Abstract
A 3-Amino-Propyl Trimethoxy Silane (APTS) functionalized silica was prepared and investigated. The functionalized silica showed a powerful removal behavior towards Chromium (III) [Cr (III)] and Cadmium (II) [Cd (II)] ions in aqueous solution. Different factors affecting the heavy metal ions adsorption on these substrates such as pH, initial concentration, contact time, and temperature were investigated. FT-IR analyses were carried out to characterize the functionalization of salicylaldehyde unto 3-aminopropyl silica. Results showed that optimum adsorption of the metal ions occurred at a pH of 7 and 6 by the pure silica and functionalized silica, respectively. Removal efficiencies of the adsorbents showed the trend: Salicylaldehyde-APTS modified > pure silica. The adsorption was described by the Langmuir adsorption isotherm. The kinetic results showed that the adsorption was described well with the pseudo second-order kinetic model. The study reveals that both pure silica and functionalized silica can be used as good adsorbents for the removal of the heavy metal pollutants from aqueous solutions and may be applied in the treatment of industrial waste waters, and they may be useful in detoxifying our already polluted environments.
1. Introduction
Heavy metal pollution has become an increasingly severe environmental problem due to rapid industrialization and urbanization [1]. The presence of toxic metals in terrestrial and aquatic ecosystems is a critical concern, as these contaminants originate from various industrial activities, including electroplating, metal finishing, battery manufacturing, mining, and pigment production [2]. Improper management of industrial waste results in the release of heavy metals into water bodies, leading to their bioaccumulation in the food chain and ultimately affecting human health. Heavy metals such as cadmium (II), mercury (II), chromium (VI), and lead (II) are particularly hazardous even at low concentrations due to their non-biodegradable nature and high toxicity. These elements tend to accumulate in living organisms, posing significant health risks, including organ damage, carcinogenic effects, and neurological disorders. Among these metals, chromium and cadmium are of particular concern due to their widespread industrial use and persistent toxic effects, necessitating efficient and sustainable removal strategies [3].
Several methods have been employed for the removal of heavy metal ions from wastewater, including chemical precipitation, ion exchange, membrane filtration, electrochemical treatment, and adsorption. Among these, adsorption is widely recognized as a highly effective, cost-efficient, and environmentally friendly approach for heavy metal removal. Adsorption offers advantages such as ease of operation, reusability of adsorbents, and minimal production of toxic byproducts [4]. However, the efficiency of the adsorption process depends on factors such as adsorbent material, surface functionalization, pH, and contact time. While conventional adsorbents like activated carbon and natural clays have been used extensively, functionalized silica-based materials have gained attention due to their high surface area, chemical stability, and tunable surface properties.
Silica gel, when immobilized with various organic compounds capable of chelating metal ions, has demonstrated significant potential as an efficient adsorbent. Compared to other organic and inorganic supports, silica-based adsorbents exhibit notable advantages, including thermal stability, ease of functionalization, and high selectivity for specific metal ions. Various studies have investigated the application of silica-based materials for heavy metal removal. For instance, Slobodanka et al. [5] developed two techniques using Saccharomyces cerevisiae and Leuconostoc mesenteroides immobilized on silica materials for cadmium ion removal, reporting maximum binding capacities of 54 mg/g and 90 mg/g, respectively. Similarly, Nnaji et al. [6] highlighted the effectiveness of eco-friendly strategies such as phytoaccumulation and phytostabilization in mitigating heavy metal contamination. Nazaripour et al. [7] provided a comparative assessment of various wastewater treatment techniques, emphasizing the efficiency of adsorption-based approaches. With an emphasis on similar adsorption techniques, Rao and Peddy [8] examined a variety of approaches for treating mining effluents.
Further research on adsorption-based removal techniques has demonstrated the potential of functionalized silica support in achieving high adsorption capacities. For example, Al-Sharani and AlSharani [9] evaluated the removal of Cr (II) and Co (II) ions using treated date pits, highlighting their effectiveness and environmental benefits. Gupta and Ali [10] reviewed real-time heavy metal adsorption methods, while Mohan and Singh [11] focused on the utilization of agricultural waste materials for metal ion adsorption. The kinetics and mechanisms of cadmium and chromium ion adsorption have also been extensively studied. Ho and McKay [12] examined the adsorption kinetics of these metals, providing insights into their interaction with various adsorbents. Kobylinska et al. [13] assessed the adsorption affinities of EDTA-functionalized samples for removing inorganic contaminants from environmental water, demonstrating Pb (II) as having the highest adsorption value (195.6 mg/g). Moreover, Nishino et al. [14] investigated the adsorption capacity of silica beads functionalized with aminosilane coupling agents, including 3-glycidyloxypropyl triethoxysilane, [3-(2-aminoethylamino) propyl] triethoxysilane (AEAPTES), and polyethyleneimines (PEIs), reporting AEAPTES as the most effective at an initial pH of 3.0. Surucic et al. [15] further explored sorbent surfaces modified with diethylene and amino propyl silane groups for chromium oxyanion removal, identifying diethylene triamine as a superior functional group for adsorption. In another study, Guevara-Lora and Wronski [16] developed amorphous adsorption material from horsetail, demonstrating its capacity for Cr (VI) ion removal following treatment with dodecylamine surfactants.
Despite these advances, there remains a need for more efficient, selective, and reusable adsorbents for heavy metal removal. This study focuses on the comparative removal of chromium and cadmium ions from aqueous solutions using silica support immobilized with 3-aminopropyl trimethoxysilane salicylate. The choice of this synthesized material is based on its expected high chelation capability, improved adsorption capacity, and selectivity for the target metal ions. By systematically evaluating its performance under various experimental conditions, this research aims to contribute to the development of improved adsorbents for heavy metal remediation.
2. Materials and Methods
2.1. Chemicals and Solutions
Pure chromatographic grade silica (mesh size 60–200 µm), 3-aminopropyl trimethoxy silane, Salicylaldehyde and Dichloroethane (Sigma-Aldrich Company, Darmstadt, Germany); cadmium (II) sulphate and chromium (III) nitrate purchased from BDH Limited, Poole, England; Ethanol (≥99.5%, Sigma-Aldrich, Darmstadt, Germany), Methanol (≥99.9%, Fisher Scientific, Waltham, MA, USA); Acetic acid (Glacial, ≥99.7%, Merck KGaA, Darmstadt, Germany); and distilled deionized water was obtained using a Milli-Q® Water Purification System were used. The chemicals are of analytical grade.
Standard solutions of 1000 mg/L of cadmium and chromium were prepared as stock solutions from their salts, Cadmium (II) Sulphate, Cd (II)SO4·8H2O (352.64 g/mol) and Chromium (III) Nitrate, Cr (NO3)3·9H2O (400.16 g/mol). This was performed by dissolving 3.14 g of the cadmium salt and 6.78 g of the chromium salt in 1000 cm3 of distilled deionized water. From the stock solution, working solutions of varying initial concentrations of metal ions were prepared by serial dilution using distilled deionized water.
2.2. Instrumentation
The concentrations of the metal ions were analyzed with a Buck Scientific Atomic Absorption spectrophotometer model 210/211 VGP Buck Scientific, East Norwalk, CT, USA). The wavelengths used for the cadmium ions was 228.9 nm and that of chromium was 357.9 nm. Fourier Transform Infra-Red (FT-IR) spectra were recorded on Shimadzu FTIR-84005 machine (Norwalk, CT, USA) using KBr pellets. The pH measurements were made using Corning Scholar 425 digital pH meter, Corning Incorporated, (New York, NY, USA). Batch adsorption processes were conducted using thermostatic water bath shaker, SHZ-82, (Shanghai Boxun Medical Biological Instrument Corp., Shanghai, China) at 150 rpm.
2.3. Synthesis of Schiff Base and Schiff Base Immobilized Silica
The Schiff base used in this work was prepared by mixing 5 mL of 10% salicylaldehyde solution with 5.0 mL of 3-aminopropyl trimethoxy silane, and the mixture stirred. This was followed with a gradual addition of 0.1 mol/dm3 NaOH to the mixture for about 30 min. The final mixture was left for about 15 min, filtered, washed with cold ethanol, and dried. The modification process was carried out according to a method described by Mortazavi et al. [17] with slight modifications. The initial step involved the production of SiO2-supported aminopropyl trimethoxy silane (APTS), by refluxing 5.0 g of pure silica powder with 5.0 mL of 3-aminopropyl trimethoxy silane in 50 mL dichloromethane for 16 h. The solid precipitate was filtered and washed off with distilled water and dried at room temperature. The second step involved the addition of 5 mL of 10% salicylaldehyde and 10 mL of acetic acid to the suspension of the silica-supported aminopropyl trimethoxy silane in 60 mL methanol and the reaction mixture refluxed for another 16 h to produce a salicylaldehyde-modified 3-aminopropyl trimethoxy silane (APTS). Both the pure silica and the salicylaldehyde modified APTS were tightly covered and carefully labeled for the adsorption studies.
The two steps involved in the modification process are shown in Scheme 1.
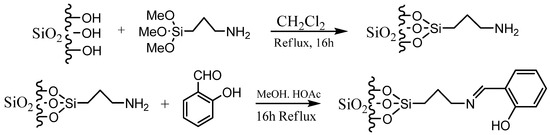
Scheme 1.
Preparation of Schiff-based immobilized silica.
2.4. Removal Studies with Batch Methods
Adsorption studies were conducted in labeled 100 cm3 beakers having 0.2 g of each of the adsorbents (silica and functionalized silica) with 25 cm3 of the adsorbates (solutions of metal ions). The flasks were agitated on a stirrer at a constant speed of 150 rpm. At the end of the experiments, residual cadmium and chromium ions concentrations were analyzed using Atomic Adsorption Spectrophotometer (AAS). The amount of metal ions adsorbed by the adsorbents (mg/g) was calculated according to Equation (1).
where qe (mg/g) is the amount adsorbed, Co (mg/L) is the first metal ion concentration in solution, Ce (mg/L) is the residual/equilibrium concentration of metal ions, v = volume of adsorbate solution in liter, while m = mass of the adsorbent used.
qe = (Co − Ce) v/m,
The best contact time was determined to be 40 min and used in all the adsorption experiments. Uptake experiments of metal ions at different pHs (i.e., 2, 4, 6, 8, and 10) were conducted by placing 0.2 g of each of the adsorbents having 25 mL solution of cadmium with an initial concentration of 50 mg/L. The pH was adjusted using 0.1 M HCl and 0.1 M NaOH. The residual concentration of cadmium and chromium ions were found using AAS. The amount of metal ions adsorbed by the adsorbents was calculated using Equation (1). The results showed that qe maximum observed was at pH 6.0 for silica and Salicylaldehyde APTS (Sal-APTS), respectively.
2.5. Adsorption Isotherms
Cadmium and chromium ions concentrations of 10, 20, 30, 40, 50, and 60 mg/L were prepared and used to obtain the adsorption isotherm data on the adsorption process. After equilibration, 5 mL of the filtrate was used for the determination of residual metal ion concentration by AAS. The amount of metal ions adsorbed by the adsorbents were calculated according to Equation (1).
2.6. Kinetic Adsorption Experiments
The kinetic study used 0.2 g of the adsorbents at their respective optimum pHs. Data was obtained after treatment of a series of 25 mL of adsorbents with 50 mg/L of metal ions solutions. These series of samples were quenched at time intervals of 20, 30, 40, 50, and 60 min by filtration. The concentration of the filtrate was analyzed by AAS. These results were used to obtain adsorption kinetics.
3. Results and Discussion
3.1. Characterization of Materials
A schematic representation of synthesis of the Schiff base is shown in Scheme 1. The FT-IR spectra (Figure 1) showed absorption bands at 3406–3226(b), 1089(vsp), 809(m) and 461(s) cm−1 corresponding to stretching vibrations of silanol -OH, asymmetric stretching vibration of Si-O, symmetric stretching vibration of Si-O-Si, and asymmetric Si-O-Si bonding, respectively. After immobilization with 3-aminopropyl trimethoxy silane (APTS) and salicylaldehyde, other characteristic vibrational frequencies corresponding to more functional groups are visible. The bonding of Cd (II) and Cr (II) ions unto silica and functionalized silica are in Figure 2, Figure 3, Figure 4 and Figure 5, respectively.
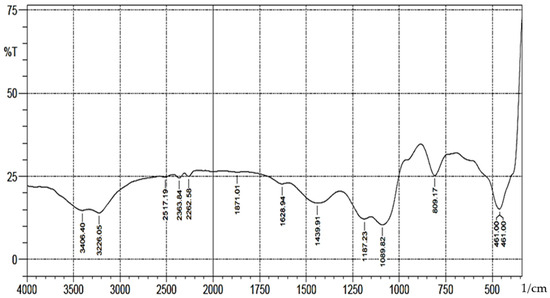
Figure 1.
FTIR analysis of pure silica.
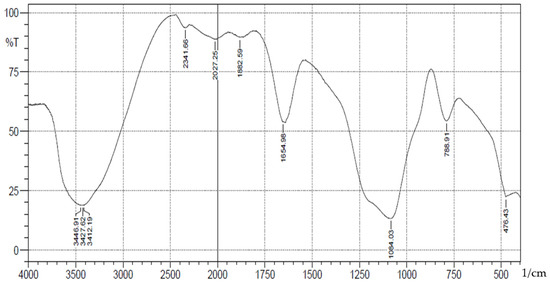
Figure 2.
FTIR analysis of Cadmium(II) ions bonded onto pure silica.
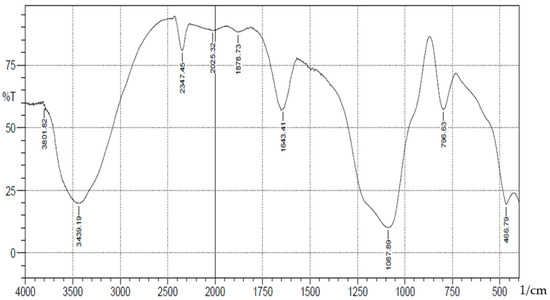
Figure 3.
FTIR analysis of Cadmium(II) ions bonded onto salicylaldehyde APTS.
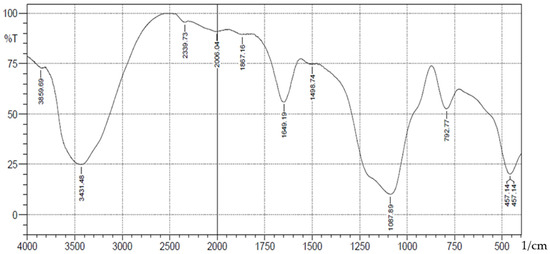
Figure 4.
FTIR analysis of Chromium(lll) ions bonded onto pure silica.
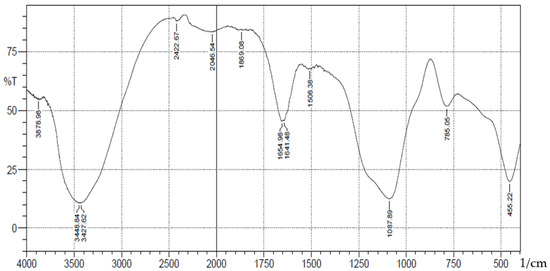
Figure 5.
FTIR analysis of Chromium(III) ions bonded onto salicylaldehyde APTS.
3.2. Adsorption Isotherms
The initial cadmium (II) and chromium (III) ion concentrations (10–60 mg/L) were used for the investigation of the adsorption isotherm. The equilibrium concentrations were obtained after 40 min of contact time. The amount of Cd (II) ions adsorbed on pure and functionalized silica were found to increase as the initial metal ions concentration increased and continued up to 40 mg/L and level off or decreased thereafter (Figure 6 and Figure 7) but Cr (III) ions adsorption continued up to 50 mg/L and decreased afterwards.
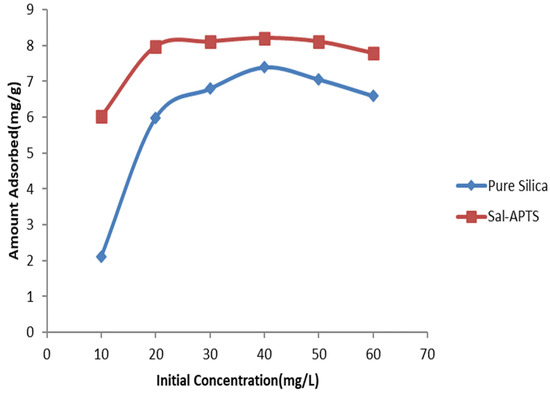
Figure 6.
Cadmium (II) ions removal by pure and modified silica at different initial concentrations.
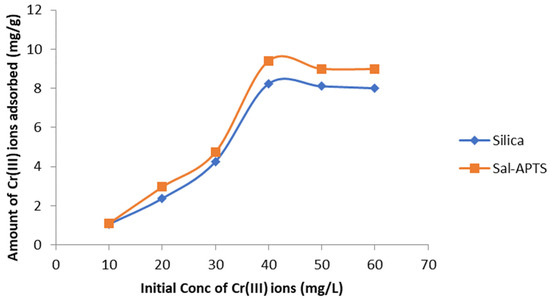
Figure 7.
Chromium (iii) ions removal by pure and modified silica (Sal-APTS) at different initial concentrations.
The Langmuir isotherm model was applied to analyze the data. The Langmuir isotherm model is based on the Equation:
where Ce (mg/L) is the equilibrium concentration, qe (mg/g) is the amount of metal ions adsorbed at equilibrium, qmax (mg/g) is the maximum adsorption capacity and KL (dm3g−1) is a constant related to adsorption/desorption energy.
Ce/qe = 1/qmax KL + Ce/qmax,
The linear plot of Ce/qe vs. Ce should yield a straight line if the Langmuir equation is obeyed by the adsorption equilibrium. The constants KL and qmax are obtained from the intercept and slope of the linear plots (Figure 8a,b and Figure 9a,b).
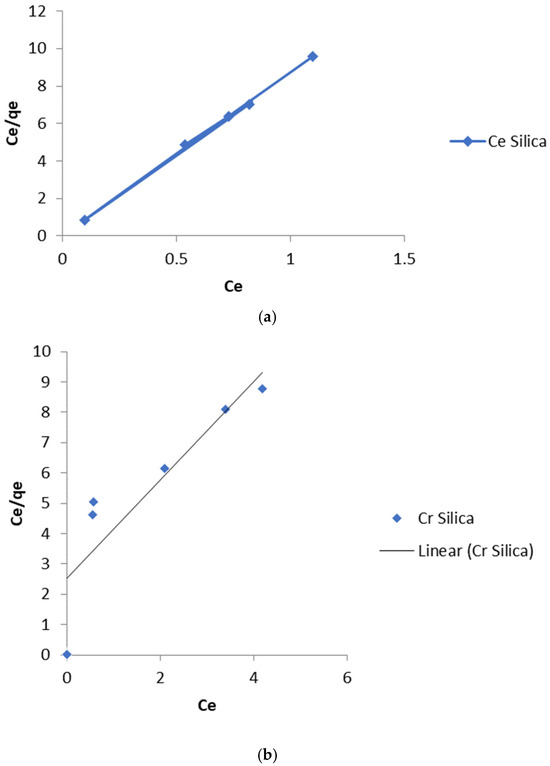
Figure 8.
(a) Langmuir isotherm for the adsorption of Cd (II) ions onto pure silica. (b) Langmuir isotherm for the adsorption of Cr (III) ions onto pure silica.
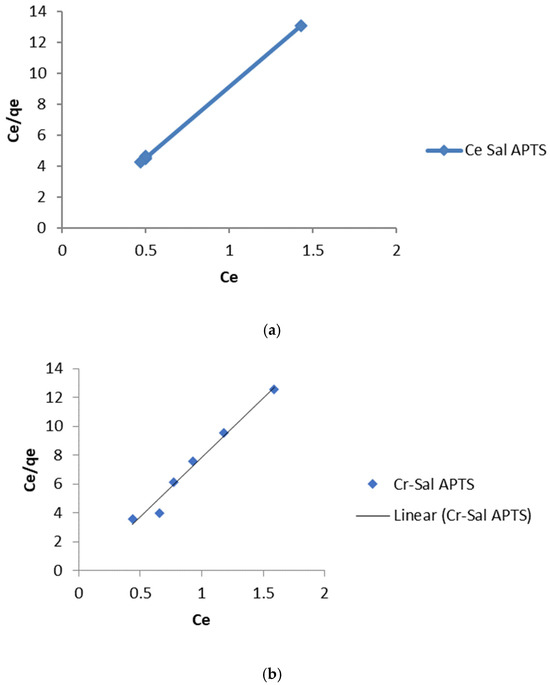
Figure 9.
(a) Langmuir isotherm for the adsorption of Cd (II) ions onto Salicylaldehyde-APTS modified silica. (b) Langmuir isotherm for the adsorption of Cr (III) ions onto Salicylaldehyde APTS modified silica.
3.3. The Langmuir Isotherm Parameters

Table 1.
Langmuir isotherm parameters of Cd (II) and Cr (III) ions on pure silica.

Table 2.
Langmuir isotherm parameters of Cd (II) ions on Salicylaldehyde-APTS modified silica.
3.4. Adsorption Kinetics
The kinetic property of the metal ions adsorbed onto the pure and functionalized silica were assessed (Figure 10 and Figure 11). The adsorption rates were found at pHs of 6 for pure silica and salicylaldehyde APTS, respectively, in the range of metal ions concentrations of 10–60 mg/L in aqueous media. Generally, the adsorption increased with increased concentration of the metal ions. At any given concentration, the metal ion adsorption quickly rose and then reached the plateau, which is the equilibrium capacity. In the two cases, the adsorption reached equilibrium capacity in 40 min.
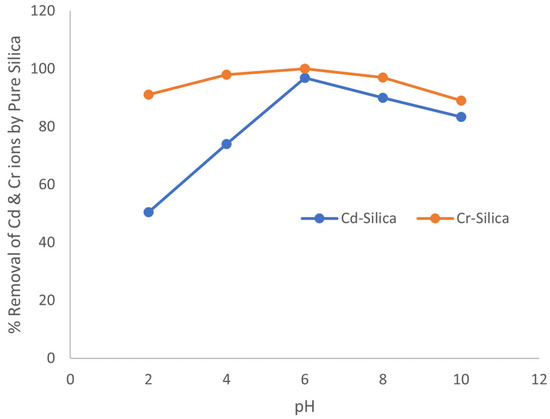
Figure 10.
Variation of % removal of Cd and Cr ions with pH unto pure silica.
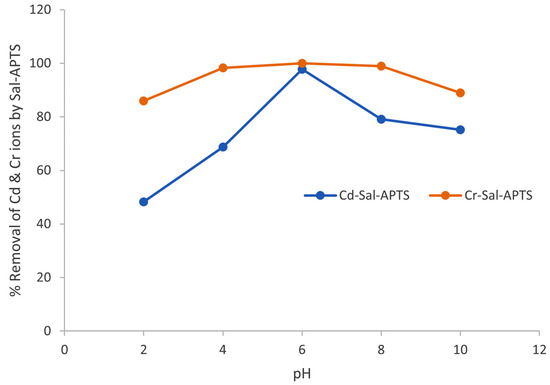
Figure 11.
Variation of % removal of Cd and Cr ions with pH unto Sal-APTS.
Three kinetic models: Elovich, pseudo first-order and pseudo second-order were applied in the analyses. In these cases, Elovich and pseudo first-order gave low correlation coefficient values. The pseudo second-order which gave a high correlation coefficient was reported for the adsorption kinetic studies. The pseudo second-order reaction is guided by the expression:
where qe is the amount of metal ions adsorbed at equilibrium, qt is the amount of metal ions on the surface of the adsorbent at time t and k2 is the rate constant of the pseudo second-order adsorption kinetics. A plot of (t/qt) against t yields a straight line if the pseudo second-order kinetics applied. The constants qe and k2 were determined from the slope and intercept of the plots, respectively. The plots are shown in Figure 12 and Figure 13.
t/qt = 1/(k2qe)2 + t/qe,
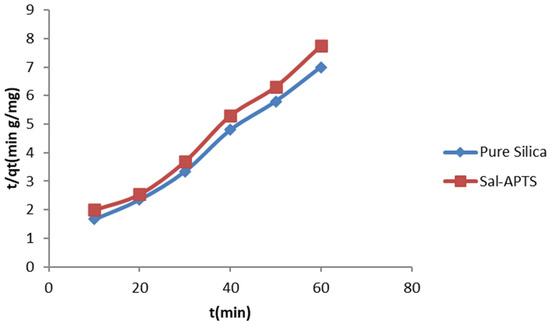
Figure 12.
Pseudo second-order kinetics of adsorption of Cd (II) ions onto pure and modified silica.
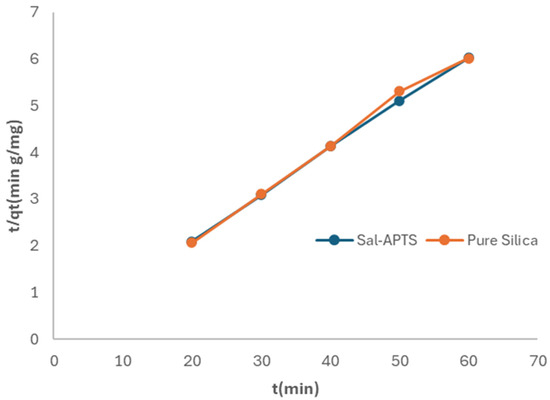
Figure 13.
Pseudo second-order kinetics of adsorption of Cr (III) ions onto pure and modified silica.

Table 3.
Pseudo second-order kinetic data of pure silica.

Table 4.
Pseudo second-order kinetic data of Salicylaldehyde-APTS modified.
4. Discussion
The results obtained showed that pure silica had an optimum pH for adsorption of the metal ions at 6.0 and with percentage removals of 96.92% and 99.94% recorded for cadmium (II) and chromium (III) ions, respectively. The salicylaldehyde-APTS modified surface also had an optimum pH of 6.0 and percentage adsorption of 97.78% and 100% were observed for cadmium (II) and chromium (III) ions, respectively.
The experimental results indicate that at low pH values (i.e., pH 1–4), the solutions are highly acidic, and this results in net positive charges on the active sites of the adsorbents. The metal ions thus compete with protons in the active sites leading to a reduction in the amount of the metal ions adsorbed by the adsorbents. But as the pH of the solutions increased, the active sites become deprotonated and are therefore free for the metal ions to bind or be associated with. The deprotonation of the active sites at higher pH thus aids in reducing competition between metal ions and protons leading to an increased adsorption with an increase in pH. Similar results have been reported elsewhere [18].
There was generally an increase in the metal ions adsorption with an increase in concentration. This is in line with the expected because at lower initial concentrations, there is a reduction in inter-ionic interactions, but at higher initial concentrations, there is more efficient utilization of active sites due to a greater mass transfer through the concentration gradient. Increased concentration leads to increased collision between the metal ions and the active sites of the adsorbents, leading to enhanced adsorption. The results obtained indicate that surface saturation is dependent on initial metal ion concentration. Other researchers have obtained similar results [19].
The values of the linear regression coefficient, R2 (>>0.96) obtained from the Langmuir isotherm parameters (Table 1 and Table 2) showed that the isotherm gave the best fit for the experimental data. The moderate values of KL obtained indicate a favorable adsorption process. The RL values indicate the shape of the isotherm as expressed in Equation (4):
RL = 1/(1 + KLCO).
RL values between 0 and 1 indicate a favorable adsorption process [20]. The values obtained in this study for Cd (II) and Cr (III) ions lie between 0 and 1. This low value that tends towards zero indicates that the adsorption process is almost irreversible [21].
The pseudo second-order kinetics also gave a high R2 (Table 3 and Table 4). The high R2 (>>0.98) values signify a perfect description of the adsorbate–adsorbent interaction at the interface. The fitting of adsorption data into pseudo second-order kinetic model generally explains that the rate-limiting step of adsorption may be by chemical reaction. The high R2 values obtained from the plots signify that the model could perfectly describe the adsorption of the metal ions onto the silica-based adsorbents in our present study. This fitting into the model therefore suggests a possible exchange of valences’ or sharing of electrons between the metal ions and the silica-based surface leading to a chemical reaction. The good fitting of the adsorption data into pseudo second-order, in addition to results obtained from Langmuir adsorption isotherm, suggest a monolayer coverage, showing that the rate-limiting step of this process may be chemical reaction and that the chelating silica-based adsorbents have most of their chelating groups on or near the surface for easy access. The results reveal the possibility of a complexation reaction between the metal ions and the chelating groups of the functionalized silica adsorbents. The obtained results show that different types of coordination mode may have taken place during the interaction of metal ions with the functionalized silica.
Removal efficiencies of the adsorbents followed the trend: Salicylaldehyde-APTS modified > Pure silica. This is because there are more functional groups in Salicylaldehyde modified silica than pure silica (Scheme 1). The trend of adsorption of the two metal ions by the adsorbents showed that Cr (III) ions adsorbed more than Cd (II) ions. This could be because of the relatively smaller ionic radius of Chromium (0.62 Å) compared to Cadmium (0.95 Å) [22]. The higher charge density of Cr (III) compared to Cd (II) means that Cr (III) can create stronger electrostatic attractions with negatively charged sites on the adsorbent. This could lead to more significant adsorption for Cr (III) [23].
The Cr3+ ligand has a coordination number of 6. The unbound electron pair from oxygen and Nitrogen atoms can be absorbed by the free chromium orbital following the cleavage of the hydrogen atom. It is possible to separate a hydrogen atom from either a carboxyl or hydroxyl group. The core Cr ion can be chelated by free electron pairs that come from carboxyl or hydroxyl groups. Consequently, silica and salicylic functional groups bind to chromium (III). The resulting chelates complexes have a high degree of stability [24].
5. Conclusions
This study has demonstrated the effectiveness of both pure and functionalized silica as adsorbents for the removal of cadmium (II) and chromium (III) ions from aqueous solutions. The results indicate that adsorption efficiency is influenced by factors such as pH, initial metal ion concentration, and surface modifications. The optimum pH for adsorption was determined to be 6.0 for both adsorbents, with the salicylaldehyde-APTS modified silica exhibiting superior adsorption capacity compared to pure silica. This enhanced performance is attributed to the presence of additional functional groups, which facilitate stronger metal–ligand interactions and improved metal ion binding affinity.
The adsorption process followed the Langmuir isotherm model, suggesting monolayer adsorption onto a homogenous surface with high affinity for metal ions. The RL values obtained in this study confirm that the adsorption process is favorable, with a trend that suggests near irreversibility. Additionally, the pseudo second-order kinetic model provided the best fit for the experimental data, indicating that chemisorption plays a significant role in the removal mechanism. This suggests that the interaction between the metal ions and the adsorbents involves valence exchange or electron sharing, leading to stable complex formation.
Comparative adsorption trends showed that chromium (III) exhibited higher adsorption efficiency than cadmium (II), likely due to its smaller ionic radius and higher charge density, which promotes stronger electrostatic attraction and coordination bonding with the functionalized silica. The ability of functionalized silica to form stable chelate complexes with metal ions further supports its potential application in wastewater treatment. These findings underscore the potential of modified silica-based materials for use in environmental remediation, particularly for the removal of toxic heavy metals from industrial effluents.
Future research should focus on optimizing the synthesis of functionalized silica adsorbents, evaluating their performance in real wastewater systems, and exploring regeneration and reuse capabilities to enhance cost-effectiveness and sustainability. Additionally, emerging hybrid materials, such as nanocomposites and bio-inspired adsorbents, have shown promise in enhancing adsorption efficiency and selectivity [25]. Such advancements could further improve the applicability of silica-based materials in water treatment technologies.
Author Contributions
Conceptualization—K.O.A. and J.O.A.; methodology—L.C.N.-O. and R.I.U.; investigation, A.I.C.E.; writing—original draft preparation—P.I.O.; writing—review and editing—G.J.O.; data curation—C.E.E. supervision—K.O.A. and J.O.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed at the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sodhi, K.K.; Mishra, L.C.; Chandra Kant Singh, C.K.; Kumar, M. Perspective on the heavy metal pollution and recent remediation strategies. Curr. Res. Microb. Sci. 2022, 3, 100166. [Google Scholar] [CrossRef]
- Osae, R.; Nukpezah, D.; Darko, D.A.; Koranteng, S.S.; Mensah, A. Accumulation of heavy metals and human health risk assessment of vegetable consumption from a farm within the Korle lagoon catchment. Heliyon 2023, 9, e16005. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Slobodanka, S.; Katarina, V.P.; Milan, P.N.; Vladimir, V.S.; Marina, S. Cerevisiae and Leuconostoc Mesenteroids immobilized in silica materials by two processing methods. Mater. Res. 2022, 25, 1–15. [Google Scholar]
- Nnaji, N.D.; Onyeaka, H.; Miri, T.; Ugwa, C. Bioaccumulation for heavy metal removal: A review. SN Appl. Sci. 2023, 5, 125. [Google Scholar] [CrossRef]
- Nazaripour, M.; Reshadi, M.M.; Mirbagheri, S.A.; Nazaripour, M.; Bazargan, A. Research trends of heavy metal removal from aqueous environments. Environ. Sci. Pollut. Res. 2021, 28, 22867–22884. [Google Scholar] [CrossRef]
- Rao, P.S.; Peddy, M.V. Different techniques for cadmium ion remediation. Int. J. Eng. Sci. Technol. 2010, 2, 81–103. [Google Scholar]
- Al-Shahrani, S.O.; Alshahrani, A.M. Removal of chromium (II) and cadmium (II) heavy metal ions from aqueous solutions using treated date seeds: An eco-friendly method. Molecules 2021, 26, 3718. [Google Scholar]
- Gupta, V.K.; Ali, I. Removal of toxic metals from wastewater by adsorption on low-cost materials: A review. J. Hazard. Mater. 2004, B112, 169–178. [Google Scholar]
- Mohan, D.; Singh, K.P. Single-binary-, and multi-component adsorption of cadmium and Zinc using activated carbon derived from biogases-an agricultural waste material. Ind. Eng. Chem. Res. 2002, 4, 3929–3938. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Kobylinska, N.G.; Kessler, V.G.; Seisenbaeva, G.A.; Dudarko, O.A. In situ functionalized mesoporous silicas for sustainable remediation strategies in removal of inorganic pollutants from contaminated environmental water. Am. Chem. Soc. Omega 2022, 7, 23576–23590. [Google Scholar] [CrossRef]
- Nishino, A.; Taki, A.; Asamotto, H.; Minamisawa, H.; Yamada, K. Kinetic isotherm and equilibrium investigation of Cr (VI) ion adsorption in amine-functionalized porous silica beads. Polymers 2022, 14, 2104. [Google Scholar] [CrossRef] [PubMed]
- Surucic, L.; Janjic, G.; Markovic, B.; Tadic, T.; Vukovic, Z.; Nastasovic, A.; Onjia, A. Speciation of hexavalent chromium in aqueous solutions using a magnetic silica-crated amino-modifiedglycidyl methacrylate. Polym. Nanocompos. Mater. 2023, 16, 2233. [Google Scholar] [CrossRef]
- Guevara-Lora, I.; Wronski, N.; Bialas, A.; Osip, H.; Czosnek, C. Efficient adsorption of chromium ions from aqueous solutions by plant-derived silica. Molecules 2022, 27, 4171. [Google Scholar] [CrossRef]
- Mortazavi, K.; Ghaedi, M.; Roosta, M.; Montazerozohori, M. Chemical functionalization of silica gel with 2-((3-silypropylimino) methyl) phenol (SPIMP) and its application for solid phase extraction and preconcentration of Fe(III), Pb(II), Cu(II), Ni(II), Co(II) and Zn(II) ions. Indian J. Sci. Technol. 2012, 5, 1893–1900. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, F.; Zeng, G.; Li, X.; Gu, Y.; Shi, L.; Liu, W.; Shi, Y. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef]
- Youssif, M.M.; El-Attar, H.G.; Hessel, V.; Wojnicki, M. Recent Developments in the Adsorption of Heavy Metal Ions from Aqueous Solutions Using Various Nanomaterials. Materials 2024, 17, 5141. [Google Scholar] [CrossRef]
- Ajenifuja, E.; Ajao, J.A.; Ajayi, E.O.B. Adsorption Isotherm Studies of Cu(ii) and Co(ii) in High Concentration Aqueous Solutions on Photocatalytically Modified Diatomacceous Ceremic Adsorbents. Appl. Water Sci. 2017, 7, 3793–3801. [Google Scholar] [CrossRef]
- Emam, A.A.; Ismail, L.F.M.; Abdelkhalek, M.A.; Rehan, A. Adsorption Study of Some Heavy Metal Ions on Modified Kaolinite Clay. Int. J. Adv. Eng. Technol. Manag. Appl. Sci. 2016, 3, 152–163. [Google Scholar]
- Baig, T.H.; Garcia, A.E.; Tiemann, K.J.; Gardea-Torresdey, J.L. Adsorption of heavy metal ions by the biomass of solanum elaeagnifolium (Silverleaf Night-Shade). In Proceedings of the 1999 Conference on Hazardous Waste Research, St. Louis, MO, USA, 24–27 May 1999; pp. 131–142. [Google Scholar]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Kaźmierczak, B.; Molenda, J.; Swat, M. The adsorption of chromium (III) ions from water solutions on biocarbons obtained from plant waste. Environ. Technol. Innov. 2021, 23, 101737. [Google Scholar] [CrossRef]
- Yu, G.; Wang, X.; Liu, J.; Jiang, P.; You, S.; Ding, N.; Guo, Q.; Lin, F. Applications of Nanomaterials for Heavy Metal Removal from Water and Soil: A Review. Sustainability 2021, 13, 713. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).