Radical and Catalyst Effect on Fenton-like Textile Dyes’ Degradation Process and Techno-Economical Consideration
Abstract
1. Introduction
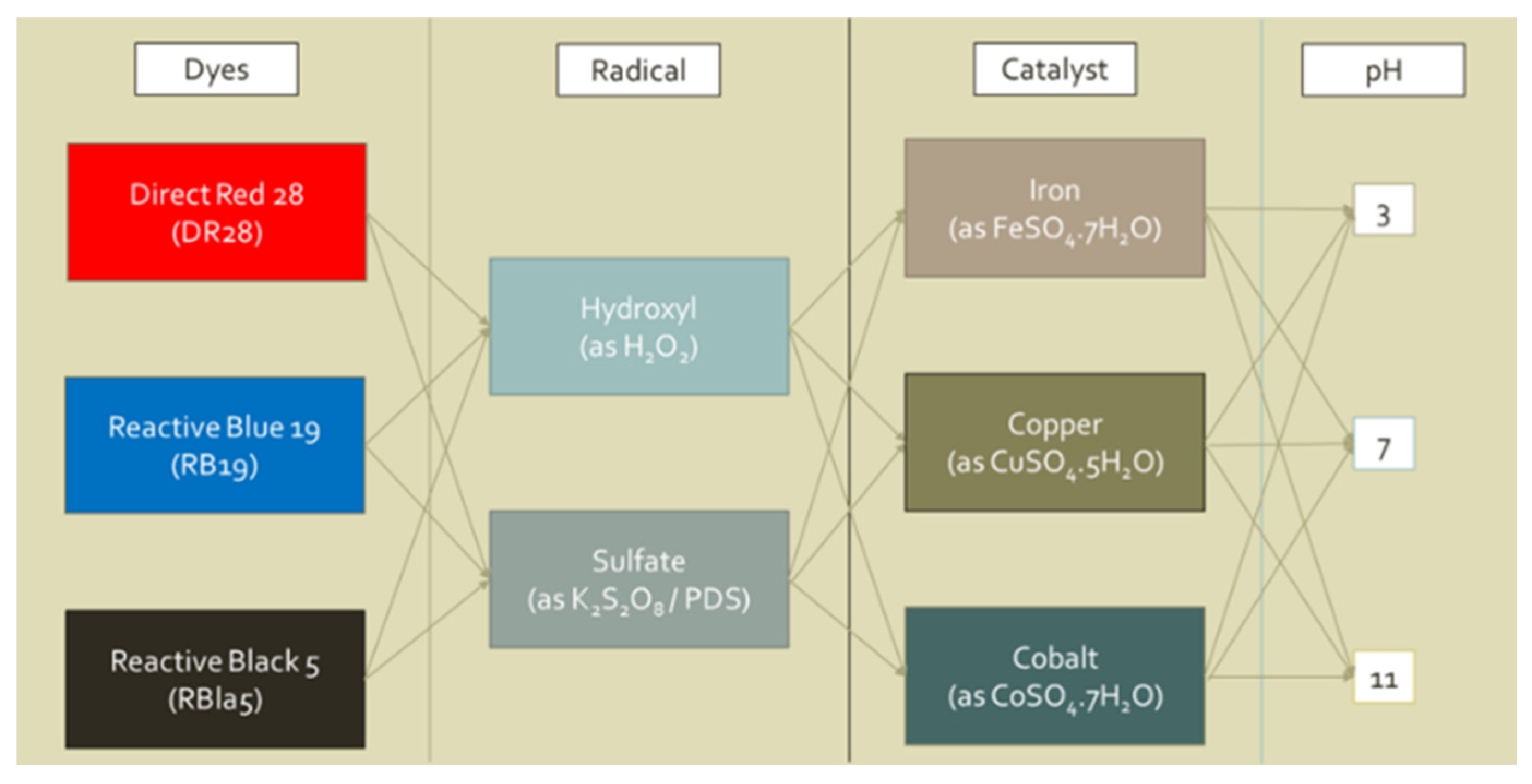
2. Materials and Methods
3. Results and Discussions
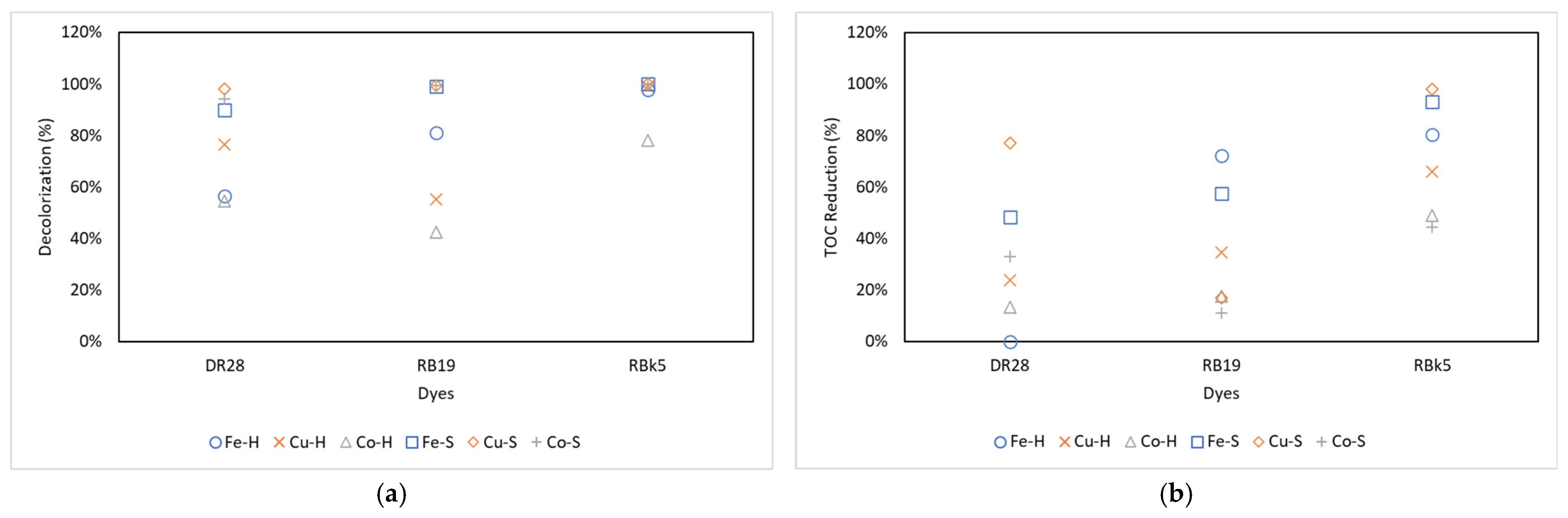
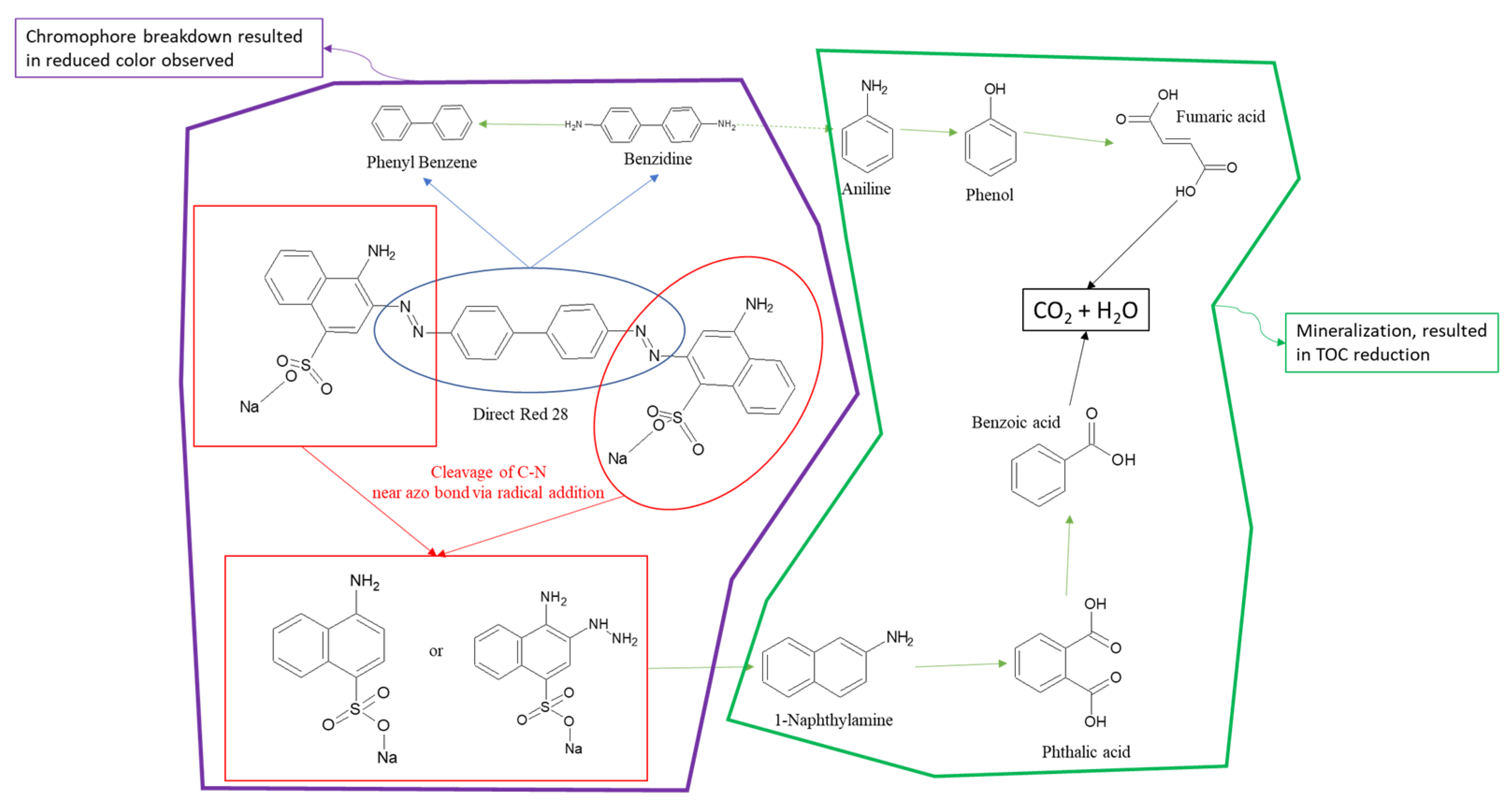
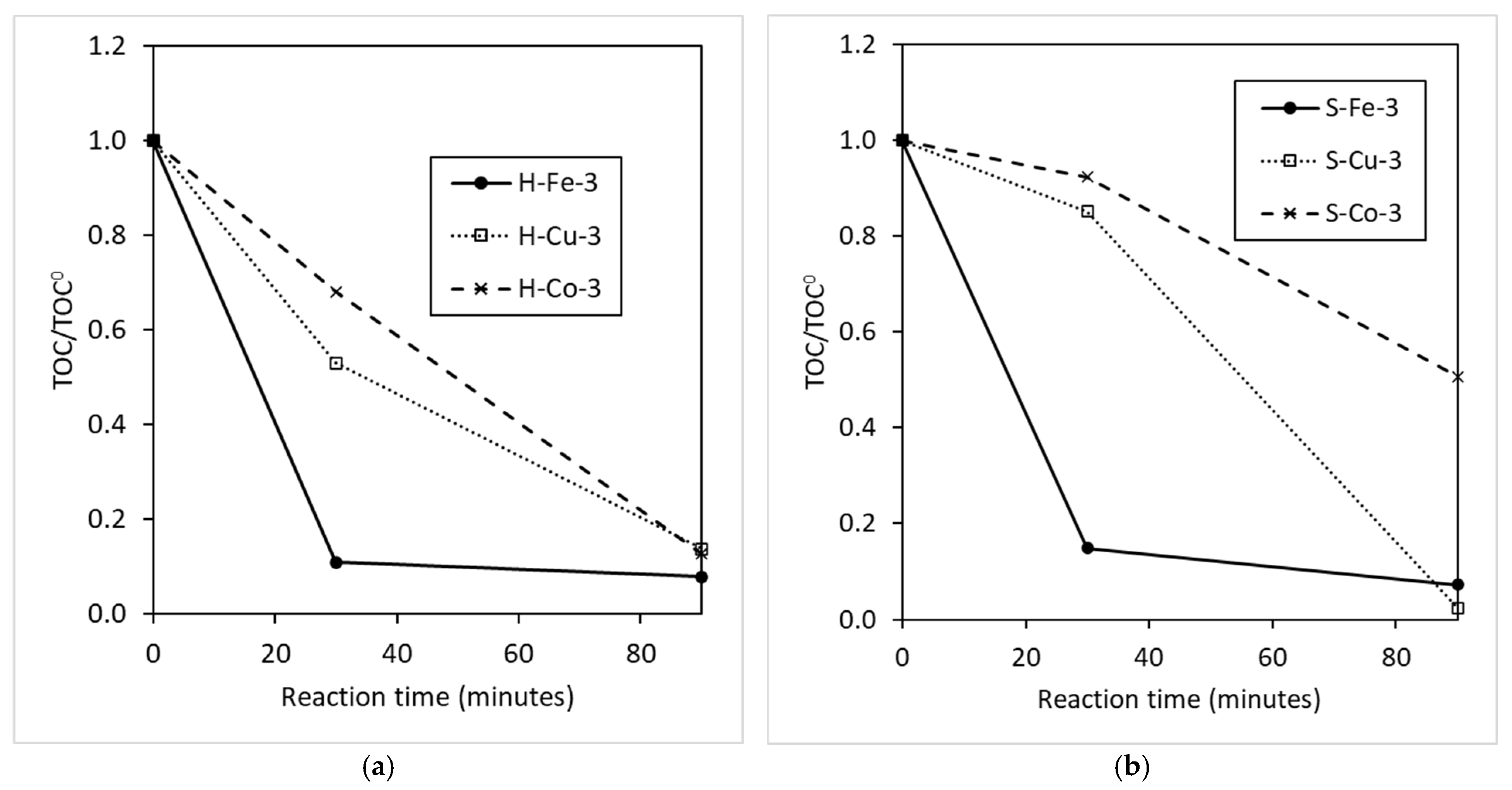
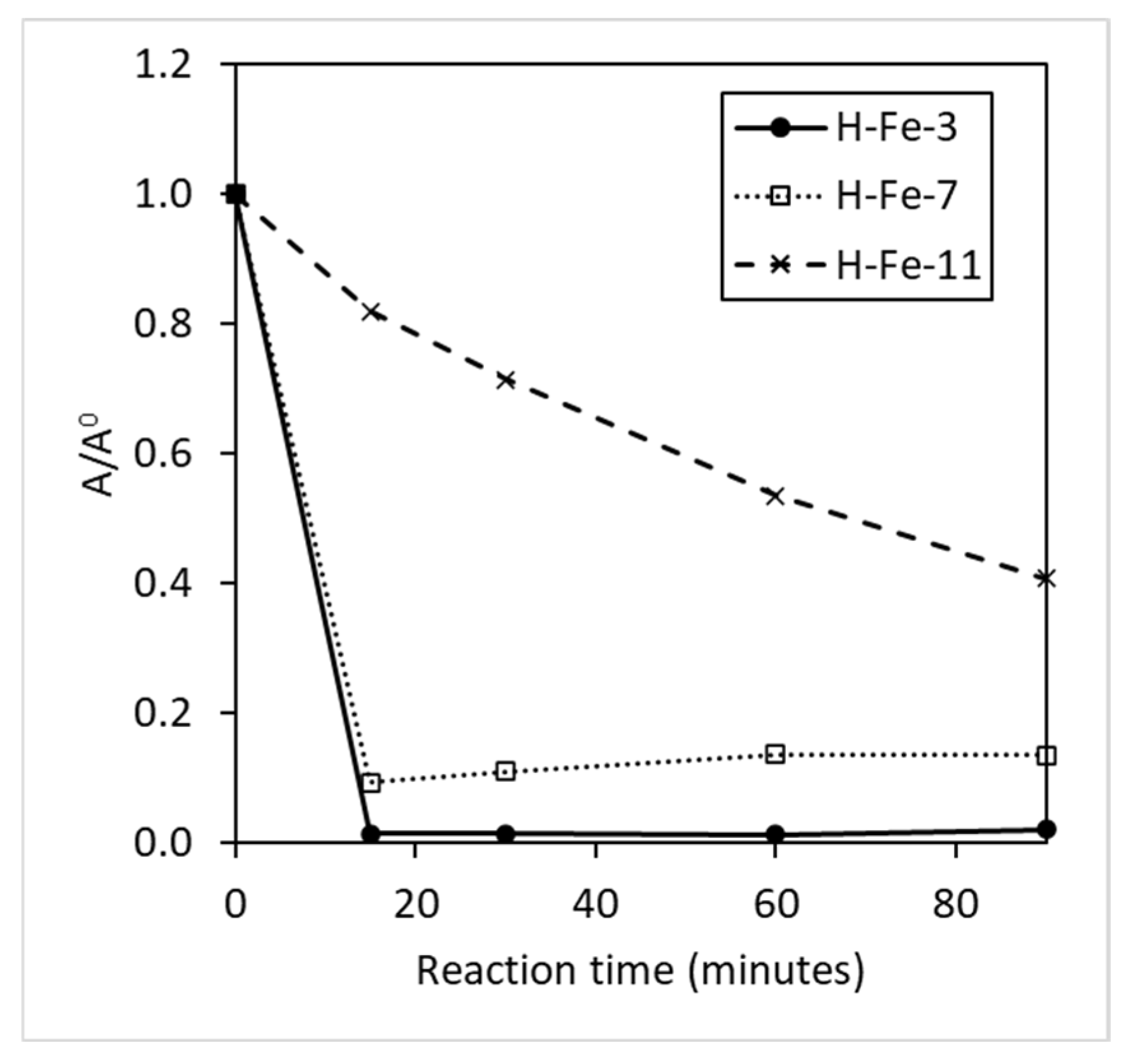
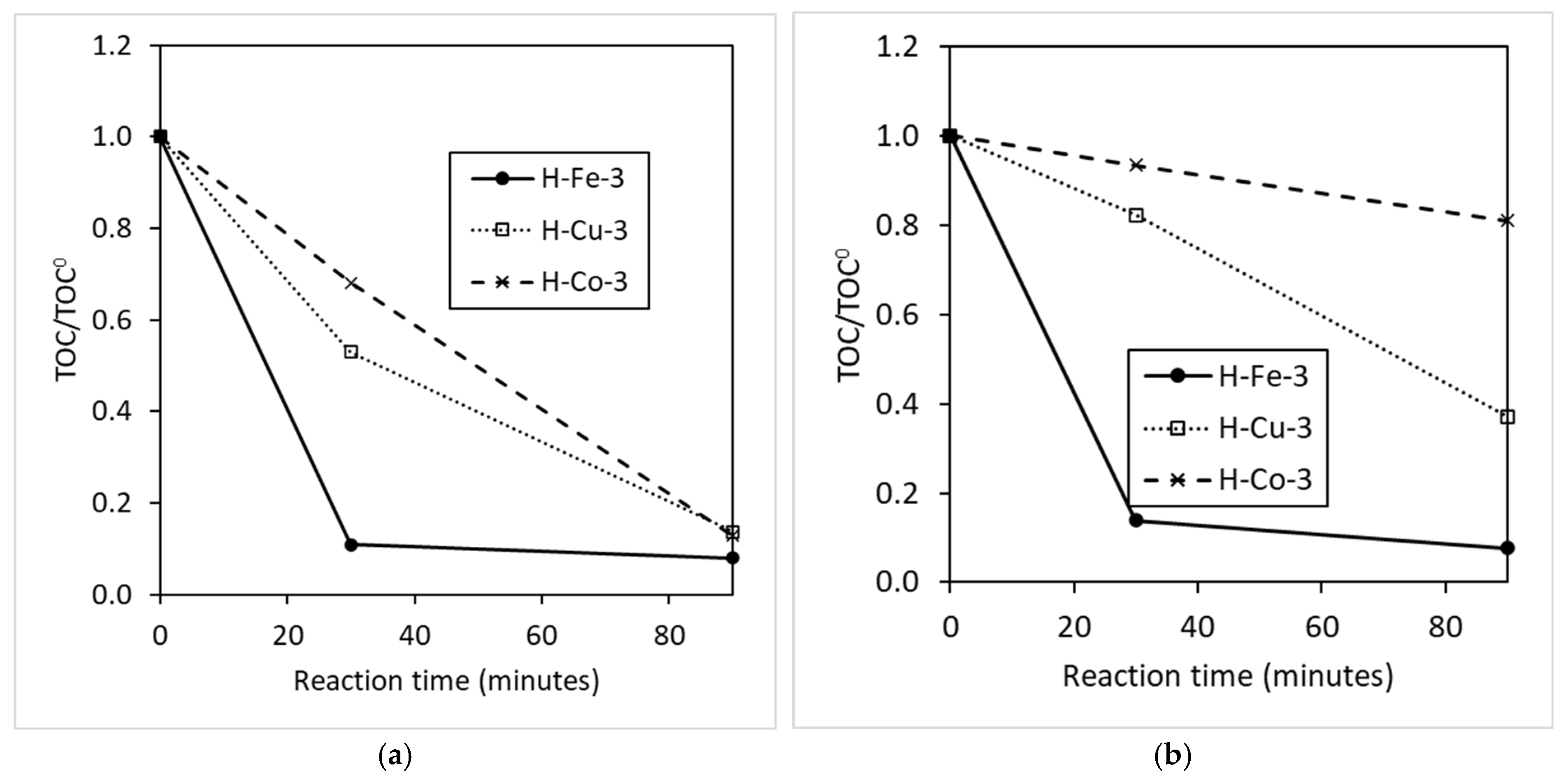
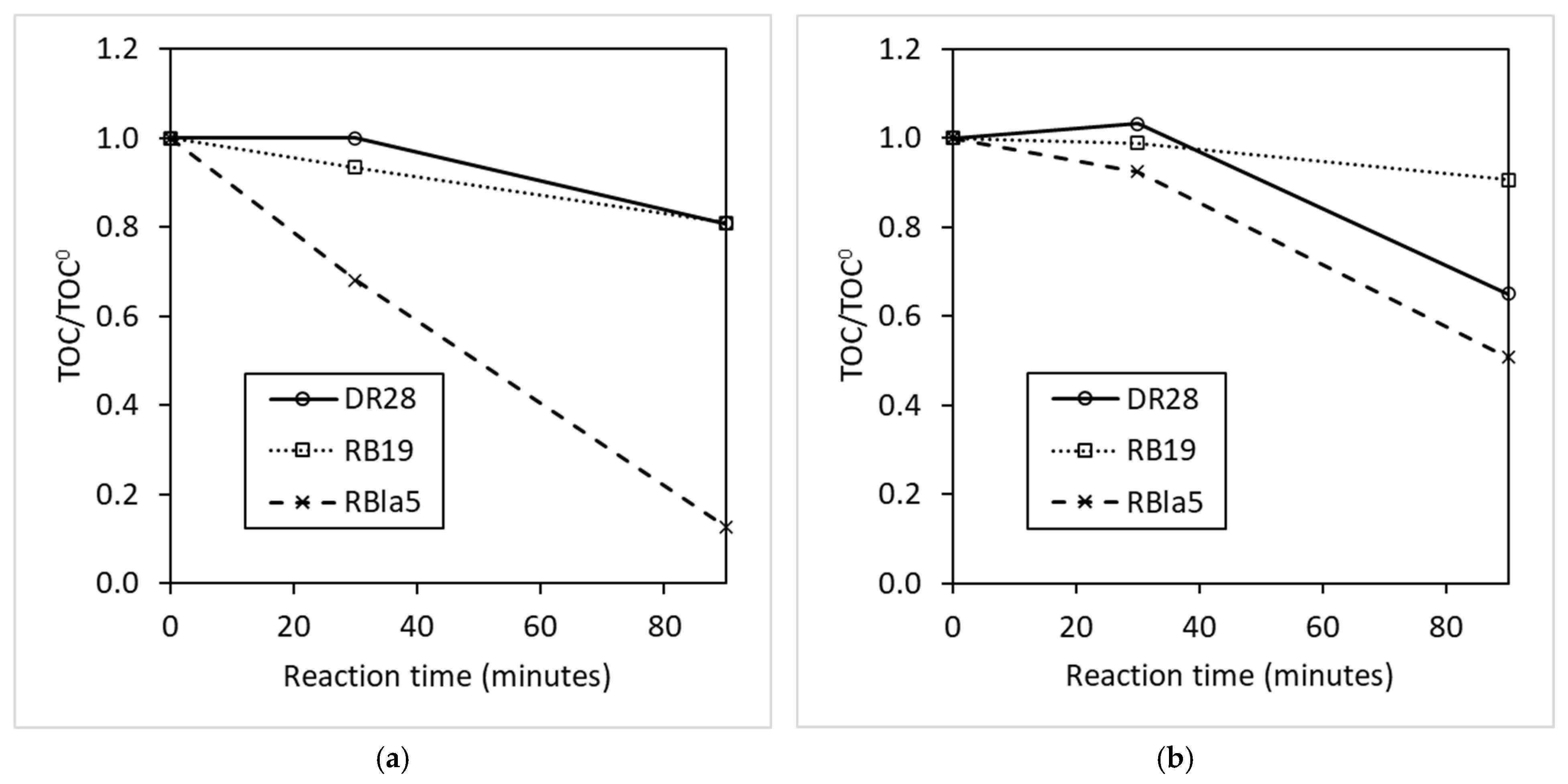
3.1. Catalyst and Radical Effect
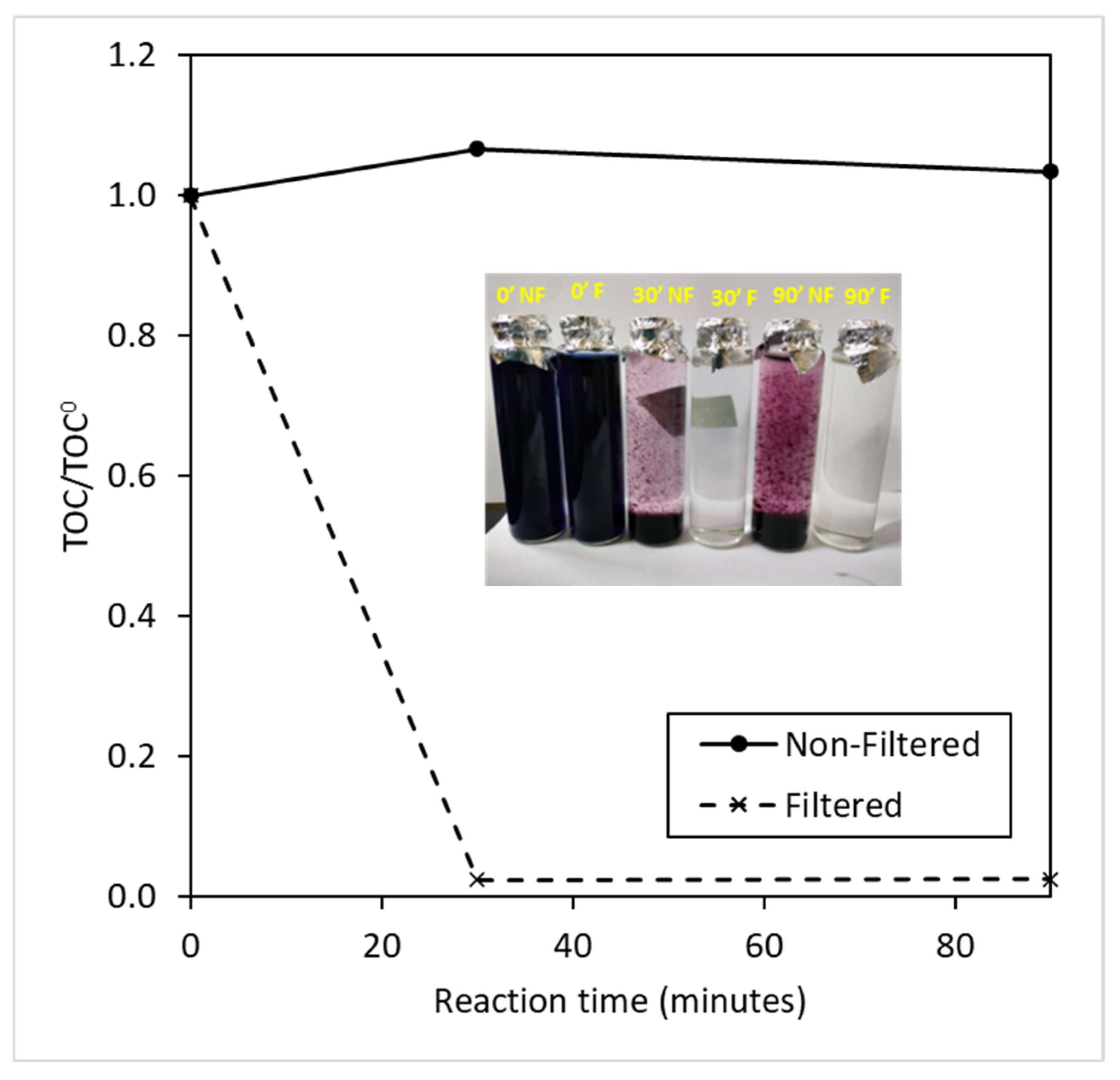
3.2. Characteristics of Catalyst and Radical Agent
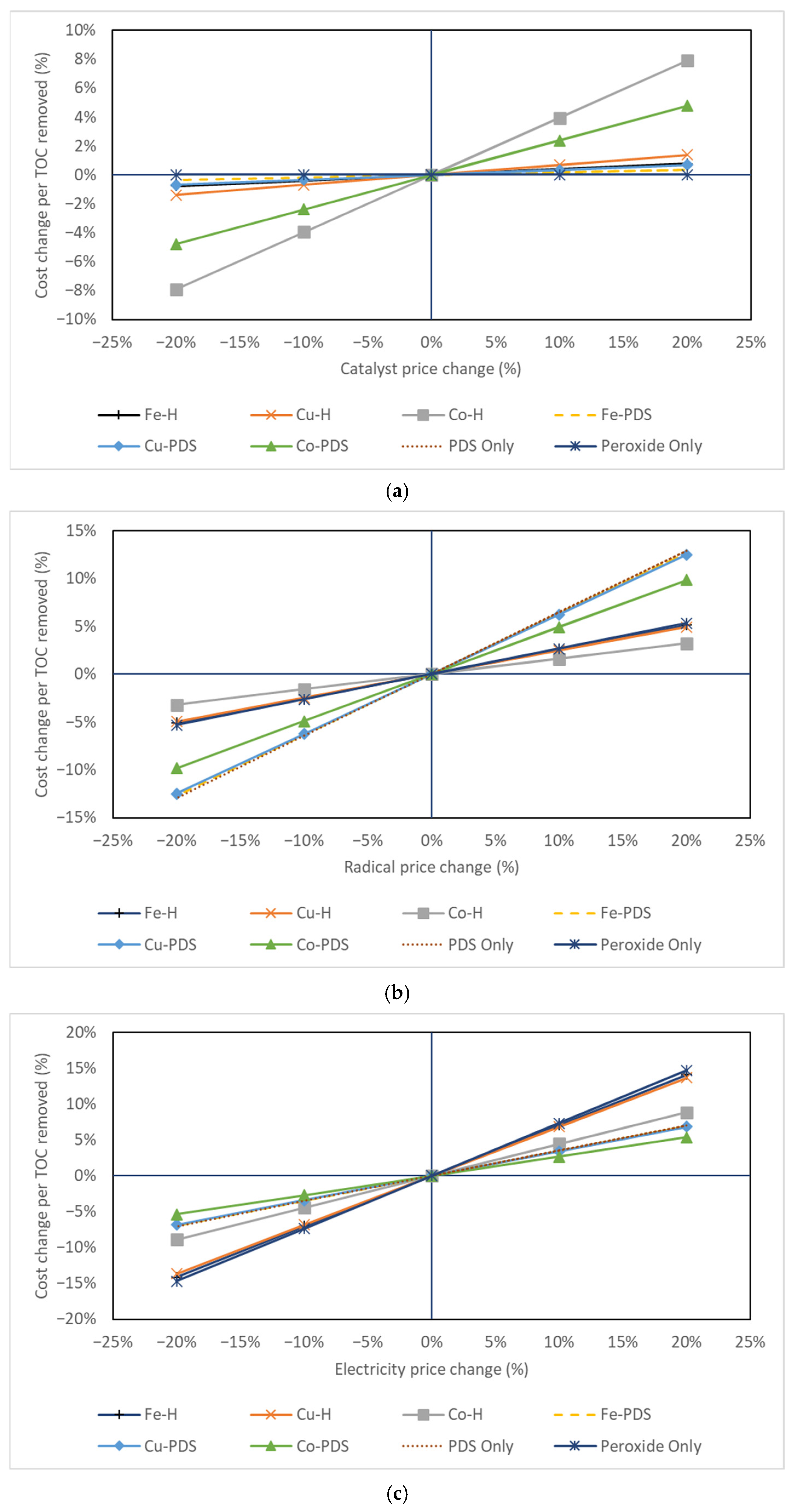
3.3. Cost Consideration for Technology Selection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DR28 | Direct Red 28 |
| RB19 | Reactive Blue 19 |
| RBk5 | Reactive Black 5 |
| TOC | Total Organic Carbon |
| PDS | Peroxydisulfate |
Appendix A
| Process | Decolorization | TOC Removal | ||||
|---|---|---|---|---|---|---|
| DR28 | RB19 | RBk5 | DR28 | RB19 | RBk5 | |
| H-Fe-3 | 49.05% ± 2.03% | 97.89% | 99.74% | 3.47% | 92.33% | 92.05% |
| H-Cu-3 | 65.22% ± 5.65% | 76.29% | 99.86% | 18.99% | 62.90% | 86.26% |
| H-Co-3 | 50.97% | 76.73% | 100.00% | 19.10% | 18.97% | 87.24% |
| H-3 | 70.72% | 81.55% | 100.00% | 41.09% | 26.26% | 73.70% |
| H-Fe-7 | 60.33% | 86.43% | 95.15% | 1.59% | 75.05% | 87.32% |
| H-Cu-7 | 86.53% | 47.09% | 100.00% | 53.42% | 15.01% | 80.05% |
| H-Co-7 | 76.29% | 40.25% | 99.88% | 21.41% | 7.52% | 61.47% |
| H-7 | 78.58% | 38.23% | 100.00% | 33.15% | 13.25% | 51.70% |
| H-Fe-11 | 58.76% | 59.12% | 98.61% | 1.85% | 49.66% | 61.57% |
| H-Cu-11 | 81.61% | 42.38% | 97.29% | 1.27% | 26.31% | 31.56% |
| H-Co-11 | 36.94% | 10.15% | 34.50% | 0.82% | 26.17% | 1.74% |
| H-11 | 73.74% | 61.68% | 99.81% | 10.53% | 9.61% | 55.98% |
| S-Fe-3 | 88.63% ± 4.87% | 98.49% | 100.00% | 52.74% | 59.60% | 92.80% |
| S-Cu-3 | 98.48% ± 1.31% | 99.07% | 100.00% | 72.32% | 16.77% | 97.53% |
| S-Co-3 | 95.93% | 99.30% | 100.00% | 35.01% | 9.43% | 49.27% |
| S-3 | 92.52% | 96.98% | 99.83% | 67.51% | 27.39% | 88.73% |
| S-Fe-7 | 90.77% | 99.46% | 100.00% | 51.35% | 65.27% | 92.43% |
| S-Cu-7 | 96.88% | 99.70% | 100.00% | 73.73% | 17.83% | 98.26% |
| S-Co-7 | 92.31% | 99.67% | 99.76% | 24.30% | 9.42% | 44.86% |
| S-7 | 93.02% | 97.14% | 99.79% | 70.46% | 20.27% | 89.70% |
| S-Fe-11 | 86.61% | 99.36% | 99.86% | 40.72% | 47.32% | 93.85% |
| S-Cu-11 | 97.59% | 99.36% | 99.89% | 85.19% | 16.35% | 98.50% |
| S-Co-11 | 94.63% | 99.11% | 99.71% | 39.77% | 14.70% | 38.99% |
| S-11 | 95.15% | 97.12% | 99.79% | 71.28% | 22.93% | 83.76% |
References
- West, A.O.; Nolan, J.M.; Scott, J.T. Optical water quality and human perceptions of rivers: An ethnohydrology study. Ecosyst. Health Sustain. 2016, 2, e01230. [Google Scholar] [CrossRef]
- Ovalle, R. A History of the Fenton Reactions (Fenton Chemistry for Beginners). In Reactive Oxygen Species; Ahmad, R., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Cardona, Y.; Węgrzyn, A.; Miśkowiec, P.; Korili, S.A.; Gil, A. Heterogeneous Fenton- and photo-Fenton-like catalytic degradation of emerging pollutants using Fe2O3/TiO2/pillared clays synthesized from aluminum industrial wastes. J. Water Process Eng. 2023, 52, 103494. [Google Scholar] [CrossRef]
- Ramos, M.D.N.; Santana, C.S.; Velloso, C.C.V.; da Silva, A.H.M.; Magalhães, F.; Aguiar, A. A review on the treatment of textile industry effluents through Fenton processes. Process Saf. Environ. Prot. 2021, 155, 366–386. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ananthashankar, R.; Alhattab, M.; Vasudevan Ramakrishnan, V. Production, characterization and treatment of textile effluents: A critical review. J. Chem. Eng. Process Technol. 2014, 5, 1–19. [Google Scholar]
- Ismail, G.A.; Sakai, H. Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere 2021, 291, 132906. [Google Scholar] [CrossRef]
- ZDHC Foundation. ZDHC Programme Textile Industry Wastewater Quality Guideline Literature Review—Revison 1. 2016. Available online: https://uploads-ssl.webflow.com/5c4065f2d6b53e08a1b03de7/5db6f553d5d32793e8e9a653_WastewaterQualityGuidelineLitReview.pdf. (accessed on 1 June 2025).
- Ding, X.; Gutierrez, L.; Croue, J.P.; Li, M.; Wang, L.; Wang, Y. Hydroxyl and sulfate radical-based oxidation of RhB dye in UV/H2O2 and UV/persulfate systems: Kinetics, mechanisms, and comparison. Chemosphere 2020, 253, 126655. [Google Scholar] [CrossRef]
- Yue, S.; Ye, W.; Xu, Z. SERS monitoring of the Fenton degradation reaction based on microfluidic droplets and alginate microparticles. Analyst 2019, 144, 5882–5889. [Google Scholar] [CrossRef]
- Soares, P.A.; Souza, R.; Soler, J.; Silva, T.F.C.V.; Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Remediation of a synthetic textile wastewater from polyester-cotton dyeing combining biological and photochemical oxidation processes. Sep. Purif. Technol. 2017, 172, 450–462. [Google Scholar] [CrossRef]
- Sharma, S.; Kapoor, S.; Christian, R.A. Effect of Fenton process on treatment of simulated textile wastewater: Optimization using response surface methodology. Int. J. Environ. Sci. Technol. 2017, 14, 1665–1678. [Google Scholar] [CrossRef]
- Ismail, G.A.; Sakai, H. Toxicity changes of dye degradation via photo-fenton treatment and the possible degradation mechanism. Case Stud. Chem. Environ. Eng. 2024, 9, 100665. [Google Scholar] [CrossRef]
- Sponza, D.T.; Işik, M. Toxicity and intermediates of C.I. Direct Red 28 dye through sequential anaerobic/aerobic treatment. Process Biochem. 2005, 40, 2735–2744. [Google Scholar] [CrossRef]
- Yakupova, E.I.; Bobyleva, L.G.; Vikhlyantsev, I.M.; Bobylev, A.G. Congo Red and amyloids: History and relationship. Biosci. Rep. 2019, 39, BSR20181415. [Google Scholar] [CrossRef]
- El Bouraie, M.; El Din, W.S. Biodegradation of Reactive Black 5 by Aeromonas hydrophila strain isolated from dye-contaminated textile wastewater. Sustain. Environ. Res. 2016, 26, 209–216. [Google Scholar] [CrossRef]
- Siddique, M.; Farooq, R.; Khan, Z.M.; Khan, Z.; Shaukat, S.F. Enhanced decomposition of reactive blue 19 dye in ultrasound assisted electrochemical reactor. Ultrason. Sonochem. 2011, 18, 190–196. [Google Scholar] [CrossRef]
- Arslan, S.; Eyvaz, M.; Gürbulak, E.; Yüksel, E. A Review of State-of-the-Art Technologies in Dye-Containing Wastewater Treatment—The Textile Industry Case. In Textile Wastewater Treatment; IntechOpen: London, UK, 2016; pp. 1–28. [Google Scholar]
- Fortunato, L.; Elcik, H.; Blankert, B.; Ghaffour, N.; Vrouwenvelder, J. Textile dye wastewater treatment by direct contact membrane distillation: Membrane performance and detailed fouling analysis. J. Memb. Sci. 2021, 636, 119552. [Google Scholar] [CrossRef]
- Ay, F.; Catalkaya, E.C.; Kargi, F. A statistical experiment design approach for advanced oxidation of Direct Red azo-dye by photo-Fenton treatment. J. Hazard. Mater. 2009, 162, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, L.A.; Shah, N.S.; Khan, H.M.; Khattak, R. Degradation of crystal violet dye by fenton and photo-fenton oxidation processes. Z. Fur Phys. Chem. 2018, 232, 1771–1786. [Google Scholar] [CrossRef]
- Bidu, J.M.; Njau, K.N.; Rwiza, M.; Van der Bruggen, B. Textile wastewater treatment in anaerobic reactor: Influence of domestic wastewater as co-substrate in color and COD removal. S. Afr. J. Chem. Eng. 2023, 43, 112–121. [Google Scholar] [CrossRef]
- Çalık, Ç.; Çifçi, D.İ. Comparison of kinetics and costs of Fenton and photo-Fenton processes used for the treatment of a textile industry wastewater. J. Environ. Manage. 2022, 304, 114234. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Marques, C.C.; Portugal, I.; Nunes, M.I. Fenton processes for AOX removal from a kraft pulp bleaching industrial wastewater: Optimisation of operating conditions and cost assessment. J. Environ. Chem. Eng. 2020, 8, 104032. [Google Scholar] [CrossRef]
- Varank, G.; Guvenc, S.Y.; Çebi, A.; Güven, E.C. Central composite design for the advanced treatment of biologically treated leachate nanofiltration concentrate using zero-valent copper and iron activated persulfate. Desalin. Water Treat. 2020, 208, 434–447. [Google Scholar] [CrossRef]
- Liu, K.; Roddick, F.A.; Fan, L. Impact of salinity and pH on the UVC/H2O2 treatment of reverse osmosis concentrate produced from municipal wastewater reclamation. Water Res. 2012, 46, 3229–3239. [Google Scholar] [CrossRef]
- Baresel, C.; Harding, M.; Junestedt, C. Removal of Pharmaceutical Residues from Municipal Wastewater Using UV/H2O2. 2019. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:ivl:diva-2828 (accessed on 15 June 2025).
- Rueda-Márquez, J.J.; Levchuk, I.; Manzano, M.; Sillanpää, M. Toxicity reduction of industrial and municipal wastewater by advanced oxidation processes (Photo-fenton, UVC/H2O2, electro-fenton and galvanic fenton): A review. Catalysts 2020, 10, 612. [Google Scholar] [CrossRef]
- EPA. Technologies and Costs Document for the Final Long Term 2 Enhanced Surface Water Treatment Rule and Final Stage 2 Disinfectants and Disinfection Byproducts Rule. 2005. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100LZ09.TXT (accessed on 15 June 2025).
- Rueda-Márquez, J.J.; Pintado-Herrera, M.G.; Martín-Díaz, M.L.; Acevedo-Merino, A.; Manzano, M.A. Combined AOPs for potential wastewater reuse or safe discharge based on multi-barrier treatment (microfiltration-H2O2/UV-catalytic wet peroxide oxidation). Chem. Eng. J. 2015, 270, 80–90. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Suntronvipart, N. Evaluation of biodegradability and oxidation degree of hospital wastewater using photo-Fenton process as the pretreatment method. J. Hazard. Mater. 2006, 138, 384–391. [Google Scholar] [CrossRef]
- Umar, M.; Roddick, F.; Fan, L. Impact of coagulation as a pre-treatment for UVC/H2O2-biological activated carbon treatment of a municipal wastewater reverse osmosis concentrate. Water Res. 2016, 88, 12–19. [Google Scholar] [CrossRef]
- Nacowong, P.; Saikrasun, S. Thermo-oxidative and weathering degradation affecting coloration performance of lac dye. Fash. Text. 2016, 3, 18. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Badii, K.; Norouzi, M.; Rashidi, A.; Montazer, M.; Sadeghi, M.; Vafaee, M. Decolorization and mineralization of an azo reactive dye using loaded nano-photocatalysts on spacer fabric: Kinetic study and operational factors. J. Taiwan Inst. Chem. Eng. 2014, 45, 2436–2446. [Google Scholar] [CrossRef]
- Seraghni, N.; Dekkiche, B.A.; Debbache, N.; Belattar, S.; Mameri, Y.; Belaidi, S.; Sehili, T. Photodegradation of cresol red by a non-iron Fenton process under UV and sunlight irradiation: Effect of the copper(II)-organic acid complex activated by H2O2. J. Photochem. Photobiol. A Chem. 2021, 420, 113485. [Google Scholar] [CrossRef]
- Bali, U.; Çatalkaya, E.; Şengül, F. Photodegradation of reactive black 5, direct red 28 and direct yellow 12 using UV, UV/H2O2 and UV/H2O2/Fe2+: A comparative study. J. Hazard. Mater. 2004, 114, 159–166. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Saroha, A.K. Decolorization of the Textile Wastewater Containing Reactive Blue 19 Dye by Fenton and Photo-Fenton Oxidation. J. Hazard. Toxic Radioact. Waste 2015, 19, 4014043. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, M.; Huang, C. Degradation of the Reactive Black 5 by Fenton and Fenton-like system. Procedia Eng. 2011, 15, 4835–4840. [Google Scholar] [CrossRef]
- Baeza, C.; Knappe, D.R.U. Transformation kinetics of biochemically active compounds in low-pressure UV Photolysis and UV/H2O2 advanced oxidation processes. Water Res. 2011, 45, 4531–4543. [Google Scholar] [CrossRef]
- Ismail, G.A.; Sakai, H. pH-Dependent Dye Protonation and the Effect of Iron on Dye Degradation During Fenton-Based Processes. Ozone Sci. Eng. 2024, 46, 294–308. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Ghaly, A.E.; Brooks, S.L. Influence of temperature and pH on the stability and colorimetric measurement of textile dyes. Am. J. Biochem. Biotechnol. 2007, 3, 33–41. [Google Scholar] [CrossRef]
- Manos, D.; Miserli, K.; Konstantinou, I. Perovskite and spinel catalysts for sulfate radical-based advanced oxidation of organic pollutants in water and wastewater systems. Catalysts 2020, 10, 1299. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Ding, Y.; Fu, L.; Peng, X.; Lei, M.; Wang, C.; Jiang, J. Copper catalysts for radical and nonradical persulfate based advanced oxidation processes: Certainties and uncertainties. Chem. Eng. J. 2022, 427, 131776. [Google Scholar] [CrossRef]
- Kadiiska, M.B.; Maples, K.R.; Mason, R.P. A comparison of cobalt(II) and iron(II) hydroxyl and superoxide free radical formation. Arch. Biochem. Biophys. 1989, 275, 98–111. [Google Scholar] [CrossRef]
- Turrà, N.; Neuenschwander, U.; Baiker, A.; Peeters, J.; Hermans, I. Mechanism of the catalytic deperoxidation of tert-butylhydroperoxide with cobalt(II) acetylacetonate. Chem.—A Eur. J. 2010, 16, 13226–13235. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kim, S.J.; Lee, J.W.; Kim, Y.C. Comparison of hydroxyl radical, peroxyl radical, and peroxynitrite scavenging capacity of extracts and active components from selected medicinal plants. Toxicol. Res. 2013, 26, 321–327. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef]
- Antoniou, M.G.; Nicolaou, P.A.; Shoemaker, J.A.; de la Cruz, A.A.; Dionysiou, D.D. Impact of the morphological properties of thin TiO2 photocatalytic films on the detoxification of water contaminated with the cyanotoxin, microcystin-LR. Appl. Catal. B Environ. 2009, 91, 165–173. [Google Scholar] [CrossRef]
- Dutta, P.; Mahjebin, S.; Sufian, M.A.; Razaya Rabbi, M.; Chowdhury, S.; Imran, I.H. Impacts of natural and synthetic mordants on cotton knit fabric dyed with natural dye from onion skin in perspective of eco-friendly textile process. Mater. Today Proc. 2021, 47, 2633–2640. [Google Scholar] [CrossRef]
- Vargas, S.; Santamaria-Holek, I.; Rodríguez, R. Photocurrent oscillations in natural dyes-based DSSCs with different mordant and assistants: Their role in oscillations and color stability. Mater. Chem. Phys. 2022, 286, 126163. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.J.; Sedlak, D.L.; Lee, C. pH-Dependent reactivity of oxidants formed by iron and copper-catalyzed decomposition of hydrogen peroxide. Chemosphere 2013, 92, 652–658. [Google Scholar] [CrossRef]
- Dianto, A.; Ridwansyah, I.; Subehi, L. Organic matter and organic carbon levels in sediments of Lake Maninjau, West Sumatra. IOP Conf. Ser. Earth Environ. Sci. 2020, 535, 012030. [Google Scholar] [CrossRef]
- Xia, H.; Li, C.; Yang, G.; Shi, Z.; Jin, C.; He, W.; Xu, J.; Li, G. A review of microwave-assisted advanced oxidation processes for wastewater treatment. Chemosphere 2022, 287, 131981. [Google Scholar] [CrossRef] [PubMed]
- Behnami, A.; Aghayani, E.; Benis, K.Z.; Sattari, M.; Pourakbar, M. Comparing the efficacy of various methods for sulfate radical generation for antibiotics degradation in synthetic wastewater: Degradation mechanism, kinetics study, and toxicity assessment. RSC Adv. 2022, 12, 14945–14956. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A Review Study on Sulfate-Radical-Based Advanced Oxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency, and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of sulfate radical-based advanced oxidation processes for water and wastewater treatment: A review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ghanbari, F.; Moradi, M. Photocatalysis assisted by peroxymonosulfate and persulfate for benzotriazole degradation: Effect of ph on sulfate and hydroxyl radicals. Water Sci. Technol. 2015, 72, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.H.; Ma, J.; Li, X.C.; Fang, J.Y.; Chen, L.W. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/Peroxymonosulfate system. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.G.; de la Cruz, A.A.; Dionysiou, D.D. Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e− transfer mechanisms. Appl. Catal. B Environ. 2010, 96, 290–298. [Google Scholar] [CrossRef]
- Nawaz, T.; Sengupta, S. Chapter 13—Wastewater: Novel treatment technologies and source for epidemiological studies. In Handbook of Water Purity and Quality, 2nd ed.; Ahuja, E., Ed.; Academic Press: Amsterdam, The Netherlands, 2021; pp. 293–337. [Google Scholar]
- Ji, Y.; Shi, Y.; Dong, W.; Wen, X.; Jiang, M.; Lu, J. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution. Chem. Eng. J. 2016, 298, 225–233. [Google Scholar] [CrossRef]
- Kamenická, B.; Weidlich, T. A Comparison of Different Reagents Applicable for Destroying Halogenated Anionic Textile Dye Mordant Blue 9 in Polluted Aqueous Streams. Catalysts 2023, 13, 460. [Google Scholar] [CrossRef]
- De la Cruz, N.; Esquius, L.; Grandjean, D.; Magnet, A.; Tungler, A.; de Alencastro, L.F.; Pulgarín, C. Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res. 2013, 47, 5836–5845. [Google Scholar] [CrossRef]
- Yildirim, R.; Eskikaya, O.; Keskinler, B.; Karagunduz, A.; Dizge, N.; Balakrishnan, D. Fabric dyeing wastewater treatment and salt recovery using a pilot scale system consisted of graphite electrodes based on electrooxidation and nanofiltration. Environ. Res. 2023, 234, 116283. [Google Scholar] [CrossRef]
- Bacardit, J.; Stötzner, J.; Chamarro, E.; Esplugas, S. Effect of salinity on the photo-fenton process. Ind. Eng. Chem. Res. 2007, 46, 7615–7619. [Google Scholar] [CrossRef]


| Component | Usage | Price | Cost (Per m3) | Reference |
|---|---|---|---|---|
| H2O2/H | 1 L/m3 | $0.962/L | 0.962 $ | Current work, [22] |
| K2S2O8/PDS | 2.7 kg/m3 | $1.804/L | 4.870 $ | Current work, [24] |
| FeSO4 | 139 g/m3 | $1.022/kg | 0.142 $ | Current work, [22] |
| CuSO4 | 125 g/m3 | $2.165/kg | 0.271 $ | Current work |
| CoSO4 | 141 g/m3 | $16.835/kg | 2.374 $ | Current work |
| Electricity | 5.5–60 (35) kWh/m3 | $0.076/kWh | 2.660 $ | [27,28,29,30,31] |
| Method | Cost ($/m3) | TOC Removed | Cost Per TOC Removed ($/g) | Cost Per 90% TOC Removed ($) | |
|---|---|---|---|---|---|
| mg/L | % | ||||
| Fe-H | 3.764 | 21.69 | 92.05 | 0.174 | 2.944 |
| Cu-H | 3.893 | 20.19 | 86.26 | 0.193 | 4.527 |
| Co-H | 5.996 | 19.77 | 87.24 | 0.303 | 7.004 |
| Peroxide only | 3.622 | 20.37 | 73.70 | 0.340 | 6.192 |
| Fe-PDS | 7.672 | 22.54 | 93.85 | 0.347 | 5.812 |
| Cu-PDS | 7.801 | 22.47 | 98.50 | 0.859 | 4.686 |
| Co-PDS | 9.904 | 11.53 | 49.27 | 0.178 | 36.161 |
| PDS only | 7.530 | 20.14 | 89.70 | 0.374 | 8.184 |
| Method | Cost ($/m3) | TOC Removed | Cost Per TOC Removed ($/g) | Cost Per 90% TOC Removed ($) | |
|---|---|---|---|---|---|
| mg/L | % | ||||
| Fe-H | 3.764 | 0.45 | 1.85 | 8.365 | 962.625 |
| Cu-H | 3.893 | 13.59 | 53.42 | 0.286 | 12.755 |
| Co-H | 5.996 | 5.70 | 21.41 | 1.052 | 63.787 |
| Peroxide only | 3.622 | 10.51 | 41.09 | 0.345 | 15.965 |
| Fe-PDS | 7.672 | 13.46 | 52.74 | 0.570 | 22.744 |
| Cu-PDS | 7.801 | 21.02 | 85.19 | 0.371 | 10.337 |
| Co-PDS | 9.904 | 9.76 | 39.77 | 1.015 | 50.662 |
| PDS only | 7.530 | 18.78 | 71.28 | 0.401 | 15.156 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, G.A.; Sakai, H. Radical and Catalyst Effect on Fenton-like Textile Dyes’ Degradation Process and Techno-Economical Consideration. Textiles 2025, 5, 37. https://doi.org/10.3390/textiles5030037
Ismail GA, Sakai H. Radical and Catalyst Effect on Fenton-like Textile Dyes’ Degradation Process and Techno-Economical Consideration. Textiles. 2025; 5(3):37. https://doi.org/10.3390/textiles5030037
Chicago/Turabian StyleIsmail, Guntur Adisurya, and Hiroshi Sakai. 2025. "Radical and Catalyst Effect on Fenton-like Textile Dyes’ Degradation Process and Techno-Economical Consideration" Textiles 5, no. 3: 37. https://doi.org/10.3390/textiles5030037
APA StyleIsmail, G. A., & Sakai, H. (2025). Radical and Catalyst Effect on Fenton-like Textile Dyes’ Degradation Process and Techno-Economical Consideration. Textiles, 5(3), 37. https://doi.org/10.3390/textiles5030037






