Conformational Structure, Infrared Spectra and Light-Induced Transformations of Thymol Isolated in Noble Gas Cryomatrices
Abstract
1. Introduction
2. Methods
2.1. Experimental Methods
2.2. Computational Methods
3. Results and Discussion
3.1. Conformers and Barriers to Internal Interconversion
3.2. Infrared Spectra of Matrix-Isolated Thymol, Annealing and IR Irradiations
3.3. UV-Induced Transformations
4. Concluding Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Paidesetty, S.K.; Padhy, R.N. The recent development of thymol derivative as a promising pharmacological scaffold. Drug Dev. Res. 2021, 82, 1079–1095. [Google Scholar] [CrossRef]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Jamali, T.; Kavoosi, G.; Jamali, Y.; Mortezazadeh, S.; Ardestani, S.K. In-vitro, in-vivo, and in-silico assessment of radical scavenging and cytotoxic activities of Oliveria decumbens essential oil and its main components. Sci. Rep. 2021, 11, 14281. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar Verma, D.; Chávez-González, M.L. Mexican oregano (Lippia graveolens Kunth) as source of bioactive compounds: A review. Molecules 2021, 26, 5156. [Google Scholar] [CrossRef]
- Nieto, G. A review on applications and uses of thymus in the food industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Pandey, S.K.; Upadhyay, S.; Tripathi, A.K. Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi (Linn) Sprague seeds against Anopheles stephensi. Parasitol. Res. 2009, 105, 507–512. [Google Scholar] [CrossRef]
- Scoralik, M.G.; Daemon, E.; de Oliveira Monteiro, C.M.; Maturano, R. Enhancing the acaricide effect of thymol on larvae of the cattle tick Rhipicephalus microplus (Acari: Ixodidae) by solubilization in ethanol. Parasitol. Res. 2012, 110, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Thozet, A.; Perrin, M. Structure of 2-isopropyl-5-methylphenol (thymol). Acta Crystallogr. Sect. B 1980, 36, 1444–1447. [Google Scholar] [CrossRef]
- Schmitz, D.; Shubert, V.A.; Giuliano, B.M.; Schnell, M. The broadband microwave spectra of the monoterpenoids thymol and carvacrol: Conformational landscape and internal dynamics. J. Chem. Phys. 2014, 141, 034304. [Google Scholar] [CrossRef]
- Sin, K.-R.; Kim, C.-J.; Ko, S.-G.; Hwang, T.-M.; Han, Y.-N.; Pak, Y.-N. Inclusion of thymol into cucurbiturils: Density functional theory approach with dispersion correction and natural bond orbital analysis. J. Inclusion Phenom. Macrocycl. Chem. 2022, 102, 533–542. [Google Scholar] [CrossRef]
- Saraiva, A.G.Q.; Saraiva, G.D.; Albuquerque, R.L.; Nogueira, C.E.S.; Teixeira, A.M.R.; Lima, L.B.; Cruz, B.G.; de Sousa, F.F. Chemical analysis and vibrational spectroscopy study of essential oils from Lippia sidoides and of its major constituent. Vib. Spectrosc. 2020, 110, 103111. [Google Scholar] [CrossRef]
- Rajkumar, P.; Selvaraj, S.; Suganya, R.; Velmurugan, D.; Gunasekaran, S.; Kumaresan, S. Vibrational and electronic spectral analysis of thymol an isomer of carvacrol isolated from Trachyspermum ammi seed: A combined experimental and theoretical study. Chem. Data Collect. 2018, 15–16, 10–31. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Kirchler, C.G.; Huck, C.W. NIR spectra simulation of thymol for better understanding of the spectra forming factors, phase and concentration effects and PLS regression features. J. Mol. Liq. 2018, 268, 895–902. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Fausto, R.; Reva, I. Conformational Space, IR-Induced, and UV-induced chemistry of carvacrol isolated in a low-temperature argon matrix. J. Phys. Chem. A 2021, 125, 8215–8229. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron-density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an approximation treatment for many-electron systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Pople, J.A.; Head-Gordon, M.; Raghavachari, K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 1987, 87, 5968–5975. [Google Scholar] [CrossRef]
- Reva, I.; Lopes Jesus, A.J.; Nunes, C.M.; Roque, J.P.L.; Fausto, R. UV-Induced Photochemistry of 1,3-Benzoxazole, 2-Isocyanophenol, and 2-Cyanophenol Isolated in Low-Temperature Ar Matrixes. J. Org. Chem. 2021, 86, 6126–6137. [Google Scholar] [CrossRef] [PubMed]
- Zhurko, G.A. Chemcraft—Graphical Program for Visualization of Quantum Chemistry Computations, Version 1.8. 2020. Available online: http://www.chemcraftprog.com (accessed on 18 May 2022).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Teixeira, F.; Cordeiro, M.N.D.S. Improving vibrational mode interpretation using bayesian regression. J. Chem. Theory Comput. 2019, 15, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Lesarri, A.; Shipman, S.T.; Neill, J.L.; Brown, G.G.; Suenram, R.D.; Kang, L.; Caminati, W.; Pate, B.H. Interplay of phenol and isopropyl isomerism in propofol from broadband chirped-pulse microwave spectroscopy. J. Am. Chem. Soc. 2010, 132, 13417–13424. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, Y.; Hao, J.; Yang, Y.; Wang, L.; Li, C.; Jia, S. Rotamers of p-isopropylphenol studied by hole-burning resonantly enhanced multiphoton ionization and mass analyzed threshold ionization spectroscopy. Spectrochim. Acta Part A 2019, 207, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.R.; Chapman, M.A.; Wilson, D.C.; Bates, S.P.; Jones, A.C. The nature of conformational preference in a number of p-alkyl phenols and p-alkyl benzenes. Phys. Chem. Chem. Phys. 2002, 4, 4910–4915. [Google Scholar] [CrossRef]
- Schaefer, T.; Addison, B.M.; Sebastian, R.; Wildman, T.A. Orientations of the hydroxyl and isopropyl groups in the cis and trans conformers of 2-isopropylphenol and 2-isopropyl-6-methylphenol. Can. J. Chem. 1981, 59, 1656–1659. [Google Scholar] [CrossRef]
- Rozenberg, M.; Fausto, R.; Reva, I. Variable temperature FTIR spectra of polycrystalline purine nucleobases and estimating strengths of individual hydrogen bonds. Spectrochim. Acta Part A 2021, 251, 119323. [Google Scholar] [CrossRef] [PubMed]
- Lopes Jesus, A.J.; Rosado, M.T.S.; Leitão, M.L.P.; Redinha, J.S. Molecular structure of butanediol isomers in gas and liquid states: Combination of DFT calculations and infrared spectroscopy studies. J. Phys. Chem. A 2003, 107, 3891–3897. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Rosado, M.T.S.; Reva, I.; Fausto, R.; Eusébio, M.E.S.; Redinha, J.S. Structure of isolated 1,4-Butanediol: Combination of MP2 calculations, NBO analysis, and matrix-isolation infrared spectroscopy. J. Phys. Chem. A 2008, 112, 4669–4678. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Rosado, M.T.S.; Reva, I.; Fausto, R.; Eusébio, M.E.; Redinha, J.S. Conformational study of monomeric 2,3-Butanediols by matrix-isolation infrared spectroscopy and DFT calculations. J. Phys. Chem. A 2006, 110, 4169–4179. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.T.S.; Lopes Jesus, A.J.; Reva, I.D.; Fausto, R.; Redinha, J.S. Conformational cooling dynamics in matrix-isolated 1,3-Butanediol. J. Phys. Chem. A 2009, 113, 7499–7507. [Google Scholar] [CrossRef][Green Version]
- Lopes Jesus, A.J.; Nunes, C.M.; Reva, I.; Pinto, S.M.V.; Fausto, R. Effects of entangled IR radiation and tunneling on the conformational interconversion of 2-Cyanophenol. J. Phys. Chem. A 2019, 123, 4396–4405. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Reva, I.; Nunes, C.M.; Roque, J.P.L.; Pinto, S.M.V.; Fausto, R. Kinetically unstable 2-isocyanophenol isolated in cryogenic matrices: Vibrational excitation, conformational changes and spontaneous tunneling. Chem. Phys. Lett. 2020, 742, 137069. [Google Scholar] [CrossRef]
- Akai, N.; Kudoh, S.; Takayanagi, M.; Nakata, M. Cis-Trans isomerization equilibrium in hydroquinone in low-temperature argon and xenon matrices studied by FTIR spectroscopy. Chem. Phys. Lett. 2002, 356, 133–139. [Google Scholar] [CrossRef]
- Akai, N.; Kudoh, S.; Nakata, M. Photoisomerization and tunneling isomerization of tetrachlorohydroquinone in a low-temperature argon matrix. J. Phys. Chem. A 2003, 107, 3655–3659. [Google Scholar] [CrossRef]
- Borden, W.T. Reactions that involve tunneling by carbon and the role that calculations have played in their study. WIREs Comput. Mol. Sci. 2016, 6, 20–46. [Google Scholar] [CrossRef]
- Nunes, C.M.; Reva, I.; Fausto, R. Direct observation of tunnelling reactions by matrix isolation spectroscopy. In Tunnelling in Molecules: Nuclear Quantum Effects from Bio to Physical Chemistry; Kästner, J., Kozuch, S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2021; pp. 1–60. [Google Scholar] [CrossRef]
- Pettersson, M.; Lundell, J.; Khriachtchev, L.; Räsänen, M. IR spectrum of the other rotamer of formic acid, cis-HCOOH. J. Am. Chem. Soc. 1997, 119, 11715–11716. [Google Scholar] [CrossRef]
- Maçôas, E.M.S.; Khriachtchev, L.; Pettersson, M.; Fausto, R.; Räsänen, M. Rotational isomerism in acetic acid: The first experimental observation of the high-energy conformer. J. Am. Chem. Soc. 2003, 125, 16188–16189. [Google Scholar] [CrossRef]
- Lapinski, L.; Reva, I.; Rostkowska, H.; Halasa, A.; Fausto, R.; Nowak, M.J. Conformational Transformation in Squaric Acid Induced by Near-IR Laser Light. J. Phys. Chem. A 2013, 117, 5251–5259. [Google Scholar] [CrossRef]
- Kuş, N.; Fausto, R. Effects of the matrix and intramolecular interactions on the stability of the higher-energy conformers of 2-fluorobenzoic acid. J. Chem. Phys. 2017, 146, 124305. [Google Scholar] [CrossRef]
- Gerbig, D.; Schreiner, P.R. Hydrogen-Tunneling in Biologically Relevant Small Molecules: The Rotamerizations of α-Ketocarboxylic Acids. J. Phys. Chem. B 2015, 119, 693–703. [Google Scholar] [CrossRef]
- Bazsó, G.; Magyarfalvi, G.; Tarczay, G. Tunneling lifetime of the ttc/VIp conformer of glycine in low-temperature matrices. J. Phys. Chem. A 2012, 116, 10539–10547. [Google Scholar] [CrossRef] [PubMed]
- Lopes Jesus, A.J.; Reva, I.; Araujo-Andrade, C.; Fausto, R. Conformational changes in matrix-isolated 6-methoxyindole: Effects of the thermal and infrared light excitations. J. Chem. Phys. 2016, 144, 124306. [Google Scholar] [CrossRef]
- Marzec, K.M.; Reva, I.; Fausto, R.; Proniewicz, L.M. Comparative matrix isolation infrared spectroscopy study of 1,3- and 1,4-diene monoterpenes (α-Phellandrene and γ-Terpinene). J. Phys. Chem. A 2011, 115, 4342–4353. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, B.M.; Reva, I.; Lapinski, L.; Fausto, R. Infrared spectra and ultraviolet-tunable laser induced photochemistry of matrix-isolated phenol and phenol-d5. J. Chem. Phys. 2012, 136, 024505. [Google Scholar] [CrossRef]
- Giuliano, B.M.; Melandri, S.; Reva, I.; Fausto, R. Conformational space and photochemistry of tyramine isolated in argon and xenon cryomatrixes. J. Phys. Chem. A 2013, 117, 10248–10259. [Google Scholar] [CrossRef]
- Barnes, A.J. Matrix isolation vibrational spectroscopy as a tool for studying conformational isomerism. J. Mol. Struct. 1984, 113, 161–174. [Google Scholar] [CrossRef]
- Breda, S.; Lapinski, L.; Reva, I.; Fausto, R. 4,6-Dimethyl-α-pyrone: A matrix isolation study of the photochemical generation of conjugated ketene, Dewar valence isomer and 1,3-dimethyl-cyclobutadiene. J. Photochem. Photobiol. A 2004, 162, 139–151. [Google Scholar] [CrossRef]
- Breda, S.; Reva, I.; Lapinski, L.; Fausto, R. Matrix isolation FTIR and theoretical study of α-pyrone photochemistry. Phys. Chem. Chem. Phys. 2004, 6, 929–937. [Google Scholar] [CrossRef]
- Tidwell, T.T. Spectroscopy and physical properties of ketenes. In Ketenes II; John Wiley and Sons: Hoboken, NJ, USA, 2006; pp. 27–53. [Google Scholar]
- Kuş, N.; Sagdinc, S.; Fausto, R. Infrared spectrum and UV-induced photochemistry of matrix-isolated 5-Hydroxyquinoline. J. Phys. Chem. A 2015, 119, 6296–6308. [Google Scholar] [CrossRef] [PubMed]
- Krupa, J.; Olbert-Majkut, A.; Reva, I.; Fausto, R.; Wierzejewska, M. Ultraviolet-tunable laser induced phototransformations of matrix isolated isoeugenol and eugenol. J. Phys. Chem. B 2012, 116, 11148–11158. [Google Scholar] [CrossRef]
- Samanta, A.K.; Pandey, P.; Bandyopadhyay, B.; Chakraborty, T. Keto–enol tautomers of 1,2-cyclohexanedione in solid, liquid, vapour and a cold inert gas matrix: Infrared spectroscopy and quantum chemistry calculation. J. Mol. Struct. 2010, 963, 234–239. [Google Scholar] [CrossRef]
- Miyazaki, J.; Toh, S.Y.; Moore, B.; Djuricanin, P.; Momose, T. UV photochemistry of 1,3-cyclohexadiene isolated in solid parahydrogen. J. Mol. Struct. 2021, 1224, 128986. [Google Scholar] [CrossRef]
- Chapman, O.L.; McIntosh, C.L.; Pacansky, J. Photochemistry of a-Pyrone in Argon at 8 °K. J. Am. Chem. Soc. 1973, 95, 244–246. [Google Scholar] [CrossRef]
- Pong, R.G.S.; Shirk, J.S. Photochemistry of α-Pyrone in solid argon. J. Am. Chem. Soc. 1973, 95, 248–249. [Google Scholar] [CrossRef]
- Breda, S.; Lapinski, L.; Fausto, R.; Nowak, M.J. Photoisomerization reactions of 4-methoxy- and 4-hydroxy-6-methyl-α-pyrones: An experimental matrix isolation and theoretical density functional theory study. Phys. Chem. Chem. Phys. 2003, 5, 4527–4532. [Google Scholar] [CrossRef]
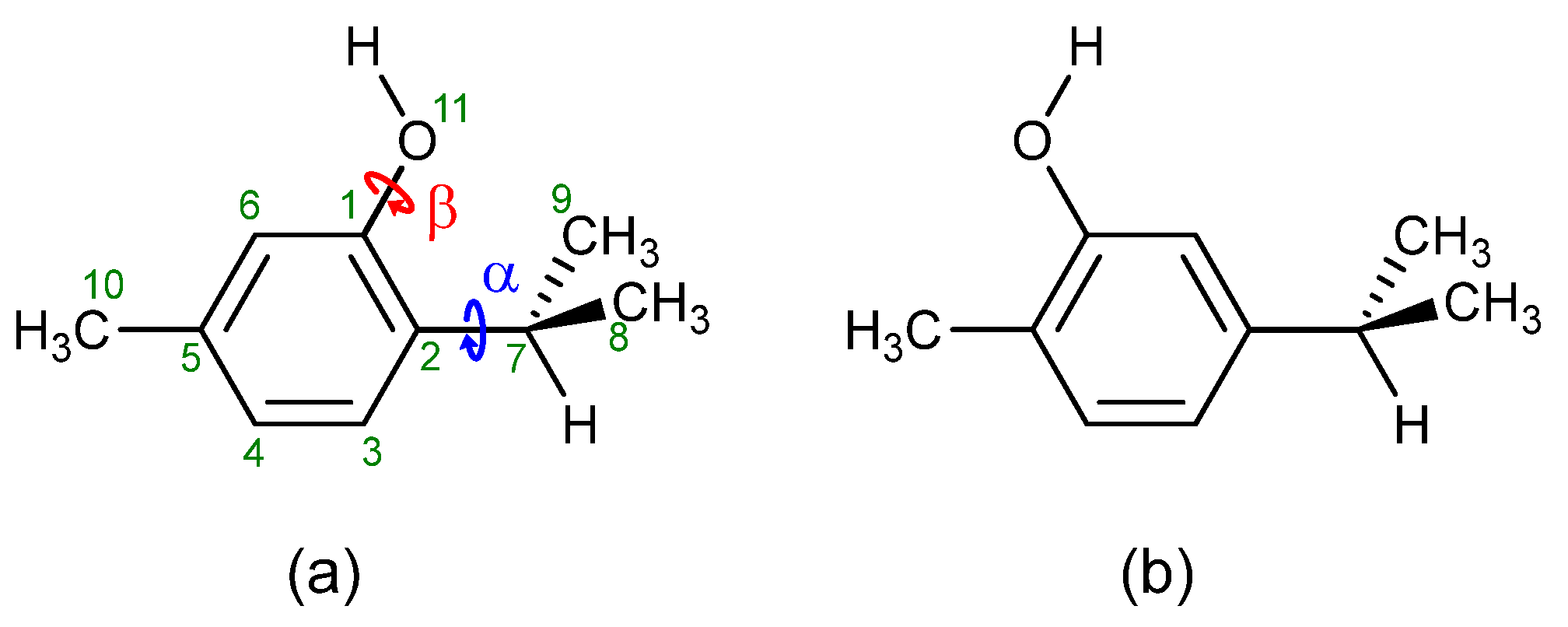
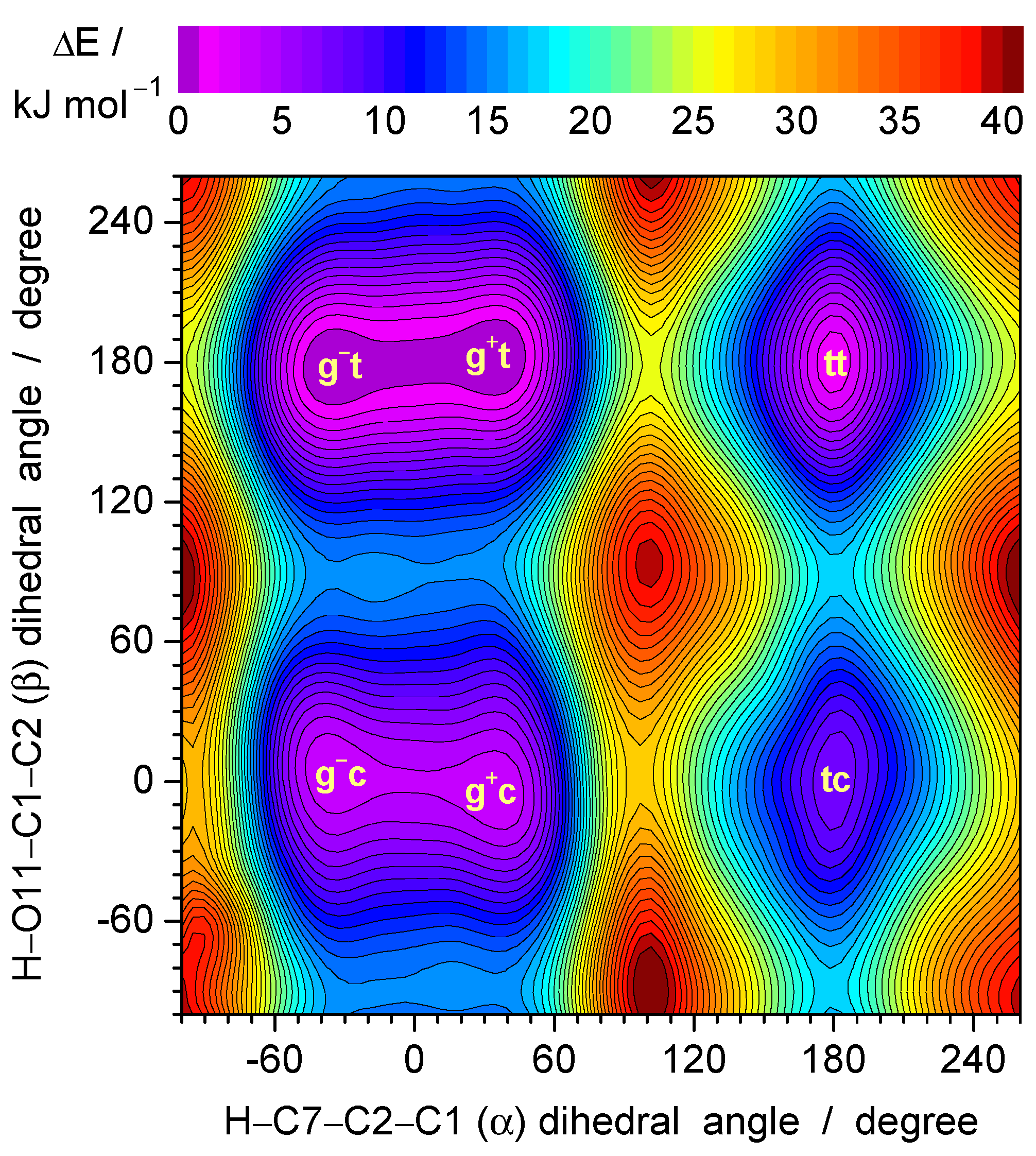
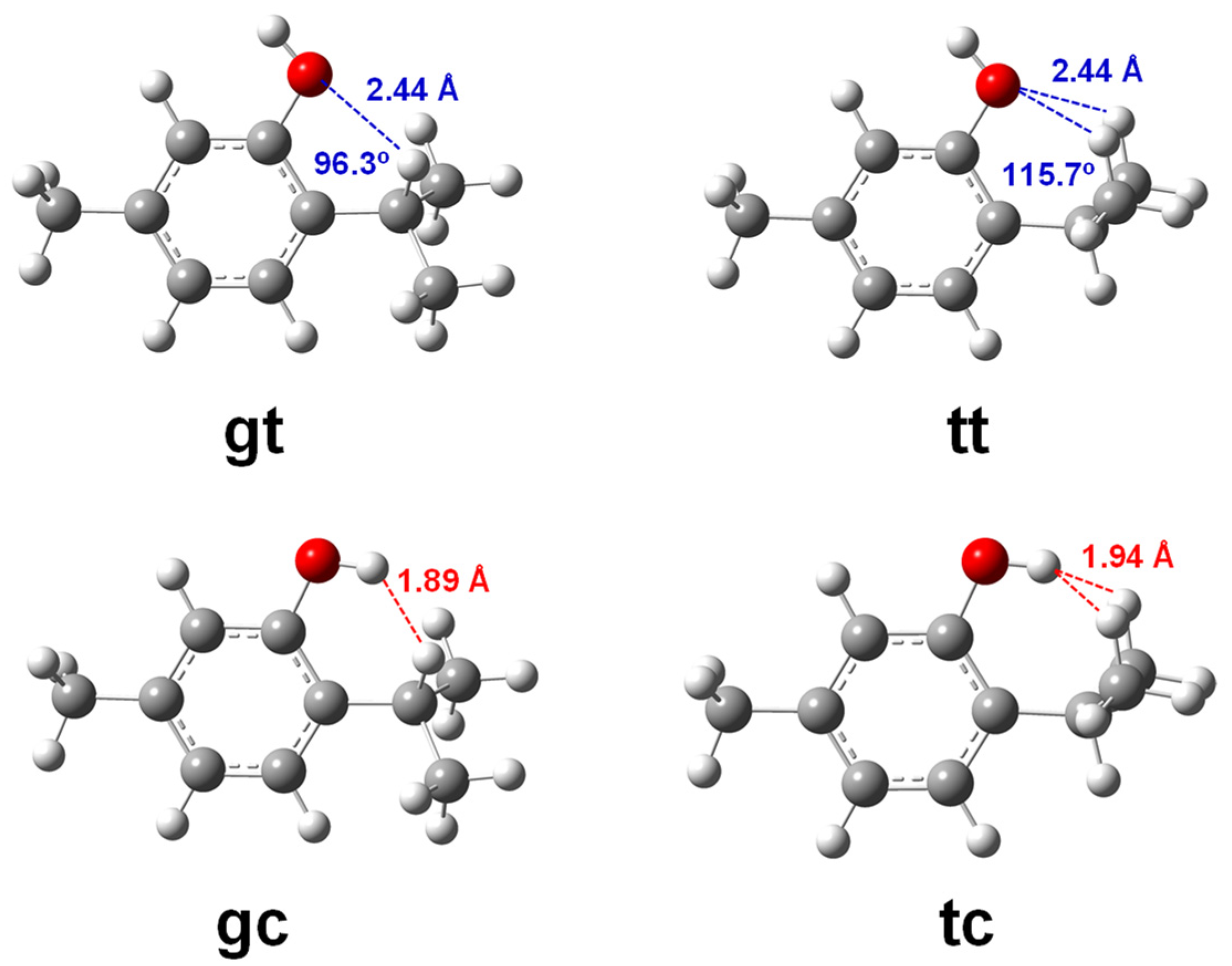
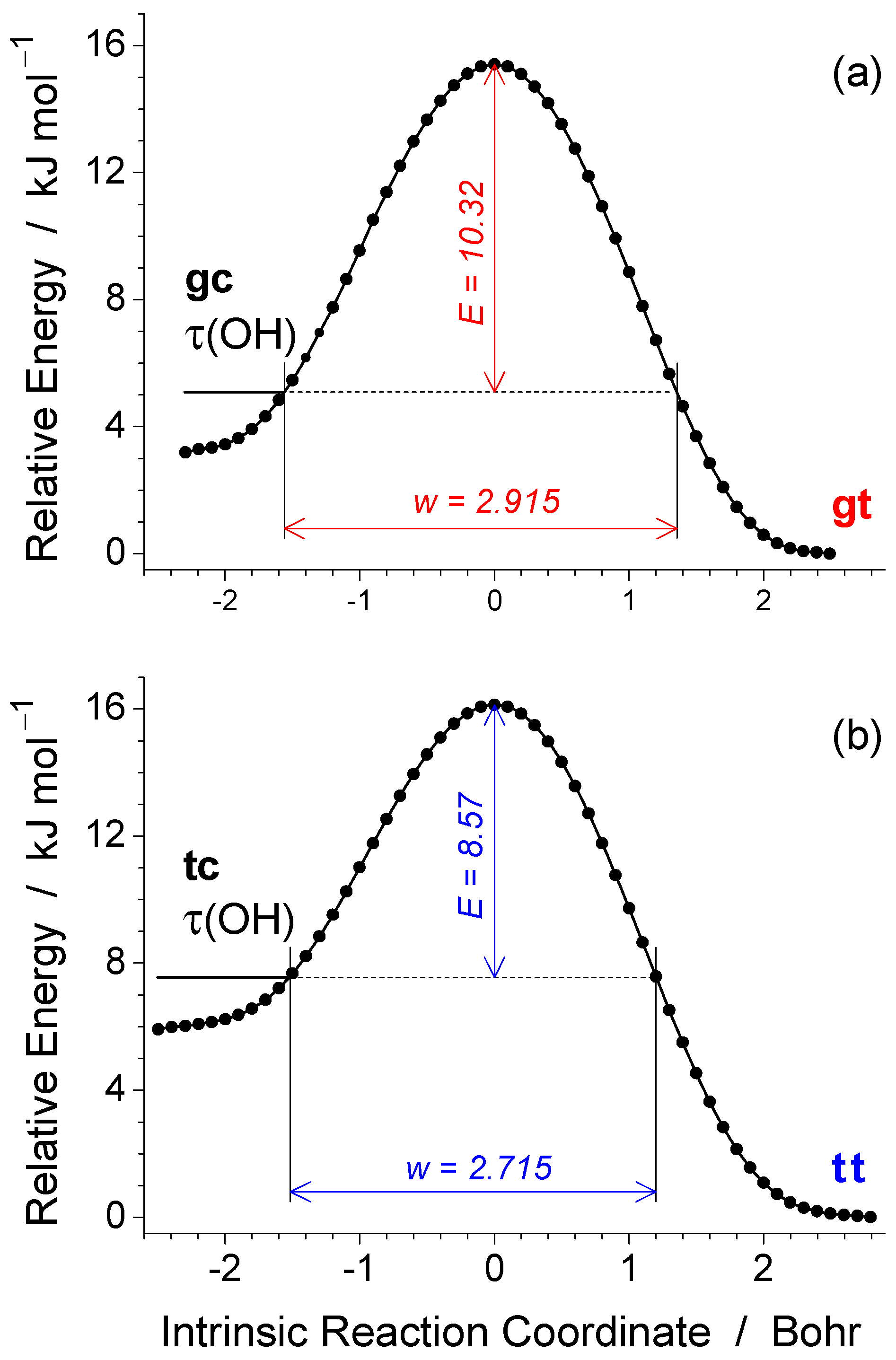
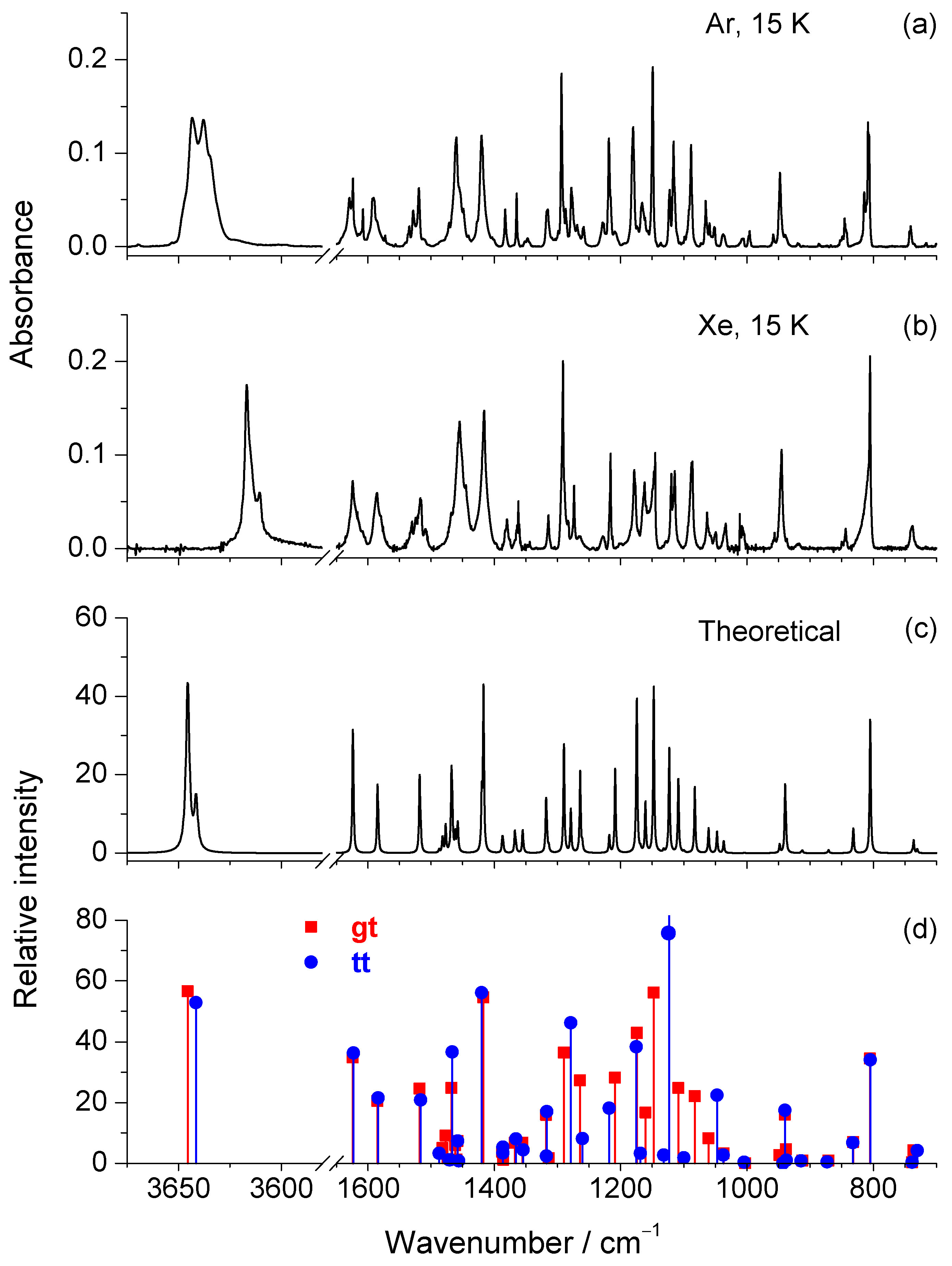
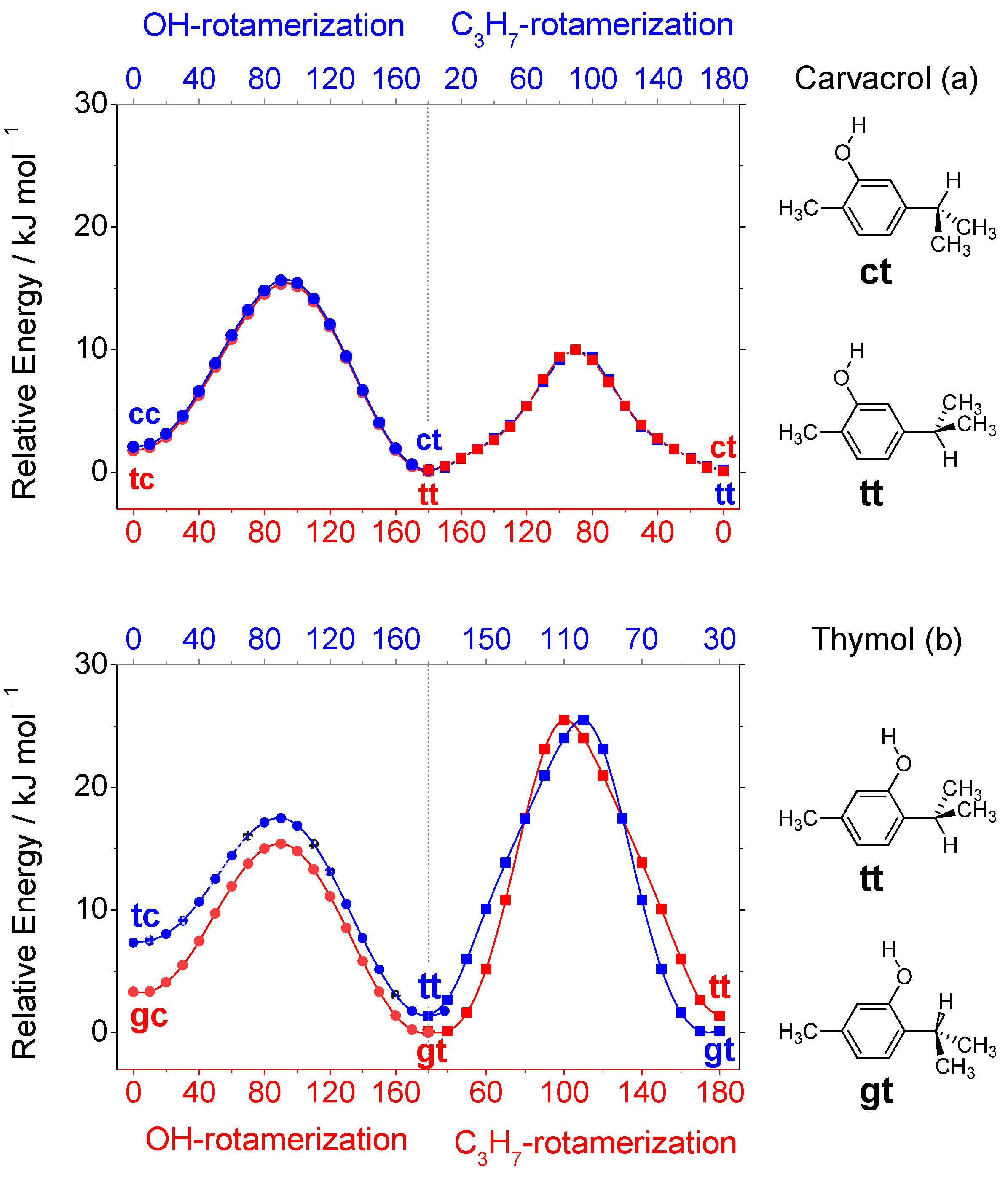

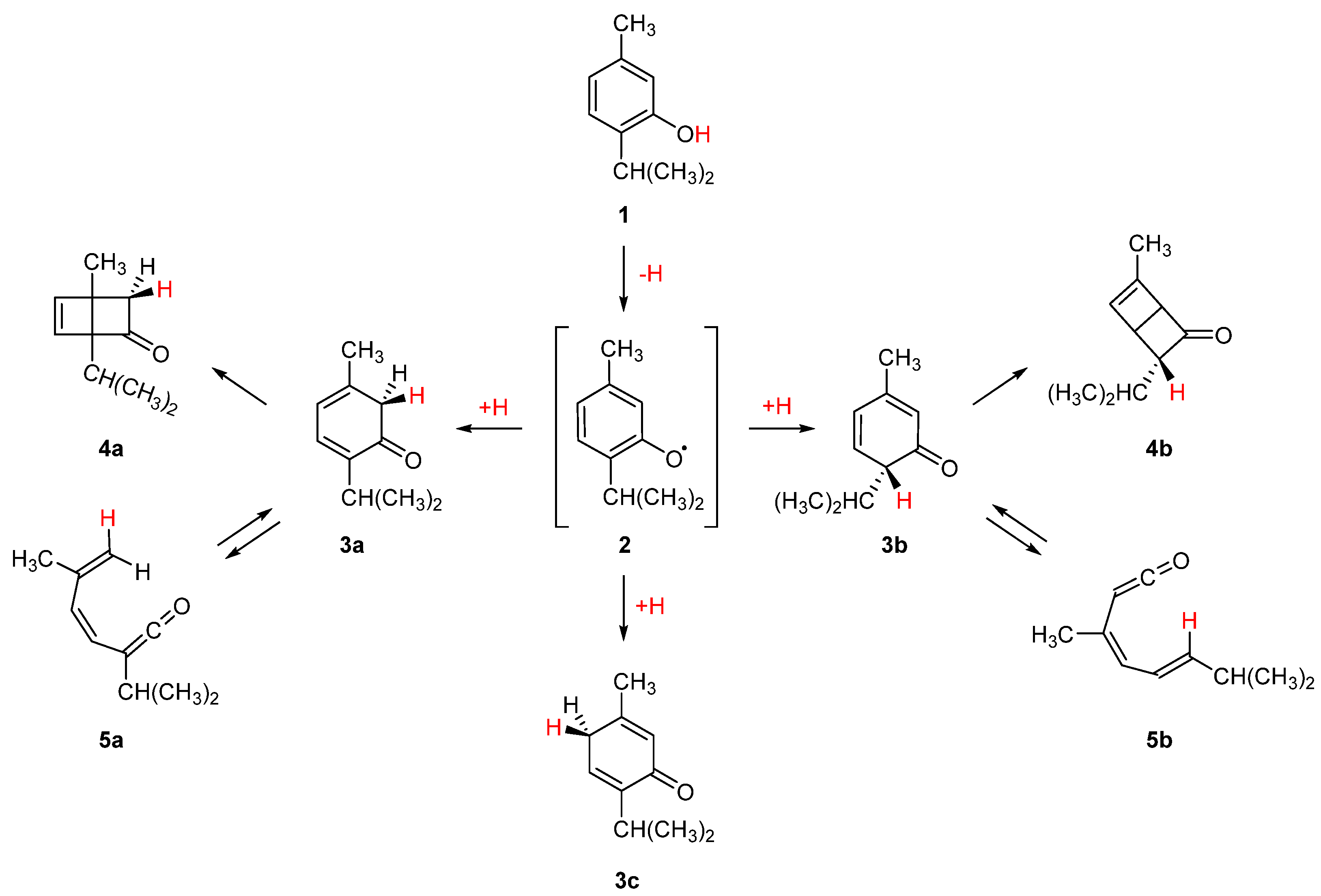
| Level of Theory | Conformer | |||
|---|---|---|---|---|
| gt | tt | gc | tc | |
| B3LYP/6-311++G(d,p) | ||||
| ΔEel | 0.00 | 1.37 | 3.24 | 7.34 |
| ΔE0 | 0.00 | 1.11 | 3.54 | 6.07 |
| ΔG (298 K) | 0.00 | 1.27 | 4.96 | 5.18 |
| Pop. (%) | 66.9 | 20.0 | 9.0 | 4.1 |
| (75.9) | (24.1) | |||
| MP2/aug-cc-pVDZ | ||||
| ΔEel | 0.00 | 2.51 | 2.54 | 7.94 |
| ΔE0 | 0.00 | 1.94 | 2.43 | 7.01 |
| ΔG (298 K) | 0.00 | 1.52 | 2.12 | 5.43 |
| Pop. (%) | 57.0 | 15.5 | 24.3 | 3.2 |
| (81.3) | (18.7) | |||
| QCISD/aug-cc-pVDZ b | ||||
| ΔEel | 0.00 | 2.29 | 2.56 | 7.30 |
| Ar, 15 K a | Xe, 15 K a | Calc. gt b | Calc. tt b | Assignment c | ||||
|---|---|---|---|---|---|---|---|---|
| ῦ | Int. | ῦ | Int. | ῦ | Ath | ῦ | Ath | |
| 3643/3638/ 3635 (sh) | vs | 3617/3611 | vs | 3645.5 | 56.6 | 3641.5 | 52.8 | ν(OH) |
| 1629 | m | 1624 | m | 1623.9 | 34.9 | 1622.9 | 36.3 | ν(CC)ring |
| 1591 | m | 1586 | m | 1584.8 | 20.6 | 1583.5 | 21.5 | ν(CC)ring |
| 1519 (split) | m | 1517 (split) | m | 1518.2 | 24.7 | 1516.5 | 20.5 | ν(CC)ring; δ(CH)ring |
| 1481.9 | 5.1 | 1487.3 | 3.3 | δ(CH3)iso,as’ (+) | ||||
| 1476.9 | 9.2 | 1471.4 | 1.1 | δ(CH3)iso,as’’ (+) | ||||
| 1460 (split) | s | 1455 (split) | s | 1467.7 | 24.8 | 1466.3 | 36.7 | δ(CH3)as’ |
| 1461.7 | 6.0 | 1456.6 | 0.8 | δ(CH3)iso,as’’ (−) | ||||
| 1459.3 | 1.3 | 1469.3 | 1.4 | δ(CH3)iso,as’ (−) | ||||
| 1457.6 | 7.3 | 1458.0 | 7.3 | δ(CH3)as’’ | ||||
| 1420 | s | 1416 | s | 1417.0 | 54.5 | 1420.0 | 56.6 | δ(CH)ring; ν(CC)ring; ν(CO) |
| 1383 | w | 1380 | w | 1387.4 | 3.8 | 1386.3 | 3.4 | δ(CH3)iso,s (+) |
| 1385.5 | 1.1 | 1386.5 | 5.3 | δ(CH3)s | ||||
| 1365 | w | 1362 | w | 1367.4 | 6.8 | 1365.8 | 8.0 | δ(CH3)iso,s (−) |
| 1347 | vw | 1344 | vw | 1355.1 | 6.8 | 1355.1 | 4.6 | δ(C7H) |
| 1315 | w | 1314 | w | 1318.0 | 16.0 | 1316.6 | 17.1 | ν(CC)ring; δ(OH) |
| 1313.7 | 1.7 | 1317.6 | 2.3 | γ(C7H) | ||||
| 1294/1287 | vs | 1291/1284 | vs | 1289.8 | 36.5 | – | – | ν(CC)ring; δ(CH)ring |
| 1278 | m | 1274 | m | – | – | 1278.9 | 46.2 | ν(CC)ring; δ(CH)ring |
| 1269/1259 | vw | 1265 | vw | 1264.0 | 27.4 | 1260.5 | 8.1 | ν(CO) + ν(C5C10); ν(CC)ring |
| 1228 | vw | 1228 | vw | – | – | 1218.4 | 18.2 | ν(C2C7) |
| 1219 | s | 1216 | s | 1208.7 | 28.2 | – | – | ν(C2C7) |
| 1180 | s | 1179 | m | 1174.4 | 42.8 | 1175.0 | 38.3 | δ(OH); δ(C–H)ring |
| – | – | 1168.7 | 3.2 | ν(CO) − ν(C5C10); δ(CH)ring | ||||
| 1166 | w | 1162 | m | 1160.8 | 16.7 | – | – | ν(CO) − ν(C5C10); δ(CH)ring |
| 1149 | vs | 1147/1145 | s | 1147.7 | 56.1 | – | – | δ(OH); δ(CH)ring; ν(CC)ring |
| 1123 | m | 1120 | m | – | – | 1123.1 | 111.8 | δ(ring); δ(OH); ν(CO) |
| 1116 | m | 1115 | m | 1108.7 | 24.8 | – | – | ν(C7C8) − ν(C7C9) |
| 1089 | m | 1087 | m | 1082.6 | 22.1 | – | – | δ(ring) |
| 1065/1059 | w | 1063 | w | 1060.7 | 8.3 | – | – | ρ(CH3)iso (+) |
| 1051 | vw | 1049 | vw | – | – | 1047.4 | 22.5 | ρ(CH3)iso (+) |
| 1037 | vw | 1034 | vw | 1036.7 | 3.2 | 1037.2 | 2.7 | ρ(CH3)’ |
| 1007/996 | vw | 1008 | vw | – | – | – | – | – |
| 959 | vw | 956 | vw | 948.3 | 2.7 | – | – | ρ(CH3)iso (−) |
| 948/940 | m | 945/937 | m | 939.6 | 16.0 | 939.7 | 17.5 | ν(CO) − ν(C5C10); δ(ring) |
| 918 | vw | 918 | vw | 912.4 | 0.9 | 914.2 | 0.8 | ρ(CH3)iso (−); γ(C7H) |
| 886 | vw | n.o. | 870.8 | 1.0 | 872.8 | 0.5 | ν(C7C8) + ν(C7C9) | |
| 849/846 | vw | 850/844 | vw | 831.6 | 7.0 | 832.4 | 6.8 | γ(C6H) |
| 814/809/807 | s | 805 | vs | 805.0 | 34.5 | 805.1 | 33.8 | γ(C3H) + γ(C4H) |
| 741 | vw | 738 | vw | 736.3 | 4.3 | 730.5 | 4.1 | δ(ring) |
| 700 | vw | n.o. | 681.3 | 1.3 | 684.0 | 4.9 | ν(C2C7) − ν(C5C10); δ(ring) | |
| 594 | w | 593 | w | 594.0 | 7.8 | 594.6 | 8.5 | γ(C5) |
| 577 | w | 577 | w | 571.5 | 12.9 | 576.9 | 6.4 | δ(ring) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes Jesus, A.J.; Nunes, C.M.; Reva, I. Conformational Structure, Infrared Spectra and Light-Induced Transformations of Thymol Isolated in Noble Gas Cryomatrices. Photochem 2022, 2, 405-422. https://doi.org/10.3390/photochem2020028
Lopes Jesus AJ, Nunes CM, Reva I. Conformational Structure, Infrared Spectra and Light-Induced Transformations of Thymol Isolated in Noble Gas Cryomatrices. Photochem. 2022; 2(2):405-422. https://doi.org/10.3390/photochem2020028
Chicago/Turabian StyleLopes Jesus, Antόnio Jorge, Cláudio M. Nunes, and Igor Reva. 2022. "Conformational Structure, Infrared Spectra and Light-Induced Transformations of Thymol Isolated in Noble Gas Cryomatrices" Photochem 2, no. 2: 405-422. https://doi.org/10.3390/photochem2020028
APA StyleLopes Jesus, A. J., Nunes, C. M., & Reva, I. (2022). Conformational Structure, Infrared Spectra and Light-Induced Transformations of Thymol Isolated in Noble Gas Cryomatrices. Photochem, 2(2), 405-422. https://doi.org/10.3390/photochem2020028







