Abstract
Poly(lactic acid) (PLA) is an aliphatic polyester considered a “green” material due to its natural-based origin and biodegradable properties. This is why PLA fibres may be compared with poly(ethylene terephthalate) (PET) fibres in an effort to partially replace the latter in industrial production. The purpose of this study is to investigate the dyeability of poly(lactic acid) fibres using six (6) commercially available disperse dyes with different energy levels, molecular weights and chemical structures, namely Disperse Red 59 (Serisol Fast Pink RFL), Disperse Red 60 (Serilene Red 2BL), Disperse Red 92 (Serilene Red TBLS), Disperse Orange 31 (Serisol Br Orange RGL), Disperse Yellow 54 (Serilene Yellow 3GL) and Disperse Blue 79 (Serilene Navy Blue GRLS). The dyeing characteristics, such as dye exhaustion, colour strength (K/S value), colorimetric values, wash fastness, light fastness and sublimation fastness of dyed fibres, were examined at dyeing temperatures of 110 and 130 °C, while the presence of carrier agent was also investigated. The dye exhaustion values of PLA fibres were found to be lower than those of PET fabrics; however, K/S values were higher than those of the corresponding PET fabrics in some cases. Dyed PLA fibres illustrated good colour fastness, light fastness and sublimation fastness properties, comparable to similarly dyed PET fibres.
1. Introduction
Synthetic fibres, such as polyester, nylon and polyacrylic, have caused increasing environmental concerns due to the use of non-renewable petroleum-based raw materials and the production of non-biodegradable wastes, especially nowadays, since “fast fashion” has significantly increased textile waste [1,2,3,4]. Given that the alternatives to recycling have not so far produced the desired results, the development of biodegradable/bio-based substitutes that have similar characteristics to conventional plastics and are more energy efficient seems to be gaining ground [5]. Currently, barriers to bio-sourced polymers include cost, which is typically higher than that of petrochemically produced polymers. However, bio-sourced polymers are expected to gain more importance, mostly due to increasing public awareness and legislative initiatives promoting them on behalf of consumers [6].
Poly(lactic acid), or PLA, is an aliphatic polyester (Figure 1) [7], which, due to its natural-sourced origin and thus biodegradability properties, is considered a “green”, eco-friendly material [1]. PLA is a synthetic fibre that is fortunately available in large commercial quantities and produced from an annually renewable raw material that is not related to oil. Lactic acid is produced in high quantities by fermentation of molasses, potato starch or dextrose from corn (often called “corn fibre”) [8,9]. Then, PLA is yielded by polymerisation of lactic acid (also known as PLLA or PDLA depending on the stereochemistry of the natural ingredient) by direct condensation or formation of the cyclic dimer intermediate (i.e., lactide) [10,11,12]. The crystallinity of PLA reaches 37%, with a Tm ranging between 173 and 178 °C, a tensile modulus of 2.7–16 GPa and a Tg between 50 and 65 °C [10]. Time, temperature, impurities and the residual catalyst concentration have a great influence on the degradation of PLA. The degree of crystallisation is important in determining the mechanical properties of PLA and its hydrophilicity and moisture retention, which finally also determine its suitability in each application.
PLA has been studied intensively with the aim, among others, of replacing classical polyester fibres, namely poly(ethylene terephthalate) (PET), as fibres, fabrics and eventually textiles [13]. PLA is well suited for melt-spinning into fibres [14] while presenting higher moisture regain, better elastic recovery (i.e., better durability; PLA has 93% recovery at 5% elongation, as opposed to 65% for PET with the rigid aromatics), a higher limiting oxygen index, a lower smoke generation than PET, a lower refractive index and slightly better moisture recovery. It also exhibits excellent crimping and retention capacity [15]. Finally, PLA displays a better UV-resistant performance than other synthetic fibres [16]. These good performance properties of PLA make its implementation in the textile sector a strong and valuable prospect. The manufacture of PLA is estimated to demand 25–55% less fossil energy and 20–50% fewer fossil fuel resources than the manufacture of petroleum-based polymers. As an example, the conversion of 10,000 polyester sports shirts to PLA shirts saves an amount of fossil fuel equal to 2000 L of gas and “greenhouse” gas emissions, which is equal to driving a car for ca. 19,000 km [2]. Around 54,000 metric tons of PLA are used in the textile industry alone. PLA is, so far, primarily used in the packaging industry for replacing PET [4]. In that sector, it is applied as pellets, flakes and thermoplastic films, not as fibres and yarn, which are necessary in the textile industry.
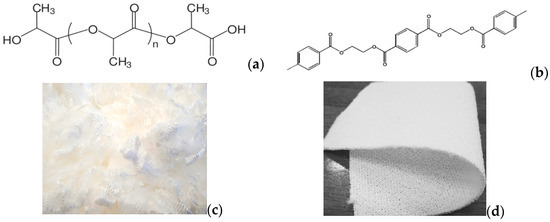
Figure 1.
Macromolecular structures of PLA (a) and PET (b) fibres, along with their macroscopic textures for PLA staple (c) and PET fabric (d), as applied in this research [14].
Figure 1.
Macromolecular structures of PLA (a) and PET (b) fibres, along with their macroscopic textures for PLA staple (c) and PET fabric (d), as applied in this research [14].
Since PLA is a relatively hydrophobic polymer compared to natural cotton, it is not unexpected that disperse dyes have been found to possess relatively high substantivity and affinity for the fibre, as is the case with synthetic fibres [17]. The alternate up and down positions of carbonyl groups (Figure 1) demonstrate that the structure is helical [7,14]. PLA is a thermoplastic polyester too, with poor hydrolytic and thermal stability when compared to industry-standard PET, so it does not always perform well during dyeing. Notably, 100% PLA yarn is used to produce certain clothing, such as activewear, underwear, medical wear and eco-fashion garments, since it provides good softness, breathability and comfort while meeting environmental sustainability standards. Often, PLA is found blended with cotton, polyesters, nylon and lyocell. As is known, PET is dyed with disperse dyes, at higher temperatures, but all disperse dyes suitable for PET dyeing may not provide levelled colour yield on PLA. However, whereas polyester is conventionally dyed at 130 °C (when carriers may not be used) so as to enable dispersed dyes to diffuse at a satisfying level into the material, PLA must be dyed at a lower temperature and over, generally, shorter times. This parallelism of PET towards PLA dyeings is investigated in this research. The optimum applying conditions for dyeing PLA are 110 °C for 30 min under an acidic pH (pH = 5) [1,16,17]. Strong alkaline conditions must be avoided during PLA dyeing. Moreover, excessive dyeing temperatures and/or times promote fibre degradation, manifested as reductions in molecular weight and, subsequently, poorer mechanical properties. In addition, processes other than dyeing, such as heat setting, bleaching and scouring, may have an influence on the physical strength of PLA fibres.
The aim of this study is using six (6) commercial disperse dyes of red, orange, yellow and blue hues (characterized as “low energy” and “high energy” dyes) to be applied to PLA and PET polyester fibres, in order to examine their performance. The novelty of this study is that on the one hand, the specific dyes have not been investigated on PLA, and on the other hand, the variation in energy demands and chemistry of the compounds may cause surprise by their overall performance. The dyeability of the commercial disperse dyes on PLA and PET fibres was examined after applying novel or conventional conditions. The colour properties and fastness of the resultant dyeing were compared, according to their exhaustion, colour measurements, wash fastness, light fastness and sublimation fastness. In that way, a well-evaluated overall performance is reported for each case.
2. Materials and Methods
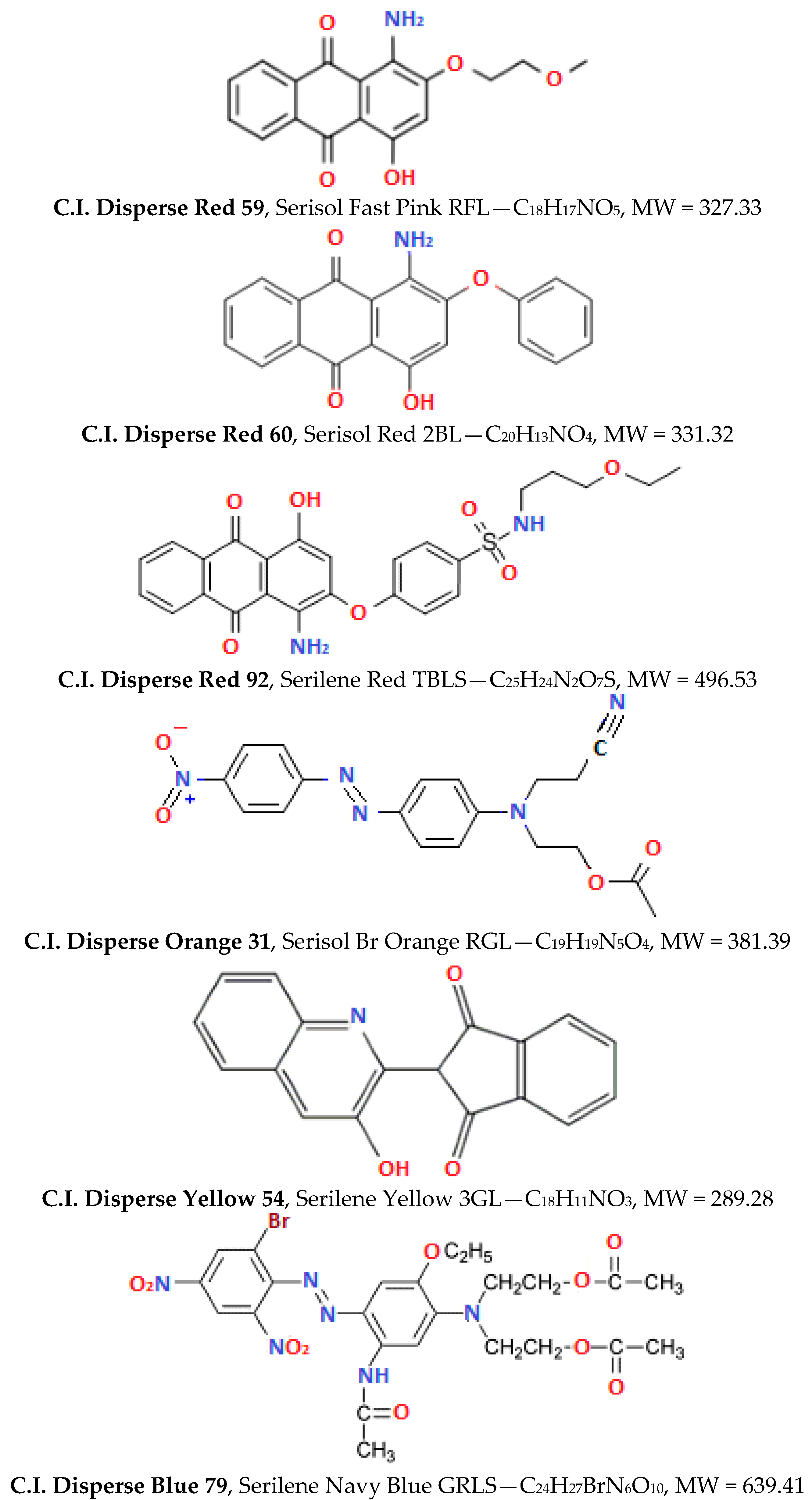
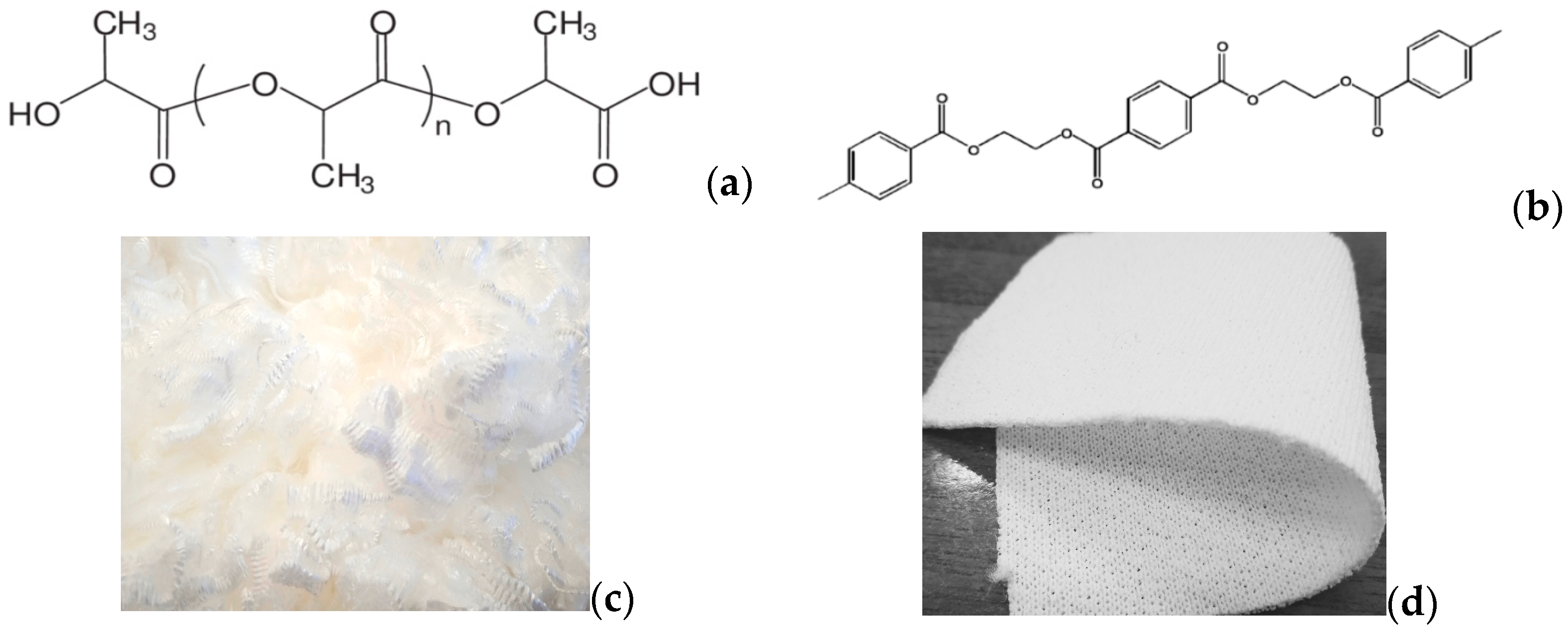
Double-jersey 100% polyester as white fabric (190 g/m2) and lightweight PLA fibre, also white, was supplied by Kyke Hellas S.A (Thessaloniki, Greece) to be dyed, as seen in Figure 1c,d. The PLA is in a staple form, i.e., short yarns, which after twisting transform to greater yarns, and this is why there is no “yarn count” in staple PLA type. Six disperse dyes were used for this study, the chemical structures of which are shown in Figure 2 and the properties in Table 1. The Colour Index (C.I.) name is sourced by the reference database, jointly maintained by the “Society of Dyers and Colourists” and the “American Association of Textile Chemists and Colorists”. Brands “Serilene” and “Serisol” dyes are produced by Yorkshire Chemicals Ltd. (Leeds, UK) [18]. Both names available are noted for each dye in Figure 2, while in the present text, the generic name is used, for convenient reading.
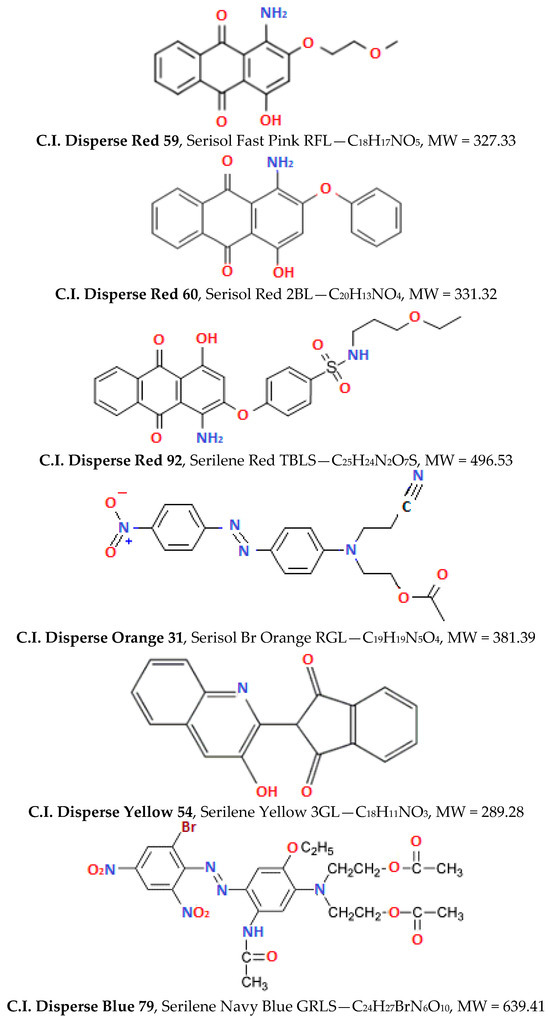
Figure 2.
Chemical structures, formulae and MW of the disperse dyes applied [19].

Table 1.
Energy level requirements and type of disperse dyes applied in research.
Chemicals such as CH3COOH, NaOH and Na2S2O4 (or hydrosulphite) (Merck pa.) were used in experiments, along with commercial auxiliary agents, such as the dispersing agent Alcoosperse AD (Dispergin KS), the cleansing agent Intratex TE 250 (non-ionic wetting agent), Kahatex TE (non-ionic wetting agent), BIOTEX PS 03 (an anionic biodegradable sugar-based dispersing/sequestering agent for all textile processes, Prochimica, Novarese) and finally the acid-reduction agent “Redusante” (a sodium formaldehyde sulphate product), all provided by Kyke Hellas S.A (Thessaloniki, Greece). The carrier agent for the polyester dyeing was Optinol (MBF).
2.1. Dyeing Procedure
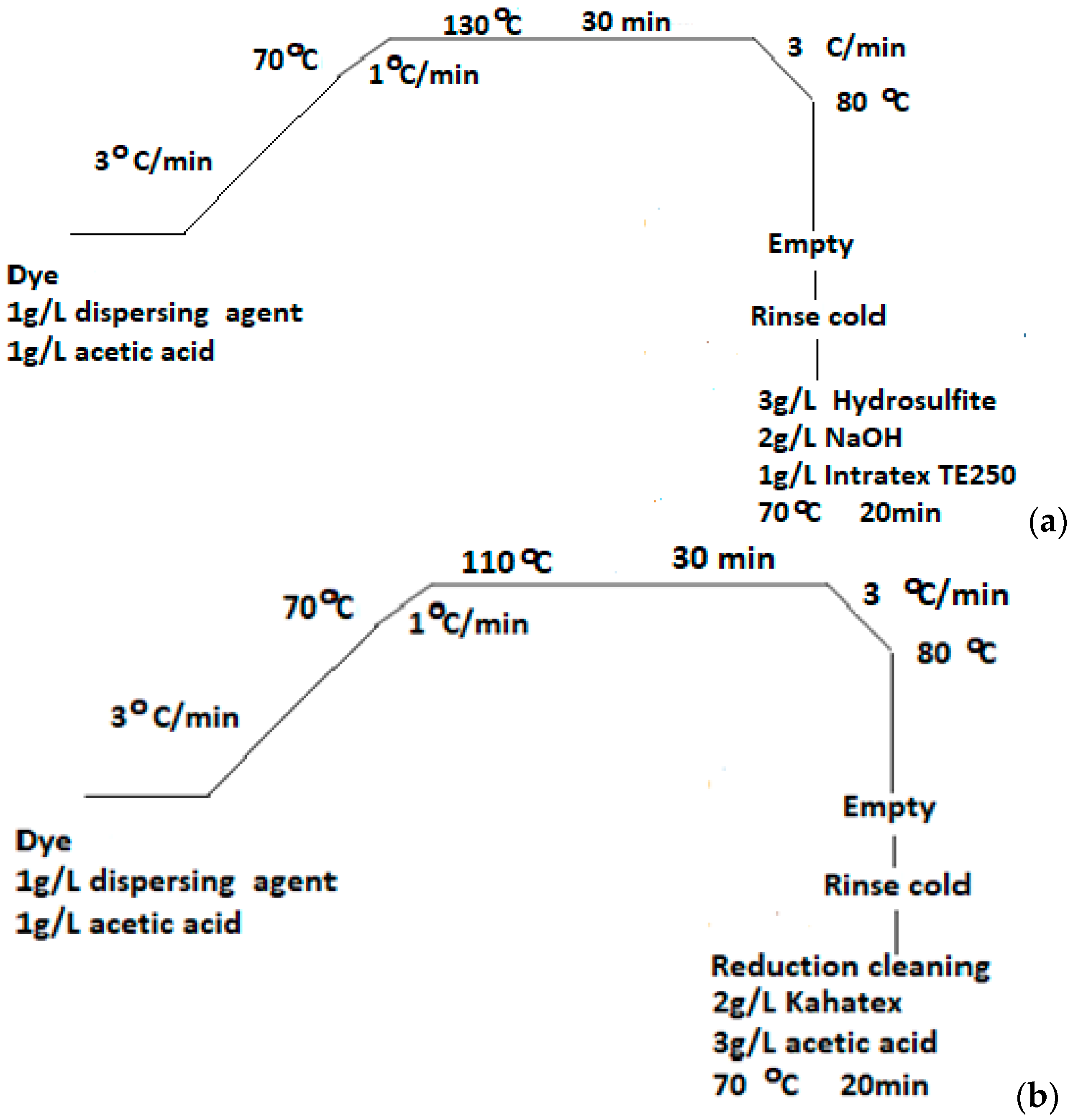
Dyeings were carried out in a Rotadyer dyeing apparatus (John Jeffreys Ltd., Rochdale Banbury, UK) set at the appropriate temperatures, equipped with the metal tubes. PΕΤ fabrics and PLA fibres, of m = 2 g each, thoroughly washed with cold deionised H2O and squeezed. These were then dyed at concentration of 2% o.w.f in all cases. The dyebath included final liquor ratio 1:30, with 2 g/L Alcoosperse, 1 g/LCH3COOH and the process line of Figure 3 was followed. The dyebath temperature was raised at 130 °C (in absence of carrier agent) and 110 °C (in presence of carrier agent) for 30 min, regarding PET fabrics. The dyebath temperature was raised at 110 °C (in both absence and presence of carrier agent) for 30 min, regarding PLA fibres. The time of 30 min was considered more than sufficient for dyeing and the system equilibrium to be achieved. The rinse, cooling and final fixing are described in Figure 3 for each case. Dyed PLA fabric was immersed in a cleansing bath at 70 °C, containing 2 g/L Kahatex, 3 g/L CH3COOH, for 20 min. For PET fabrics, the treatment involved immersion in a dyebath containing 3 g/L Na2S2O4, 2 g/L NaOH, 1 g/L Intratex TE 250 at 70 °C, for 20 min too. The finishing treatments after dyeing are important for “securing” the dye molecules onto the fibres and then evaluating their performance by fastness tests. Samples dyed were allowed to dry, then eventually used for the fastness tests, exhaustion calculations, colour measurements and visual estimations.
Figure 3.
Polyester dyeing processes followed (a) at 130 °C on PET fabric and (b) at 110 °C on PLA fibres.
2.2. UV Absorption Spectrum Measurement and Determination of Exhaustion Yield
The absorption spectra were collected on a Shimadzu UV-1800 UV-Vis spectrophotometer, working with the UVProbe ver. 2.61 software (Shimadzu, Japan). The spectra scan range was 700–400 nm with a slit width of 1 nm and peak threshold of 0.001. The Starna glass cuvettes (type 1, material G, Hainault Industrial Estate England) had a 10 mm path length. The baseline was collected beforehand, regarding the respective fine solution.
Dye exhaustion values were determined for medium-depth shades in each case (at 2% o.w.f. dye concentration). Absorbance values of the dyebaths were measured at the beginning and end of each dyeing process, so to calculate the exhaustion value of the disperse dyes using Equation (1). Dyebath liquor was examined before and after dyeing once diluted with dimethylformamide (Merck pa), at 1 and 5% v/v on dyebath liquor, always protected in dark-glass vials. The absorbance values were measured at each λmax for each case. Below, Equation (1) is applied:
where A0 and A1 are the absorbance values of dyebath liquors at λmax before and after the dyeing operation, respectively. The liquor was checked in all cases to be transparent, despite the presence of disperse dyestuffs, since DMF was used as solvent.
2.3. Colour Measurements
A Macbeth CE 3000 spectrophotometer under D65 illumination, 10° standard observer with UV included and specular component included was elaborated for colour index measurements. The samples were folded twice (i.e., opaque) and four measurements were performed each time with the incident light beam. Dyed specimens were examined for their colour strength and evaluated by the light reflectance technique, using the Kubelka–Munk Equation (2):
where S is the scattering coefficient; K is the absorption coefficient of the dyed fabrics (forming the ratio of colour strength) and R is the reflectance of the dyed fabrics at the maximum absorption wavelength. Reproducibility was checked by taking four measurements and calculating the st. variation in %R values over the range of 400–700 nm. It was found to be satisfactory in all cases.
2.4. Tests on Fastness Properties
Wash fastness tests were carried out according to the BS 1006:1990 C06 standard (colour fastness of domestic and laundering washing) [20], using a Zeltex Vistacolor dyeing apparatus. Initially, dyed samples were cut into 4 cm × 10 cm dimensions and stapled with the same dimensions of multi-fastness witness fabric, consisting of 6 different types of fabric in the following order: wool, acrylic, polyester, nylon, cotton and cellulose acetate. Next, in beakers of 250 mL, dyed samples remained in 100 mL of soap water solution of 4 g/L concentration (as set by the standards ISO 105:1989: Parts C01 and C02 Colour fastness to washing: Tests 1 and 2 [21] and BS EN 20105:1993: Colour fastness to washing: Test 2 [22]), for 30 min after the temperature reached 40 °C. After the washing was completed, the samples were removed, separated from the multi-fastness witness fabric, which was rinsed twice with deionized water and allowed to dry.
Light fastness was determined according to the BS 1006:1990 B02 standard (Methods of test for colour fastness of textiles and leather [20]), using a Q-SUN Xe-1-B xenon light fastness machine. In this case, the dyed samples were cut to 1 cm × 6 cm dimensions, secured in a metal case, half-masked, in order to separate two individual areas, where one is covered and the other is in direct contact with light. At the same time, a “blue” scale witness fabric and the woollen standards supplied by James H. Head Co. Ltd., Calderdale, UK (batch No. 000932), were introduced alongside the samples in the ageing device, on the 10°-slanted tray, cut in the same dimensions and placed beside the witness fabric. The exposure was for 48 h, at 340 nm, 0.50 W/m2, in an air-ventilated chamber at 50 °C.
The machine used for the sublimation fastness test was Heraeus Original Hanau. The samples were coated on one side with a white polyester control and on the other side with a white polyamide control and exposed to 150 °C for 30 s. Then, the white control fabrics were evaluated with the scale of greyscale, in terms of contrast changes.
Colour shades for all samples were examined visually under standard conditions, in a Mulder’s VeriVide® observation chamber, equipped with a D65 lamp (which mimics the average natural daylight) under 45° observation angle. In addition to the blue wool standards’ observation, a visual observation of the samples also took place, in comparison to the greyscale contrast scale (the scale 5 to 1 indicates gradual contrast increase) as described in ISO 105-A02: Colour fastness-Grey scale for assessing change in colour [21] (BS 1006 A02:1989: Methods of test for colour fastness of textiles and leather. Grey scale for assessing change in colour, SDC Standard Methods 4th edition A02 of the British Standards Institution [20]), in order to detect the extent of colour changes. The observations/comparisons between the masked and unmasked samples, or towards the blue witness fabric changes, were made 48 h after their ageing in the Q-SUN chamber. In all cases, the same experienced experimenter performed the naked-eye visual observations, following the same pattern, under the same conditions.
3. Results and Discussion
Despite the standardised tests, which have been developed to describe the dyeing using disperse dyes on PET fibres, individual dye-makers/providers commonly classify commercial dyestuffs according to, for instance, their diffusion profile, fastness endurance, sublimation behaviour, etc. One such common classification method categorises dyes as colourants of low-, medium- or high energy. According to this, the energy level increases as the molecular size and polarity of the dye molecules increase, and, simultaneously, volatility decreases (i.e., this is when the sublimation fastness increases) and wet fastness increases. Usually, low-energy (small molecular size, low polarity) dyes display good levelling and rapid adsorption rates, while higher energy (larger size, more polar) dye molecules exhibit low dyeing rates and poor migration [23]. In our case, readers may find it useful to keep in mind the information provided in Table 1 and Figure 2, when evaluating the results that unfold beyond, and the correlations made to substrates (Figure 1). D. Red 59 (MW = 327) and 60 (MW = 331) along with D. Yellow 54 (MW = 289) are dyestuffs of low energy, while D. Red 92 (MW = 496), D. Orange 31 (MW = 381) and D. Blue 79 (MW = 639) are dyestuffs of high energy. All molecules include O, N heteroatoms. For the last one, Br is an electronegative element, a stable leaving substitute.
3.1. Dye Exhaustion After the PET and PLA Dyeings
Disperse dyes are soluble in DMF, and this is how we measure their concentration via UV-Vis. Thus, we evaluate their strength and determine accurately their exhaustion profiles. The UV-Vis spectra taken from each dyebath illustrate a maximum absorption for each disperse dye. The data recorded are summarised in Table 2. Notice that D. Red 60, Red 92 dyes’ spectra occurred showing a split peak; however, the highest values are reported. High absorptions on several samples are attributed to the high concentration of the dyestuff in solutions measured, in spite of the dissolution taking place in all cases. So, special care is taken for recording the λmax values and filling (Table 2). The presence of carrier agent shifts the max absorption of the dyebath to higher wavelengths, since the surfactant compounds that the dyebath solutions contain produce slightly “duller” results. Their use is obligatory though, since often, in synthetic fibres, the performance of dyeing is improved by the use of carriers, who “carry” the dyestuff molecules among the fibrous macromolecules, by providing distance between them, to facilitate the dyestuff molecules getting into and among the fibres. Carriers are usually phenolic compounds, primarily amines, hydrocarbons and ethers, e.g., o- and p-hydroxydiphenyl, methylnaphthalene and methyl salicylic acid ester, dedicated to polyester dyeing, to improve levelness, penetration and bare coverage [24]. Regarding PLA samples, the λmax absorptions are identical with the ones listed in Table 2 of the PET dyeing, in the absence of a carrier agent. In fact, the carrier is mandatory mostly for PET dyeings, since the stiff alipharomatic macromolecular skeleton is harder to “open” to dyestuff molecules; this is not the case for PLA linear macromolecules.

Table 2.
The λmax recorded in each case, regarding the dyebath solutions (for PET dyeing).
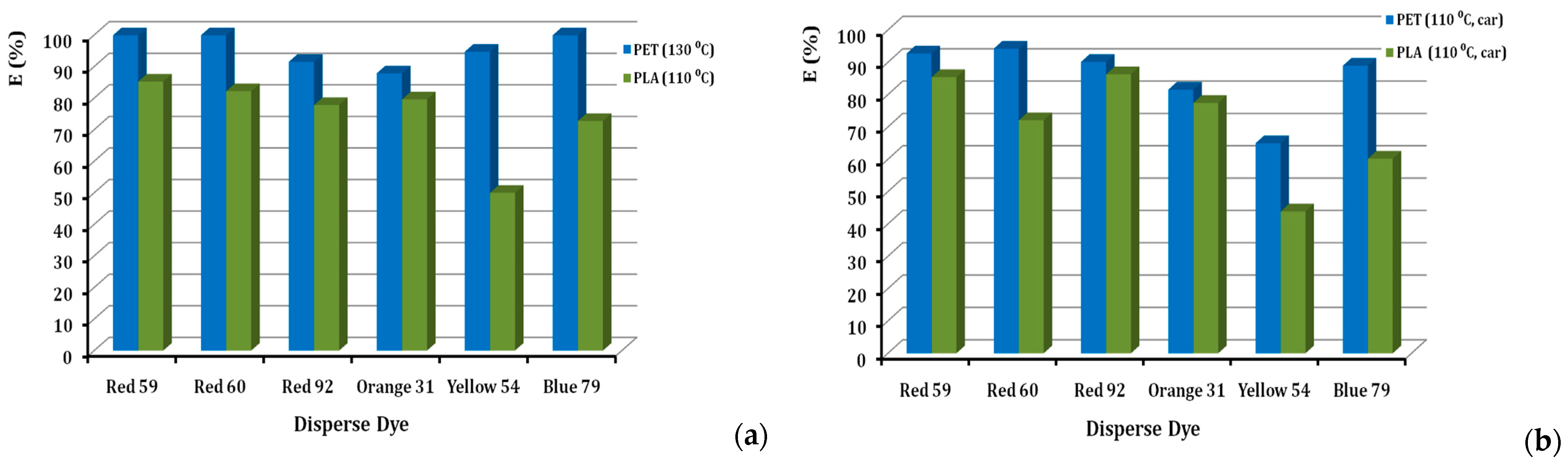
Percentages of dye exhaustion (%E) for each of the six dyes, when applied to PLA and PET, are given in Figure 4. The value of the exhaustion of the dyebath is an important parameter, both as a sign of colour resilience and at the same time, as a direct relation to the cost of the dyeing process and the control of its sewage [25]. The exhaustion of all disperse dyestuffs is higher on polyester that is dyed at 130 °C, when compared to the exhaustion of the same dye on PLA at 110 °C, in the absence of a carrier agent (Figure 4a). This can be attributed to the more accessible polyester structure at the higher temperature, allowing the absorption and diffusion of more dye molecules in the polymeric substrate of polyester. The temperature of 130 °C cannot be applied to PLA, due to the low durability of the macromolecules at that temperature. In addition, exhaustion values for each of the six dyes, when applied to PLA and PET at a dyeing temperature of 110 °C with a carrier agent, are given in Figure 4b. The same pattern is observed in dyeing at 110 °C (in the presence of a carrier for both PET and PLA), as can be seen in Figure 4b, where all disperse dyes exhaust more on polyester rather than the PLA fibres. Meanwhile, all classes of disperse dyes seem to become exhausted on both substrates to acceptable levels; the only exception is the D. Yellow 54, which shows the lowest exhaustion rate on PLA. This can possibly be attributed to the lower molecular size of its quinoline type, MW = 289. E(%) values range widely, being as low as 50% for D. Yellow 54 and as high as 85% for D. Red 59. The rest of the dyes have exhaustion values above 70% at 2% o.w.f. According to the literature, the D. Yellow 54 has a low %sorption [26], which explains the low %E of this colour. In Figure 4b, almost all the dyes, except D. Yellow 54 (43.9%), have exhaustion values above 50%. In this case, for the D. Red 92, the %E is higher for PET than PLA, and generally higher than that of PLA (110 °C) without a carrier. On the other hand, the D. Blue 79 has a lower %E (50.2%) on PET than PLA (110 °C) without a carrier (72.8%), and similarly, the D. Red 60 showed %E (52.1%) lower than PLA (at 110 °C) without a carrier (82.2%). Generally, it is shown that the dye-uptake value of the PET fabric is higher than the PLA fibre, due to its high dyeing temperature [27]. In a more thorough investigation, considering the detailed chemical structures of the commercial dyes, it is perhaps necessary to understand the dye affinity to PLA. Lunt & Bone stated that medium-energy dyes were the most suitable ones for PLA [15,28]. Linear dyes were found to give good exhaustion rates on high crystallinity spun-bonded PLA [4].
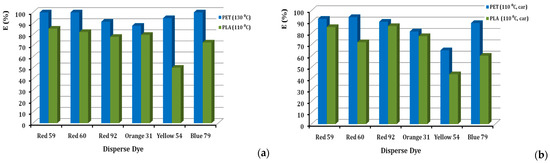
Figure 4.
Comparison of % exhaustion of disperse dyes on PLA fibres and PET fabrics, dyed at (a) 110 °C and 130 °C, respectively, and (b) at 110 °C in the presence of carrier, at 2% o.w.f.
High dye-uptake is important, since it reduces the dye consumption required to obtain a given shade, especially for a dark shade. Lower dye consumption leads to less pollution, due to less waste and lower dyeing costs. High dye uptake indicates that the dye has a high affinity for the fibre and, therefore, good colour fastness. The light tone of 2% o.w.f. facilitates an easier judgement of these issues. Furthermore, D. Red 59 dye which belongs to the anthraquinone class, was expected to show low interaction with PLA [7]. The high depletion rates of D. Red 59 (85%, with or without carrier) compared to D. Red 60 (70%, with carrier) are probably justified by the presence of the alkyl chain of the former, versus the aromatic ring of the latter [7]. On the contrary, the dye D. Blue 79 presents the lowest depletion rate, both in the absence of a carrier (73%) and in the presence of a carrier (50%). This fact may be connected to the bromine substituent (-Br), linked by a single bond to the carbon skeleton, which forms weak interactions with PLA [29]. Therefore, we can estimate that our experimental results are in affinity with the literature.
Apart from the different chemistry among the dyestuff molecules and between the substrates (mainly the presence of the aromatic ring in PET), the form of the substrates may affect the thermodynamic of the dyebath. The diffusion may be easier in PLA staples but delocation is also probable. It is considered that little variations are observed, attributable to the substrate’s form: fibre, yarn, staple, bulk and fabric. PLA staples, whether used alone or blended with various fibres, such as cotton, linen, silk, wool, viscose and lyocell, can create a wide range of clothing fabrics. PET is a common candidate to co-exist with PLA, since a similar behaviour facilitates their dyeing.
3.2. Colour Properties of the PET and PLA Dyeing
The colour durability and the shades of dyed PLA fibres are slightly different from those of the PET fabric, under the same conditions. These properties are influenced by the dye chemical structures and polyester macromolecular structures [4]. Colorimetric data using the CIELAB system are given in Table 3, for dyeing PET at 130 °C and PLA at 110 °C, showing the greatest temperature applied, without a carrier agent. It is interesting to note that PLA shows a higher uptake for D. Blue 79 and D. Red 59, whereas PET shows a higher uptake for D. Red 60, D. Red 92 and D. Yellow 54. It is evident that dye structure plays an important role in the uptake on both substrates. The highly hydrophobic, high MW anthraquinone D. Red 92, D. Red 60 and simple quinoline D. Yellow 54 are absorbed to a higher extent on the more hydrophobic polyester fibre, rather than on the aliphatic, less hydrophobic, PLA fibre. The D. Red 59, being smaller than D. Red 60 and D. Red 92 anthraquinone dyes, is more easily absorbed by PLA. The azo dye D. Blue 79 shows a higher uptake on PLA than on PET, possibly due to the presence of a number of ionizable groups in the structure, making it more substantiative to the less hydrophobic aliphatic PLA. In the case of the smaller azo dye D. Orange 31 with less “ionizable” groups to D. Blue 79, the dye uptake is almost the same on both fibres.

Table 3.
Colorimetric values of the PET (at 130 °C) and PLA (at 110 °C) dyeing.
The same pattern is shown in Table 4, which shows the colorimetric data of the dyeing performed at 110 °C for PET and PLA, in the presence of a carrier. The anthraquinone type D. Red 60, D. Red 92 and quinoline type D. Yellow 54 show a higher uptake on PET. The less hydrophobic azo dyes D. Blue 79, D. Orange 31 and the smaller anthraquinone dye D. Red 59 show a higher uptake on PLA. Through analysis of the absorbance and uptake capability, eventually, colours of a “heavier” shade are the result. Brighter dyeing and higher C* values were generally obtained on PLA for D. Blue 79, D. Red 59 and D. Orange 61; meanwhile, for PET fabric, the colours D. Red 60, D. Red 92 and D. Yellow 54 can be explained in the same terms as above.

Table 4.
Colorimetric values of the PET and PLA dyed at 110 °C, in presence of carrier.
3.3. Colour Yield
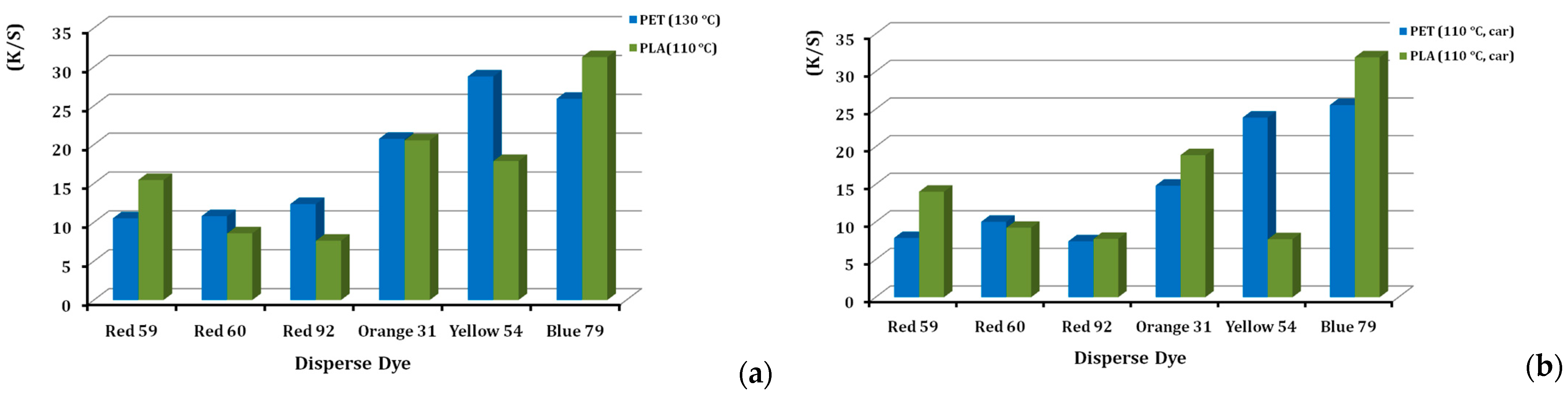
Colour yield is a term used to describe colour efficiency and strength. The colour yield for each of the six dyes, when applied to PLA and PET, are given in Figure 5a,b, either at 130 and 110 °C or in the presence and absence of a carrier agent. As shown in Figure 5a, the colour yield on PLA is higher than on PET in both cases, although disperse dye exhaustion on PLA is lower than that on PET [25], which was attributed to the lower temperature applied. The explanation was the higher reflectance of PLA [4,14,25]. The final rinse and setting in polyester dyeing are of great importance (Figure 3), in order to immerse the final shade; after this, the disperse dyestuff that is not retained goes away. Moreover, the way to maintain the colour achieved, over time, demands the appropriate surfactant chemistry. PET polyester usually is finalized with alkali reduction, but for other synthetic fibres, like polyamide, Lycra®, polyacrylic and acetates, alkali reduction is too harsh, so acidic reduction cleansing is preferred (Figure 3). We chose this type for PLA too, as a more sensitive fibre compared to PET. “Redusante” agent is applied for this reason, along with the other non-ionic wetting agents described in Figure 3. Regarding the differences in the physical form of the PLA fibres and PET fabric, we consider that this slightly affects the colour result, because the equilibrium conditions were achieved upon dyeing. This means that we are certain the best colour yield possible was achieved in each case.
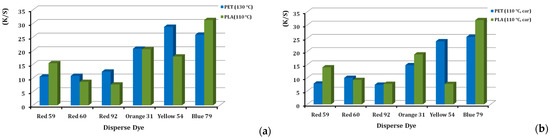
Figure 5.
Comparison of colour strength (K/S) of disperse dyes on PLA fibres and PET fabrics, dyed at (a) 110 °C and 130 °C, respectively, and (b) at 110 °C, with the presence of carrier, at 2% o.w.f.
The colorimetric coordinators illustrated in Table 3 may be verified visually via the images in Figure 6, where the red colours vary a bit to more pink or bright shades. The PLA samples were dyed brighter and as more “vivid” colours than those of PET, as shown in Figure 6: the higher reflectance for PLA is verified again. The polymer environment affects the light absorption properties of dyes. The brightness of PET and PLA can be explained based on Fresnel reflection (i.e., the reflection of light at the interface between two different media with different refractive indexes). Since surface reflection decreases with decreasing refractive index, it is logical that PLA dyes will appear brighter than PET dyes when dyed at the same concentration, meaning the same number of dye molecules per unit volume of the fibre [7].

Figure 6.
Visual result of the PLA fibres (top row) and PET fabrics (low row) dyed with D. Red 59, D. Red 60, D. Red 92, D. Orange 32, D. Yellow 54 and D. Blue 79, as received after dyeing, without carrier at 2% o.w.f.
3.4. Fastness Outcomes
The wash and light fastness properties of PLA and PET dyeing are shown in Table 5, when they were treated in the absence of a carrier, and in Table 6 similarly, in the presence of a carrier. Thus, wash tolerance results of the disperse dyes on PLA and PET, dyed at 110 and 130 °C correspondingly, without a carrier are shown, on the grey scale (1–5 out of 5). A very experienced experimenter visually judged the fastness results, under similar standard conditions in each case.

Table 5.
Wash and light fastness of dyed PET (130 °C) fabrics and PLA (110 °C) fibres.

Table 6.
Wash and light fastness of dyed PET and PLA fabrics at 110 °C, in presence of carrier agent.
Table 5 illustrates that the conventional dyeing conditions of both fibres are the same for the small molecular and low-energy disperse dyes, such as D. Red 59, D. Red 60 and D. Orange 31, whereas on PLA, they are worse for the high-molecular, high-energy D. Red 92 and D. Blue 79. This can be explained in terms of the polymeric structure of both fibres; PET having a higher Tg, more crystalline compact structure, compared to lower Tg and less crystalline structure of PLA, makes desorption of disperse dyes easier from PLA fibres. In Table 6, dyeing at 110 °C for both fibres in the presence of a carrier proved that the wash fastness is generally worse again for PLA, with the exception of the more hydrophobic dyes D. Red 60 and 92 which are slightly worse on PET. This is common when a carrier is involved to lower the dyeing temperature of PET since a “ring dyeing” of the PET occurs, which shows inferior wash fastness properties. The light fastness is generally the same or lower on PLA for the azo dyes D. Blue 79 and D. Orange 31, the same or slightly worse for the azo disperse dyes D. Blue 79 and D. Orange 3, inferior for the anthraquinone dyes D. Red 59, 60 and 92 and much worse for the quinoline type D. Yellow 54. Wash fastness is judged on the multifibre witness fabric correlation, thus, in comparison to white diacetate, cotton, polyamide (nylon), polyester, poly(cellulose acetate) and wool, when dyed PLA or PET are washed along with them. The existence of coloured staining or a light hue on the white comparison fabrics is evaluated in each case.
On the other hand, as seen in Table 6, the blue woollen witness fabric, scaled 1–8 out of 8, helps us with the characterization of colour tolerance against light exposure. It seems that the more hydrophobic dyes are affected to a higher extent as far as fastness to light is concerned. The light fastness of PLA dyeing was lower than on PET. One of the reasons for this may be attributed to the lower dye exhaustion on PLA than on PET [17,25]. Generally, the light fastness was very good in both PLA and PET. The PLA macromolecules are more transparent to light and UV radiation in the 370–240 nm range than PET, because of the linear structure (absence of aromatic rings); the “glow” seen in some regions of the PLA fibres (Figure 1) verify that. The UV radiation penetrating the PLA macrochains may result in poor light fastness of disperse dyes on PLA, possibly due to the destruction of the dye chromophore. The interaction between the dye and fibre, the exhaustion, the distribution of the dye in the fibre along with the dye crystallization agglomeration, all have impact on light fastness [7,15,25]. Scale 1 means being the least resistant and 8 being the highest resistance, towards 340 nm irradiation.
Sublimation fastness is commonly associated with the dyeing of polyester disperse dyes. Sublimation is the process of phase change from solid to gas, without going into a liquid phase. Fastness to sublimation is probably the most important requirement of dyed polyester, apart from fastness to light. When the temperature rises above a certain point (setting above 180 °C, ironing above 200 °C), the disperse dyes in the polyester fibres directly convert from powdery solids to gas and escape, changing the colour of the polyester. Regarding the thermosetting that some fibres go through, in this case, there is no need, since there is no Lycra® to stabilize. PLA with low Tg and Tm cannot be stabilized (even if containing Lycra®). The sublimation fastness against staining (CS) and shade change values (CC) of PLA and PET (dyed at 110 and 130 °C, respectively) in the absence of a carrier agent are shown in Table 7. Similarly, the results observed on greyscale, when polyester is dyed in the presence of a carrier agent, are seen in Table 8.

Table 7.
Sublimation fastness results on greyscale for dyed PET at 130 °C and PLA at 110 °C.

Table 8.
Sublimation fastness results on greyscale for dyed PET and PLA at 110 °C, in the presence of a carrier.
The sublimation fastness results of PLA and PET fibres were very good, with grey scale ratings at 5 out of 5, without any colour change and without any staining on the polyester and polyamide fibres. The only exception is that of D. Red 60 on PLA, in both the presence and absence of a carrier, causing staining of the polyester and the polyamide witness fabrics. It is important to note that the sublimation fastness was performed at 150 °C temperature for both PLA and PET. This temperature is a bit high for PLA, higher to PLA’s Tg temperature and at the same time, much lower to PET’s Tm temperature. Despite this, PLA showed very good sublimation fastness characteristics. This temperature is high for PLA and this is essential. From the literature, PLA exhibits no dye loss by sublimation at 130 °C, due to this low heat-setting temperature [30]. In this study, the sublimation temperature for PLA was 20 °C higher (150 °C) than the literature [31,32]. Sublimation for polyester and polyamide alone could rise to 175–205 °C or 165–170 °C, but the bio-sourced PLA does not allow those ranges.
Due to the high thermal stability of PET, extra thermosetting occurs for the PET feedstock which is destinated for the textile industry. In fact, it is given that PET is one of the most important raw materials used in the textile industry. It is also known that this is the case due to its great properties, such as mechanical and chemical resistance, low moisture absorption, good tensile values and thermal resistance. The above properties practically imbue the fabrics with resistance to processes such as washing, ironing and resistance to ultraviolet radiation [33]. At the same time, recycled PET (rPET), already induced in the textile industry as co-weaved fibre, and later launched by great firms as a good solution for diminishing cloth-waste, raises concerns regarding its degraded mechanical properties (like reduction in the strength, lower flexibility) and clarity of the material [34]. PLA has its own advantages and, certainly, the crucial environmental low-impact factor. As a mimic of PET, PLA fibres or fabrics may cover several textiles’ uses and demands, with no lag in dyeing properties, applying, of course, similar disperse dyes.
This research proved that D. Red 59, 60 and 92 (the last one with greater MW than the first two; all of high-energy demands) dyed PET fabric and PLA fibres at good levels. Between the two, PET was dyed in better terms as anticipated, due to anthraquinone type and hydrophobic yarns applied [35,36]. However, the impressive element is that D. Blue 79 with the great MW = 639, a compound of azo type, managed to become dispersed and to dye the PLA fibre to a significant degree with the aliphatic macromolecular structure, which is less hydrophobic than PET. The D. Orange 31 is of the same impressive pattern, an azo type colourant, of MW = 381, and showed great results on PLA. As for quinoline type D. Yellow 54, a rather peculiar structure, it performed satisfactorily on K/S values, even in the PET dyeing, where the conditions were more favourable. All in all, the findings of this research are evaluated as worthy for further applications on an industrial scale, for the dyeing of PLA fibres and fabrics.
The limitations of this research study include the small sample sizes and dyebath volumes applied, considered to be able to promote more successful dyeing under the same temperatures and times. The experiments were planned in an industrial manner; additionally, some crucial ingredients of the baths were industrially supplied to mimic the actual process. However, it is difficult to study and discuss the thermodynamics and diffusion mechanisms in disperse systems.
4. Conclusions
In this work, the dyeability was studied of PLA fibres and PET fabric with six commercial disperse dyes at 130 °C for PET and 110 °C for PLA, without a carrier agent, and at 110 °C for both PET and PLA, in the presence of a carrier. The fastness properties, such as against wash, light and sublimation, were evaluated. The exhaustion of dyes was generally higher on PET, whereas the colour uptake was similar or higher on PLA, with the exception of D. Yellow 54, which was substantially higher on PET. The PLA dyeing exhibited greater L* and C* values in almost all samples. Wash fastness of the PLA dyeings was generally similar or slightly worse than the corresponding PET dyeings. The light fastness of PLA dyeing was lower than that of corresponding PET dyeing for all samples. The sublimation fastness results of PLA and PET fibres were excellent, with grey scale ratings at 5, without any shade change and without considerable staining of the polyester and polyamide fibres in almost all samples. The only exception was D. Red 60, which was worse on PLA. It can be concluded that the six commercially available disperse dyes, originally developed for PET, also dye PLA to an acceptable level, without any detrimental effect on fastness properties.
Author Contributions
Conceptualization, N.F.N.; methodology, S.S.L.; validation, L.P., E.T. and S.S.L.; formal analysis, L.P., E.T. and S.S.L.; investigation, L.P., E.T. and S.S.L.; data curation, S.S.L. and E.C.V.; writing—original draft preparation, S.S.L. and E.C.V.; writing—review and editing, E.C.V.; visualization, E.C.V.; supervision, N.F.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suesat, J.; Suwanruji, P. Dyeing and Fastness Properties of Disperse Dyes on Poly(Lactic Acid) Fiber; InTech: Rijeka, Croatia, 2011; pp. 351–372. [Google Scholar]
- Avinc, O.; Bakan, E.; Demirçalı, A.; Gedik, G.; Karcı, F. Dyeing of poly (lactic acid) fibres with synthesised novel heterocyclic disazo disperse dyes. Color. Technol. 2020, 136, 356–369. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, W.Y. Coloration of poly (lactic acid) with disperse dyes. 1. Comparison to poly(ethylene terephthalate) of dyeability, shade and fastness. Fibers Polym. 2006, 7, 270–275. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Ju, Z.; Tam, P.Y.; Hua, T.; Younas, M.W.; Hasan, K.; Hu, H. Poly(lactic acid) fibers, yarns and fabrics: Manufacturing, properties and applications. Text. Res. J. 2021, 91, 1641–1669. [Google Scholar] [CrossRef]
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for food packaging. Trends Food Sci. Technol. 2022, 125, 66–80. [Google Scholar] [CrossRef]
- Payne, J.; McKeown, P.; Jones, M.D. A circular economy approach to plastic waste. Polym. Degrad. Stabil. 2019, 165, 170–181. [Google Scholar] [CrossRef]
- Baig, G.A. Coloration of poly (lactic acid) based textiles–A review. Polimery 2020, 65, 415–429. [Google Scholar]
- Nandakumar, A.; Chuah, J.A.; Sudesh, K. Bioplastics: A boon or bane? Renew. Sust. Energ. Rev. 2021, 147, 111237. [Google Scholar] [CrossRef]
- Avinc, O.; Khoddami, A. Overview of poly (lactic acid)(PLA) fibre: Part II: Wet processing; pretreatment, dyeing, clearing, finishing, and washing properties of poly (lactic acid) fibres. Fibre Chem. 2010, 42, 68–78. [Google Scholar] [CrossRef]
- Abdelrazek, S.; Abou Taleb, E.; Mahmoud, A.S.; Hamouda, T. Utilization of polylactic acid (PLA) in textile food packaging: A review. Egypt. J. Chem. 2022, 65, 725–738. [Google Scholar] [CrossRef]
- Idumah, C.I.; Nwachukwu, A. Comparative analysis of the effects of time of heat setting and wet processing on tensile properties of treated and untreated knitted PLA fabric. Am. J. Mater. Sci. Eng. 2013, 1, 40–45. [Google Scholar]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and biological application of polylactic acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Xu, S.; Chen, J.; Wang, B.; Yang, Y. Sustainable and hydrolysis-free dyeing process for polylactic acid using nonaqueous medium. ACS Sustain. Chem. Eng. 2015, 3, 1039–1046. [Google Scholar] [CrossRef]
- Dugan, J.S. Novel properties of PLA fibers. Int. Nonwovens J. 2001, 3, 1558925001OS-01000308. [Google Scholar] [CrossRef]
- Hussain, T.; Tausif, M.; Ashraf, M. A review of progress in the dyeing of eco-friendly aliphatic polyester-based polylactic acid fabrics. J. Clean. Prod. 2015, 108, 476–483. [Google Scholar] [CrossRef]
- Sisodia, N.; Parmar, M.S. Dyeing behavior and fastness properties of corn (PLA) fiber. IOSR J. Polym. Text. Eng. 2014, 1, 1–7. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, M.H.; Park, J.S.; Jeon, J.M.; Kim, D.O.; Towns, A.D. Coloration of poly (lactic acid) with disperse dyes. II. Dyeing characteristics and color fastness. Fibers Polym. 2007, 8, 37–42. [Google Scholar] [CrossRef]
- Disperse Dyestuffs. Available online: https://yorkshire-farben.eu/textile/disperse-dyestuffs/ (accessed on 16 February 2025).
- World Dye Variety. Available online: https://www.worlddyevariety.com/ (accessed on 16 February 2025).
- British Standard BS1006:1990. Available online: https://yiqi-oss.oss-cn-hangzhou.aliyuncs.com/aliyun/900102963/solution/527233.pdf (accessed on 16 February 2025).
- International Standard Organization ISO105:1989. Available online: https://www.iso.org/standard/3801.html (accessed on 16 February 2025).
- British Standard BS EN 20105:1993. Available online: https://knowledge.bsigroup.com/products/textiles-tests-for-colour-fastness-colour-fastness-to-washing-test-2 (accessed on 16 February 2025).
- Burkinshaw, S.M. The roles of elevated temperature and carriers in the dyeing of polyester fibres using disperse dyes: Part 1 fundamental aspects. Color. Technol. 2024, 140, 149–207. [Google Scholar] [CrossRef]
- Serisol and Serilene Dyes for Polyester. Available online: https://yorkshire-espana-sa.com/wp-content/uploads/2019/02/SERISOL-SERILENE-DYES.pdf (accessed on 16 February 2025).
- Yang, Y.; Huda, S. Comparison of disperse dye exhaustion, color yield, and colorfastness between polylactide and poly (ethylene terephthalate). J. Appl. Polym. Sci. 2003, 90, 3285–3290. [Google Scholar] [CrossRef]
- Karst, D.; Hain, M.; Yang, Y. Care of PLA textiles. Res. J. Text. Appar. 2009, 13, 69–74. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, W.S. Dyeing properties on polylactic acid (PLA) fabrics by disperse dyes. J. Korean Soc. Cloth. Text. 2013, 37, 952–961. [Google Scholar] [CrossRef][Green Version]
- Lunt, J.; Bone, J. Properties and dyeability of fibers and fabrics produced from polylactide (PLA) polymers. AATCC Rev. 2001, 1, 20–23. [Google Scholar]
- Karst, D.; Nama, D.; Yang, Y. Effect of disperse dye structure on dye sorption onto PLA fiber. J. Colloid Interface Sci. 2007, 310, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Suesat, J.; Taylor, J.A.; Wilding, M.; Farrington, D.; Bone, J.; Dervan, S. Thermal migration of selected disperse dyes on poly(ethylene terephthalate) and poly(lactic acid)(Ingeo†) fibres. Color. Technol. 2004, 120, 260–264. [Google Scholar] [CrossRef]
- Avinc, O.; Phillips, D.; Wilding, M. Influence of different finishing conditions on the wet fastness of selected disperse dyes on polylactic acid fabrics. Color. Technol. 2009, 125, 288–295. [Google Scholar] [CrossRef]
- Avinc, O.; Wilding, M.; Bone, J.; Phillips, D.; Farrington, D. Evaluation of colour fastness and thermal migration in softened polylactic acid fabrics dyed with disperse dyes of differing hydrophobicity. Color. Technol. 2010, 126, 353–364. [Google Scholar] [CrossRef]
- Palacios-Mateo, C.; Van der Meer, Y.; Seide, G. Analysis of the polyester clothing value chain to identify key intervention points for sustainability. Environ. Sci. Eur. 2021, 33, 2. [Google Scholar] [CrossRef]
- Chilton, T.; Burnley, S.; Nesaratnam, S. A life cycle assessment of the closed-loop recycling and thermal recovery of post-consumer PET. Resour. Conserv. Recycl. 2010, 54, 1241–1249. [Google Scholar] [CrossRef]
- Liang, H.E.; Zhang, S.; Tang, B.; Wang, L.; Yang, J. Dyeability of polylactide fabric with hydrophobic anthraquinone dyes. Chin. J. Chem. Eng. 2009, 17, 156–159. [Google Scholar] [CrossRef]
- Ovil, F.; Mahmud, S. Sensitivity analysis of medium & high energy disperse dyes in polyester dyeing. Int. J. Text. Sci. 2021, 10, 27–38. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).