Fluorescence and Phosphorescence of Flavylium Cation Analogues of Anthocyanins
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cruz, L.; Basílio, N.; Mateus, N.; De Freitas, V.; Pina, F. Natural and Synthetic Flavylium-Based Dyes: The Chemistry behind the Color. Chem. Rev. 2022, 122, 1416–1481. [Google Scholar] [CrossRef] [PubMed]
- Quina, F.H.; Bastos, E.L. Chemistry Inspired by the Colors of Fruits, Flowers and Wine. An. Acad. Bras. Cienc. 2018, 90, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Pina, F.; Alejo-armijo, A.; Clemente, A.; Mendoza, J.; Seco, A.; Basílio, N.; Parola, A.J. Evolution of Flavylium-based Color Systems in Plants: What Physical Chemistry Can Tell Us. Int. J. Mol. Sci. 2021, 22, 3833. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Laia, C.A.T.; Parola, A.J.; Lima, J.C. Chemistry and Applications of Flavylium Compounds: A Handful of Colours. Chem. Soc. Rev. 2012, 41, 869–908. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.F.; Giestas, L.; Yihwa, C.; Vautier-Giongo, C.; Quina, F.H.; Maçanita, A.L.; Lima, J.C. Ground- and Excited-State Proton Transfer in Anthocyanins: From Weak Acids to Superphotoacids. J. Phys. Chem. A 2003, 107, 4203–4210. [Google Scholar] [CrossRef]
- Silva, V.O.; Freitas, A.A.; Maçanita, A.L.; Quina, F.H. Chemistry and Photochemistry of Natural Plant Pigments: The Anthocyanins. J. Phys. Org. Chem. 2016, 29, 594–599. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [Green Version]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Oancea, S. A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Silva, G.T.M.; Quina, F.H.; Aquino, A.J.A. Quantum Chemical Investigation of the Intramolecular Copigmentation Complex of an Acylated Anthocyanin. J. Braz. Chem. Soc. 2019, 30, 492–498. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue Flower Color Development by Anthocyanins: From Chemical Structure to Cell Physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.T.M.; Silva, K.M.; Silva, C.P.; Gonçalves, J.M.; Quina, F.H. Hybrid Pigments from Anthocyanin Analogues and Synthetic Clay Minerals. ACS Omega 2020, 5, 26592–26600. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mu, B.; Wang, X.; Kang, Y.; Wang, A. A Comparative Study on Color Stability of Anthocyanin Hybrid Pigments Derived from 1D and 2D Clay Minerals. Materials 2019, 12, 3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Held, B.; Tang, H.; Natarajan, P.; Da Silva, C.P.; De Oliveira Silva, V.; Bohne, C.; Quina, F.H. Cucurbit[7]Uril Inclusion Complexation as a Supramolecular Strategy for Color Stabilization of Anthocyanin Model Compounds. Photochem. Photobiol. Sci. 2016, 15, 752–757. [Google Scholar] [CrossRef] [Green Version]
- Basílio, N.; Pina, F. Chemistry and Photochemistry of Anthocyanins and Related Compounds: A Thermodynamic and Kinetic Approach. Molecules 2016, 21, 1502. [Google Scholar] [CrossRef]
- Pradhan, P.C.; Mandal, A.; Dutta, A.; Sarkar, R.; Kundu, A.; Saha, S. Delineating the Behavior of Berberis Anthocyanin/β-Cyclodextrin Inclusion Complex in Vitro: A Molecular Dynamics Approach. LWT 2022, 157, 113090. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Ramos, M.; Viqueira, V.; Luzi, F.; Dominici, F.; Terenzi, A.; Maron, E.; Hamzaoui, M.; Kohnen, S.; Torre, L.; et al. Anthocyanin Hybrid Nanopigments from Pomegranate Waste: Colour, Thermomechanical Stability and Environmental Impact of Polyester-Based Bionanocomposites. Polymers 2021, 13, 1966. [Google Scholar] [CrossRef]
- Quina, F.H.; Moreira, P.F.; Vautier-Giongo, C.; Rettori, D.; Rodrigues, R.F.; Freitas, A.A.; Silva, P.F.; Maçanita, A.L. Photochemistry of Anthocyanins and Their Biological Role in Plant Tissues. Pure Appl. Chem. 2009, 81, 1687–1694. [Google Scholar] [CrossRef]
- Freitas, A.A.; Quina, F.H.; Fernandes, A.C.; Maçanita, A.A.L. Picosecond Dynamics of the Prototropic Reactions of 7-Hydroxyflavylium Photoacids Anchored at an Anionic Micellar Surface. J. Phys. Chem. A 2010, 114, 4188–4196. [Google Scholar] [CrossRef]
- Ferreira da Silva, P.; Lima, J.C.; Freitas, A.A.; Shimizu, K.; Maçanita, A.L.; Quina, F.H. Charge-Transfer Complexation as a General Phenomenon in the Copigmentation of Anthocyanins. J. Phys. Chem. A 2005, 109, 7329–7338. [Google Scholar] [CrossRef]
- Rodrigues, R.F.; Da Silva, P.F.; Shimizu, K.; Freitas, A.A.; Kovalenko, S.A.; Ernsting, N.P.; Quina, F.H.; Maçanita, A. Ultrafast Internal Conversion in a Model Anthocyanin-Polyphenol Complex: Implications for the Biological Role of Anthocyanins in Vegetative Tissues of Plants. Chem. Eur. J. 2009, 15, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Massari, S.; Desantis, J.; Nannetti, G.; Sabatini, S.; Tortorella, S.; Goracci, L.; Cecchetti, V.; Loregian, A.; Tabarrini, O. Efficient and Regioselective One-Step Synthesis of 7-Aryl-5-Methyl- and 5-Aryl-7-Methyl-2-Amino-[1,2,4]Triazolo[1,5-a] Pyrimidine Derivatives. Org. Biomol. Chem. 2017, 15, 7944–7955. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Zhao, H.; Gong, L.; Jiang, H.; Zhang, M. Direct Access to Functionalized Indoles via Single Electron Oxidation Induced Coupling of Diarylamines with 1,3-Dicarbonyl Compounds. Org. Lett. 2019, 21, 6736–6740. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Saito, K.; Suto, S.; Aihara, H.; Sugawara, A.; Tamura, S.; Kawano, T. Synthesis of 5-Aryl-3(2 H)-Furanones Using Intramolecular Cyclization of Sulfonium Salts. J. Org. Chem. 2018, 83, 13834–13846. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, S.; Isorez-Mahler, G.; Kueny-Stotz, M.; Brouillard, R. Aged Red Wine Pigments as a Source of Inspiration for Organic Synthesis-The Cases of the Color-Stable Pyranoflavylium and Flavylium-(4→8)-Flavan Chromophores. Tetrahedron 2015, 71, 3066–3078. [Google Scholar] [CrossRef]
- Chen, J.-R.; Wong, J.-B.; Kuo, P.-Y.; Yang, D.-Y. Synthesis and Characterization of Coumarin-Based Spiropyran Photochromic Colorants. Org. Lett. 2008, 10, 4823–4826. [Google Scholar] [CrossRef]
- Werbovetz, K.; George, T.; Richard, J.; Barszcz, T.; Capers, J. Flavylium Salts as Precursors of Biologically Active Chalcones. Lett. Drug Des. Discov. 2008, 5, 69–73. [Google Scholar] [CrossRef]
- Jurd, L. Anthocyanins and Related Compounds. IV. The Synthesis of Coumestrol and Related Coumarinobenzofurans from Flavylium Salts. J. Org. Chem. 1964, 29, 3036–3038. [Google Scholar] [CrossRef]
- Kueny-stotz, M.; Chassaing, S.; Brouillard, R.; Nielsen, M.; Goeldner, M. Flavylium Salts as in Vitro Precursors of Potent Ligands to Brain GABA-A Receptors. Bioorg. Med. Chem. Lett. 2008, 18, 4864–4867. [Google Scholar] [CrossRef]
- Jurd, L. Anthocyanins and Related Compounds. I. Structural Transformations of Flavylium Salts in Acidic Solutions. J. Org. Chem. 1963, 28, 987–991. [Google Scholar] [CrossRef]
- Jurd, L.; Geissman, T.A. Anthocyanins and Related Compounds. II. Structural Transformations of Some Anhydro Bases. J. Org. Chem. 1963, 28, 2394–2397. [Google Scholar] [CrossRef]
- Da Silva, C.P.; Pioli, R.M.; Liu, L.; Zheng, S.; Zhang, M.; Silva, G.T.D.M.; Carneiro, V.M.T.; Quina, F.H. Improved Synthesis of Analogues of Red Wine Pyranoanthocyanin Pigments. ACS Omega 2018, 3, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, R.; Iacobucci, G.A.; Sweeny, J.G. Chemistry of Anthocyanin Pigments. 9. UV-Visible Spectrophotometric Determination of the Acidity Constants of Apigeninidin and Three Related 3-Deoxyflavylium Salts. J. Am. Chem. Soc. 1982, 104, 7585–7590. [Google Scholar] [CrossRef]
- Gavrilyuk, I.M.; Ishchenko, A.A.; Kudinova, M.A.; Tolmachev, A.I. Pyrylocyanines. 22. Styrils Derived from Methoxy-Substituted 4-Methylflavylium Salts. Chem. Heterocycl. Compd. 1986, 22, 703–707. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Nad, S.; Pal, H. Unusual Photophysical Properties of Coumarin-151. J. Phys. Chem. A 2001, 105, 1097–1106. [Google Scholar] [CrossRef]
- Siddique, F.; Silva, C.P.; Silva, G.T.M.; Lischka, H.; Quina, F.H.; Aquino, A.J.A. The Electronic Transitions of Analogs of Red Wine Pyranoanthocyanin Pigments. Photochem. Photobiol. Sci. 2019, 18, 45–53. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic Structure Calculations on Workstation Computers: The Program System Turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Weigend, F.; Häser, M.; Patzelt, H.; Ahlrichs, R. RI-MP2: Optimized Auxiliary Basis Sets and Demonstration of Efficiency. Chem. Phys. Lett. 1998, 294, 143–152. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Hättig, C. Structure Optimizations for Excited States with Correlated Second-Order Methods: CC2 and ADC(2). Adv. Quantum Chem. 2005, 50, 37–60. [Google Scholar] [CrossRef]
- Hättig, C. Geometry Optimizations with the Coupled-Cluster Model CC2 Using the Resolution-of-the-Identity Approximation. J. Chem. Phys. 2003, 118, 7751–7761. [Google Scholar] [CrossRef]
- Martin, R.L. Natural Transition Orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Plasser, F.; Wormit, M.; Dreuw, A. New Tools for the Systematic Analysis and Visualization of Electronic Excitations. I. Formalism. J. Chem. Phys. 2014, 141, 024106. [Google Scholar] [CrossRef] [Green Version]
- Plasser, F.; Lischka, H. Analysis of Excitonic and Charge Transfer Interactions from Quantum Chemical Calculations. J. Chem. Theory Comput. 2012, 8, 2777–2789. [Google Scholar] [CrossRef]
- Klamt, A.; Jonas, V. Treatment of the Outlying Charge in Continuum Solvation Models. J. Chem. Phys. 1996, 105, 9972–9981. [Google Scholar] [CrossRef]
- Silva, C.P.; Silva, G.T.M.; Costa, T.D.S.; Carneiro, V.M.T.; Siddique, F.; Aquino, A.J.A.; Freitas, A.A.; Clark, J.A.; Espinoza, E.M.; Vullev, V.I.; et al. Chromophores Inspired by the Colors of Fruit, Flowers and Wine. Pure Appl. Chem. 2019, 92, 255–263. [Google Scholar] [CrossRef]
- Freitas, A.A.; Maçanita, A.A.L.; Quina, F.H. Improved Analysis of Excited State Proton Transfer Kinetics by the Combination of Standard and Convolution Methods. Photochem. Photobiol. Sci. 2013, 12, 902–910. [Google Scholar] [CrossRef]
- Silva, G.T.M.; Silva, C.P.; Gehlen, M.H.; Oake, J.; Bohne, C.; Quina, F.H. Organic/Inorganic Hybrid Pigments from Flavylium Cations and Palygorskite. Appl. Clay Sci. 2018, 162, 478–486. [Google Scholar] [CrossRef]
- Silva, G.T.M.; Da Silva, K.M.; Silva, C.P.; Rodrigues, A.C.B.; Oake, J.; Gehlen, M.H.; Bohne, C.; Quina, F.H. Highly Fluorescent Hybrid Pigments from Anthocyanin- and Red Wine Pyranoanthocyanin-Analogs Adsorbed on Sepiolite Clay. Photochem. Photobiol. Sci. 2019, 18, 1750–1760. [Google Scholar] [CrossRef]
- Penfold, T.J.; Gindensperger, E.; Daniel, C.; Marian, C.M. Spin-Vibronic Mechanism for Intersystem Crossing. Chem. Rev. 2018, 118, 6975–7025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marian, C.M. Understanding and Controlling Intersystem Crossing in Molecules. Annu. Rev. Phys. Chem. 2021, 72, 617–640. [Google Scholar] [CrossRef] [PubMed]
- Lower, S.K.; El-Sayed, M.A. The Triplet State and Molecular Electronic Processes in Organic Molecules. Chem. Rev. 1966, 66, 199–241. [Google Scholar] [CrossRef]
- Solovyov, K.N.; Borisevich, E.A. Intramolecular Heavy-Atom Effect in the Photophysics of Organic Molecules. Physics-Uspekhi 2005, 48, 231–253. [Google Scholar] [CrossRef]
- Baryshnikov, G.; Minaev, B.; Ågren, H. Theory and Calculation of the Phosphorescence Phenomenon. Chem. Rev. 2017, 117, 6500–6537. [Google Scholar] [CrossRef]
- Aqdas, A.; Siddique, F.; Nieman, R.; Quina, F.H.; Aquino, A.J.A. Photoacidity of the 7-Hydroxyflavylium Cation. Photochem. Photobiol. 2019, 95, 1339–1344. [Google Scholar] [CrossRef]
- Feng, R.; Yu, X.; Autschbach, J. Spin-Orbit Natural Transition Orbitals and Spin-Forbidden Transitions. J. Chem. Theory Comput. 2021, 17, 7531–7544. [Google Scholar] [CrossRef]
- Quina, F.H.; Silva, G.T.M. The photophysics of photosensitization: A brief overview. J. Photochem. Photobiol. 2021, 7, 100042. [Google Scholar] [CrossRef]
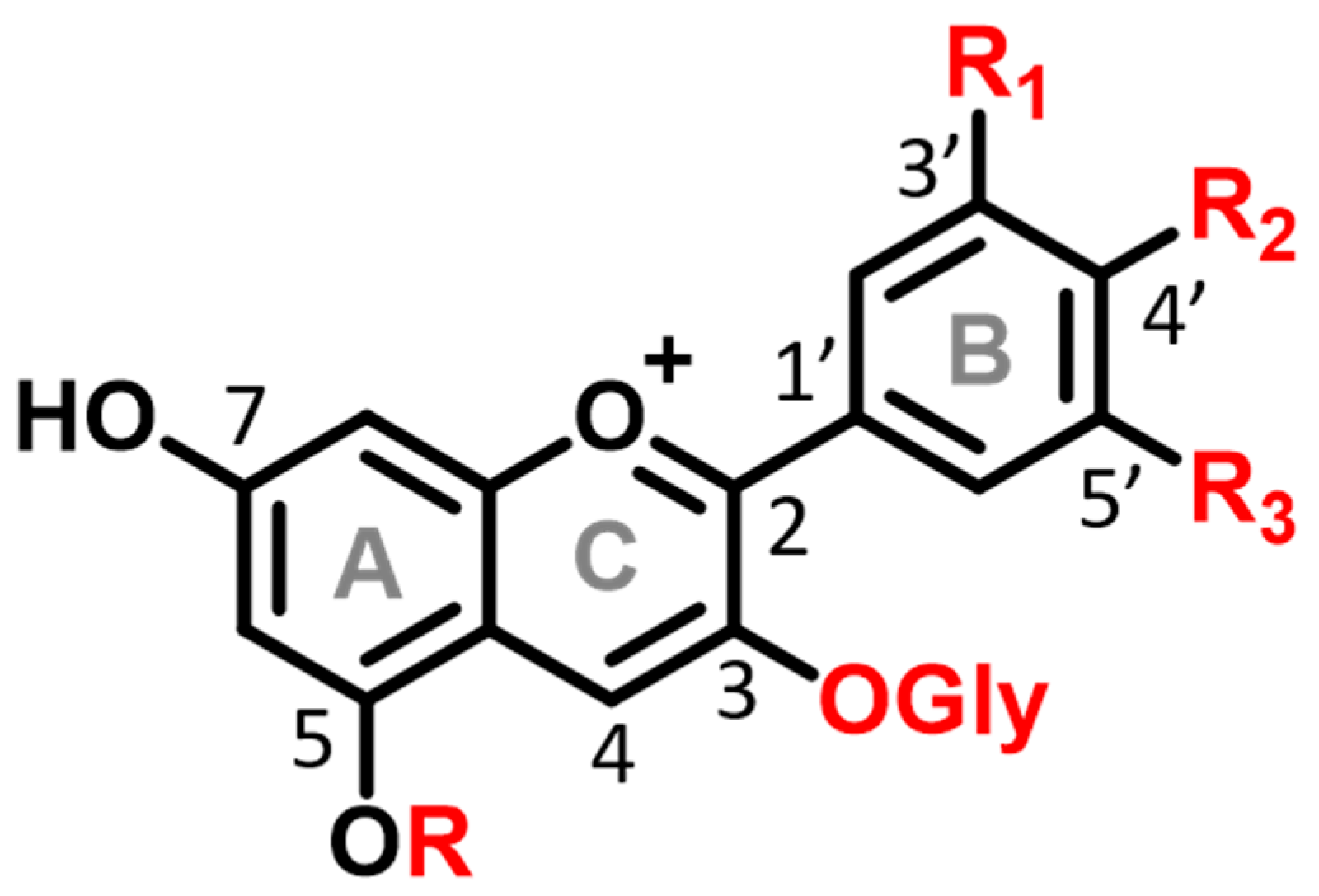
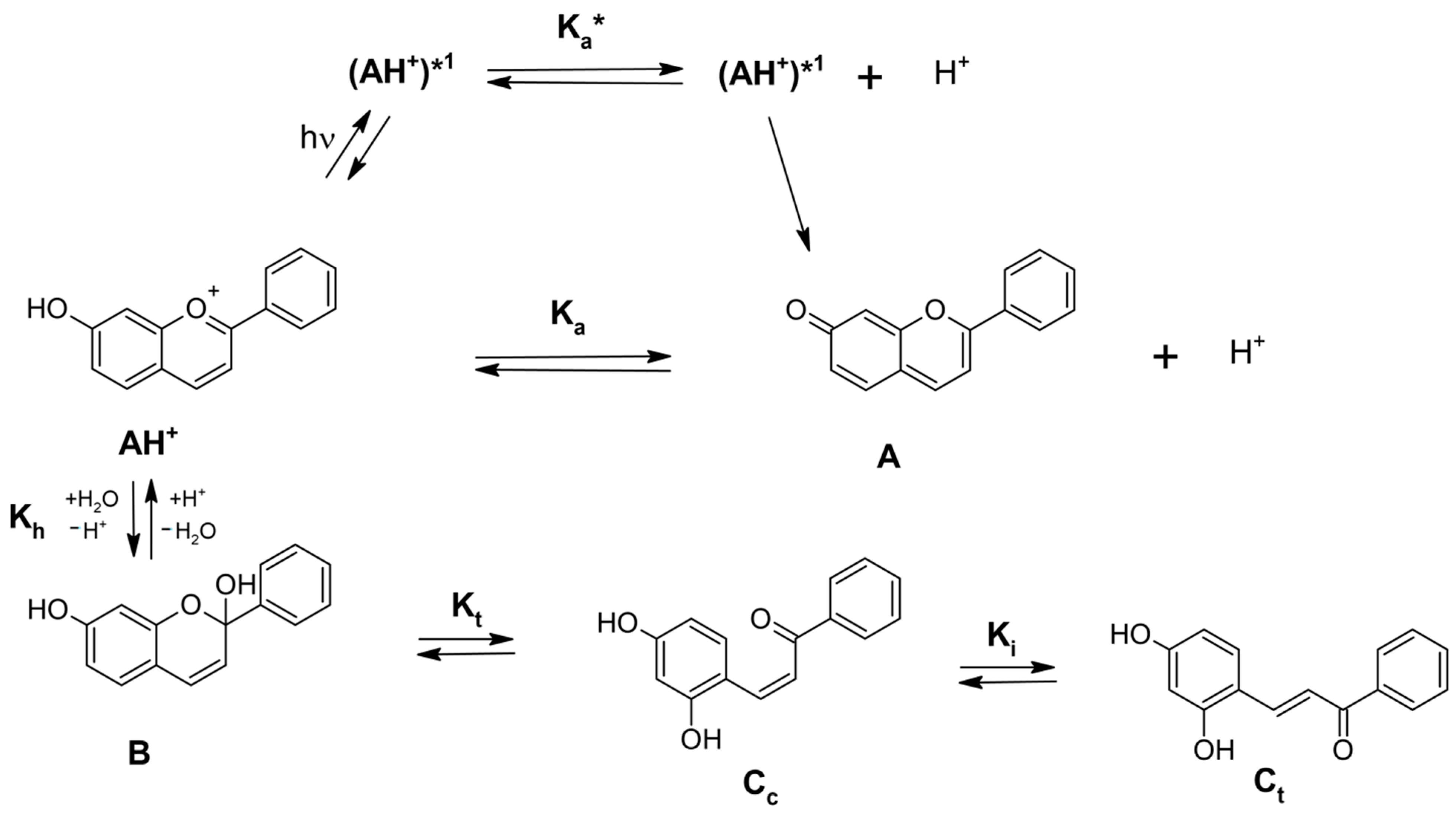

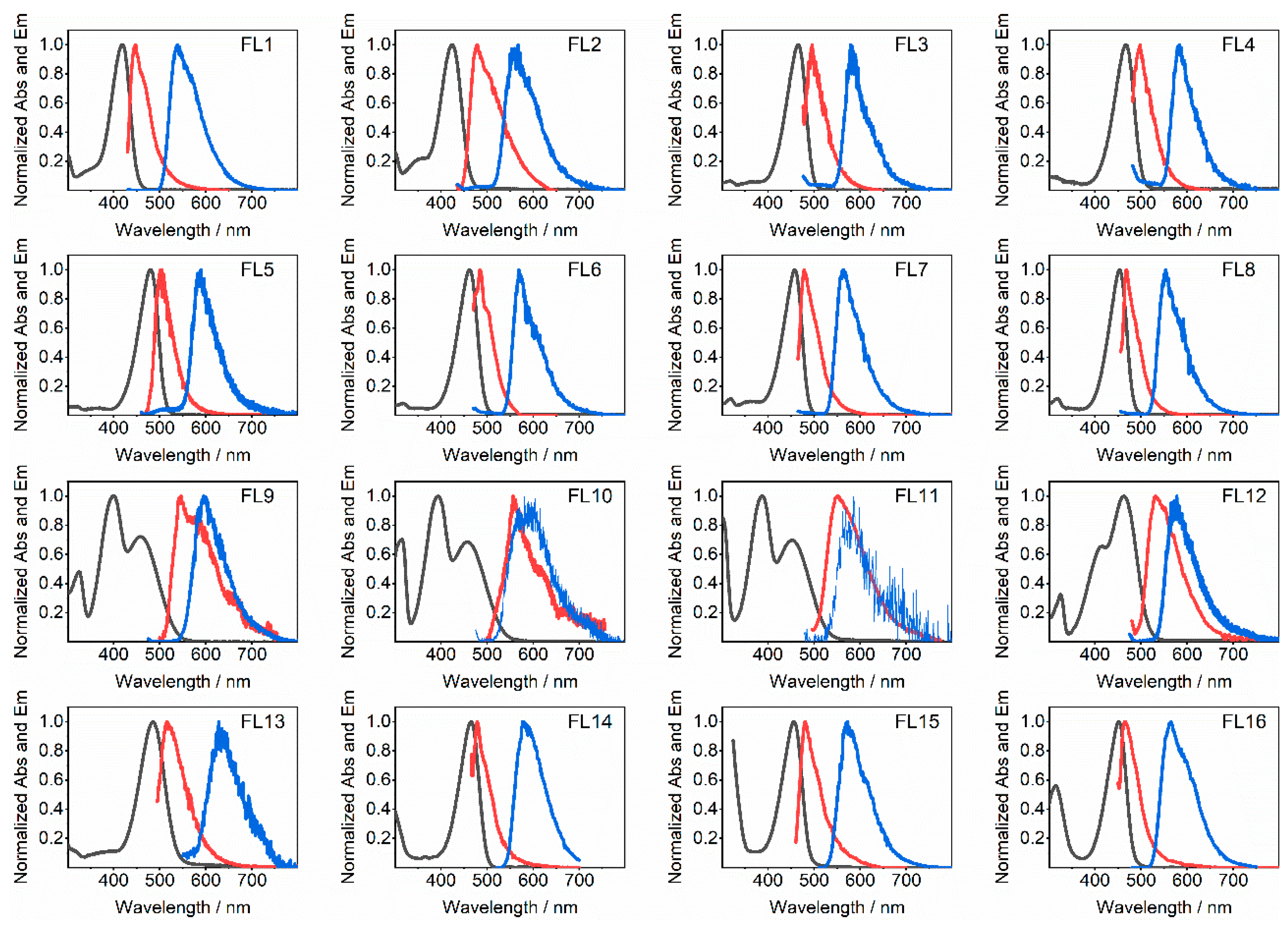

| Flavylium Cation | pKap | pKa | λmax | εmax |
|---|---|---|---|---|
| ±0.1 | ±0.1 | nm | L mol−1 cm−1 | |
| FL1 | - | - | 417 | 28,300 |
| FL2 | - | 4.4 | 418 | 33,900 |
| FL3 | 2.9 | 3.9 | 461 | 51,800 |
| FL4 | 3.7 | 5.1 | 460 | 47,300 |
| FL5 | 3.5 | 1st 4.2; 2nd 7.4 | 459 | 52,600 |
| FL6 | 3.4 | - | 461 | 44,800 |
| FL7 | - | 4.8 | 447 | 43,600 |
| FL8 | - | - | 446 | 51,000 |
| FL9 | - | 1st 4.9; 2nd 9.0 | 390 & 444 | 18,400 & 14,200 |
| FL10 | - | 1st 5.2; 2nd 8.7 | 385 & 444 | 19,400 & 13,900 |
| FL11 | - | 1st 4.2; 2nd 7.2 | 383 & 445 | 19,000 & 14,300 |
| FL12 | - | 1st 4.7; 2nd 7.4 | 452 | 31,900 |
| FL13 | 3.2 | - | 478 | 37,800 |
| FL14 | 3.2 | - | 460 | 21,400 |
| FL15 | - | 4.3 | 446 | 27,700 |
| FL16 | - | - | 446 | 39,000 |
| Dry CH3CN + 0.1 mol L−1 TFA, 20 °C | Isopropanol + 0.1 mol L−1 TFA, 77 K | |||||||
|---|---|---|---|---|---|---|---|---|
| Flavylium Cation | Φf | τf, ns | 10−7kf, s−1 | ES, eV a | ES, eV a | ET, eV b | ∆EST, eV | τT, s |
| FL1 | 0.53 | 5.4 | 9.8 | 2.83 | 2.91 | 2.31 | 0.60 | 0.67 |
| FL2 | 0.26 | 2.6 | 10 | 2.79 | 2.76 | 2.22 | 0.54 | 0.39 |
| FL3 | 0.42 | 1.8 | 23 | 2.61 | 2.67 | 2.14 | 0.53 | 0.76 |
| FL4 | 0.055 | 0.2 | 28 | 2.62 | 2.65 | 2.14 | 0.51 | 0.42 |
| FL5 | 0.065 | 0.24 | 27 | 2.62 | 2.61 | 2.13 | 0.48 | 1.13 |
| FL6 | 0.29 | 1.5 | 20 | 2.61 | 2.76 | 2.18 | 0.58 | 1.3 |
| FL7 | 0.48 | 3.0 | 16 | 2.70 | 2.70 | 2.20 | 0.50 | 1.6 |
| FL8 | 0.68 | 3.0 | 22 | 2.70 | 2.73 | 2.24 | 0.49 | 2.4 |
| FL9 | 0.0013 | <0.2 | - | 2.41 c | 2.41 | 2.08 | 0.33 | 0.009 |
| FL10 | 0.00075 | <0.2 | - | 2.41 c | 2.43 | 2.09 | 0.34 | 0.07 |
| FL11 | 0.0011 | <0.2 | - | 2.56 | 2.42 | 2.14 | 0.28 | 0.15 |
| FL12 | 0.005 | <0.2 | - | 2.55 | 2.52 | 2.15 | 0.37 | 0.35 |
| FL13 | 0.0014 | <0.2 | - | 2.36 c | 2.57 | 1.96 | 0.61 | 0.30 |
| FL14 | 0.76 | 2.2 | 34 | 2.61 | 2.72 | 2.14 | 0.58 | 0.08 |
| FL15 | 0.79 | 2.8 | 28 | 2.70 | 2.71 | 2.17 | 0.54 | 0.11 |
| FL16 | 1.00 | 2.7 | 37 | 2.70 | 2.79 | 2.20 | 0.59 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.T.M.; Silva, C.P.; Silva, K.M.; Pioli, R.M.; Costa, T.S.; Marto, V.V.; Freitas, A.A.; Rozendo, J.; Martins, L.M.O.S.; Cavalcante, V.F.; et al. Fluorescence and Phosphorescence of Flavylium Cation Analogues of Anthocyanins. Photochem 2022, 2, 423-434. https://doi.org/10.3390/photochem2020029
Silva GTM, Silva CP, Silva KM, Pioli RM, Costa TS, Marto VV, Freitas AA, Rozendo J, Martins LMOS, Cavalcante VF, et al. Fluorescence and Phosphorescence of Flavylium Cation Analogues of Anthocyanins. Photochem. 2022; 2(2):423-434. https://doi.org/10.3390/photochem2020029
Chicago/Turabian StyleSilva, Gustavo T. M., Cassio P. Silva, Karen M. Silva, Renan M. Pioli, Tássia S. Costa, Vinícius V. Marto, Adilson A. Freitas, Jennifer Rozendo, Lucas M. O. S. Martins, Victor F. Cavalcante, and et al. 2022. "Fluorescence and Phosphorescence of Flavylium Cation Analogues of Anthocyanins" Photochem 2, no. 2: 423-434. https://doi.org/10.3390/photochem2020029
APA StyleSilva, G. T. M., Silva, C. P., Silva, K. M., Pioli, R. M., Costa, T. S., Marto, V. V., Freitas, A. A., Rozendo, J., Martins, L. M. O. S., Cavalcante, V. F., Sun, L., Aquino, A. J. A., Carneiro, V. M. T., & Quina, F. H. (2022). Fluorescence and Phosphorescence of Flavylium Cation Analogues of Anthocyanins. Photochem, 2(2), 423-434. https://doi.org/10.3390/photochem2020029






