Application of Textile Technology in Vascular Tissue Engineering
Abstract
1. Introduction
2. Architecture of Native Vessels
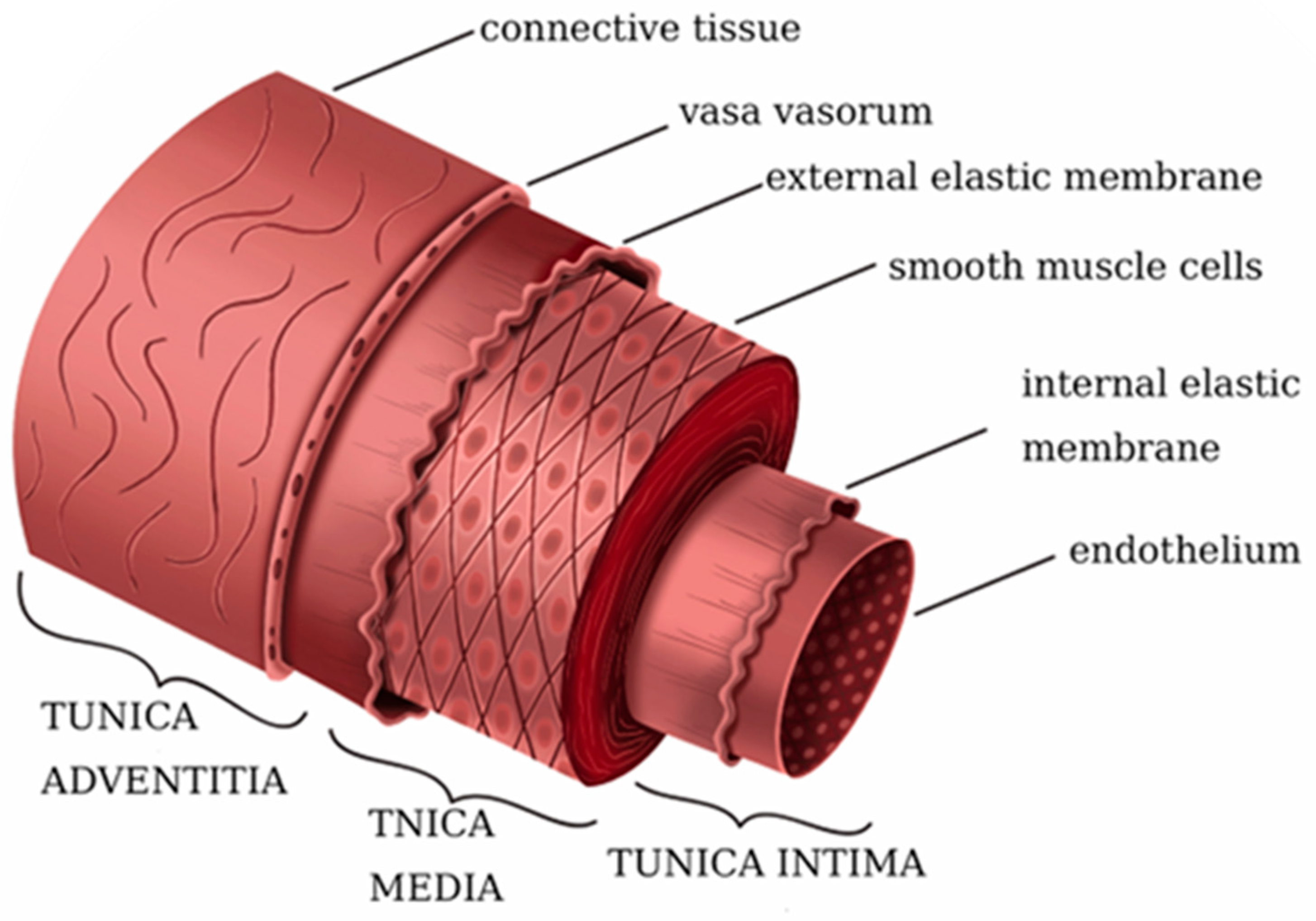
| Mechanical Property | Saphenous Vein | Internal Mammary Artery | Artificial Blood Vessel Benchmarking |
|---|---|---|---|
| Young’s modulus (MPa) | 4.2 (circ) [37,41] 23.7 (long) [37,41] | 8.0 (circ) [37,41] 16.8 (long) [37,41] | >1 (circ) [41,42] |
| Burst pressure (mmHg) | 1599 ± 877 [20,43] | 3196 ± 1264 [20,44] | >1000 [20,42] |
| Compliance (%/100 mmHg) | 4.4 [37,45] | 11.5 [20,37] | 10–20 [20,42] |
| Wall thickness (µm) | 180 to 650 [43,46] | 180 to 430 [43,46] | 500 [43] |
| Suture retention (g) | 196 ± 2 [43,47] | 138 ± 50 [20,43] | >100 [20,42] |
3. Application of Textile Technology in Artificial Blood Vessels
3.1. Electrospinning for Vascular Grafts
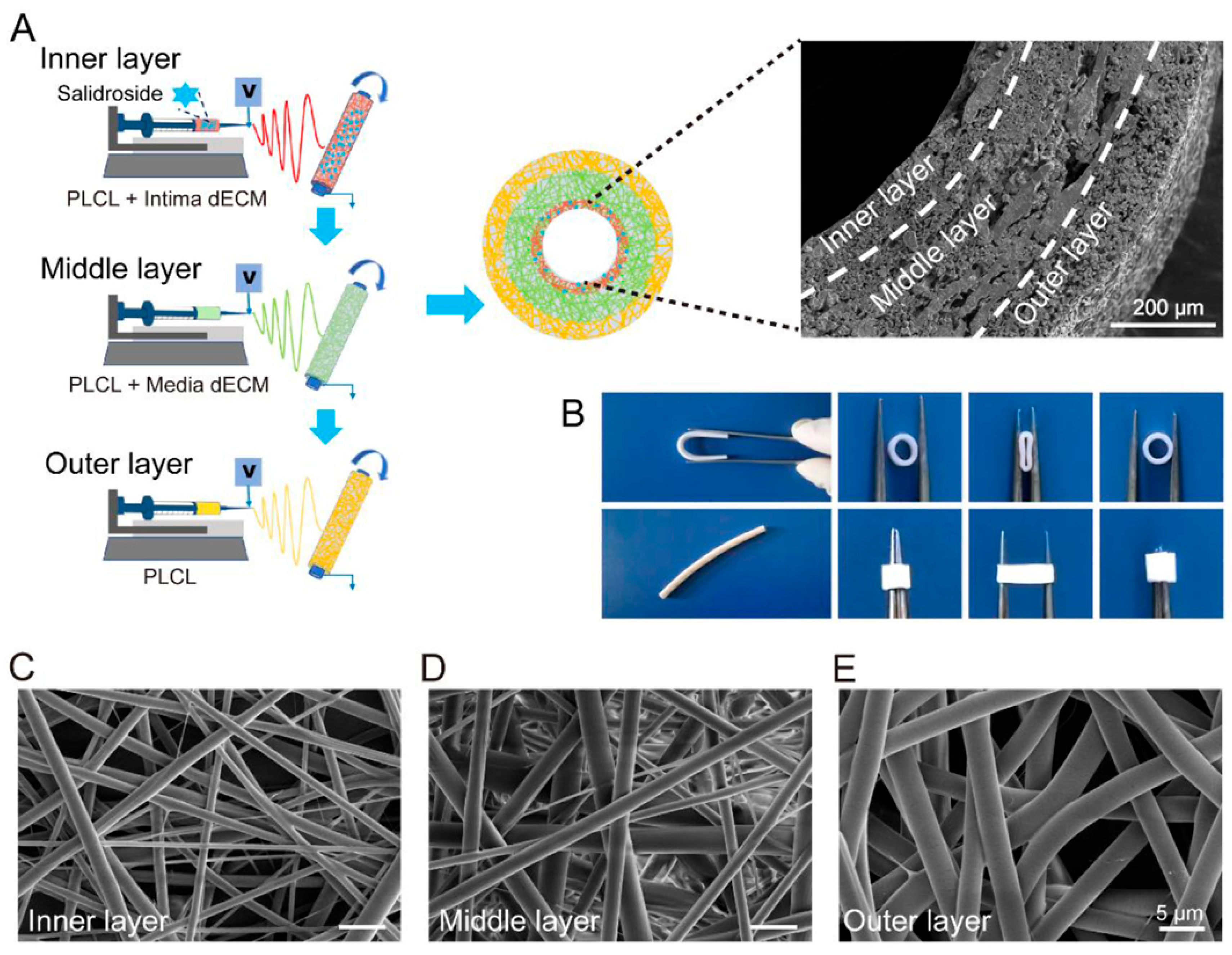
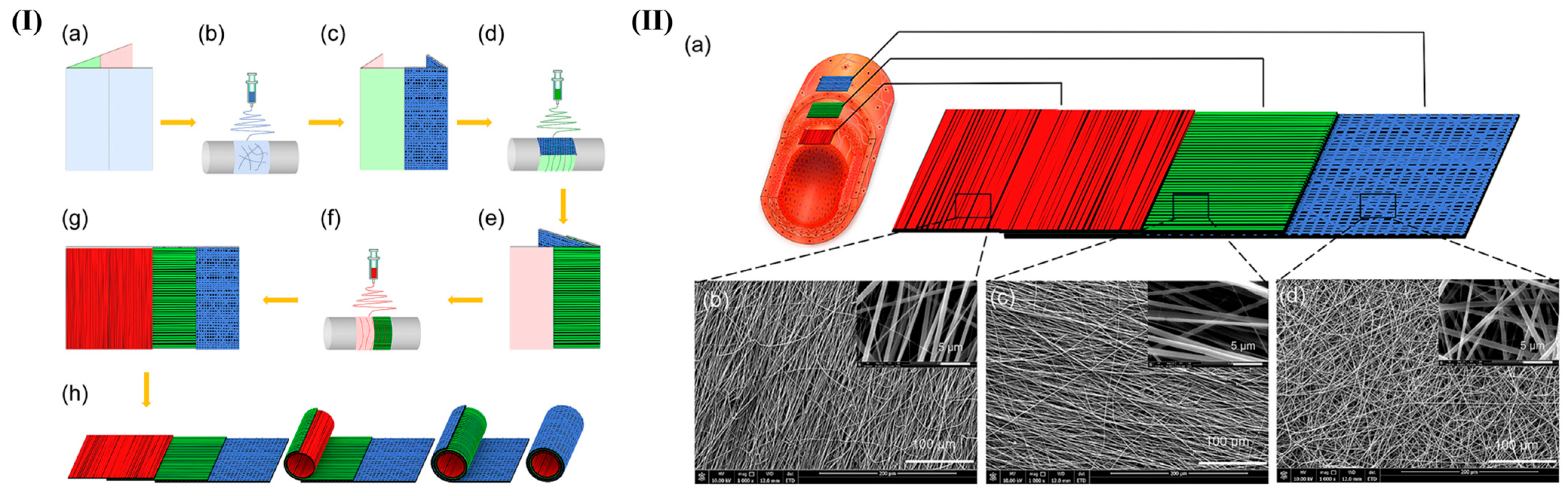
3.1.1. Mimicking the Multilayered Architecture of Native Vessels
3.1.2. Recapitulating Native Fibrous ECM: Fiber Morphology and Alignment
3.2. Controlled Fabrication of Vascular Grafts
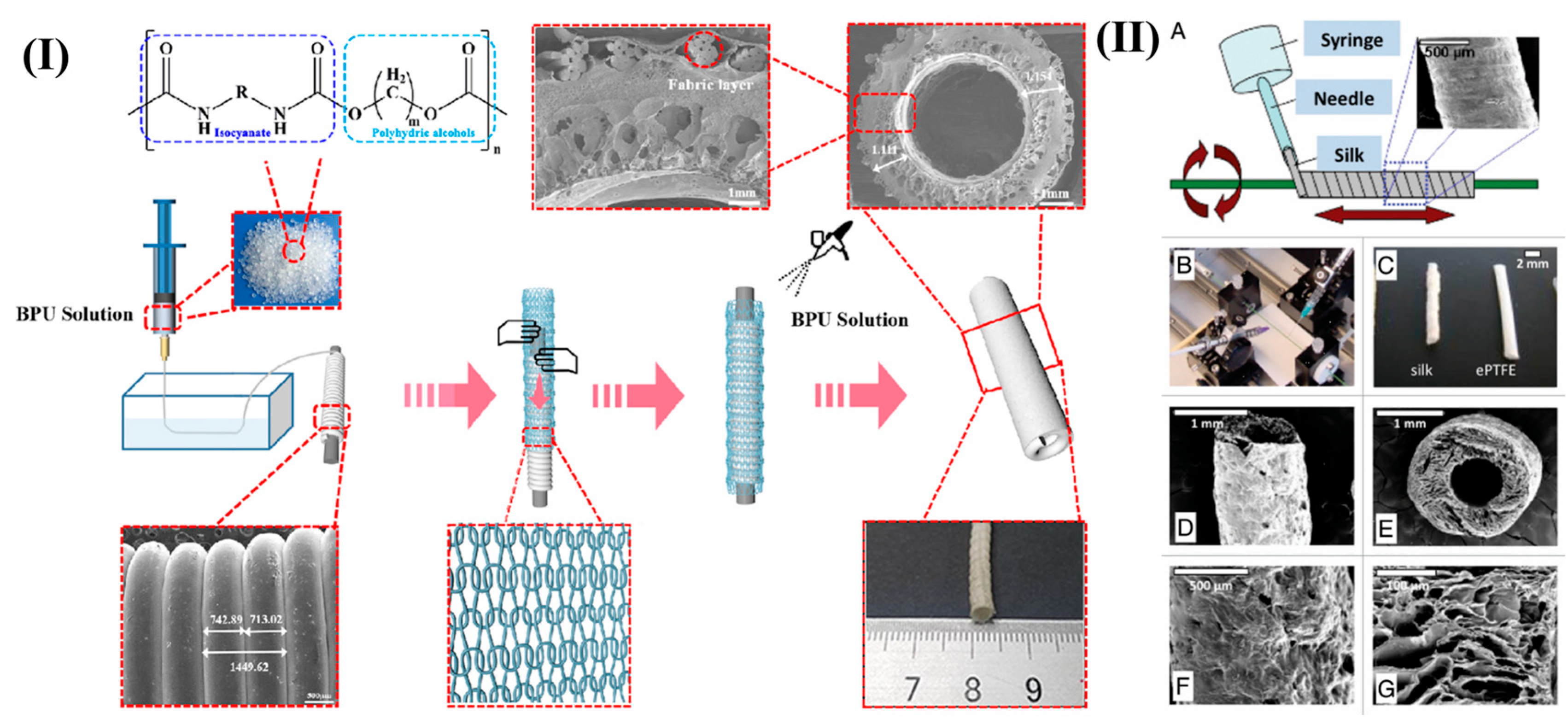
3.2.1. Wet Spinning and Gel Spinning for Vascular Grafts
3.2.2. Melt Electrowriting (MEW) for Vascular Grafts
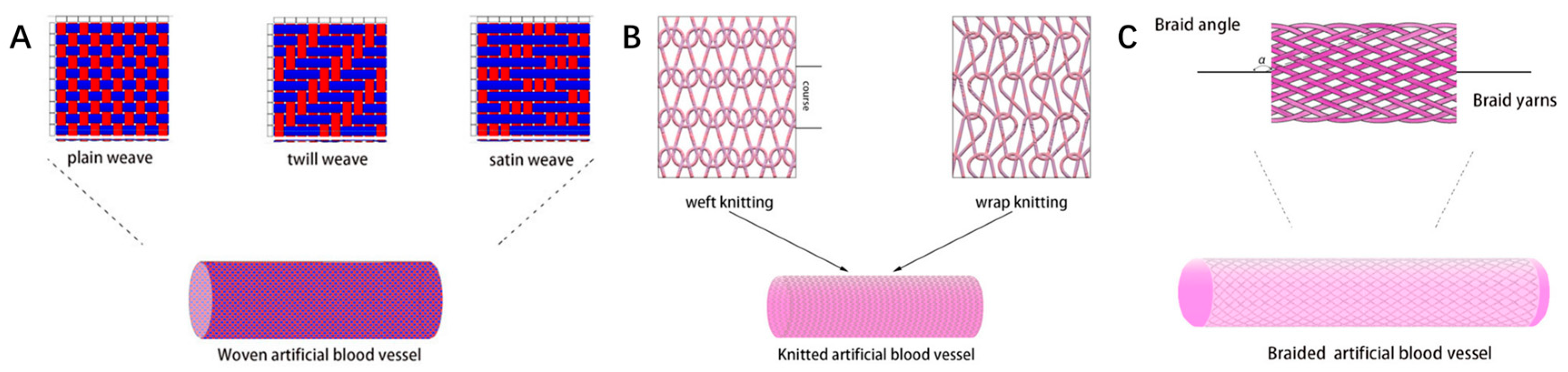
3.3. Traditional Textile Technologies for Vascular Tissue Engineering
3.3.1. Weaving: Structurally Stable Vascular Grafts with Tailored Permeability and Mechanical Strength
3.3.2. Knitting: Elastic and Compliant Vascular Grafts with Controllable Loop Architectures

3.3.3. Braiding: Flexible, Anisotropic Vascular Grafts with Tunable Porosity and Regeneration Potential
4. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- West-Livingston, L.; Lim, J.W.; Lee, S.J. Translational tissue-engineered vascular grafts: From bench to bedside. Biomaterials 2023, 302, 122322. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, D.; Comtois-Bona, M.; Munoz, M.; Ruel, M.; Suuronen, E.J.; Alarcon, E.I. Manufacturing and validation of small-diameter vascular grafts: A mini review. iScience 2024, 27, 109845. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Shahriari-Khalaji, M.; Shafiq, M.; Cui, H.; Cao, R.; Zhu, M. Advancements in the fabrication technologies and biomaterials for small diameter vascular grafts: A fine-tuning of physicochemical and biological properties. Appl. Mater. Today 2023, 31, 101778. [Google Scholar] [CrossRef]
- Weekes, A.; Bartnikowski, N.; Pinto, N.; Jenkins, J.; Meinert, C.; Klein, T.J. Biofabrication of small diameter tissue-engineered vascular grafts. Acta Biomater. 2022, 138, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Skovrind, I.; Harvald, E.B.; Juul Belling, H.; Jorgensen, C.D.; Lindholt, J.S.; Andersen, D.C. Concise Review: Patency of Small-Diameter Tissue-Engineered Vascular Grafts: A Meta-Analysis of Preclinical Trials. Stem Cells Transl. Med. 2019, 8, 671–680. [Google Scholar] [CrossRef]
- Saito, J.; Kaneko, M.; Ishikawa, Y.; Yokoyama, U. Challenges and Possibilities of Cell-Based Tissue-Engineered Vascular Grafts. Cyborg Bionic Syst. 2021, 2021, 1532103. [Google Scholar] [CrossRef]
- Bakare, A.; Mohanadas, H.P.; Tucker, N.; Ahmed, W.; Manikandan, A.; Faudzi, A.A.M.; Mohamaddan, S.; Jaganathan, S.K. Advancements in textile techniques for cardiovascular tissue replacement and repair. APL Bioeng. 2024, 8, 041503. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, K.; Liu, Y.; Zhang, C.; Wang, B. Application of textile technology in tissue engineering: A review. Acta Biomater. 2021, 128, 60–76. [Google Scholar] [CrossRef]
- Doersam, A.; Tsigkou, O.; Jones, C. A Review: Textile Technologies for Single and Multi-Layer Tubular Soft Tissue Engineering. Adv. Mater. Technol. 2022, 7, 2101720. [Google Scholar] [CrossRef]
- Liu, C.; Dai, J.; Wang, X.; Hu, X. The Influence of Textile Structure Characteristics on the Performance of Artificial Blood Vessels. Polymers 2023, 15, 3003. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112373. [Google Scholar] [CrossRef]
- Kawecki, F.; L’Heureux, N. Current biofabrication methods for vascular tissue engineering and an introduction to biological textiles. Biofabrication 2023, 15, 022004. [Google Scholar] [CrossRef]
- Yuan, Q.; Ma, C.; Ma, M.-G. Biotextile-based scaffolds in tissue engineering. In Medical Textiles from Natural Resources; Woodhead: Cambridge, UK, 2022; pp. 285–313. [Google Scholar]
- Di Francesco, D.; Pigliafreddo, A.; Casarella, S.; Di Nunno, L.; Mantovani, D.; Boccafoschi, F. Biological Materials for Tissue-Engineered Vascular Grafts: Overview of Recent Advancements. Biomolecules 2023, 13, 1389. [Google Scholar] [CrossRef]
- Jockenhoevel, S.; Zund, G.; Hoerstrup, S.P.; Chalabi, K.; Sachweh, J.S.; Demircan, L.; Messmer, B.J.; Turina, M. Fibrin gel– advantages of a new scaffold in cardiovascular tissue engineering. Eur. J. Cardiothorac. Surg. 2001, 19, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Hajjaji, R.; Abdessalem, S.B.; Ganghoffer, J.F. The influence of textile vascular prosthesis crimping on graft longitudinal elasticity and flexibility. J. Mech. Behav. Biomed. Mater. 2012, 16, 73–80. [Google Scholar] [CrossRef]
- Konig, G.; McAllister, T.N.; Dusserre, N.; Garrido, S.A.; Iyican, C.; Marini, A.; Fiorillo, A.; Avila, H.; Wystrychowski, W.; Zagalski, K.; et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 2009, 30, 1542–1550. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Li, Q.; Turng, L.S. Artificial small-diameter blood vessels: Materials, fabrication, surface modification, mechanical properties, and bioactive functionalities. J. Mater. Chem. B 2020, 8, 1801–1822. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, R.; Zhang, Y.; Kannan, P.R.; Li, Y.; Lv, Y.; Zhao, R.; Kong, X. Silk-based biomaterials for tissue engineering. Adv. Colloid. Interface Sci. 2025, 338, 103413. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Mandal, B.B. Silk biomaterials for vascular tissue engineering applications. Acta Biomater. 2021, 134, 79–106. [Google Scholar] [CrossRef]
- Coimbra, P.; Santos, P.; Alves, P.; Miguel, S.P.; Carvalho, M.P.; de Sa, K.D.; Correia, I.J.; Ferreira, P. Coaxial electrospun PCL/Gelatin-MA fibers as scaffolds for vascular tissue engineering. Colloids Surf. B Biointerfaces 2017, 159, 7–15. [Google Scholar] [CrossRef]
- Hyun, G.W.; Park, S.H. 3D freeform fabrication of fiber-reinforced artificial vascular grafts with biomimetic softness and enhanced mechanical strength. J. Manuf. Process. 2025, 133, 1237–1248. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.U.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef]
- Wu, W.; Allen, R.A.; Wang, Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 2012, 18, 1148–1153. [Google Scholar] [CrossRef]
- Guo, Y.; Wusiman, H.; Zhao, L.; James-ocloo, O.s.; Han, X.; Miao, Y.; Nie, J.; Wang, L.; Du, J.; Wei, Y.; et al. Engineering endothelialized small-diameter artificial blood vessels: Strategies, advances and applications. Compos. Part B Eng. 2025, 301, 112505. [Google Scholar] [CrossRef]
- Cao, X.; Maharjan, S.; Ashfaq, R.; Shin, J.; Zhang, Y.S. Bioprinting of Small-Diameter Blood Vessels. Engineering 2021, 7, 832–844. [Google Scholar] [CrossRef]
- Nwokoye, P.N.; Abilez, O.J. Bioengineering methods for vascularizing organoids. Cell Rep. Methods 2024, 4, 100779. [Google Scholar] [CrossRef] [PubMed]
- Bouten, C.V.; Dankers, P.Y.; Driessen-Mol, A.; Pedron, S.; Brizard, A.M.; Baaijens, F.P. Substrates for cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wise, S.G.; Kovacic, J.C.; Rnjak-Kovacina, J.; Lord, M.S. Biomaterials containing extracellular matrix molecules as biomimetic next-generation vascular grafts. Trends Biotechnol. 2024, 42, 369–381. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; Yuan, Y.; Zhang, N.; Zou, Z.; Wang, Y.; Yan, S.; Li, X.; Zhao, P.; Li, Q. Nonlinear Elasticity of Blood Vessels and Vascular Grafts. ACS Biomater. Sci. Eng. 2024, 10, 3631–3654. [Google Scholar] [CrossRef]
- Breuer, T.; Jimenez, M.; Humphrey, J.D.; Shinoka, T.; Breuer, C.K. Tissue Engineering of Vascular Grafts: A Case Report from Bench to Bedside and Back. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 399–409. [Google Scholar] [CrossRef]
- Krawiec, J.T.; Vorp, D.A. Adult stem cell-based tissue engineered blood vessels: A review. Biomaterials 2012, 33, 3388–3400. [Google Scholar] [CrossRef]
- Camasao, D.B.; Mantovani, D. The mechanical characterization of blood vessels and their substitutes in the continuous quest for physiological-relevant performances. A critical review. Mater. Today Bio 2021, 10, 100106. [Google Scholar] [CrossRef] [PubMed]
- Isella, B.; Sallustio, F.; Acosta, S.; Andre, D.; Jockenhovel, S.; Fernandez-Colino, A.; Rodriguez-Cabello, J.C.; Vaughan, T.J.; Kopp, A. A new approach for small-diameter vascular grafts using combined dip-coating of silk fibroin and elastin-like recombinamers. Biomater. Adv. 2025, 174, 214312. [Google Scholar] [CrossRef]
- Weekes, A.; Davern, J.W.; Pinto, N.; Jenkins, J.; Li, Z.; Meinert, C.; Klein, T.J. Enhancing compliance and extracellular matrix properties of tissue-engineered vascular grafts through pulsatile bioreactor culture. Biomater. Adv. 2025, 175, 214346. [Google Scholar] [CrossRef] [PubMed]
- Carrabba, M.; Fagnano, M.; Ghorbel, M.T.; Rapetto, F.; Su, B.; De Maria, C.; Vozzi, G.; Biglino, G.; Perriman, A.W.; Caputo, M.; et al. Development of a Novel Hierarchically Biofabricated Blood Vessel Mimic Decorated with Three Vascular Cell Populations for the Reconstruction of Small-Diameter Arteries. Adv. Funct. Mater. 2024, 34, 2300621. [Google Scholar] [CrossRef]
- Stekelenburg, M.; Rutten, M.C.M.; Snoeckx, L.H.E.H.; Baaijens, F.P.T. Dynamic straining combined with fibrin gel cell seeding improves strength of tissue-engineered small diameter vascular grafts. Tissue Eng. Part A 2009, 15, 1081–1089. [Google Scholar] [CrossRef]
- Moore, M.J.; Tan, R.P.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering artificial blood vessels from natural materials. Trends Biotechnol. 2022, 40, 693–707. [Google Scholar] [CrossRef]
- Nash, K.M.; Boe, B.A.; Carrillo, S.A.; Harrison, A.; Iwaki, R.; Kelly, J.; Kirkton, R.D.; Krishnamurthy, R.; Lawson, J.H.; Matsuzaki, Y.; et al. Evaluation of tissue-engineered human acellular vessels as a Blalock-Taussig-Thomas shunt in a juvenile primate model. JTCVS Open 2023, 15, 433–445. [Google Scholar] [CrossRef]
- Shen, N.; Zhang, Y.; Raza, A.; Chang, L.; Wang, J.Y. Effects of the micro/nanostructure of electrospun zein fibres on cells in simulated blood flow environment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111900. [Google Scholar] [CrossRef]
- Walden, R.; L’Italien, G.J.; Megerman, J.; Abbott, W.M. Matched elastic properties and successful arterial grafting. Arch. Surg. 1980, 115, 1166–1169. [Google Scholar] [CrossRef]
- Canham, P.B.; Finlay, H.M.; Boughner, D.R. Contrasting structure of the saphenous vein and internal mammary artery used as coronary bypass vessels. Cardiovasc. Res. 1997, 34, 557–567. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, N.; Dusserre, N.; Konig, G.; Victor, B.; Keire, P.; Wight, T.N.; Chronos, N.A.F.; Kyles, A.E.; Gregory, C.R.; Hoyt, G.; et al. Human Tissue Engineered Blood Vessel For Adult Arterial Revascularization. Nat. Med. 2006, 12, 361–365. [Google Scholar] [CrossRef]
- Scholpp, S.; Hoffmann, L.A.; Schatzlein, E.; Gries, T.; Emonts, C.; Blaeser, A. Interlacing biology and engineering: An introduction to textiles and their application in tissue engineering. Mater. Today Bio 2025, 31, 101617. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Recent advances in modification strategies of pre- and post-electrospinning of nanofiber scaffolds in tissue engineering. React. Funct. Polym. 2022, 172, 105202. [Google Scholar] [CrossRef]
- Keerthii, R.; Vinotha Sre, V.; Khan, S.S. Biopolymer-based electrospinning nanoarchitectonics for advancement in tissue regeneration. Surf. Interfaces 2025, 72, 107031. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Karkan, S.F.; Davaran, S.; Rahbarghazi, R.; Salehi, R.; Akbarzadeh, A. Electrospun nanofibers for the fabrication of engineered vascular grafts. J. Biol. Eng. 2019, 13, 83. [Google Scholar] [CrossRef]
- Shi, J.; Teng, Y.; Li, D.; He, J.; Midgley, A.C.; Guo, X.; Wang, X.; Yang, X.; Wang, S.; Feng, Y.; et al. Biomimetic tri-layered small-diameter vascular grafts with decellularized extracellular matrix promoting vascular regeneration and inhibiting thrombosis with the salidroside. Mater. Today Bio 2023, 21, 100709. [Google Scholar] [CrossRef]
- Han, F.; Jia, X.; Dai, D.; Yang, X.; Zhao, J.; Zhao, Y.; Fan, Y.; Yuan, X. Performance of a multilayered small-diameter vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials 2013, 34, 7302–7313. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, J.; Wang, Y.; Li, D.; Sun, B.; El-Hamshary, H.; Yin, M.; Mo, X. Fabrication and preliminary study of a biomimetic tri-layer tubular graft based on fibers and fiber yarns for vascular tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 121–129. [Google Scholar] [CrossRef]
- Ma, F.; Huang, X.; Wang, Y. Fabrication of a Triple-Layer Bionic Vascular Scaffold via Hybrid Electrospinning. J. Funct. Biomater. 2024, 15, 140. [Google Scholar] [CrossRef]
- Han, D.G.; Ahn, C.B.; Lee, J.H.; Hwang, Y.; Kim, J.H.; Park, K.Y.; Lee, J.W.; Son, K.H. Optimization of Electrospun Poly(caprolactone) Fiber Diameter for Vascular Scaffolds to Maximize Smooth Muscle Cell Infiltration and Phenotype Modulation. Polymers 2019, 11, 643. [Google Scholar] [CrossRef]
- Kew, S.J.; Gwynne, J.H.; Enea, D.; Abu-Rub, M.; Pandit, A.; Zeugolis, D.; Brooks, R.A.; Rushton, N.; Best, S.M.; Cameron, R.E. Regeneration and repair of tendon and ligament tissue using collagen fibre biomaterials. Acta Biomater. 2011, 7, 3237–3247. [Google Scholar] [CrossRef] [PubMed]
- Grace Chao, P.H.; Hsu, H.Y.; Tseng, H.Y. Electrospun microcrimped fibers with nonlinear mechanical properties enhance ligament fibroblast phenotype. Biofabrication 2014, 6, 035008. [Google Scholar] [CrossRef]
- He, W.; Hu, Z.; Xu, A.; Liu, R.; Yin, H.; Wang, J.; Wang, S. The preparation and performance of a new polyurethane vascular prosthesis. Cell Biochem. Biophys. 2013, 66, 855–866. [Google Scholar] [CrossRef]
- Fusaro, L.; Gualandi, C.; Antonioli, D.; Soccio, M.; Liguori, A.; Laus, M.; Lotti, N.; Boccafoschi, F.; Focarete, M.L. Elastomeric Electrospun Scaffolds of a Biodegradable Aliphatic Copolyester Containing PEG-Like Sequences for Dynamic Culture of Human Endothelial Cells. Biomolecules 2020, 10, 1620. [Google Scholar] [CrossRef]
- Tan, Z.; Gao, X.; Liu, T.; Yang, Y.; Zhong, J.; Tong, C.; Tan, Y. Electrospun vein grafts with high cell infiltration for vascular tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Napiwocki, B.; Xu, Y.; Zhang, J.; Zhang, X.; Wang, X.; Crone, W.C.; Li, Q.; Turng, L.S. Wavy small-diameter vascular graft made of eggshell membrane and thermoplastic polyurethane. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110311. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.Y.; Jing, X.; Yu, E.; Wang, X.; Li, Q.; Turng, L.S. Manipulating the structure and mechanical properties of thermoplastic polyurethane/polycaprolactone hybrid small diameter vascular scaffolds fabricated via electrospinning using an assembled rotating collector. J. Mech. Behav. Biomed. Mater. 2018, 78, 433–441. [Google Scholar] [CrossRef]
- Yu, E.; Mi, H.Y.; Zhang, J.; Thomson, J.A.; Turng, L.S. Development of biomimetic thermoplastic polyurethane/fibroin small-diameter vascular grafts via a novel electrospinning approach. J. Biomed. Mater. Res. A 2018, 106, 985–996. [Google Scholar] [CrossRef]
- Elsayed, Y.; Lekakou, C.; Labeed, F.; Tomlins, P. Fabrication and characterisation of biomimetic, electrospun gelatin fibre scaffolds for tunica media-equivalent, tissue engineered vascular grafts. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 473–483. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, Y.; Jiao, J.; Shi, Y.; Zhu, T.; Li, L. Electrospun gelatin-based biomimetic scaffold with spatially aligned and three-layer architectures for vascular tissue engineering. Int. J. Biol. Macromol. 2023, 242 Pt 3, 125039. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhu, M.; Wang, L.; Xiao, N.; Kong, D. Wet-spun poly(ε-caprolactone) microfiber scaffolds for oriented growth and infiltration of smooth muscle cells. Mater. Lett. 2014, 132, 59–62. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Ma, S.; Wang, L.; Liu, C.; Xu, W.; Shi, J.; Qiao, W.; Yang, H. Small-diameter polyurethane vascular graft with high strength and excellent compliance. J. Mech. Behav. Biomed. Mater. 2021, 121, 104614. [Google Scholar] [CrossRef]
- Lovett, M.; Eng, G.; Kluge, J.A.; Cannizzaro, C.; Vunjak-Novakovic, G. Tubular silk scaffolds for small diameter vascular grafts. Organogenesis 2010, 6, 217–224. [Google Scholar] [CrossRef]
- Rodriguez, M.; Kluge, J.A.; Smoot, D.; Kluge, M.A.; Schmidt, D.F.; Paetsch, C.R.; Kim, P.S.; Kaplan, D.L. Fabricating mechanically improved silk-based vascular grafts by solution control of the gel-spinning process. Biomaterials 2020, 230, 119567. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D. Melt electrowriting with additive manufacturing principles. Curr. Opin. Biomed. Eng. 2017, 2, 49–57. [Google Scholar] [CrossRef]
- Fleischer, S.; Tavakol, D.N.; Vunjak-Novakovic, G. From Arteries to Capillaries: Approaches to Engineering Human Vasculature. Adv. Funct. Mater. 2020, 30, 1910811. [Google Scholar] [CrossRef]
- Mueller, K.M.A.; Mansi, S.; De-Juan-Pardo, E.M.; Mela, P. Advances in melt electrowriting for cardiovascular applications. Front. Bioeng. Biotechnol. 2024, 12, 1425073. [Google Scholar] [CrossRef]
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef]
- Saidy, N.T.; Shabab, T.; Bas, O.; Rojas-Gonzalez, D.M.; Menne, M.; Henry, T.; Hutmacher, D.W.; Mela, P.; De-Juan-Pardo, E.M. Melt Electrowriting of Complex 3D Anatomically Relevant Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 793. [Google Scholar] [CrossRef]
- Paxton, N.C.; Lanaro, M.; Bo, A.; Crooks, N.; Ross, M.T.; Green, N.; Tetsworth, K.; Allenby, M.C.; Gu, Y.; Wong, C.S.; et al. Design tools for patient specific and highly controlled melt electrowritten scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 105, 103695. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.C.; Daley, R.; Forrestal, D.P.; Allenby, M.C.; Woodruff, M.A. Auxetic tubular scaffolds via melt electrowriting. Mater. Des. 2020, 193, 108787. [Google Scholar] [CrossRef]
- Bas, O.; D’Angella, D.; Baldwin, J.G.; Castro, N.J.; Wunner, F.M.; Saidy, N.T.; Kollmannsberger, S.; Reali, A.; Rank, E.; De-Juan-Pardo, E.M.; et al. An Integrated Design, Material, and Fabrication Platform for Engineering Biomechanically and Biologically Functional Soft Tissues. ACS Appl. Mater. Interfaces 2017, 9, 29430–29437. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Yao, Y.; Yim, E.K.F. Current understanding of intimal hyperplasia and effect of compliance in synthetic small diameter vascular grafts. Biomater. Sci. 2020, 8, 4383–4395. [Google Scholar] [CrossRef]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Direct writing by way of melt electrospinning. Adv. Mater. 2011, 23, 5651–5657. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Somszor, K.; Bas, O.; Karimi, F.; Shabab, T.; Saidy, N.T.; O’Connor, A.J.; Ellis, A.V.; Hutmacher, D.; Heath, D.E. Personalized, Mechanically Strong, and Biodegradable Coronary Artery Stents via Melt Electrowriting. ACS Macro Lett. 2020, 9, 1732–1739. [Google Scholar] [CrossRef]
- Sanchez Diaz, R.; Park, J.R.; Rodrigues, L.L.; Dalton, P.D.; De-Juan-Pardo, E.M.; Dargaville, T.R. Highly Elastic Scaffolds Produced by Melt Electrowriting of Poly(L-lactide-co-ε-caprolactone). Adv. Funct. Mater. 2021, 7, 2100508. [Google Scholar] [CrossRef]
- Kade, J.C.; Dalton, P.D. Polymers for Melt Electrowriting. Adv. Healthc. Mater. 2021, 10, e2001232. [Google Scholar] [CrossRef]
- Jungst, T.; Pennings, I.; Schmitz, M.; Rosenberg, A.J.W.P.; Groll, J.; Gawlitta, D. Heterotypic Scaffold Design Orchestrates Primary Cell Organization and Phenotypes in Cocultured Small Diameter Vascular Grafts. Adv. Funct. Mater. 2019, 29, 1905987. [Google Scholar] [CrossRef]
- Bartolf-Kopp, M.; de Silva, L.; Rosenberg, A.J.W.P.; Groll, J.; Gawlitta, D.; Jungst, T. Hybrid Co-Spinning and Melt Electrowriting Approach Enables Fabrication of Heterotypic Tubular Scaffolds Resembling the Non-Linear Mechanical Properties of Human Blood Vessels. Adv. Funct. Mater. 2024, 34, 2311797. [Google Scholar] [CrossRef]
- Federici, A.S.; Tornifoglio, B.; Lally, C.; Garcia, O.; Kelly, D.J.; Hoey, D.A. Melt electrowritten scaffold architectures to mimic tissue mechanics and guide neo-tissue orientation. J. Mech. Behav. Biomed. Mater. 2024, 150, 106292. [Google Scholar]
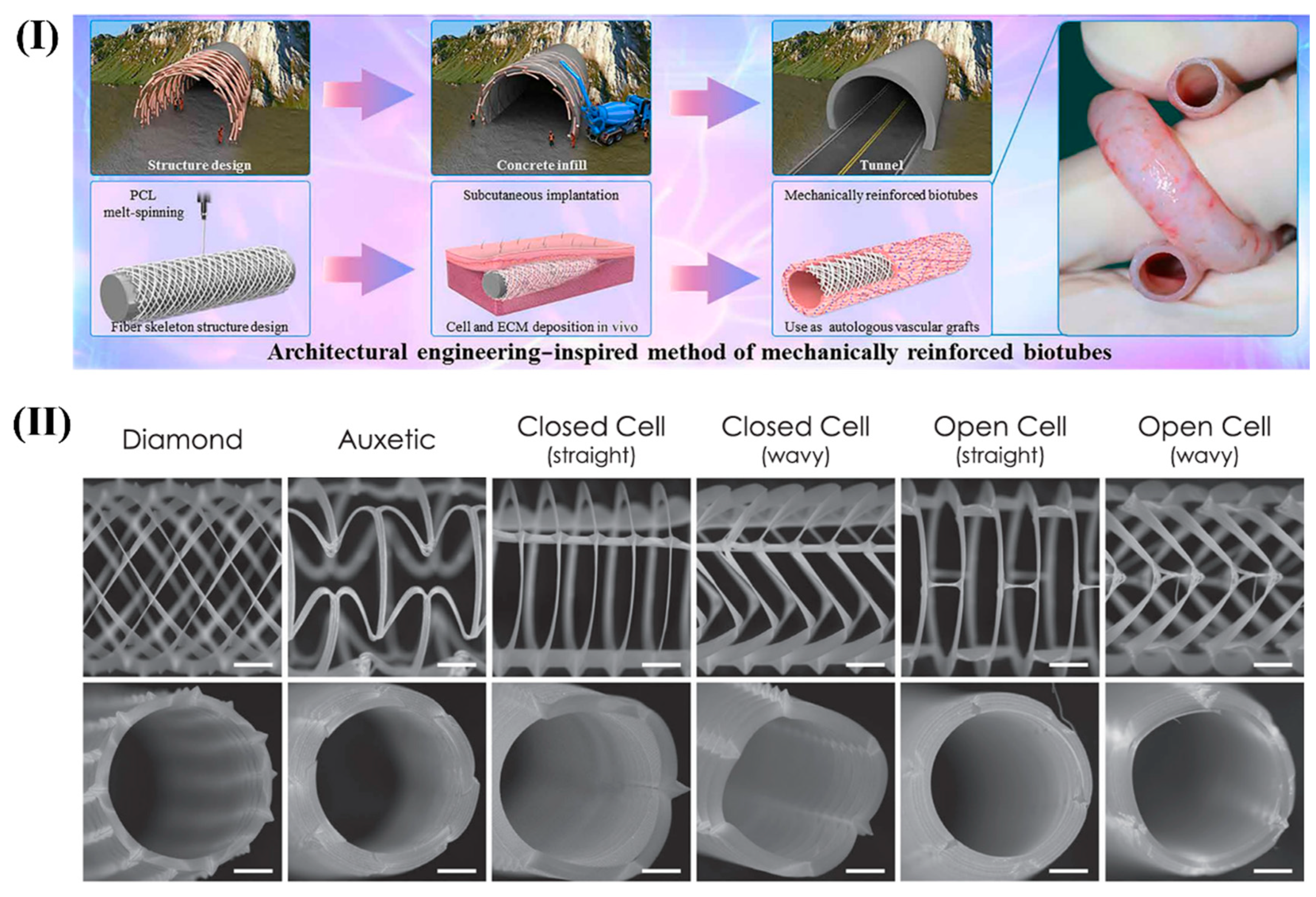
- Zhi, D.; Cheng, Q.; Midgley, A.C.; Zhang, Q.; Wei, T.; Li, Y. Mechanically reinforced biotubes for arterial replacement and arteriovenous grafting inspired by architectural engineering. Sci. Adv. 2022, 8, eabl3888. [Google Scholar] [CrossRef]
- McCosker, A.B.; Snowdon, M.E.; Lamont, R.; Woodruff, M.A.; Paxton, N.C. Exploiting Nonlinear Fiber Patterning to Control Tubular Scaffold Mechanical Behavior. Adv. Mater. Technol. 2022, 7, 2200259. [Google Scholar] [CrossRef]
- Weekes, A.; Wehr, G.; Pinto, N.; Jenkins, J.; Li, Z.; Meinert, C.; Klein, T.J. Highly compliant biomimetic scaffolds for small diameter tissue-engineered vascular grafts (TEVGs) produced via melt electrowriting (MEW). Biofabrication 2023, 16, 015017. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Richards, T.L.; Paxton, N.C.; Allenby, M.C.; Woodruff, M.A. Dissolvable 3D printed PVA moulds for melt electrowriting tubular scaffolds with patient-specific geometry. Mater. Des. 2022, 215, 110466. [Google Scholar] [CrossRef]
- Ding, H.; Cao, K.; Zhang, F.; Boettcher, W.; Chang, R.C. A Fundamental Study of Charge Effects on Melt Electrowritten Polymer Fibers. Mater. Des. 2019, 178, 107857. [Google Scholar] [CrossRef]
- Kim, J.; Bakirci, E.; O’Neill, K.L.; Hrynevich, A.; Dalton, P.D. Fiber Bridging during Melt Electrowriting of Poly(ε-Caprolactone) and the Influence of Fiber Diameter and Wall Height. Macromol. Mater. Eng. 2021, 306, 2000685. [Google Scholar] [CrossRef]
- Mueller, K.M.A.; Unterrainer, A.; Rojas-González, D.M.; De-Juan-Pardo, E.; Willner, M.S.; Herzen, J.; Mela, P. Introducing Controlled Microporosity in Melt Electrowriting. Adv. Mater. Technol. 2023, 8, 2201158. [Google Scholar] [CrossRef]
- Neuhaus, B.; Loewner, S.; Heymann, H.; Webering, F.; Synofzik, J.; Blume, H.; Blume, C. Fiber deviation and optimized toolpath strategies in melt electrowriting of tubular scaffolds. Mater. Des. 2025, 254, 114147. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, C.; Liu, L.; Wang, F.; Liu, X.; Mao, J.; Wang, L. Construction and application of textile-based tissue engineering scaffolds: A review. Biomater. Sci. 2020, 8, 3574–3600. [Google Scholar] [CrossRef]
- Fernández-Colino, A.; Jockenhoevel, S. Textile-Reinforced Scaffolds for Vascular Tissue Engineering. In Tissue-Engineered Vascular Grafts; Springer International: Cham, Switzerland, 2020; pp. 339–363. [Google Scholar]
- Akbari, M.; Tamayol, A.; Bagherifard, S.; Serex, L.; Mostafalu, P.; Faramarzi, N.; Mohammadi, M.H.; Khademhosseini, A. Textile Technologies and Tissue Engineering: A Path Toward Organ Weaving. Adv. Healthc. Mater. 2016, 5, 751–766. [Google Scholar] [CrossRef]
- Kun, M.; Chan, C.; Ramakrishna, S. Textile-based scaffolds for tissue engineering. In Advanced Textiles for Wound Care; Woodhead: Cambridge, UK, 2009; pp. 289–321. [Google Scholar]
- Hu, X.; Hu, T.; Guan, G.; Yu, S.; Wu, Y.; Wang, L. Control of weft yarn or density improves biocompatibility of PET small diameter artificial blood vessels. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 954–964. [Google Scholar] [CrossRef]
- Hu, K.; Li, Y.; Ke, Z.; Yang, H.; Lu, C.; Li, Y.; Guo, Y.; Wang, W. History, progress and future challenges of artificial blood vessels: A narrative review. Biomater. Transl. 2022, 3, 81–98. [Google Scholar]
- Joseph, J.; Krishnan, A.G.; Cherian, A.M.; Rajagopalan, B.; Jose, R.; Varma, P.; Maniyal, V.; Balakrishnan, S.; Nair, S.V.; Menon, D. Transforming Nanofibers into Woven Nanotextiles for Vascular Application. ACS Appl. Mater. Interfaces 2018, 10, 19449–19458. [Google Scholar] [CrossRef]
- Guan, G.; Yu, C.; Fang, X.; Guidoin, R.; King, M.W.; Wang, H.; Wang, L. Exploration into practical significance of integral water permeability of textile vascular grafts. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211014007. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Z.; Chen, K.; Li, Y.; Wang, X.; Li, G. A heparin-functionalized woven stent graft for endovascular exclusion. Colloids Surf. B Biointerfaces 2019, 180, 118–126. [Google Scholar] [CrossRef]
- Roudier, G.; Hourques, M.; Da Silva, N.; Gluais, M.; Binyet, E.; Olive, J.M.; L’Heureux, N. Effects of weaving parameters on the properties of completely biological tissue-engineered vascular grafts. Biofabrication 2023, 16, 015015. [Google Scholar] [CrossRef]
- Magnan, L.; Labrunie, G.; Fenelon, M.; Dusserre, N.; Foulc, M.P.; Lafourcade, M.; Svahn, I.; Gontier, E.; Velez, V.J.; McAllister, T.N.; et al. Human textiles: A cell-synthesized yarn as a truly “bio” material for tissue engineering applications. Acta Biomater. 2020, 105, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K. Advances in Textile Structural Composites II. Polymers 2025, 17, 1603. [Google Scholar] [CrossRef]
- Zhang, F.; Bambharoliya, T.; Xie, Y.; Liu, L.; Celik, H.; Wang, L.; Akkus, O.; King, M.W. A hybrid vascular graft harnessing the superior mechanical properties of synthetic fibers and the biological performance of collagen filaments. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ao, Q.; Wang, A.; Lu, G.; Kong, L.; Gong, Y.; Zhao, N.; Zhang, X. A sandwich tubular scaffold derived from chitosan for blood vessel tissue engineering. J. Biomed. Mater. Res. A 2006, 77, 277–284. [Google Scholar] [CrossRef]
- Torikai, K.; Ichikawa, H.; Hirakawa, K.; Matsumiya, G.; Kuratani, T.; Iwai, S.; Saito, A.; Kawaguchi, N.; Matsuura, N.; Sawa, Y. A self-renewing, tissue-engineered vascular graft for arterial reconstruction. J. Thorac. Cardiovasc. Surg. 2008, 136, 37–45.e1. [Google Scholar] [CrossRef][Green Version]
- Rama, E.; Mohapatra, S.R.; Sugimura, Y.; Suzuki, T.; Siebert, S.; Barmin, R.; Hermann, J.; Baier, J.; Rix, A.; Lemainque, T.; et al. In vitro and in vivo evaluation of biohybrid tissue-engineered vascular grafts with transformative 1H/19F MRI traceable scaffolds. Biomaterials 2024, 311, 122669. [Google Scholar] [CrossRef]
- Lou, C.-W.; Huang, C.-L.; Hsieh, C.-T.; Lu, P.-C.; Hsieh, C.-T.; Lin, J.-H. Polylactic acid tubular knits used as vascular grafts: Mechanical property evaluation. Fiber Polym. 2016, 16, 2593–2600. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, Y.; Celik, H.; Akkus, O.; Bernacki, S.H.; King, M.W. Engineering small-caliber vascular grafts from collagen filaments and nanofibers with comparable mechanical properties to native vessels. Biofabrication 2019, 11, 035020. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.W.; Lu, P.C.; Hu, J.J.; Lin, J.H. Effects of yarn types and fabric types on the compliance and bursting strength of vascular grafts. J. Mech. Behav. Biomed. Mater. 2016, 59, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Kiritani, S.; Kaneko, J.; Ito, D.; Morito, M.; Ishizawa, T.; Akamatsu, N.; Tanaka, M.; Iida, T.; Tanaka, T.; Tanaka, R.; et al. Silk fibroin vascular graft: A promising tissue-engineered scaffold material for abdominal venous system replacement. Sci. Rep. 2020, 10, 21041. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liang, M.; Zhang, B.; Li, W.; Huang, X.; Zhang, X.; Chen, K.; Li, G. Advances in artificial blood vessels: Exploring materials, preparation, and functionality. J. Mater. Sci. Technol. 2025, 219, 225–256. [Google Scholar] [CrossRef]
- Emonts, C.; Grigat, N.; Merkord, F.; Vollbrecht, B.; Idrissi, A.; Sackmann, J.; Gries, T. Innovation in 3D Braiding Technology and Its Applications. Textiles 2021, 1, 185–205. [Google Scholar] [CrossRef]
- Lin, M.C.; Lou, C.W.; Lin, J.Y.; Lin, T.A.; Chen, Y.S.; Lin, J.H. Biodegradable Polyvinyl Alcohol Vascular Stents: Structural Model and Mechanical and Biological Property Evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 404–413. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jiang, Y.; Jing, X.; Enriquez, E.; Li, H.; Li, Q.; Turng, L.S. Fabrication of triple-layered vascular grafts composed of silk fibers, polyacrylamide hydrogel, and polyurethane nanofibers with biomimetic mechanical properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 241–249. [Google Scholar] [CrossRef]
- Fukunishi, T.; Ong, C.S.; Lui, C.; Pitaktong, I.; Smoot, C.; Harris, J.; Gabriele, P.; Vricella, L.; Santhanam, L.; Lu, S.; et al. Formation of Neoarteries with Optimal Remodeling Using Rapidly Degrading Textile Vascular Grafts. Tissue Eng. Part A 2019, 25, 632–641. [Google Scholar] [CrossRef]
- Guan, G.; Yu, C.; Xing, M.; Wu, Y.; Hu, X.; Wang, H.; Wang, L. Hydrogel Small-Diameter Vascular Graft Reinforced with a Braided Fiber Strut with Improved Mechanical Properties. Polymers 2019, 11, 810. [Google Scholar] [CrossRef]
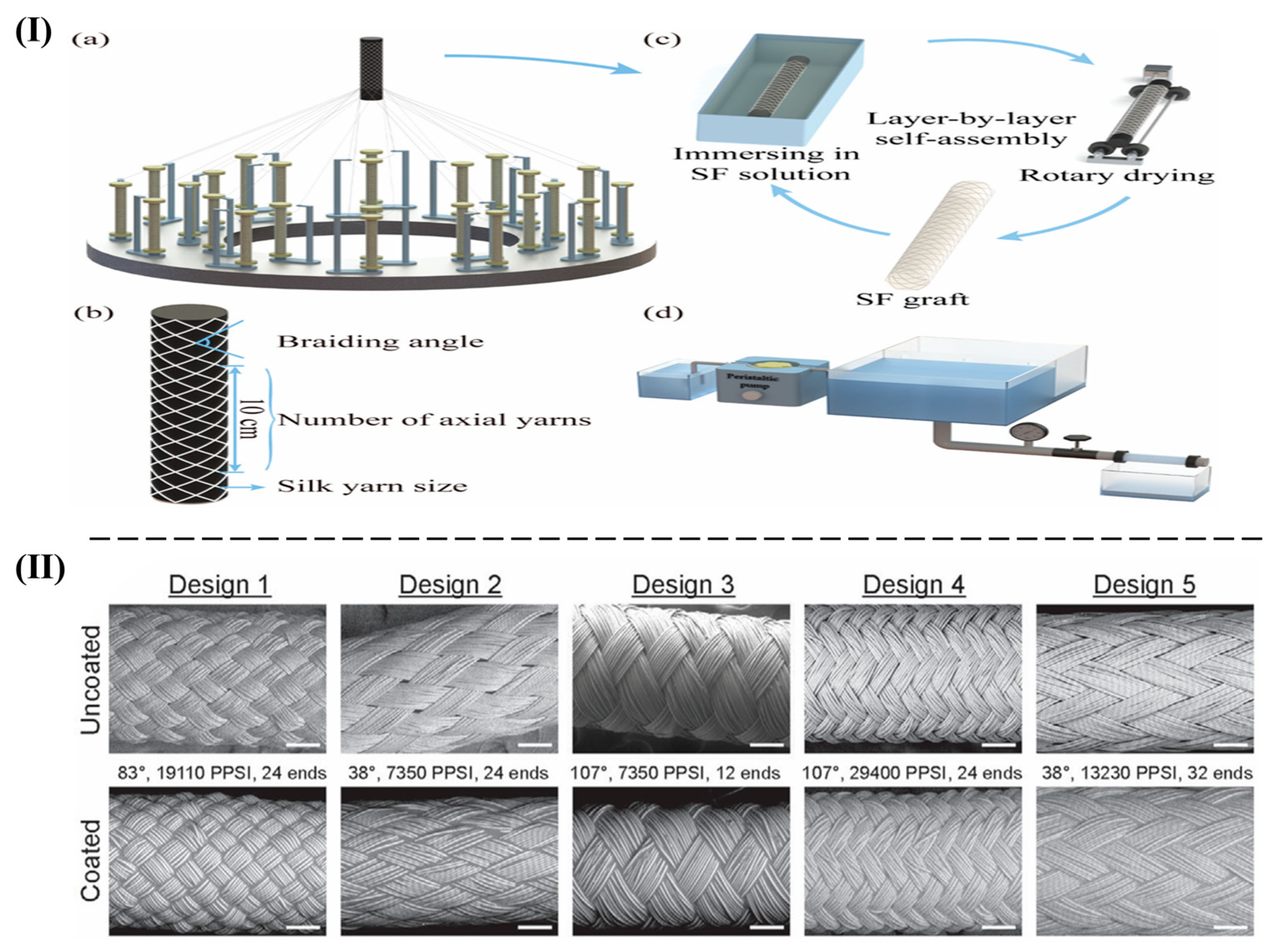
- Yu, Y.; Song, G.; Dai, M.; Meng, L.; Xu, J.; Fan, Q.; Dong, F.; Yin, Y.; Wang, J. Silk Fibroin/Silk Braiding Fabric Composite Grafts via Layer-by-Layer Self-Assembly: Water Permeability and Cytocompatibility. J. Nat. Fibers 2023, 20, 2163028. [Google Scholar] [CrossRef]
- Zbinden, J.C.; Blum, K.M.; Berman, A.G.; Ramachandra, A.B.; Szafron, J.M.; Kerr, K.E.; Anderson, J.L.; Sangha, G.S.; Earl, C.C.; Nigh, N.R.; et al. Effects of Braiding Parameters on Tissue Engineered Vascular Graft Development. Adv. Healthc. Mater. 2020, 9, e2001093. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, W.; Xu, P.; Feng, W.; Tang, X.; Yang, X.; Wang, L.; Li, L.; Huang, Y.; Ji, J.; et al. The Regulatory Effect of Braided Silk Fiber Skeletons with Differential Porosities on In Vivo Vascular Tissue Regeneration and Long-Term Patency. Research 2022, 2022, 9825237. [Google Scholar] [CrossRef] [PubMed]

| Textile Technology | Mechanical Strength | Mechanical Stretch | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|---|
| Weaving | High | Low | Stable structure and excellent mechanical properties | Poor compliance | [9,16,99] |
| Knitting | High | Low | Excellent elasticity and compliance | Large pores and poor mechanical properties | [11,100,101] |
| Braiding | Low | High | Good ductility, flexibility, and compliance | Large pore structure, which can cause serious blood leakage | [10,15,102] |
| Category | Material | ES | WS | GS | MEW | Weaving | Knitting | Braiding |
|---|---|---|---|---|---|---|---|---|
| Natural biomaterials | Collagen | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Elastin | ✓ | ✓ | ||||||
| Fibrin | ✓ | |||||||
| Chitosan | ✓ | |||||||
| Silk and silk fibroin | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Bacterial cellulose | ✓ | |||||||
| Gelatin | ✓ | ✓ | ||||||
| Degradable synthetic materials | Polycaprolactone (PCL) | ✓ | ✓ | ✓ | ||||
| Polylactic acid (PLA) | ✓ | ✓ | ✓ | |||||
| Poly(L-lactide-co-ε-caprolactone) (PLCL) | ✓ | |||||||
| Polyglycolic acid (PGA) | ✓ | ✓ | ||||||
| Poly(lactic-co-glycolic) acid (PLGA) | ✓ | |||||||
| Polyvinyl alcohol (PVA) | ✓ | |||||||
| Polyglycerol sebacate (PGS) | ✓ | |||||||
| Non-degradable synthetic materials | Polyurethanes (PU) | ✓ | ✓ | ✓ | ✓ | |||
| Polyethylene terephthalate (PET) | ✓ | ✓ | ||||||
| Thermoplastic polyurethane (TPU) | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Yang, H.; Li, Z. Application of Textile Technology in Vascular Tissue Engineering. Textiles 2025, 5, 38. https://doi.org/10.3390/textiles5030038
Ji H, Yang H, Li Z. Application of Textile Technology in Vascular Tissue Engineering. Textiles. 2025; 5(3):38. https://doi.org/10.3390/textiles5030038
Chicago/Turabian StyleJi, Hua, Hongjun Yang, and Zehao Li. 2025. "Application of Textile Technology in Vascular Tissue Engineering" Textiles 5, no. 3: 38. https://doi.org/10.3390/textiles5030038
APA StyleJi, H., Yang, H., & Li, Z. (2025). Application of Textile Technology in Vascular Tissue Engineering. Textiles, 5(3), 38. https://doi.org/10.3390/textiles5030038





