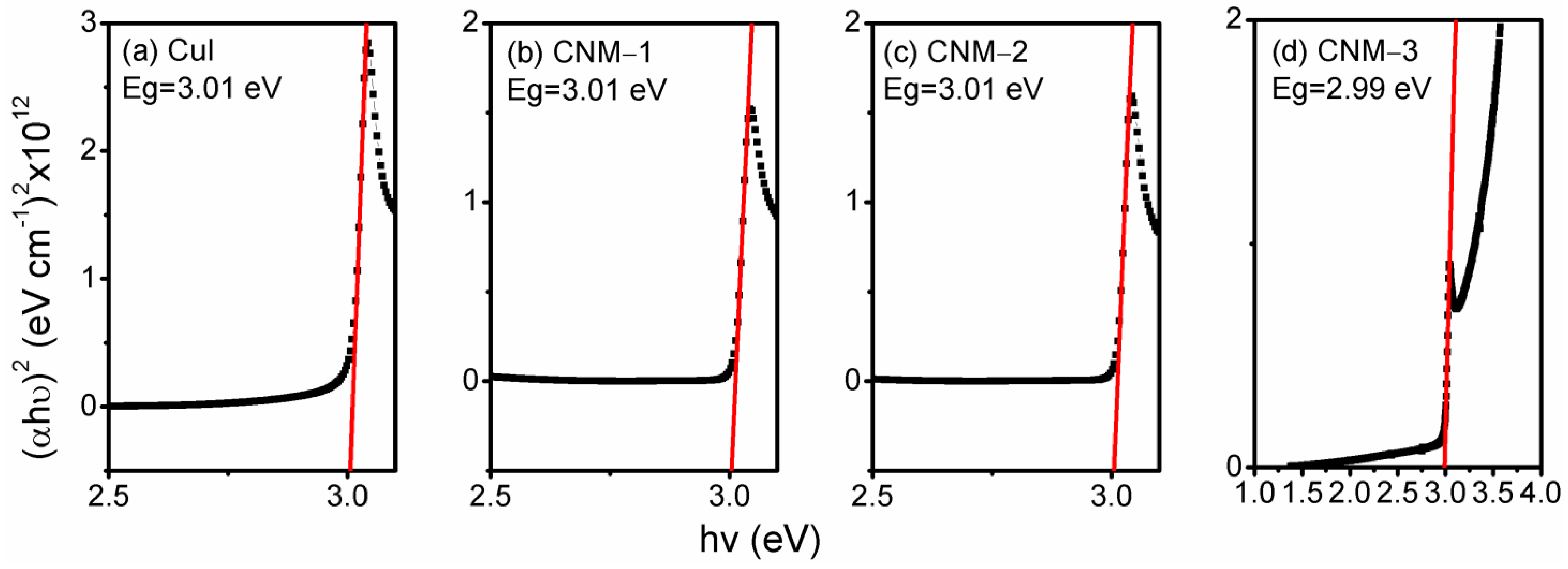
3.4. Optical Properties
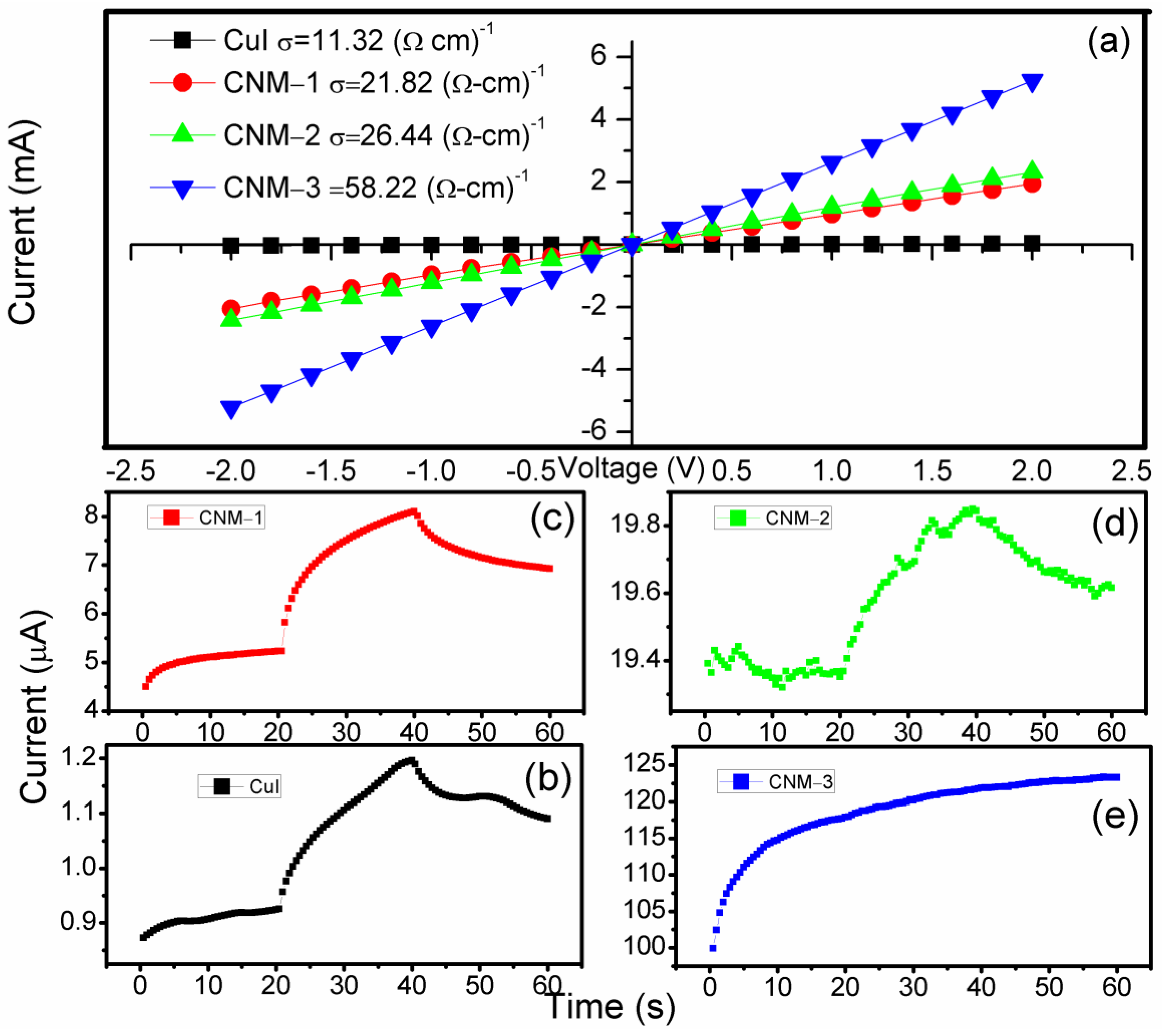
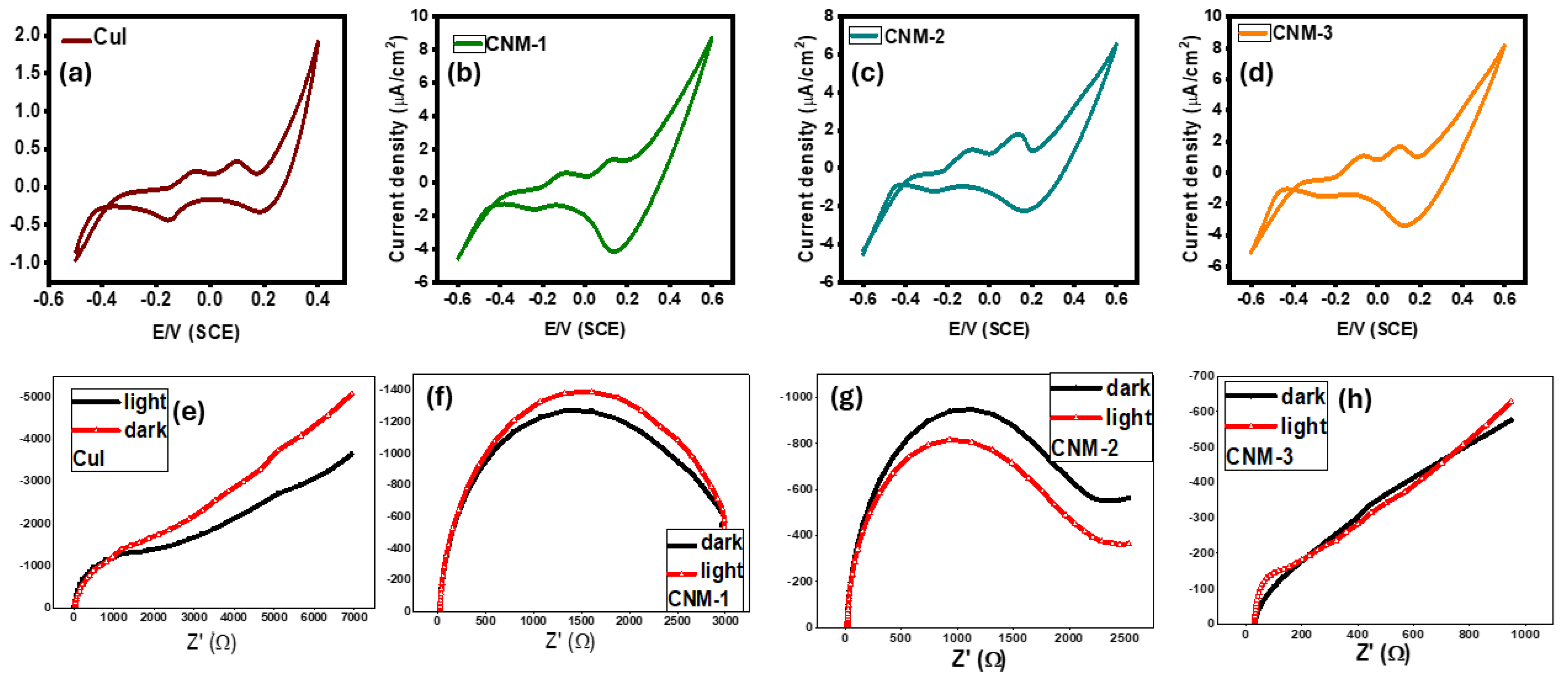
3.5. Photoelectrochemical Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, H.; Shin, E.-S.; Liu, A.; Ji, D.; Xu, Y.; Noh, Y.-Y. Printable Semiconductors for Backplane TFTs of Flexible OLED Displays. Adv. Funct. Mater. 2020, 30, 1904588. [Google Scholar] [CrossRef]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. P-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Sato, H.; Minami, T.; Takata, S.; Yamada, T. Transparent conducting p-type NiO thin films prepared by magnetron sputtering. Thin Solid Film 1993, 236, 27–31. [Google Scholar] [CrossRef]
- Moreira, M.; Afonso, J.; Crepelliere, J.; Lenoble, D.; Lunca-Popa, P. A review on the p-type transparent Cu–Cr–O delafossite materials. J. Mater. Sci. 2022, 57, 3114–3142. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, J.; Gong, H. Transparent p-Type Semiconductors: Copper-Based Oxides and Oxychalcogenides. Coatings 2019, 9, 137. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, H.; Noh, Y.-Y. Molecule Charge Transfer Doping for p-Channel Solution-Processed Copper Oxide Transistors. Adv. Funct. Mater. 2020, 30, 2002625. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, H.; Kim, M.-G.; Kim, J.; Noh, Y.-Y. Engineering Copper Iodide (CuI) for Multifunctional p-Type Transparent Semiconductors and Conductors. Adv. Sci. 2021, 8, 2100546. [Google Scholar] [CrossRef]
- Storm, P.; Bar, M.S.; Selle, S.; von Wenckstern, H.; Grundmann, M.; Lorenz, M. p-Type Doping and Alloying of CuI Thin Films with Selenium. Phys. Status Solidi (RRL) Rapid Res. Lett. 2021, 15, 2100214. [Google Scholar] [CrossRef]
- Kaushik, D.K.; Selvaraj, M.; Ramu, S.; Subrahmanyam, A. Thermal evaporated Copper Iodide (CuI) thin films: A note on the disorder evaluated through the temperature dependent electrical properties. Sol. Energy Mater. Sol. Cells 2017, 165, 52–58. [Google Scholar] [CrossRef]
- Ayhan, M.E.; Shinde, M.; Todankar, B.; Desai, P.; Ranade, A.K.; Tanemura, M.; Kalita, G. Ultraviolet radiation-induced photovoltaic action in γ-CuI/β-Ga2O3 heterojunction. Mater. Lett. 2020, 262, 127074. [Google Scholar] [CrossRef]
- Kim, T.; Son, C.; Lee, J.; Oh, E.; Li, S.; Chang, C.-H.; Lim, S. Interfacial ZnS passivation for improvement of transparent ZnO/CuI diode characteristics. Appl. Surf. Sci. 2021, 536, 147645. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, W.-J.; Kim, T.H.; Lee, T.; Hong, S.; Kim, K.H. Transparent p-CuI/n-BaSnO3−δ heterojunctions with a high rectification ratio. J. Phys. Condens. Matter 2017, 29, 384004. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.H.; Kang, J.; Diware, M.; Jeon, K.; Jeong, C.; Lee, S.Y.; Kim, K.H. Characteristics and Electronic Band Alignment of a Transparent p-CuI/n-SiZnSnO Heterojunction Diode with a High Rectification Ratio. Nanomaterials 2021, 11, 1237. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-H.; Jung, D.-Y. Air-Stable Transparent Silver Iodide–Copper Iodide Heterojunction Diode. ACS Appl. Mater. Interfaces 2017, 9, 43807–43813. [Google Scholar] [CrossRef]
- Annadi, A.; Zhang, N.; Boon Kiang Lim, D.; Gong, H. New Transparent Magnetic Semiconductor NixCu1–xI which Can Perform as Either P-type or N-type and Success in the P–N Homojunction Diode. ACS Appl. Mater. Interfaces 2020, 12, 6048–6055. [Google Scholar] [CrossRef]
- Antoniadou, M.; Arfanis, M.K.; Ibrahim, I.; Falaras, P. Bifunctional g-C3N4/WO3 Thin Films for Photocatalytic Water Purification. Water 2019, 11, 2439. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z.; Yu, J.; Li, W. A novel application of g-C3N4 thin film in photoelectrochemical anticorrosion. Electrochim. Acta 2013, 88, 294–300. [Google Scholar] [CrossRef]
- Nagal, V.; Kumar, V.; Ahmad, R.; Khan, M.; Khan, Z.H.; Singh, K.; Furukawa, H.; Khosla, A.; Hahn, Y.B.; Hafiz, A.K. Review—Emerging Applications of g-C3N4 Films in Perovskite-Based Solar Cells. ECS J. Solid State Sci. Technol. 2021, 10, 065001. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Tuci, G.; Sanson, A.; Peruzzini, M.; Giambastiani, G. Metal-free carbon-based materials for electrocatalytic and photo-electrocatalytic CO2 reduction. Rend. Lincei. Sci. Fis. E Nat. 2019, 30, 497–513. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Zheng, G.; Qu, L.; Han, Q. Few-layer carbon nitride photocatalysts for solar fuels and chemicals: Current status and prospects. Chin. J. Catal. 2022, 43, 1216–1229. [Google Scholar] [CrossRef]
- Chen, L.; Yan, G.; Liu, X.; Ying, S.; Xia, Y.; Ning, S.; Wang, X. Phosphorus doped and defect modified graphitic carbon nitride for boosting photocatalytic hydrogen production. Phys. Chem. Chem. Phys. 2023, 25, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.-N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2022, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Muchuweni, E.; Mombeshora, E.T.; Martincigh, B.S.; Nyamori, V.O. Graphitic carbon nitride-based new-generation solar cells: Critical challenges, recent breakthroughs and future prospects. Sol. Energy 2022, 239, 74–87. [Google Scholar] [CrossRef]
- Acuña Leal, D.A.; Santiago Ramírez, C.R.; Ramos Delgado, N.A.; Shaji, S.; Gracia Pinilla, M.A. Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials. Nanotechnol. Rev. 2024, 13, 20240127. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Shi, L.; He, Z.; Liu, S. MoS2 quantum dots embedded in g-C3N4 frameworks: A hybrid 0D-2D heterojunction as an efficient visible-light driven photocatalyst. Appl. Surf. Sci. 2018, 457, 30–40. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Simple and Large Scale Construction of MoS2-g-C3N4 Heterostructures Using Mechanochemistry for High Performance Electrochemical Supercapacitor and Visible Light Photocatalytic Applications. Sci. Rep. 2017, 7, 43055. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, X.; Liu, Y.; Jiang, S.; Wang, D.; Fan, Y.; Hu, L.; Zhang, D.; Yao, W.; Zhang, L. New g-C3N4/GO/MoS2 composites as efficient photocatalyst for photocathodic protection of 304 stainless steel. Water Sci. Technol. 2021, 84, 499–511. [Google Scholar] [CrossRef]
- Ye, L.; Wang, D.; Chen, S. Fabrication and Enhanced Photoelectrochemical Performance of MoS2/S-Doped g-C3N4 Heterojunction Film. ACS Appl. Mater. Interfaces 2016, 8, 5280–5289. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Liu, X.; Qian, T.; Yao, M.; Liu, W.; Ji, H. Tuning band structure of graphitic carbon nitride for efficient degradation of sulfamethazine: Atmospheric condition and theoretical calculation. Chin. Chem. Lett. 2022, 33, 1385–1389. [Google Scholar] [CrossRef]
- Tan, X.; Kou, L.; Tahini, H.A.; Smith, S.C. Conductive Graphitic Carbon Nitride as an Ideal Material for Electrocatalytically Switchable CO2 Capture. Sci. Rep. 2015, 5, 17636. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; He, Z.-L.; Yuan, C.; Wang, X.; Zhai, C.; Zhu, M. Heterostructures Based on g-C3N4/CuI as a Photoactivated Support for Pt Nanoparticles toward Efficient Photoelectrocatalytic Methanol Oxidation. Ind. Eng. Chem. Res. 2021, 60, 762–770. [Google Scholar] [CrossRef]
- Meftahi, A.; Reisi-Vanani, A.; Shabani-Nooshabadi, M. Comparison of performance of CuI/g-C3N4 nanocomposites synthesized on Ni-foam and graphitic substrates as suitable electrode materials for supercapacitors. Fuel 2023, 331, 125683. [Google Scholar] [CrossRef]
- Rugma, T.P.; Lakhera, S.K.; Sahoo, T.; Neppolian, B. Phosphorus modified and CuI incorporated polymeric g-C3N4 photocatalyst for efficient photocatalytic hydrogen production under direct solar light irradiation. Mater. Sci. Eng. B 2022, 284, 115873. [Google Scholar] [CrossRef]
- Peng, W.; Li, L.; Yu, S.; Zheng, H.; Yang, P. Structure, binding energy and optoelectrical properties of p-type CuI thin films: The effects of thickness. Appl. Surf. Sci. 2020, 502, 144424. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural Investigation of Graphitic Carbon Nitride via XRD and Neutron Diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Wang, J.; Yang, L.; Xin, L.; Li, G.; Lin, G.; Niu, S.; Yan, J.; Zhao, F. Hydrothermal growth and their optoelectronic device application of CuI nanostructure. Mater. Res. Express 2019, 6, 045048. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, D.; Jung, S.; Bae, M.-H.; Yun, Y.J.; Yi, S.N.; Yu, J.-S.; Kim, J.-H.; Ha, D.H. Changes in the Raman spectra of monolayer MoS2 upon thermal annealing. J. Raman Spectrosc. 2018, 49, 1938–1944. [Google Scholar] [CrossRef]
- Bagnall, A.G.; Liang, W.Y.; Marseglia, E.A.; Welber, B. Raman studies of MoS2 at high pressure. Phys. B+C 1980, 99, 343–346. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Allred, A.L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]
- Posopa, N.; Sakulkalavek, A.; Chanlek, N.; Kaewkhao, J.; Sakdanuphab, R. Room-temperature rapid synthesis of CuI thin films via liquid iodination method. Superlattices Microstruct. 2020, 141, 106501. [Google Scholar] [CrossRef]
- Chinnakutti, K.K.; Panneerselvam, V.; Govindarajan, D.; Soman, A.K.; Parasuraman, K.; Thankaraj Salammal, S. Optoelectronic and electrochemical behaviour of γ-CuI thin films prepared by solid iodination process. Prog. Nat. Sci. Mater. Int. 2019, 29, 533–540. [Google Scholar] [CrossRef]
- Sharma, P.; Sarngan, P.P.; Lakshmanan, A.; Sarkar, D. One-step synthesis of highly reactive g-C3N4. J. Mater. Sci. Mater. Electron. 2022, 33, 9116–9125. [Google Scholar] [CrossRef]
- Li, B.; Jiang, L.; Li, X.; Ran, P.; Zuo, P.; Wang, A.; Qu, L.; Zhao, Y.; Cheng, Z.; Lu, Y. Preparation of Monolayer MoS2 Quantum Dots using Temporally Shaped Femtosecond Laser Ablation of Bulk MoS2 Targets in Water. Sci. Rep. 2017, 7, 11182. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Ahn, K.; Kim, G.H.; Kim, S.-J.; Kim, J.; Ryu, G.-S.; Lee, P.; Ryu, B.; Cho, J.Y.; Kim, Y.-H.; Kang, J.; et al. Highly Conductive p-Type Transparent Conducting Electrode with Sulfur-Doped Copper Iodide. Chem. Mater. 2022, 34, 10517–10527. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Li, C. A comparison of graphitic carbon nitrides synthesized from different precursors through pyrolysis. J. Photochem. Photobiol. A Chem. 2017, 332, 32–44. [Google Scholar] [CrossRef]
- Gowri, V.M.; Ajith, A.; John, S.A. Systematic Study on Morphological, Electrochemical Impedance, and Electrocatalytic Activity of Graphitic Carbon Nitride Modified on a Glassy Carbon Substrate from Sequential Exfoliation in Water. Langmuir 2021, 37, 10538–10546. [Google Scholar] [CrossRef]
- Nasir, S.N.S.; Mohamed, N.A.; Tukimon, M.A.; Noh, M.F.M.; Arzaee, N.A.; Teridi, M.A.M. Direct extrapolation techniques on the energy band diagram of BiVO4 thin films. Phys. B Condens. Matter 2021, 604, 412719. [Google Scholar] [CrossRef]
- Wang, M.; Zhuang, M.; Ge, L.; Yuan, Y.; Wang, Z.; Liu, W.; Li, H.; Song, A. Tunable photoresponse properties of CuI/Si self-powered photodetectors through Zn doping engineering. Appl. Surf. Sci. 2024, 654, 159400. [Google Scholar] [CrossRef]
- Geng, F.; Yang, L.; Dai, B.; Guo, S.; Gao, G.; Xu, L.; Han, J.; Bolshakov, A.; Zhu, J. Enhanced transmittance and mobility of p-type copper iodide thin films prepared at room temperature via a layer-by-layer approach. Surf. Coat. Technol. 2019, 361, 396–402. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, J.; Gao, G.; Xu, J.; Zhao, L.; Zhou, W.; Wang, Q.; Yuan, S.; Sun, J. Transparent p-type CuI film based self-powered ultraviolet photodetectors with ultrahigh speed, responsivity and detectivity. J. Mater. Chem. C 2022, 10, 13040–13046. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, X.; Qin, J.; Liu, R. Constructing MoS2/g-C3N4 heterojunction with enhanced oxygen evolution reaction activity: A theoretical insight. Appl. Surf. Sci. 2020, 510, 145489. [Google Scholar] [CrossRef]
- Li, Z.H.; He, J.X.; Lv, X.H.; Chi, L.F.; Egbo, K.O.; Li, M.-D.; Tanaka, T.; Guo, Q.X.; Yu, K.M.; Liu, C.P. Optoelectronic properties and ultrafast carrier dynamics of copper iodide thin films. Nat. Commun. 2022, 13, 6346. [Google Scholar] [CrossRef]
- Senthilnathan, S.; Kumar, K.G.; Sugunraj, S.; Dhanalakshmi, M.A.; Rajendraprasad, M.; Suganthi, M.; Kesavan, K.S.; Sasikumar, P.; Abbas, M.; Dator, W.L.T. MoS2 modified g-C3N4 composite: A potential candidate for photocatalytic applications. J. Saudi Chem. Soc. 2023, 27, 101717. [Google Scholar] [CrossRef]
- Bosch Ojeda, C.; Sanchez Rojas, F. Recent developments in derivative ultraviolet/visible absorption spectrophotometry. Anal. Chim. Acta 2004, 518, 1–24. [Google Scholar] [CrossRef]
- Trenczek-Zajac, A.; Synowiec, M.; Zakrzewska, K.; Zazakowny, K.; Kowalski, K.; Dziedzic, A.; Radecka, M. Scavenger-Supported Photocatalytic Evidence of an Extended Type I Electronic Structure of the TiO2@Fe2O3 Interface. ACS Appl. Mater. Interfaces 2022, 14, 38255–38269. [Google Scholar] [CrossRef]
- Moditswe, C.; Muiva, C.M.; Luhanga, P.; Juma, A. Effect of annealing temperature on structural and optoelectronic properties of γ-CuI thin films prepared by the thermal evaporation method. Ceram. Int. 2017, 43, 5121–5126. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Mao, S.; Hao, X.; Duan, X.; Wen, Y. Different Morphology MoS2 Over the g-C3N4 as a Boosted Photo-Catalyst for Pollutant Removal Under Visible-Light. J. Inorg. Organomet. Polym. Mater. 2021, 31, 32–42. [Google Scholar] [CrossRef]
- Rahman, M.; Newaz, M.A.; Mondal, B.K.; Kuddus, A.; Karim, M.A.; Rashid, M.M.; Rubel, M.H.K.; Hossain, J. Unraveling the electrical properties of solution-processed copper iodide thin films for CuI/n-Si solar cells. Mater. Res. Bull. 2019, 118, 110518. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, R.; Yang, Y.; Zheng, Y.; Wei, B.; Zhang, W.; Zou, M.; Han, J.; Liu, Y.; Lan, J.; et al. Synergistic effects of CuI doping on enhancing thermoelectric performance for n-type Bi2O2Se fabricated by mechanical alloying. Scr. Mater. 2023, 225, 115163. [Google Scholar] [CrossRef]
- Zuo, C.; Cai, S.; Li, Z.; Fang, X. A transparent, self-powered photodetector based on p-CuI/n-TiO2 heterojunction film with high on–off ratio. Nanotechnology 2022, 33, 105202. [Google Scholar] [CrossRef]
- Mahyavanshi, R.D.; Desai, P.; Ranade, A.; Tanemura, M.; Kalita, G. Observing Charge Transfer Interaction in CuI and MoS2 Heterojunction for Photoresponsive Device Application. ACS Appl. Electron. Mater. 2019, 1, 302–310. [Google Scholar] [CrossRef]
- Konakov, A.O.; Dremova, N.N.; Khodos, I.I.; Koch, M.; Zolotukhina, E.V.; Silina, Y.E. One-Pot Synthesis of Copper Iodide-Polypyrrole Nanocomposites. Chemosensors 2021, 9, 56. [Google Scholar] [CrossRef]
- Kosta, I.; Azaceta, E.; Yate, L.; Cabañero, G.; Grande, H.; Tena-Zaera, R. Cathodic electrochemical deposition of CuI from room temperature ionic liquid-based electrolytes. Electrochem. Commun. 2015, 59, 20–23. [Google Scholar] [CrossRef]
- Ghazal, N.; Madkour, M.; Abdel Nazeer, A.; Obayya, S.S.A.; Mohamed, S.A. Electrochemical capacitive performance of thermally evaporated Al-doped CuI thin films. RSC Adv. 2021, 11, 39262–39269. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acuña Leal, D.A.; Santiago Ramírez, C.R.; Ramos Delgado, N.A.; Shaji, S.; Krishnan, B.; Avellaneda Avellaneda, D.; Aguilar Martínez, J.A.; Lugo Loredo, S.; Espinosa Roa, A.; Gracia Pinilla, M.Á. Hybrid CuI@g-C3N4/MoS2 Thin Films for Energy Conversion Applications: A Photoelectrochemical Characterization. Photochem 2025, 5, 9. https://doi.org/10.3390/photochem5010009
Acuña Leal DA, Santiago Ramírez CR, Ramos Delgado NA, Shaji S, Krishnan B, Avellaneda Avellaneda D, Aguilar Martínez JA, Lugo Loredo S, Espinosa Roa A, Gracia Pinilla MÁ. Hybrid CuI@g-C3N4/MoS2 Thin Films for Energy Conversion Applications: A Photoelectrochemical Characterization. Photochem. 2025; 5(1):9. https://doi.org/10.3390/photochem5010009
Chicago/Turabian StyleAcuña Leal, Daniel Arturo, Claudia Rosa Santiago Ramírez, Norma A. Ramos Delgado, Sadasivan Shaji, Bindu Krishnan, David Avellaneda Avellaneda, Josue Amilcar Aguilar Martínez, Shadai Lugo Loredo, Arian Espinosa Roa, and Miguel Ángel Gracia Pinilla. 2025. "Hybrid CuI@g-C3N4/MoS2 Thin Films for Energy Conversion Applications: A Photoelectrochemical Characterization" Photochem 5, no. 1: 9. https://doi.org/10.3390/photochem5010009
APA StyleAcuña Leal, D. A., Santiago Ramírez, C. R., Ramos Delgado, N. A., Shaji, S., Krishnan, B., Avellaneda Avellaneda, D., Aguilar Martínez, J. A., Lugo Loredo, S., Espinosa Roa, A., & Gracia Pinilla, M. Á. (2025). Hybrid CuI@g-C3N4/MoS2 Thin Films for Energy Conversion Applications: A Photoelectrochemical Characterization. Photochem, 5(1), 9. https://doi.org/10.3390/photochem5010009