Abstract
In power transformers, insulating liquids are essential for cooling, insulation, and condition monitoring. However, the environmental impact and biodegradability issues of traditional hydrocarbon-based liquids have spurred interest in green alternatives like natural esters. Despite their benefits, natural esters are highly prone to oxidation, limiting their broader use. This study explores a novel blend of two plant-based oils, canola oil and methyl ester derived from palm kernel oil, enhanced with two antioxidants, Tert-butylhydroquinone (TBHQ) and 2,6-Di-tert-butyl-4-methyl-phenol (BHT), to improve oxidation resistance. The performance of this antioxidant-infused oil was evaluated in terms of its interaction with Kraft paper insulation through accelerated thermal aging over periods of 10, 20, 30, and 40 days. Key properties, including the viscosity, breakdown voltage, conductivity, and FTIR spectra of oils, were analyzed before and after aging. Additionally, the degradation of the Kraft paper was investigated using scanning electron microscopy (SEM), optical microscopy, and dielectric strength tests. The results show that the antioxidant-treated oil exhibits significantly enhanced molecular stability, reduced viscosity, lower conductivity, and improved breakdown voltage (53.16 kV after 40 days). Notably, the oil mixture maintained the integrity of the Kraft paper insulation better than traditional natural esters, demonstrating superior dielectric properties and a promising potential for more sustainable and reliable power transformer applications.
1. Introduction
Sustainable, renewable, and environmentally friendly energy sources have increasingly gained attention in the industry due to strict environmental regulations aimed at reducing reliance on fossil-based materials. Traditionally, fossil-based materials such as mineral insulating oils have been used for transformer insulation since the late 19th century [1]. However, in response to environmental concerns, green alternatives like natural ester-insulating liquids, which are plant-based materials, have emerged as a promising solution to mitigate the ecological impact of mineral-based insulating oils. Natural esters provide multiple advantages as insulating liquids, such as a high flash point, elevated fire point, non-toxic nature, and minimal environmental impact [2]. Moreover, natural esters exhibit excellent compatibility with insulating paper and help preserve its integrity due to their high hydrophilicity [3]. However, natural esters face significant challenges, including lower oxidation stability and weak ionization resistance [4]. Natural esters are susceptible to oxidation due to their fatty acids composition containing unsaturated carbon chains [5]. The challenge of oxidation stability has been addressed through additives manufacturing, which has progressively improved the quality of natural esters by incorporating antioxidants into the base liquids, leading to what is commonly known as inhibited insulating liquids [6]. It is to be mentioned that both natural and synthetic antioxidants have been widely reported in the literature for enhancing the oxidation stability of mineral oils and natural esters [7,8,9]. However, synthetic antioxidants are generally considered more stable at elevated temperatures due to their superior chemical stability. Commonly used antioxidants include 2,6-di-tert-butyl-paracresol (DBPC), Tert-Butylhydroquinone (THBQ), Propyl Gallate (PG), and 1,2,3-trihydroxybenzene (Pyrogallol), which are limited to 0.4% of the total weight of the product in IEC 60296 and 0.3% in ASTM D3487 [6]. Insulating liquids are used alongside solid insulators in power transformers for insulation purposes, making it essential to understand the compatibility between the paper and the insulating liquids. An example of a solid insulator is unbleached Kraft paper, which consists of 78–80 wt.% cellulose, 10–20 wt.% hemicellulose, and 2–6 wt.% lignin [10]. Insulating papers are a critical component of transformers, providing essential insulation and mechanical support to the windings [11]. The primary source of cellulose, Figure 1, used for industrial applications is wood pulp and cotton lint. Cellulose is a homopolymer of D-anhydroglucose units, which are bonded by a C1–C4 glycosidic oxygen linkage [12]. The degree of polymerization, which is the average number of glucose rings in a polymeric chain, is the fundamental characterization property of cellulose [13]. They are semi-crystalline materials with both highly crystalline nano-scale domains and amorphous regions [14]. Unlike insulating liquids, paper insulation is nearly impossible to replace once damaged, meaning the operational lifespan of a transformer is directly dependent on the condition of the insulating paper. In transformer insulation applications, Kraft papers are preferred because of the monoclinic lattice structure of the cellulose chains [13]. It is crucial to recognize that the quality of the insulating paper is influenced by the oxidation stability of the insulating liquids, as oxidation by-products can accelerate the degradation of the paper [15]. Furthermore, mechanical and electrical stresses also contribute to this degradation. The primary degradation mechanisms in paper insulation systems include hydrolysis, oxidation, and pyrolysis. Due to the presence of polar groups in its molecules, cellulose exhibits strong hydrophilic behavior, resulting in a high affinity for moisture. Increased moisture content accelerates hydrolytic aging and significantly reduces the lifespan of power transformers, with every 0.5 wt.% rise in moisture content contributing to faster degradation [16,17]. The oxidation of insulating paper is a slow process compared to insulating liquids. It occurs when oxygen attacks the cellulose, weakening the glycosidic linkages by targeting the hydroxyl groups in the cellulose structure. This degradation gradually compromises the mechanical integrity of the paper [18]. Furthermore, in the absence of moisture and oxidizing agents, but in the presence of heat, the chemical properties of the paper change, a process known as pyrolysis. Pyrolysis leads to the decomposition of the paper’s organic materials, producing by-products such as carbonyl compounds, water, solid carbon residues, and carbon oxides [18]. This process alters the mechanical and insulating properties of the paper, contributing to its degradation over time.
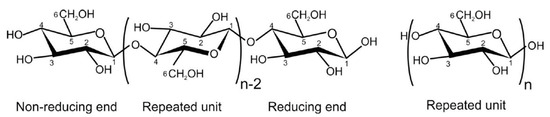
Figure 1.
Cellulose structure.
The compatibility of natural esters with insulating Kraft paper has been reported as excellent, particularly in terms of dielectric and physical properties [19,20,21,22]. In addition, the report made by Yang et al. [23] using molecular dynamics analysis reveals that vegetable oil protects Kraft paper by strongly attracting water molecules through electrostatic interactions, thereby reducing hydrolytic degradation. Additionally, ester groups in the oil bind water tightly and align along cellulose chains to form a water barrier, further minimizing moisture-related damage. However, it was concluded by Fernández et al. [24] that due to the low cooling capacity of natural ester, they created a hotspot that could rapidly degrade the paper quality in the hotspot regions. In addition, enhancing the oxidation stability of natural esters remains a significant challenge in the field of transformer insulation. This is crucial because the oxidation by-products of natural esters can directly degrade the quality of the insulating paper, impacting the overall performance and lifespan of the transformer.
This study investigates a 1:1 mixture of two different biodegradable insulating liquids to reduce the viscosity of natural ester oil to mitigate previously reported hotspot issues stated by [24], enhance the oxidation stability of the mixed oil using antioxidants, and assess its compatibility with Kraft paper. These two liquids were chosen for their distinctive properties; canola oil, rich in monounsaturated fatty acids, is ideal for insulating applications in subzero environments, while palm kernel oil, dominated by saturated fatty acids, provides enhanced oxidation stability. Given the trade-offs inherent in natural esters [25,26,27], a composite mixture of the two liquids was formulated as a single base oil to achieve both good oxidation stability and favorable low-temperature flow properties, and also a liquid with low viscosity. The low-viscosity property was achieved by transesterifying the palm kernel oil for the production of methyl ester. The combination of two different antioxidants, Tert-butylhydroquinone and 2,6-Di-tert-butyl-4-methyl-phenol, was considered following the recommendation by the CIGRE (Council on Large Electric Systems) working group, which recommended the addition of two or more antioxidants for the effective enhancement of natural esters [28]. Furthermore, the compatibility of the synthesized oil with insulating Kraft paper has also been investigated and compared with existing insulating natural ester.
2. Materials and Methods
2.1. Materials and Chemicals
Kraft paper of 0.255 mm thickness, palm kernel oil, and canola-based commercial insulating liquid are the basic insulating materials considered in this work. Citric acid (≥99.5% purity), anhydrous NaOH pellets (≥98% purity), distilled water, concentrated sulfuric acid (100% purity), methanol (100% purity), isopropyl alcohol (99.8% purity), potassium hydroxide pellets, phenolphthalein, copper catalyst, and filter paper are all of industrial standard and procured from Sigma-Aldrich, Ontario, Canada. The bleaching clay, Tonsil Standard 310 FF was procured from the TER chemical distribution group in Germany. The two donor antioxidants used in this work are Tert-butylhydroquinone (TBHQ) (97% purity) and 2,6-Di-tert-butyl-4-methyl-phenol (≥99% purity), which were obtained from Sigma-Aldrich, Ontario, Canada.
2.2. Samples Preparation and Accelerated Thermal Aging
Canola-based oil, a commercially purified insulating liquid, was first degassed and dehumidified in an Isotemp Vacuum Oven Model 285A at 60 °C for 48 h to eliminate dissolved gases and moisture. Palm kernel oil, obtained from a local producer, underwent a multi-step purification process. Initially, the raw oil was heated to 60 °C using an SH-3 hotplate with a magnetic stirrer. To remove phospholipids and other impurities, a citric acid solution (30% w/w) was introduced at a ratio of 7.5 mL per 1000 mL of oil, followed by continuous stirring for 30 min. The acidic content in the oil was then neutralized using a sodium hydroxide solution (8% w/w), which was added at the same temperature and stirred for another 30 min. Since the neutralization process introduced moisture, the oil was subsequently dehumidified in a vacuum oven at 60 °C for one hour. After dehumidification, the oil was subjected to bleaching using Tonsil Standard 310 FF bleaching clay, which effectively removed pro-oxidants and ionic impurities. Any residual impurities were then filtered out using Whatman filter papers No. 1 and 42 with a porosity of 2.5 µm. Before proceeding with transesterification, the acidity of the oil was measured to prevent saponification, which could occur if the free fatty acid (FFA) content exceeded 1% by weight of the oil. Since the purified oil had an FFA value above this threshold, an esterification process was carried out using methanol and sulfuric acid. The methanol used was 20% of the oil’s weight, while the sulfuric acid was added at 5% of the FFA value. The purified oil was preheated before adding the methanol and acid, allowing the reaction to proceed at 60 °C for one hour. The sample was then transferred into a separatory funnel, where the methanol waste was removed. This process successfully reduced the FFA content to 0.833%, making it suitable for transesterification.
Following the reduction in FFA, the transesterification process was carried out to produce methyl ester. The esterified oil was heated to 60 °C, while NaOH was dissolved in methanol to form sodium methoxide. This methoxide solution was then added to the preheated oil and stirred continuously for one hour. Afterward, the reaction mixture was placed in a separatory funnel, where glycerol was removed from the methyl ester. The methyl ester was washed several times using warm distilled water until the water phase became clear, indicating the complete removal of any residual NaOH. Finally, the purified methyl ester was transferred into a vacuum oven and dehumidified at 60 °C for 48 h to ensure a moisture-free insulating oil.
The dehumidified liquids, canola oil and methyl ester were mixed in equal proportions to form the base liquid used in this study. This equal volume ratio was chosen to balance the properties of both liquids [25,26]. The mixed liquid was then degassed and dehydrated in a vacuum oven at 60 °C for 48 h, reducing the moisture content to 20 ppm. Following the recommendations of the CIGRE working group [28], two different antioxidants, Tert-butylhydroquinone (TBHQ) and 2,6-Di-tert-butyl-4-methyl-phenol, were each added at a concentration of 0.25 wt.%. This level of antioxidant addition was selected to ensure the total additive concentration remains within the range recommended by the CIGRE, as exceeding this limit may enhance the oxidation stability but could potentially compromise the dielectric properties of the oil [29]. The antioxidants were directly introduced into the base liquid and stirred for 30 min to ensure uniform dispersion. As a result, three liquid samples were considered in this study: the mixed oil, the mixed oil with antioxidants, and an existing canola-based insulating liquid for comparison.
The insulating Kraft papers were separately dried in a vacuum oven at 110 °C for 48 h before being impregnated with the three insulating liquids: the canola-based oil (C), the composite liquid containing antioxidants (CPA), and the composite liquid without antioxidants (CP). The oil-to-paper ratio was maintained at 20:1, with 3 g/L of a copper catalyst added to facilitate the aging process. The samples were then subjected to accelerated thermal aging at 130 °C for 40 days, with oil and paper samples collected every 10 days for analysis. The sample descriptions are detailed in Table 1 and a schematic illustration of the sample aging process is presented in Figure 2.

Table 1.
Sample description.
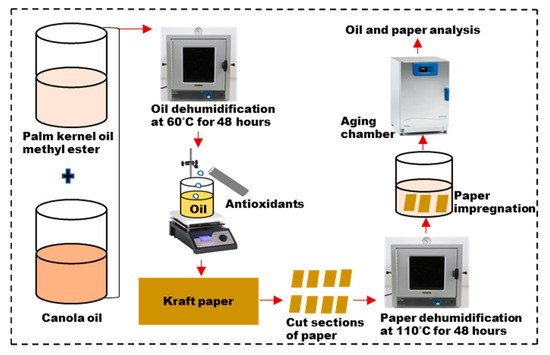
Figure 2.
Sample aging process.
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
The molecular deformation in both the oils and Kraft papers was analyzed as they aged using a Benchtop Agilent Cary 630 FTIR (Agilent, Santa Clara, CA, USA) spectrometer equipped with attenuated total reflectance (ATR). The measurement was made in the transmission mode with the wave number spanning from 450 to 4000 cm−1. The prominent peak regions that described the aging process were selected and presented graphically.
2.4. Optical Microscopy
Structural changes in the insulating Kraft papers, particularly those associated with the fiber line entanglement, were examined using an optical microscope (Nikon Eclipse ME600, Nikon Co., Tokyo, Japan). The optical microscope was initially used to capture the micrographs of the aged samples at 40 days, which provides information about the physical degradation and fiber structure of the aged paper samples.
2.5. Scanning Electron Microscopy (SEM)
A scanning electron microscope (JEOL JSM-6480LV, JEOL USA, Inc., Peabody, MA, USA) was used to further analyze the microstructural changes in the insulating Kraft paper during the aging period. Before SEM analysis, the samples were sputter-coated with a thin layer of gold using a Bio-Rad SEM coating system to render the insulating material conductive and suitable for electron imaging. The SEM images provided high-resolution visualization of surface morphology and fiber deterioration due to thermal aging. In addition, during the paper degradation, the degree of polymerization reduces, implying that the cellulose content reduces from the paper. Therefore, the elemental composition of the papers was monitored during the aging process through energy-dispersive X-ray (EDX).
2.6. Viscosity Measurement
The viscosity of the oil samples was measured following ASTM D445 using the KV3000 Series kinematic viscosity water bath. Measurements were conducted at five different temperatures: 40 °C, 50 °C, 60 °C, 70 °C, and 80 °C. The temperature–viscosity relationship of the aged oils was analyzed using the Arrhenius equation for thermally activated processes, as expressed in Equation (1):
where η is the viscosity (Pa·s), KB is the Boltzmann constant, T is the temperature in Kelvin, A is the pre-exponential factor, and is the activation energy.
2.7. AC Conductivity Measurement
The AC conductivity of both the oil and insulating Kraft paper samples was measured using a Novocontrol Alpha-A High-Performance Frequency Analyzer (Novocontrol Technologies, Montabaur, Germany). For the oil samples, a cylindrical liquid sample holder was used, and conductivity was recorded across a frequency range of 10−3 Hz to 103 Hz. For the oil-impregnated paper, the sample was positioned between 30 mm diameter electrodes, with an automated temperature control system ensuring stability. All measurements were conducted at a controlled temperature of 25 °C ± 0.1 °C.
2.8. AC Breakdown Voltage Measurement
The AC breakdown voltage of the oil samples and impregnated Kraft papers was determined following ASTM D877 and ASTM D149-20 standards [30,31]. The breakdown voltage analysis provided insights into the dielectric strength of the insulating liquids and their compatibility with paper insulation over the aging period.
3. Results and Discussion
3.1. Optical Microscopy of Impregnated Papers
The microstructure of oil-impregnated Kraft papers was examined after 40 days of accelerated thermal aging to assess the physical deformation of the cellulose fibers. Figure 3 illustrates the microstructure of paper impregnated with the canola-based oil, composite liquid without antioxidants, and composite liquid with antioxidants. During the thermal degradation of cellulose paper, the disruption of the β-1,4 glycosidic bond in the cellulose leads to a reduction in the degree of polymerization, causing a loss of mechanical strength and increased brittleness. In Figure 3a,b, the prominence of the fiber lines decreases, with evidence of microcracks and a rougher surface texture. Additionally, the entanglement of the fiber lines is reduced, indicating a loss of fiber stacking and disorganization of the crystalline cellulose chain. However, in Figure 3c, the crystalline cellulose chain remains prominent, showing a smooth and glossy morphology, with clear fiber lines, suggesting minimal degradation. The microstructure analysis indicates that the composite liquid containing antioxidants better protects the integrity of the cellulose paper at high temperatures, compared to the commercial canola-based insulating liquid. This enhanced protection can likely be attributed to the high oxidation stability of the oil, which helps to prevent the rapid degradation of the cellulose.

Figure 3.
Micrographs captured using an optical microscope showing the microstructure of impregnated papers after 40 days of accelerated thermal aging: (a) KC paper; (b) KCP paper; (c) KCPA paper.
3.2. Scanning Electron Microscopy and Energy-Dispersive Spectroscopy of Aged Papers
The SEM micrograph and EDS results of dry Kraft paper are presented in Figure 4a,b, respectively. In Figure 4a, the cellulose fibers appear clean, well-defined, and interwoven, with no visible contamination or additional structures on the surface. This represents the inherent structure of dry Kraft paper. The corresponding EDS analysis in Figure 4b reveals the elemental composition of the Kraft paper, which primarily consists of carbon (C) and oxygen (O). Although Kraft paper contains hydrogen (H) as part of its cellulose structure, it does not appear in the spectrum because EDS cannot detect hydrogen due to its low atomic number. The quantitative data for carbon and oxygen content, expressed as weight and atomic percentages, are summarized in Table 2. These results align with previously reported values for new Kraft paper [32,33]. The impregnation of Kraft paper with natural ester oil leads to a notable increase in carbon content, as shown in Table 2. This increase is attributed to the presence of long-chain hydrocarbons and esters in the oil, which elevate the overall carbon content. Simultaneously, a relative decrease in oxygen content is observed, likely due to the lower oxygen-to-carbon ratio of the natural ester oil compared to the cellulose structure in Kraft paper. After 40 days of accelerated thermal aging, significant changes are observed. The oxygen content increases substantially, which can be attributed to the oxidation and or hydrolysis of cellulose, resulting in the formation of carbonyl and carboxyl groups. Concurrently, a reduction in carbon content is noted, likely due to cellulose decomposition and the subsequent release of volatile organic compounds such as CO and CO2.
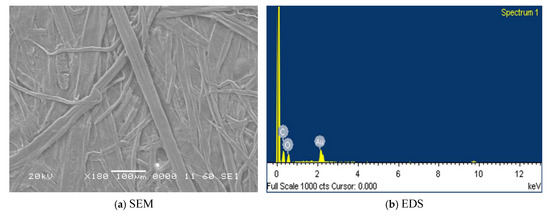
Figure 4.
(a) SEM and (b) EDS of dry Kraft paper.

Table 2.
Elemental composition of dry, freshly impregnated, and 40 days aged papers.
The SEM images of new and aged oil-impregnated papers for samples KC, KCP, and KCPA are presented in Figure 5a,b, Figure 6a,b and Figure 7a,b, respectively. The micrographs reveal distinct aging characteristics. For sample KC, Figure 5a,b, after 40 days of aging, the paper shows clear signs of delamination and surface degradation. Although the surface appears relatively smooth, the presence of cracks in Figure 5b indicates a loss of mechanical stability caused by the aging process. In the case of sample KCP, Figure 6a,b, no significant structural damage is observed; however, the surface appears rougher when compared to the fresh sample, suggesting moderate degradation. In contrast, for sample KCPA, Figure 7a,b, the aged paper in Figure 7b appears smoother, more cohesive, and free of visible cracks. This indicates superior structural preservation and mechanical stability, suggesting that the paper impregnated with CPA exhibits better resistance to aging effects. The presence of quasi-spherical shapes in the SEM micrographs of oil-impregnated papers, Figure 5, Figure 6 and Figure 7, is likely due to interactions between the natural ester oil and the Kraft paper fibers. These structures were not observed in the dry Kraft paper, further confirming their origin in the oil-impregnated samples.
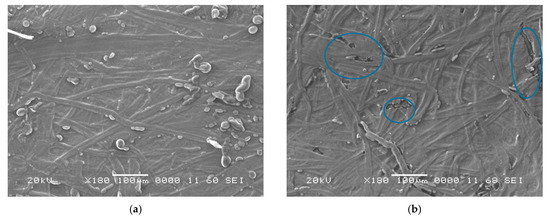
Figure 5.
(a) SEM of unaged; (b) 40 days aged impregnated paper KC.

Figure 6.
(a) SEM of unaged; (b) 40 days aged impregnated paper KCP.
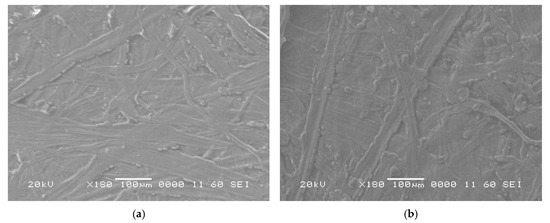
Figure 7.
SEM of (a) unaged; (b) 40 days aged impregnated paper KCPA.
Furthermore, after 40 days of aging, the reduction in carbon content and the increase in oxygen content are minimal for sample KCPA compared to samples KC and KCP. This indicates that Kraft paper impregnated with sample CPA exhibits greater chemical stability and experiences fewer degradation processes under accelerated thermal aging.
3.3. Conductivity of Oils and Impregnated Papers
The conductivity of the oil samples, commercial oil (C), mixed oil (CP), and mixed oil with antioxidants (CPA), at power frequency and over different aging durations is presented in Figure 8a. A noticeable difference in the conductivity of the three fresh oil samples was observed, which may be attributed to variations in sample purity. During the sample preparation process, including purification and transesterification, the oil samples underwent several stages that could have introduced impurities. It is important to note that the lower viscosity of the prepared samples in this study might also account for their higher conductivity compared to the commercial oil sample C. The electrical conductivity of all three insulating liquids increases with the aging time, likely due to the effect of oxidation by-products such as aldehydes and ketones or the formation of acidic by-products through hydrolysis, which contribute to the buildup of ionic impurities in the oils over time. Among the samples, CP exhibits the highest electrical conductivity as aging progresses, suggesting that CP is more susceptible to aging or degradation processes, leading to a faster accumulation of conductive species. In contrast, the C and CPA samples display lower electrical conductivity, indicating greater stability against aging and degradation at high temperatures. This enhanced stability is attributed to the presence of antioxidants in these oils, which slow the degradation rate. Additionally, CP shows a sharp rise in conductivity between 20 and 30 days of aging, a trend likely caused by the absence of antioxidants in the sample. Both the CPA and C samples show a more gradual, linear increase in conductivity, implying that their aging mechanisms are slower or that they better resist the formation of conductive by-products over time. The more stable slope of the CPA sample, in particular, suggests a consistent increase in conductivity, which could be due to the antioxidants’ ability to preserve the integrity of the oil.

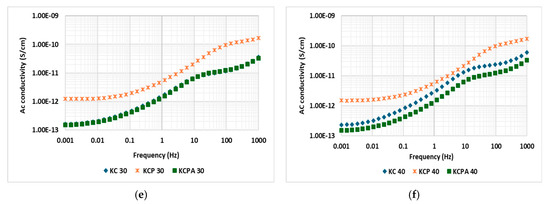
Figure 8.
(a) Conductivity of aged oils; (b–f) conductivity of aged impregnated Kraft papers.
Figure 8b–f compares the electrical conductivity of paper impregnated with three different oils. In Figure 8b, for unaged impregnated papers, the paper impregnated with CPA exhibits higher conductivity at low frequencies, with a slope close to zero, indicating a near-DC conduction behavior [5], which could be attributed to antioxidant molecule polarization at low frequencies under the influence of an electric field. As the frequency increases, AC conductivity also rises, suggesting that the system is not purely DC conduction but experiences a very slow, low-frequency dispersion. Figure 8c–e compares the electrical conductivity of aged impregnated papers at 10, 20, 30, and 40 days, respectively. As aging progresses, the electrical conductivity of the impregnated papers increases, with a noticeable upward shift in the conductivity values shown in Figure 8c–e. After 30 and 40 days of aging, the paper impregnated with CPA demonstrates lower conductivity values compared to the other samples, suggesting greater stability of the KCPA oil-impregnated Kraft paper. This stability could be due to the presence of antioxidants in the oil, which prevents the generation of radicals that could otherwise attack the insulating paper.
3.4. Fourier Transform Infrared Spectroscopy of Oil and Impregnated Paper
3.4.1. FTIR of Fresh and Aged Oils
The molecular deformation and development of aging by-products due to thermal degradation in the oils were monitored using Fourier Transform Infrared (FTIR) spectroscopy, as shown in Figure 9. In the FTIR spectrum, peaks between 400 and 1400 cm−1 represent various aromatic C-H and C=O functional groups, with the C=O groups primarily being ketones and aldehydes, which are typical by-products of oxidation [34]. In Figure 9, the prominent peak at 1158 cm−1 can be attributed to the out-of-plane bending vibrations of methylene groups (CH2), indicating an increase in ester groups. Additionally, the medium peak around 1100 cm−1 may correspond to C–O–C antisymmetric stretching in esters [35]. Figure 9a–c illustrate the spectra of samples C, CP, and CPA, respectively.

Figure 9.
(a–c) Spectra of sample C, CP, and CPA; (d–h) spectra comparing the properties of oils at all aging stages.
At the 1158 cm−1 peak in Figure 9a, a noticeable intensity shift is observed after 10 days of aging, which can be attributed to structural changes in the ester linkages, possibly due to oxidation. The distinct change in the C40 curve suggests a higher degree of ester degradation, leading to the formation of smaller molecules such as acids or alcohols. However, in Figure 9b,c, no significant variation is observed at the same peak, likely indicating greater stability of the CP and CPA oil samples. Figure 9d–h compare the spectra of the oils at different stages of thermal aging. Over the aging period from 0 to 40 days, sample CPA consistently shows a narrower peak compared to samples C and CP, indicating greater stability in its molecular structure. At 40 days of aging, sample C exhibits a broader peak at 1100 cm−1, which suggests a higher degree of ester degradation. This broadening is indicative of increased oxidation and molecular breakdown, leading to the formation of by-products such as acids and alcohols. In contrast, the narrower peak in CPA points to the effectiveness of antioxidants in slowing down the degradation process.
3.4.2. FTIR of Fresh and Aged Impregnated Papers
The FTIR spectra of unaged impregnated Kraft papers are shown in Figure 10a–c. The multiple peaks observed between 1022 and 1159.66 cm−1 correspond to the C–O–C stretching vibration, which is characteristic of cellulose backbone vibrations or ether linkages. The peak at 1457 cm−1 is associated with C–C absorption, indicating the presence of carbon-carbon bonds within the paper structure. Additionally, the peak at 2919 cm−1 is attributed to C–H (alkane) absorption, which suggests the presence of aliphatic hydrocarbons. Lastly, the peak at 3294 cm−1 corresponds to –OH stretching vibration absorption, likely from hydroxyl groups, indicating moisture content or cellulose structure within the Kraft paper [36,37,38]. Over the aging period, subtle shifts and changes in the intensity of the aged samples were observed, likely due to the formation of aging by-products, the breakdown of hydrocarbon chains, or alterations in cellulose bonds caused by thermal or oxidative aging. In the 1022–1159.66 cm−1 region, the peaks broaden, indicating structural changes in the cellulose over time. Figure 10d–h compare the spectra of all samples at various stages of aging. In Figure 10d, the unaged sample (KCP) displays stable peaks in the 1022–1159.66 cm−1 and 3294 cm−1 regions, likely due to the absence of antioxidants in the oil. As aging progresses, KCPA exhibits greater stability compared to other samples, particularly in the cellulosic peak region. This can be attributed to the enhanced oxidative stability of the oil due to the presence of antioxidants, which slow down the formation of free radicals, which would otherwise attack and degrade the cellulose structure.
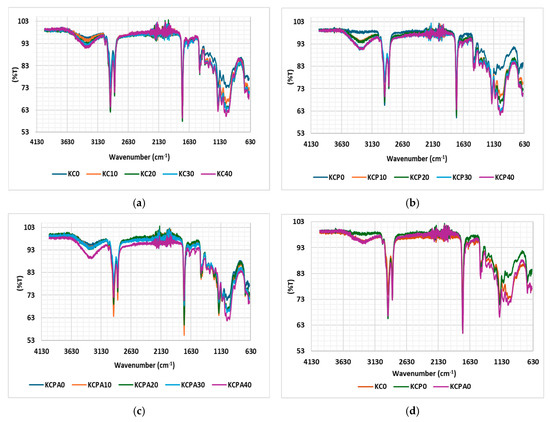
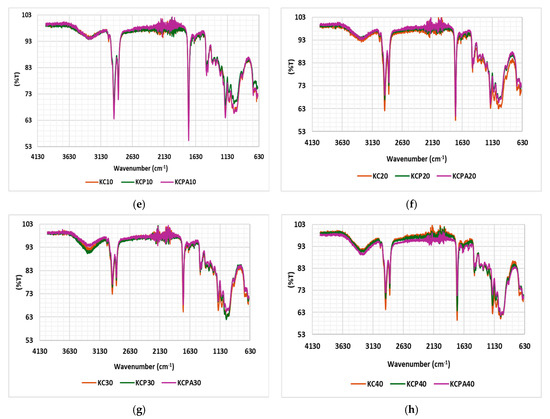
Figure 10.
(a–c) Spectra of KC, KCP, KCPA; (d–h) spectra comparing the properties of impregnated papers at all aging stages.
3.5. Viscosity and Activation Energy
The viscosity of insulating oil is a crucial parameter in transformer cooling, playing a significant role in the performance of modern power transformers. In particular, the viscosity of natural esters is highly influenced by oxidation reactions due to their chemical composition. Over time, as oxidation progresses in an operational transformer, the oil’s viscosity increases, leading to polymerization, which can adversely affect the cooling system of the transformer. The viscosity of the oils, denoted as C, CP, and CPA, was measured across varying temperatures. Figure 11, Figure 12 and Figure 13 illustrate the viscosity-temperature relationship for C, CP, and CPA, respectively, at different aging time intervals. At 40 °C for the fresh samples, the measured viscosities were 37.96 cSt for C, 12.99 cSt for CP, and 12.51 cSt for CPA. The addition of antioxidants slightly reduced the viscosity of the base sample CP, likely due to the dispersant effect of the antioxidants [9]. Since the heat transfer coefficient is inversely proportional to the viscosity of an insulating liquid [39], it can be inferred that CPA, with the lowest viscosity, offers higher cooling efficiency compared to the other samples, particularly the commercial oil, sample C. As the accelerated thermal aging period progressed, a significant increase in the viscosity of all the oils was observed. After 40 days, the percentage increase in viscosity was 85.72% for C, 83% for CP, and 66.61% for CPA. This increase is attributed to the formation of polymeric materials during the degradation of insulating liquids. The lowest percentage increase observed for CPA suggests the least oxidative degradation, which can be attributed to the effectiveness of antioxidants in the oil. The viscosities after 40 days of aging were measured as 70.5 cSt for C, 23.8 cSt for CP, and 20.844 cSt for CPA. Low-viscosity liquids are preferred for efficient transformer cooling due to their higher Reynolds number and heat transfer coefficient, as supported by the established literature [40]. Therefore, CPA emerges as a promising insulating liquid for transformer cooling and insulation applications.
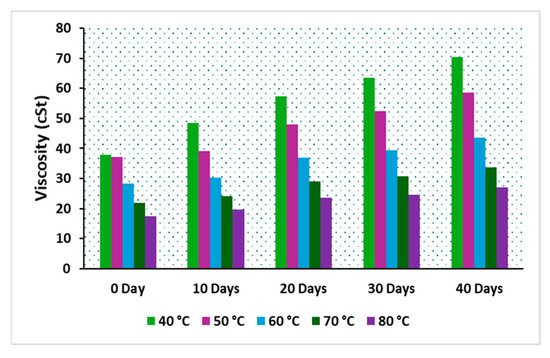
Figure 11.
Viscosity of oil, sample C.
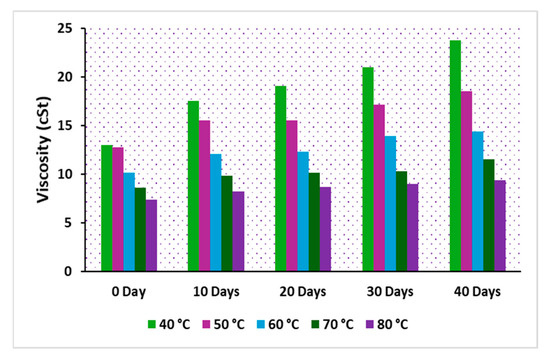
Figure 12.
Viscosity of oil, sample CP.
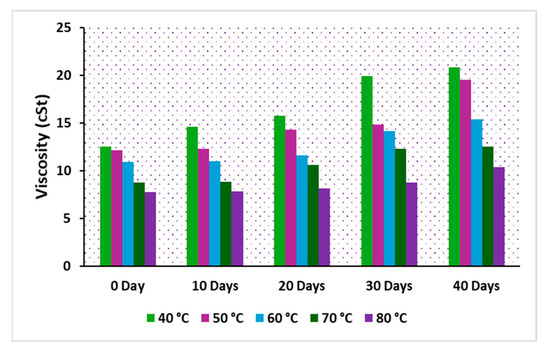
Figure 13.
Viscosity of oil, sample CPA.
During transformer operation, variations in operating temperature can occur, making it crucial to understand how the oil behaves under changing temperatures. The activation energy (Ea) for viscosity characterizes a fluid’s sensitivity to temperature changes. A high activation energy indicates significant viscosity changes with temperature, while a low activation energy implies relative stability. The activation energy of the oils was determined by measuring viscosity across various temperatures. Figure 14, Figure 15 and Figure 16 show that the oils exhibit similar Arrhenius behavior, with thermally activated responses and R2 values ranging from 0.9255 to 0.9998. The activation energy values at different aging periods are presented in Table 3. The commercial insulating liquid (sample C) has a higher activation energy compared to the prepared oils, CP and CPA. This difference is likely due to the absence of glycerol in CP and CPA, which reduces intermolecular interactions. Research has shown that removing glycerol decreases the activation energy because it significantly reduces viscosity and the strong intermolecular forces provided by glycerol [5]. The addition of antioxidants further decreases the activation energy, likely due to their dispersant behavior, which diminishes intermolecular attraction between oil molecules. The low activation energy of CPA indicates consistent flow characteristics across a wide temperature range, ensuring efficient cooling at both low and high temperatures, an essential trait for transformers where temperatures fluctuate significantly. In contrast, the high activation energy of sample C suggests a strong temperature dependence. At low temperatures, sample C may become overly viscous, impairing cooling efficiency, while at high temperatures, its viscosity may drop excessively, compromising dielectric performance. Considering temperature stability, dielectric integrity, and heat transfer efficiency, sample CPA emerges as a superior insulating liquid for transformer applications.

Figure 14.
Arrhenius plot for sample C.

Figure 15.
Arrhenius plot for sample CP.
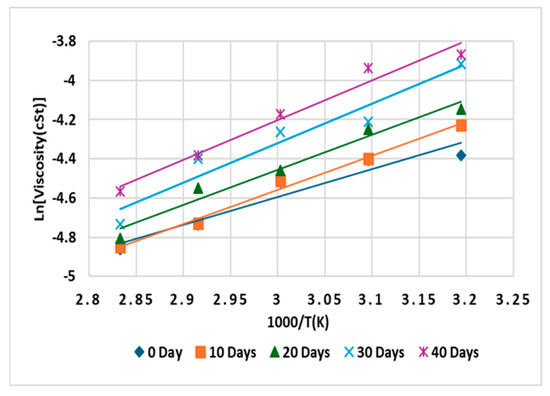
Figure 16.
Arrhenius plot for sample CPA.

Table 3.
Activation energy in J/mol.
3.6. Breakdown Voltage of Oils and Impregnated Papers
The AC breakdown voltage of the oils is shown in Figure 17. Before accelerated thermal aging, there is no significant difference in breakdown voltage between the commercial oil and the mixed oil without antioxidants, with breakdown values of 54.2 kV and 54.18 kV for C and CP, respectively. However, the fresh sample containing antioxidants, CPA, exhibits a higher breakdown voltage, with a 4.2% increase compared to the base oil and the commercial oil. This improvement is attributed to the presence of antioxidants in CPA. Antioxidants, being mono-aromatic compounds, enhance the AC breakdown voltage of natural esters by increasing their gas-absorbing capacity under electric stresses [29]. After 10 days of accelerated thermal aging, a slight increase in the breakdown voltage was observed. This could be attributed to initial chemical changes in the oils, potentially enhancing their resistivity and making the liquids more resistant to electrical breakdown. As the aging period progresses, the breakdown voltage of all the oils decreases to 49.6 kV, 47.16 kV, and 53.16 kV for C, CP, and CPA, respectively, at 40 days. The degradation of oil molecules during accelerated thermal aging leads to the formation of polymeric materials and potential charge carriers within the oil. This increases the oil’s conductivity and, consequently, reduces its breakdown strength. Nonetheless, CPA consistently maintains the highest breakdown voltage throughout the aging process, as shown in Figure 17. This demonstrates that the antioxidants in CPA effectively preserve the oil’s integrity by mitigating the impact of radicals on the oil molecules, consequently enhancing its dielectric performance over time.
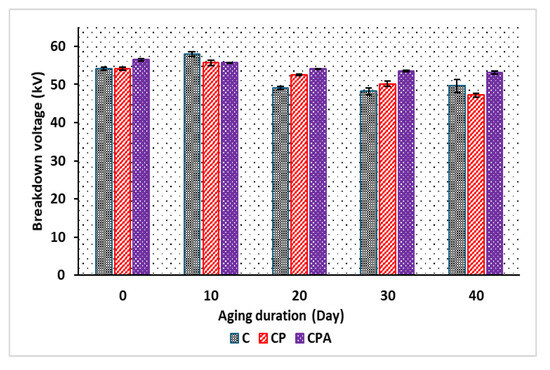
Figure 17.
AC breakdown voltage of oil samples before and after aging.
Figure 18 illustrates the dielectric breakdown strength of oil-impregnated papers throughout the accelerated thermal aging period. Initially, the vacuum-dried impregnated paper exhibited a breakdown voltage of 3.3 kV. After oil impregnation, the breakdown voltage increased, with no significant differences observed among papers impregnated with samples C, CP, and CPA. This suggests that, before thermal aging, oil impregnation provided a consistent insulation performance across all samples. As aging progressed, the dielectric strength of all paper samples converged to approximately 10 kV. However, the paper impregnated with CP showed the lowest average values, registering 9.8 kV at 30 days and 9.5 kV at 40 days. This reduction in KCP’s performance could be attributed to moisture ingress resulting from the hydrolysis of methyl esters in the oil, potentially caused by steric hindrance effects [26,41]. The relatively small variations in the breakdown voltage of all impregnated papers throughout aging suggest that dielectric strength degradation occurs at a slower rate compared to other molecular changes reported in the previous sections. Therefore, breakdown voltage may not serve as an effective early detection parameter for assessing the degradation of Kraft paper insulation.

Figure 18.
AC breakdown voltage of impregnated papers before and after aging.
4. Conclusions
The degradation and structural changes in oils and insulating papers play a critical role in maintaining the health and reliability of transformers. This study investigated the effects of two antioxidants on the thermal degradation of mixed oil and compared the results with an already existing insulating liquid. The key findings are summarized as follows:
- i.
- After 40 days of accelerated thermal aging, the microstructure of oil-impregnated papers revealed that paper impregnated with CPA exhibited the highest cellulose chain crystallinity, along with a smooth and glossy morphology. The AC conductivity of oils with antioxidants showed similar trends to already existing oil but with a more stable slope for CPA, indicating the effectiveness of antioxidants. Furthermore, CPA-impregnated paper demonstrated the lowest conductivity across the frequency range of 10−3 Hz to 103 Hz at 40 days of aging.
- ii.
- The FTIR analysis of both oils and papers with antioxidants revealed superior stability over the aging period, particularly at 40 days. Papers with antioxidants showed higher stability in the cellulosic peak region, highlighting the protective role of antioxidants against degradation.
- iii.
- The viscosity of all oils increased over the aging period, with CPA showing the lowest viscosity, making it a promising candidate as a cooling liquid for transformers. The viscosity–temperature relationship yielded activation energy values of 22.75 J/mol, 21.50 J/mol, and 16.83 J/mol for C, CP, and CPA, respectively. CPA’s lower activation energy ensures stable viscosity and reliable performance across the operational temperature range, reducing the likelihood of becoming overly viscous at low temperatures or excessively thin at high temperatures.
- iv.
- The dielectric breakdown strength of oils decreased over the aging period, with CPA maintaining the highest breakdown voltage (53.16 kV) after 40 days of aging. Additionally, no significant differences in the AC breakdown voltage of impregnated papers were observed among the samples over the aging period, particularly between the commercial oil and the base sample with antioxidants.
In conclusion, the antioxidant-enhanced, renewable-based insulating oil prepared in this work outperformed the already existing insulating oil in preserving the quality of paper insulation during accelerated aging. It exhibited superior molecular stability, lower electrical conductivity, reduced viscosity, and better activation energy, and maintained a high dielectric breakdown voltage, ensuring better overall performance and reliability. These attributes position this oil as a viable alternative to conventional mineral-based oils in power transformers, particularly where enhanced performance and sustainability are prioritized.
Author Contributions
S.O.O.: conceptualization, methodology, validation, formal analysis, investigation, visualization, writing—original draft. I.F.: validation, writing—review and editing, resources, funding acquisition, supervision. R.J.: validation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Fonds de recherche du Québec—Nature et Technologies (FRQNT), grant number V1-334706, https://doi.org/10.69777/334706 and supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), grant number RGPIN-2021-03232.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Farade, R.A.; Wahab, N.I.A.; Mansour, D.-E.A.; Soudagar, M.E.M. The Effect of Nanoadditives in Natural Ester Dielectric Liquids: A Comprehensive Review on Stability and Thermal Properties. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 1478–1492. [Google Scholar] [CrossRef]
- Rao, M.U.; Fofana, I.; Sarathi, R. Alternative Liquid Dielectrics for High Voltage Transformer Insulation Systems: Performance Analysis and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Lyutikova, M.N.; Korobeynikov, S.M.; Rao, U.M.; Fofana, I. Mixed Insulating Liquids with Mineral Oil for High-Voltage Transformer Applications: A Review. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 454–461. [Google Scholar] [CrossRef]
- Raymon, A. Transesterification Approaches to Natural Esters for Transformer Insulating Fluids–a Review. IEEE Trans. Dielectr. Electr. Insul. 2023, 31, 607–614. [Google Scholar] [CrossRef]
- Abdelmalik, A.A. The Feasibility of Using a Vegetable Oil-Based Fluid as Electrical Insulating Oil. Ph.D. Thesis, University of Leicester, Leicester, UK, 2012. [Google Scholar]
- Wolmarans, C.; Pahlavanpour, B. Insulating Liquid Properties Impacting Transformer Performance. Transform. Mag. 2019, 6, 104–110. [Google Scholar]
- Thangaraj, H.; David, P.W.; Balachandran, G.B.; Sivasekar, G.K. Performance Evaluation of Natural Olea Europaea (Olive Oil)-Based Blended Esters with Butylated Hydroxyanisole and Butylated Hydroxytoluene: Optimization Using Response Surface Methodology. Environ. Sci. Pollut. Res. 2024, 31, 4985–5000. [Google Scholar] [CrossRef]
- Samikannu, R.; Raj, R.A.; Karuppiah, D.; Dasari, N.R.; Subburaj, S.K.; Murugesan, S.; Akbar, S.S. Reclamation of Natural Esters Using Nanocarriers as the Biodegradable Choice for the Transformer Insulation. Environ. Technol. Innov. 2021, 23, 101634. [Google Scholar] [CrossRef]
- Raymon, P.P.S.; Rajamani, M.P.; Karthik, R. Enhancing the Critical Characteristics of Natural Esters with Antioxidants for Power Transformer Applications. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 899–912. [Google Scholar] [CrossRef]
- Adekunle, A.A.; Samson, O.O.; Fofana, I. Performance Assessment of Cellulose Paper Impregnated in Nanofluid for Power Transformer Insulation Application: A Review. Energies 2023, 16, 2002. [Google Scholar] [CrossRef]
- Gao, S.; Yang, L.; Ke, T. Failure Mechanism of Transformer Oil-Immersed Cellulosic Insulation Induced by Sulfur Corrosion. Cellulose 2020, 27, 7157–7174. [Google Scholar] [CrossRef]
- French, A.D. Glucose, Not Cellobiose, Is the Repeating Unit of Cellulose and Why That Is Important. Cellulose 2017, 24, 4605–4609. [Google Scholar] [CrossRef]
- Kapila, B.; Ekanayake, C.; Saha, T.K.; Annamalai, P.K. Understanding the Ageing Aspects of Natural Ester Based Insulation Liquid in Power Transformer. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 246–257. [Google Scholar]
- Lukic, J.; Deville, K.; Lessard, M.C.; Dreier, L.; Hohlein, I.A.; Vrsaljko, D.; Peixoto, A.; Melzer, L.; Lewand, L.; Ding, H. Changes of New Unused Insulating Kraft Paper Properties During Drying-Impact on Degree of Polymerization. Electra 2020, 312, 76–81. [Google Scholar]
- Garcia, D.F.; Garcia, B.; Burgos, J.C. A Review of Moisture Diffusion Coefficients in Transformer Solid Insulation-Part 1: Coefficients for Paper and Pressboard. IEEE Electr. Insul. Mag. 2013, 29, 46–54. [Google Scholar] [CrossRef]
- Zhu, M.; Liao, R.; Du, X.; Zhou, J.; Zhu, W. Simulation of Diffusion of Moisture in Insulation Paper and the Effect on Mechanical Properties of the Paper. Trans. China Electrotech. Soc 2015, 30, 338–345. [Google Scholar]
- Pavel, T.; Hornak, J.; Prosr, P.; Michal, O.; Wang, F. Various Aging Processes in a Paper-Natural Ester Insulation System in the Presence of Copper and Moisture. IEEE Access 2020, 8, 61989–61998. [Google Scholar]
- Gengadevi, K.; Rengaraj, M. Aging Assessment of Transformer Solid Insulation: A Review. Mater. Today Proc. 2021, 47, 272–277. [Google Scholar]
- Noofa, S.; Jacob, J.; Sindhu, T.K.; Preetha, P. Compatibility Analysis of Paper Insulation with Natural Ester. In Proceedings of the 2019 IEEE 4th International Conference on Condition Assessment Techniques in Electrical Systems (CATCON), Chennai, India, 21–23 November 2019. [Google Scholar]
- Abdelghaffar, A.A. Analysis of Thermally Aged Insulation Paper in a Natural Ester-Based Dielectric Fluid. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2408–2414. [Google Scholar]
- Gengadevi, K.; Madavan, R. Aging Analysis of Non-Edible Natural Ester Oil–Paper Insulation under Various Conditions. Ind. Crops Prod. 2023, 205, 117528. [Google Scholar] [CrossRef]
- Przybylek, P. Thermal Ageing of Dry Cellulose Paper Impregnated with Different Insulating Liquids—Comparative Studies of Materials Properties. Energies 2024, 17, 784. [Google Scholar] [CrossRef]
- Yang, L.; Liao, R.; Sun, C.; Yin, J.; Zhu, M. Influence of Vegetable Oil on the Thermal Aging Rate of Kraft Paper and Its Mechanism. In Proceedings of the 2010 International Conference on High Voltage Engineering and Application (ICHVE 2010), New Orleans, LA, USA, 19–22 October 2010; pp. 381–384. [Google Scholar]
- Inmaculada, F.; Delgado, F.; Ortiz, F.; Ortiz, A.; Fernández, C.; Renedo, C.J.; Santisteban, A. Thermal Degradation Assessment of Kraft Paper in Power Transformers Insulated with Natural Esters. Appl. Therm. Eng. 2016, 104, 129–138. [Google Scholar]
- Oparanti, S.O.; Yapi, K.M.L.; Fofana, I.; Rao, U.M. Preliminary Studies on Improving the Properties of Canola Oil by Addition of Methyl Ester from a Saturated Vegetable Oil. In Proceedings of the 2023 IEEE Electrical Insulation Conference (EIC 2023), Quebec City, QC, Canada, 18–21 June 2023; pp. 1–4. [Google Scholar]
- Oparanti, S.O.; Fofana, I.; Zarrougui, R.; Jafari, R.; Yapi, K.M.L. Improving Some Physicochemical Characteristics of Environmentally Friendly Insulating Liquids for Enhanced Sustainability in Subpolar Transformer Applications. Sustain. Mater. Technol. 2024, 41, e00996. [Google Scholar] [CrossRef]
- Muhammad, R.; Shafique, M.; Azam, A.; Ateeq, M.; Khan, I.A.; Hussain, A. Sustainable, Renewable and Environmental-Friendly Insulation Systems for High Voltages Applications. Molecules 2020, 25, 3901. [Google Scholar] [CrossRef]
- CIGRÉ Working Group D1.30. Oxidation Stability of Insulating Fluids; CIGRÉ Technical Brochure No. 526; CIGRÉ: Paris, France, 2013; ISBN 978-2-85873-219-7. [Google Scholar]
- Sharin, A.G.; Muhamad, N.A.; Noorden, Z.A.; Zainuddin, H.; Bakar, N.A.; Talib, M.A. Methods for Improving the Workability of Natural Ester Insulating Oils in Power Transformer Applications: A Review. Electr. Power Syst. Res. 2018, 163, 655–667. [Google Scholar]
- ASTM D877-02; Standard Test Method for Dielectric Breakdown Voltage of Insulating Liquids Using Disk Electrodes. ASTM Internationa: West Conshohocken, PA, USA, 2012; 40, pp. 244–248.
- Valcimar, S.A.; Boaventura, W.C.; Neves, M.A.; Granados, X. Dielectric Characterization of Nkn in Ln2 Using a Proposed Arrangement. IEEE Trans. Appl. Supercond. 2024, 34, 1–5. [Google Scholar]
- Auglius, P.R. Thermal Aging of Mineral Oil-Paper Composite Insulation for High Voltage Transformer. Int. J. Electr. Eng. Inform. 2016, 8, 4. [Google Scholar]
- Alvin, R. Effects of Thermal Aging on the Characteristics of Kraft Paper in Various Liquid Insulating Materials. In Proceedings of the 2020 International Symposium on Electrical Insulating Materials (ISEIM), Tokyo, Japan, 13–17 September 2020. [Google Scholar]
- Nasirul, H.; Medya, R. Assessment of Degradation of Oil-Paper Insulation Caused by Thermal Ageing in the Presence of Acids. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 2779–2787. [Google Scholar]
- Anna, F.; Vlachou, S.; Boyatzis, S.C. Fatty Acids and Their Metal Salts: A Review of Their Infrared Spectra in Light of Their Presence in Cultural Heritage. Molecules 2021, 26, 6005. [Google Scholar] [CrossRef]
- Fang, X.; Zhong, L.; Xu, Y.; Feng, S.; Zhang, C.; Zhang, F.; Zhang, G. Highly Efficient Flame-Retardant Kraft Paper. J. Mater. Sci. 2019, 54, 1884–1897. [Google Scholar]
- Abi, M.; Subroto, C.S. Fourier Transform Infrared (Ftir) Spectroscopy Analysis of Transformer Paper in Mineral Oil-Paper Composite Insulation under Accelerated Thermal Aging. Energies 2018, 11, 364. [Google Scholar] [CrossRef]
- Erina, S.Y.; Al-Ghifary, F.; Dewi, T.I.D. Chemical and Physical Performance of Kraft Paper Immersed in Natural Ester from Palm Oil under Accelerated Thermal Aging. Int. J. Electr. Eng. Inform. 2019, 11, 408–426. [Google Scholar]
- Hadi, P.; Heris, S.Z.; Mohammadpourfard, M. The Effect of Tio2 Doped Multi-Walled Carbon Nanotubes Synthesis on the Thermophysical and Heat Transfer Properties of Transformer Oil: A Comprehensive Experimental Study. Case Stud. Therm. Eng. 2023, 41, 102607. [Google Scholar]
- Rafid, M.; Azad, A.K.; Prottoy, S.M.; Alam, S.; Rahman, M.; Miah, M.J.; Hossain, M.S.; Rahman, M.M. Augmentation of Heat Exchanger Performance with Hybrid Nanofluids: Identifying Research Gaps and Future Indications-a Review. Int. Commun. Heat Mass Transf. 2024, 155, 107537. [Google Scholar] [CrossRef]
- Nurliyana, A.R.; Yunus, R.; Rashid, U.; Azis, N.; Yaakub, Z. Effect of Molecular Structure on Oxidative Degradation of Ester Based Transformer Oil. Tribol. Int. 2019, 140, 105852. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).