1. Introduction
Basalt continuous fibers are widely used in various industries, such as aircraft manufacturing, construction, and heat insulation, due to their low production costs, rapid molding capabilities, and excellent damage tolerance. In recent years, there has been growing interest in the development of Arctic regions, where materials based on basalt fibers must operate at temperatures as low as −50 °C, and even more extreme conditions, such as in facilities for the production, storage, and transportation of liquid gases, where temperatures can reach those of liquid nitrogen (down to −196 °C).
However, when exposed to cryogenic temperatures, GFRC (glass fiber reinforced composite) materials develop residual thermal stresses due to differences in thermal expansion coefficients, leading to micro-cracking, which can affect their performance. Therefore, understanding the thermomechanical behavior of glass/epoxy laminates at low temperatures is crucial for ensuring their structural integrity in future materials [
1,
2,
3].
At the same time, the effect of low temperatures on GFRC components has been studied to a much lesser extent. Most research focuses on the behavior of the polymer matrix under cryogenic conditions, while the stability of reinforcing fillers—such as glass and basalt fibers—remains largely unexplored. This issue is particularly relevant for applications where these materials are used outside of composites, for instance, as lining materials for thermal insulation covers.
Basaltic glasses are intricate aluminosilicate systems. Basalt (basalt-like) glasses are multicomponent systems with the following composition and mass %: SiO
2 43–58, Al
2O
3 11–20, CaO 7–13, FeO + Fe
2O
3 8–16, MgO 4–12, R
2O up to 4. TiO
2 is often present. Properties and production conditions [
4] of basalt continuous fibers strongly depend on their composition [
5,
6]. The chemical composition of basalt continuous fibers (BCFs) can vary [
7,
8] significantly. The mechanical properties and manufacturing processes of these fibers depend on their specific composition [
9]. E-glass fibers, which are made from aluminoborosilicate glass and contain less than 2 wt% alkaline materials, exhibit lower chemical resistance compared to basalt fibers.
Most studies in this field have focused on investigating the properties of basalt fibers at elevated temperatures, which have now been studied quite extensively [
10]. Crystallization processes at temperatures near or above the glass transition temperature, which lead to a decrease in mechanical properties, have been studied [
11].
Existing studies predominantly analyze natural basalts with significant compositional variations between different deposits, making it difficult to establish clear composition–property relationships. This work presents the first systematic investigation of cryogenic temperature effects on basalt fibers derived from a single deposit but with intentionally varied chemistry. This unique approach eliminates geochemical variability factors, enabling us to reveal fundamental temperature-induced transformation mechanisms specific to the basalt fibers.
2. Materials and Methods
2.1. Sample Preparation
Basalt fibers with various oxide contents were produced in two stages. The first stage included obtaining lithium- and sodium-rich basalt bulk glasses. The bulk glasses were prepared by adding reagent-grade chemicals Na
2CO
3, SiO
2, CaCO
3, TiO
2, Al
2O
3 to milled basalt batches. Each batch of mixture placed in a platinum crucible was heated in a high-temperature furnace at a rate of 300 °C/h to 1200 °C and at 50 °C/h in a range of 1200–1550 °C and then held at 1550 °C for 20 h. The bulk glass was quenched from 1550 °C to 20 °C by rapidly pouring the melt into water. Continuous fibers were produced using a laboratory-scale system [
4]. The temperature of fiber manufacturing was measured with an accuracy of ± 5 °C. The fiber diameter was controlled by varying the rotation rate of the reel. Lower temperature limit (T
ll) was taken to be the temperature at which a continuous fiber with a diameter less than 20 μm was manufactured within 30 min. The upper temperature limit (T
ul) was determined as the temperature at which the die field started to be flooded and drop formation ceased. Images of the process of obtaining fibers on a nozzle and measuring the fiber diameter are shown in
Figure 1.
2.2. XRD Studies
X-ray diffraction (XRD) was performed at room temperature on a Thermo ARL X’TRA powder diffractometer (Thermo Scientific, Switzerland), CuKα1 radiation, λ = 1.54060 Å; CuK α2 radiation, λ = 1.54443 Å). XRD patterns were collected in an angular range 2θ = 10–60° at a scan step 2θ = 0.02° and a scan rate of 1° (2θ)/min. The phases were identified using the Crystallographica Search-Match (CSM) software (Version 2.0.3.1) and the International Center for Diffraction Data (ICDD) database.
2.3. X-Ray Fluorescence Analysis
X-ray fluorescence analysis of the specimens was performed on a PAN Analytical Axios Advanced spectrometer (PANalytical B.V., Almelo, The Netherlands). Characteristic X-rays were excited using a 4 kW Rh-anode X-ray tube. The excited radiation was recorded by a wavelength-dispersive spectrometer (PANalytical B.V., Almelo, The Netherlands) with five exchangeable analyzer crystals (PE_002_C, PX_1, GEIII_C, LIF_200, LIF_220) and a detector (flow proportional and scintillation). Measurements were made in transmission geometry in vacuum.
The concentration of elements was expressed as weight percentage (wt %) of the corresponding oxides. The accuracy of XRF determinations of elements was controlled by analyses of standard samples OOKO 301 and OOKO 201 (State certified reference material 5358-90/5367-90) and was within 0.6% for SiO2, within 0.4% for Al2O3, within 0.2% for Fe2O3, within 0.3% for CaO, within 0.1% for MgO, within 0.1% for K2O, within 0.2% for Na2O, within 0.1% for TiO2, and within 0.3% for P2O5.
2.4. Infrared Spectroscopy
Samples for IR spectroscopy were prepared by compression of a mixture of powdered glass and KBr in a weight ratio of 1:20, respectively. FTIR spectra were measured using FTIR spectrometer Bruker Tensor 27 (Bruker Optik GmbH, Ettlingen, Germany). The absorption spectrum was recorded in the range of 100–4000 cm−1.
2.5. Mechanical Properties
The mechanical properties of the fibers were determined on a Hounsfield H100K-S universal tensile testing machine (Hounsfield Test Equipment Ltd., Redhill, UK). Specimens were mounted in paper support frames using epoxy. The gauge length was 10 mm, and the crosshead speed was 5 mm/min (ISO 5079 [
12]). Fibers for mechanical testing were produced at the midpoint of each composition’s fiber-forming temperature range (ΔTff) to ensure consistent processing conditions. Statistical analysis included 50 independent measurements per composition, with narrow confidence intervals (95% CI < ±5% of mean strength) confirming data robustness.
2.6. Low-Temperature Treatment
Low-temperature treatment at −50 °C was conducted in a VESTFROST Solutions VT 308 climate chamber (A/S Vestfrost, Esbjerg, Denmark), with a temperature stability of ± 2 °C. For liquid nitrogen exposure (LN), samples were placed in a polystyrene foam container filled with liquid nitrogen. In both cases, samples were treated for 4 h.
2.7. Optical Analysis
Optical analysis of the fibers and fiber diameter measurements were performed at magnifications of 200× to 1000× on an Olympus BX51TRF modular optical microscope (12V100WHAL lamp (Philips 7724) in transmission, and U-LH75XEAPO xenon lamp in reflection) equipped with an Olympus C-5060 camera (Olympus Optical Co., Tokyo, Japan). The linear dimensions of the fibers were determined by analyzing their images using ImageScope Color software (ver. 10.1).
4. Discussion
In this study, samples of basalt fibers with different compositions were investigated. Unlike most research in this field, the fibers were produced using a laboratory-scale setup, and after formation, they did not undergo any thermal treatment.
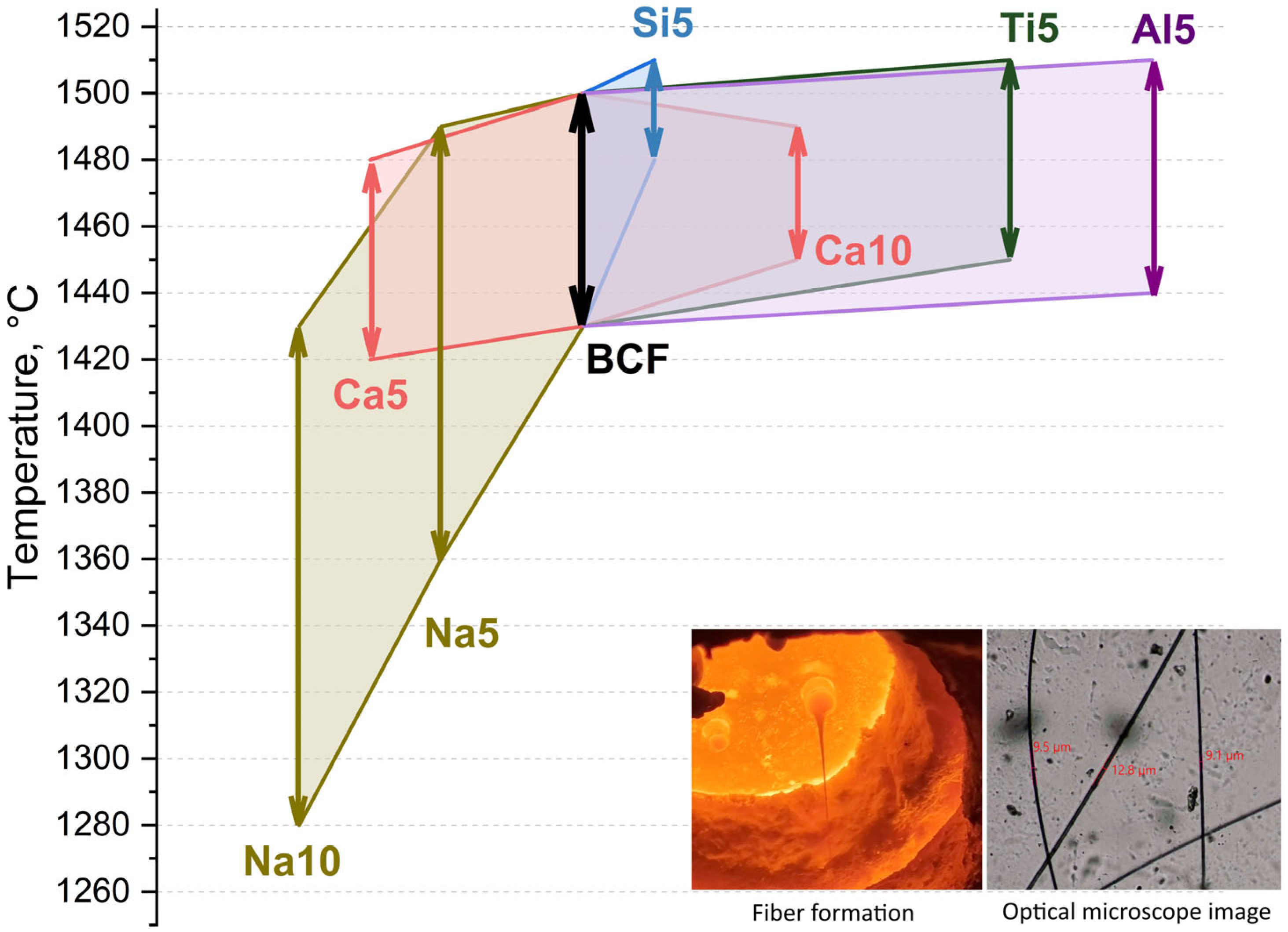
Figure 1 shows the temperature ranges for fiber production. This parameter is critical for the manufacturing technology of continuous basalt fibers, as it directly affects process productivity.
The typical production temperature range for basalt fibers derived from natural rocks is between 70 and 100 degrees [
14,
15]. Few studies have explored composition modifications of natural rocks due to the complexity and high cost of the production process. Based on the obtained results, it was concluded that adding silicon, calcium, titanium, and aluminum oxides has little to no effect on the temperature range—it either remains unchanged or even narrows. This may be attributed to increased structural connectivity in the aluminosilicate system or enhanced crystallization ability [
6,
16].
At the same time, our findings demonstrate that introducing alkali oxides can either lower the fiber production temperature or widen the processing range. This effect is associated with reduced structural connectivity in the aluminosilicate system and altered viscosity–temperature dependence [
6,
17]. The obtained results may be valuable for specialists working in continuous basalt fiber production, particularly in raw material selection or modification to optimize the manufacturing process.
All fiber samples, without additional treatment, were first dried at 106 °C until reaching constant weight and then exposed to temperatures of −50 °C and −196 °C (in liquid nitrogen) for 4 h. The selected temperatures were based on practical considerations: −50 °C represents conditions similar to those in northern construction applications, while −196 °C corresponds to cryogenic processing environments, such as those used in liquefied gas production. As shown in
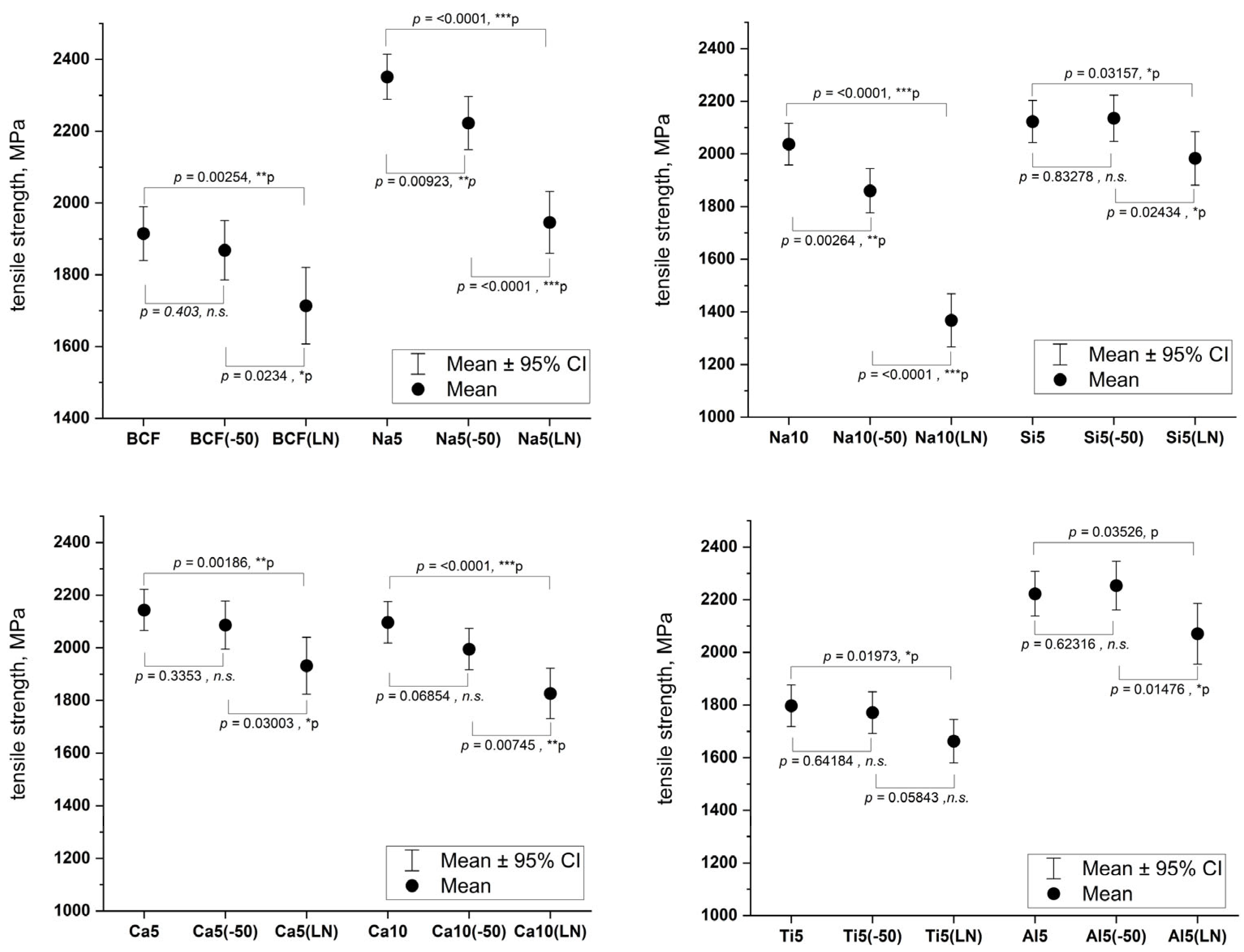
Table 2, the strength of both natural and modified basalt fibers decreases after low-temperature treatment, with the extent of reduction varying depending on the fiber composition.
To verify these results, we conducted two statistical analyses: confidence interval estimation to assess the reliability of the strength measurements and a statistical independence test to confirm that the strength data samples were not influenced by external factors. The results are presented in
Figure 4.
For all compositions except Na5 and Na10, the confidence intervals overlap after exposure to −50 °C. At the same time, the t-test analysis shows that the statistical significance levels for most samples are not significant. Based on these findings, it can be concluded that treatment at −50 °C does not significantly affect fiber strength. However, for sodium-enriched compositions (Na5 and Na10)—despite their potential for cost reduction and improved process stability (as they lower production temperatures and widen the processing window, as previously demonstrated)—strength decreases significantly even at −50 °C, as confirmed by statistical methods.
More pronounced changes in mechanical properties were observed after cryogenic treatment at −196 °C. Although some compositions show minor overlaps in confidence intervals, statistical analysis reveals highly significant changes: in most cases, the t-test yields p-values < 0.01, often much lower. The exception is modified fibers containing silicon, aluminum, and titanium—for these samples, while the significance level remains below the threshold (p < 0.05), it is less pronounced compared to other compositions.
As shown in
Figure 4, all tested fibers exhibit a statistically significant reduction in strength after liquid nitrogen treatment. The most substantial changes occur in sodium-containing compositions (Na5 and Na10), where
p-values reach < 0.0001, fully consistent with their behavior at −50 °C described earlier. This confirms the particular sensitivity of these compositions to low-temperature exposure.
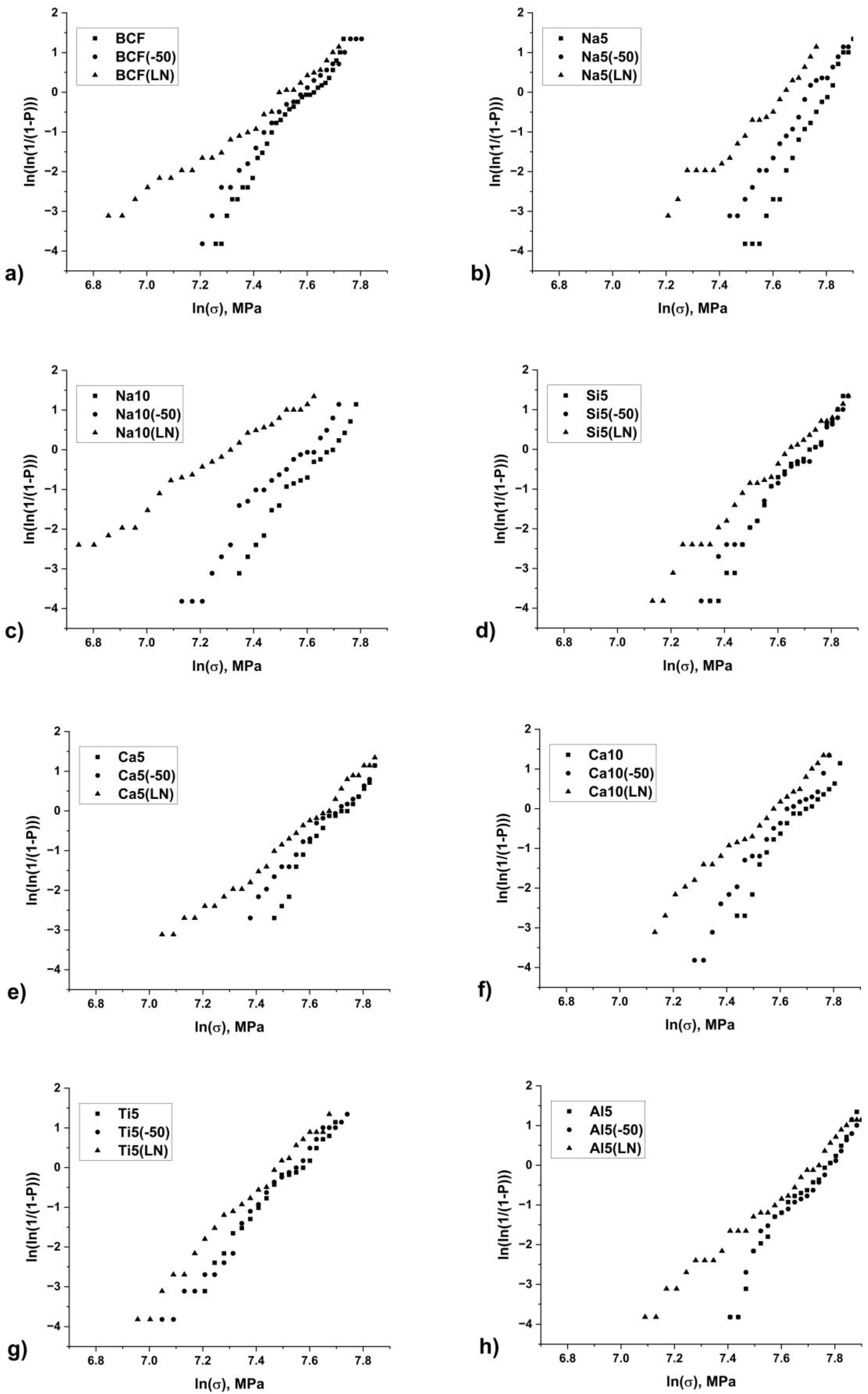
The obtained Weibull distribution parameters (
Table 3) reveal a clear correlation between fiber chemical composition, low-temperature treatment conditions, and their mechanical reliability characteristics. Particularly indicative is the behavior of the shape parameter m, which reflects the strength value scatter and, consequently, the material’s structural homogeneity.
For sodium-containing compositions (Na5, Na10), the most pronounced degradation of mechanical properties is observed after cryogenic treatment. The initially high m = 13.5 value for Na5 indicates high structural homogeneity, but after −196 °C treatment, it drops to 6.9, suggesting significant strength variability. This fully aligns with our earlier conclusions about the structural instability of these compositions under low-temperature exposure. The possible degradation mechanism involves sodium ion migration at cryogenic temperatures, leading to localized structural defects. Silicon-modified fibers (Si5) exhibit fundamentally different behavior. Despite the m decrease from 10.0 to 6.8 after liquid nitrogen treatment, the scale parameter σ0 changes minimally (from 2280 to 2126 MPa). This confirms our hypothesis about silicon’s stabilizing role, forming a robust tetrahedral SiO4 network less susceptible to low-temperature degradation.
Of particular interest are the results for titanium- and aluminum-containing fibers. Ti5 shows a significant σ0 reduction (from 1927 to 1663 MPa) with relatively stable m (7.7→7.2). This behavior is well explained by titanium ions’ amphoteric nature, capable of both network formation and structural modification. A similar, though less pronounced, effect is observed for aluminum-containing fibers.
Notably, −50 °C treatment causes less substantial Weibull parameter changes compared to liquid nitrogen exposure. However, even this moderate cooling significantly reduces m for sodium-containing compositions (from 13.5 to 10.0 for Na5), further underscoring their exceptional sensitivity to low temperatures. High determination coefficients R2 (0.94–0.99) in all cases confirm the Weibull distribution’s adequacy for describing experimental data. The observed parameter changes correlate well with presumed structural transformations in modified aluminosilicate systems, opening prospects for targeted design of compositions with specified low-temperature performance.
In our view, these statistical characteristics and strength changes stem from structural transformations in aluminosilicate systems upon chemical modification. Silicon cations act exclusively as network formers, while aluminum and titanium cations can perform both network-forming and modifying roles [
1,
2,
3]. This likely explains why silicon oxide introduction causes less dramatic property changes compared to other modifiers. When alkaline and alkaline earth oxides are added, the strength of the fibers may slightly increase. This is primarily due to the decrease in viscosity of such glasses, which helps reduce immiscibility in the glasses and crystallization in the temperature range of fiber formation. These results are consistent with other aluminosilicate glasses with a high sodium oxide content [
18]. However, an increased calcium oxide content in the glasses may initiate immiscibility processes, which negatively affect strength.
The studies of [
19,
20] has shown that oxygen in alkali and alkaline earth silicate glasses exists in two primary forms—bridging oxygen (BO) and non-bridging oxygen (NBO)—both of which are two-fold-coordinated. Bridging oxygen connects two silicon atoms (Si–O–Si), while non-bridging oxygen connects a silicon atom to a modifier cation, such as sodium (Si–O–Na). Additionally, a third type of bridging oxygen is identified in crystalline sodium disilicates and sodium metasilicate, which is bonded to two silicon atoms and a sodium atom, resulting in a triply coordinated structure. This is well aligned with the results of determining the temperature range for the production of Na5 and Na10 fibers. The addition of sodium oxide leads to a significant decrease in viscosity, which shifts the lower boundary of the production temperature range to 1280 °C and expands the temperature range by 150 °C. Although the polymerization of the glass structure decreases, the strength of the fibers increases to 2351 MPa for Na5. This is attributed to improved homogenization of the melt, which is critically important for the production of continuous basalt fibers [
21,
22]. The influence of calcium oxide on the structure of silicate glasses has been studied quite well. Calcium cations occupy CaO
6 distorted octahedra surrounded by chains of SiO
4 tetrahedra [
23,
24]. All calcium cations introduced in the glasses act as modifiers of the silicate network, i.e., each calcium cation creates two non-bridging oxygens [
25]. At the same time, the homogenization of the melt in this case is somewhat lower than for samples with a high sodium content. In addition, a high calcium oxide content can lead to glass stratification [
26].
Silicon and aluminum oxides in basalt systems act as glass-forming oxides [
27,
28]. An increase in the content of silicon and aluminum oxides leads to an increase in the temperature of the obtained glass fibers and to an increase in the degree of polymerization of their structure [
20]. Titanium oxide can simultaneously act as both a modifier oxide and a network-forming oxide, depending on the coordination it occupies in the structure of silicate glasses. It is known from studies that TiO
2 has a slightly reducing effect on the viscosity and melting temperature [
29]. At high TiO
2 contents, titanium ions enter the network as TiO
4 groups which require more non-bridging oxygen atoms [
30]. The tetrahedral form of titanium in the structural units of TiO
4 predominates in glass with low boron content and higher alkali-metal content. The formation of these structural units is accompanied by the destruction of mixed silicon-borate rings and the formation of a disordered glass network with a uniform titanium distribution [
31].
The findings derived from the analysis of experimental data regarding the tensile strength of fibers, utilizing the Weibull distribution, show a good correlation with the existing literature. The average tensile strength value obtained from Weibull statistics is slightly lower than that of natural basalts, which could be attributed to the absence of sizings during the fiber production process. Typically these values are around 2500 MPa [
32]. It is shown that fibers have a distinct knee in the Weibull data before low-temperature treatment. The results (
Figure 2) present larger scatter in strength and noticeable knee in fiber strength distribution after low-temperature treatment.
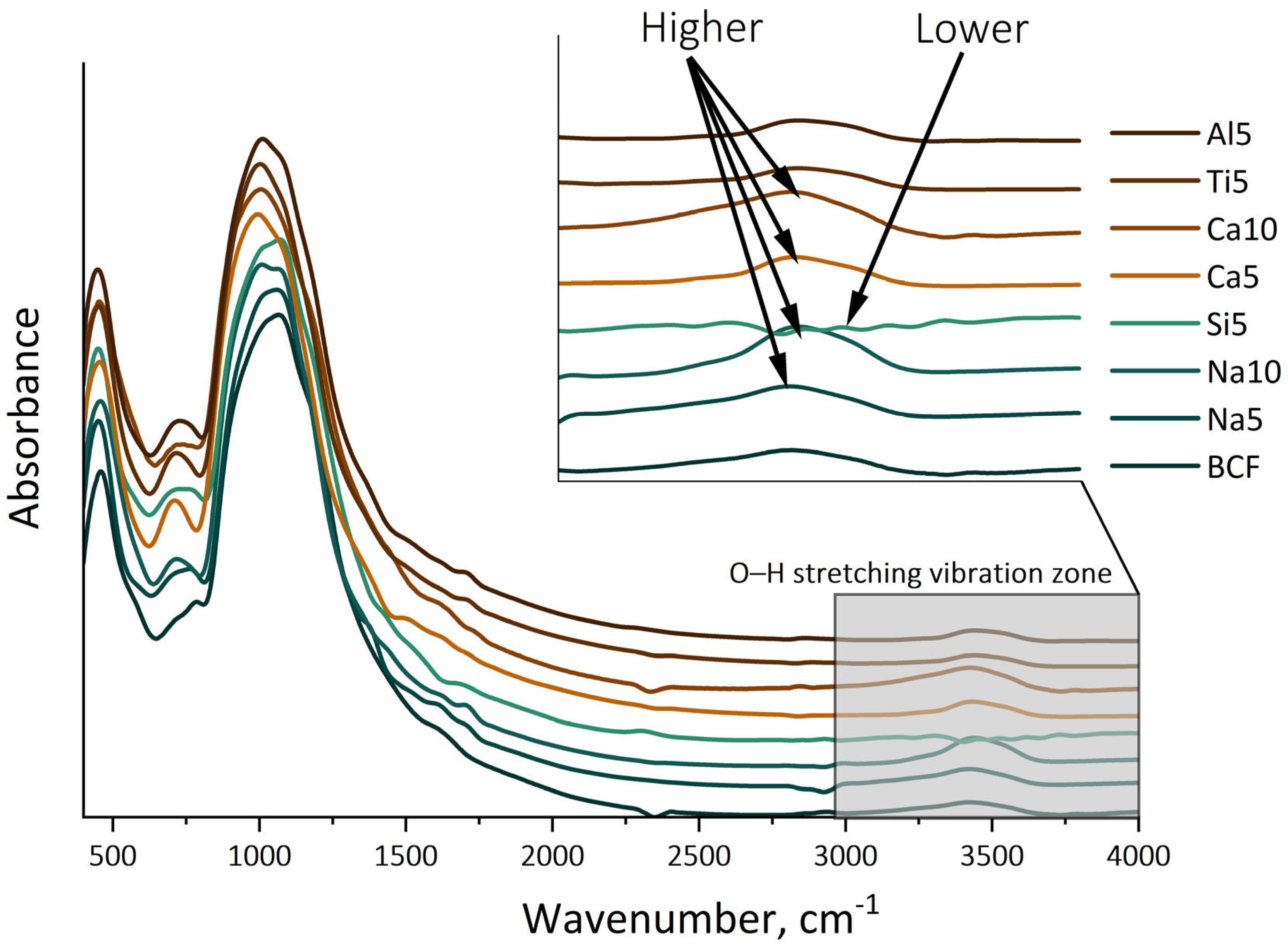
This difference in the alteration of mechanical properties is, in our opinion, linked to the varying content of OH groups on the fiber surface. As illustrated in
Figure 3, the addition of alkali and alkaline earth metals leads to a significant increase in the band around 3500–3300 cm
−1. The bands associated with Si–O stretching, Si–O–Si bending, and SiO
4 tetrahedral deformation are similar across samples, indicating a consistent silicate chain structure. Near-IR data reveal a progressive loss of Si–OH environments, shedding light on the charge-balancing substitutions necessary for depolymerization.
The IR spectrum of typical basaltic glass fibers displays three prominent bands related to the vibrations of bridging oxygen atoms: stretching (900–1100 cm
−1), bending (700–800 cm
−1), and rocking (420–500 cm
−1). The most intense band, assigned to the asymmetric vibration of the silicon sublattice against the oxygen sublattice, is observed at 1100 cm
−1, while a shoulder at 1150 cm
−1 indicates the presence of non-bridging oxygen distinct from the Q
3 species [
9,
18]. Bands in the spectral region between 4000 and 3300 cm
−1 are indicative of hydroxyl groups (O–H) associated with water molecules and silanol groups (Si–OH) primarily found at the surface of silica particles. These vibrations reflect the presence of these functional groups and their role in the chemical structure of silica. The identification of these bands is crucial for understanding the moisture interactions in silica-based materials.
The mechanism behind the change in strength in the studied samples is, in our opinion, related to the formation of varying amounts of OH groups on the surface of the fibers, particularly notable in fibers with high contents of alkali and alkaline earth metal oxides. The addition of network-forming oxides appears to decrease the number of these groups. This can lead to the formation of defects on the surface during freezing and thawing cycles, resulting in reduced strength. In cases where silicon, aluminum, and titanium oxides are added, these elements may serve as network-forming oxides in small quantities, as indirectly indicated by the increase in their processing temperature. At the same time, this can lead to a reduction in OH group content, which has a lesser impact on strength changes during freezing and thawing.
The results obtained may be of interest for the application of basalt continuous fibers as a reinforcing filler in polymer composite materials intended for use in low (for example, in Arctic regions) and ultra-low temperatures (in industries utilizing liquid nitrogen). We believe that for such conditions, it would be advisable to use additives that reduce the number of OH groups in basalt glass fibers. However, excessive additives may lead to immiscibility in glasses, which will lead to a decrease in strength.
5. Conclusions
In this study, laboratory-scale experiments established the fiber-forming temperature ranges for basalt glasses modified with Na2O, SiO2, CaO, TiO2, and Al2O3. The observed cryogenic strength variations represent intrinsic material responses to low-temperature exposure, analyzed independently of the production process.
Tensile strength of the BCF exhibited significant variation from 1915 MPa at room temperature to 1714 MPa at −196 °C, indicating a substantial reduction in strength due to low-temperature exposure. Notably, the addition of sodium oxide not only broadened the fiber-forming temperature range—from 70 °C to 130 °C for Na5—but also improved the initial tensile strength to 2351 MPa. However, as the cryogenic temperatures were applied, particularly for Na10, a dramatic reduction in strength was observed, with a decrease of 32.8%.
Infrared spectroscopy revealed that variations in hydroxyl (OH) group content on the fiber surface could significantly affect mechanical properties, reinforcing the importance of careful oxide selection during fiber production. The results indicate that basalt fibers are promising candidates for reinforcing composites intended for use in ultra-low temperatures, although care must be taken to avoid excessive additives that could lead to immiscibility and reduced strength. The results indicate that basalt fibers are promising candidates for reinforcing composites intended for use in ultra-low temperatures. However, it is crucial to avoid excessive additives that could lead to immiscibility and reduced strength. These findings lay the groundwork for future research aimed at optimizing basalt fiber compositions for enhanced performance in extreme environmental conditions. Additionally, further studies could explore the long-term effects of low-temperature exposure on the structural integrity and durability of basalt fibers, providing valuable insights for their application in various industries.