The Application of Zeolites for Fixation of Cr(VI) Ions in Sediments
Abstract
1. Introduction
2. Materials and Methods
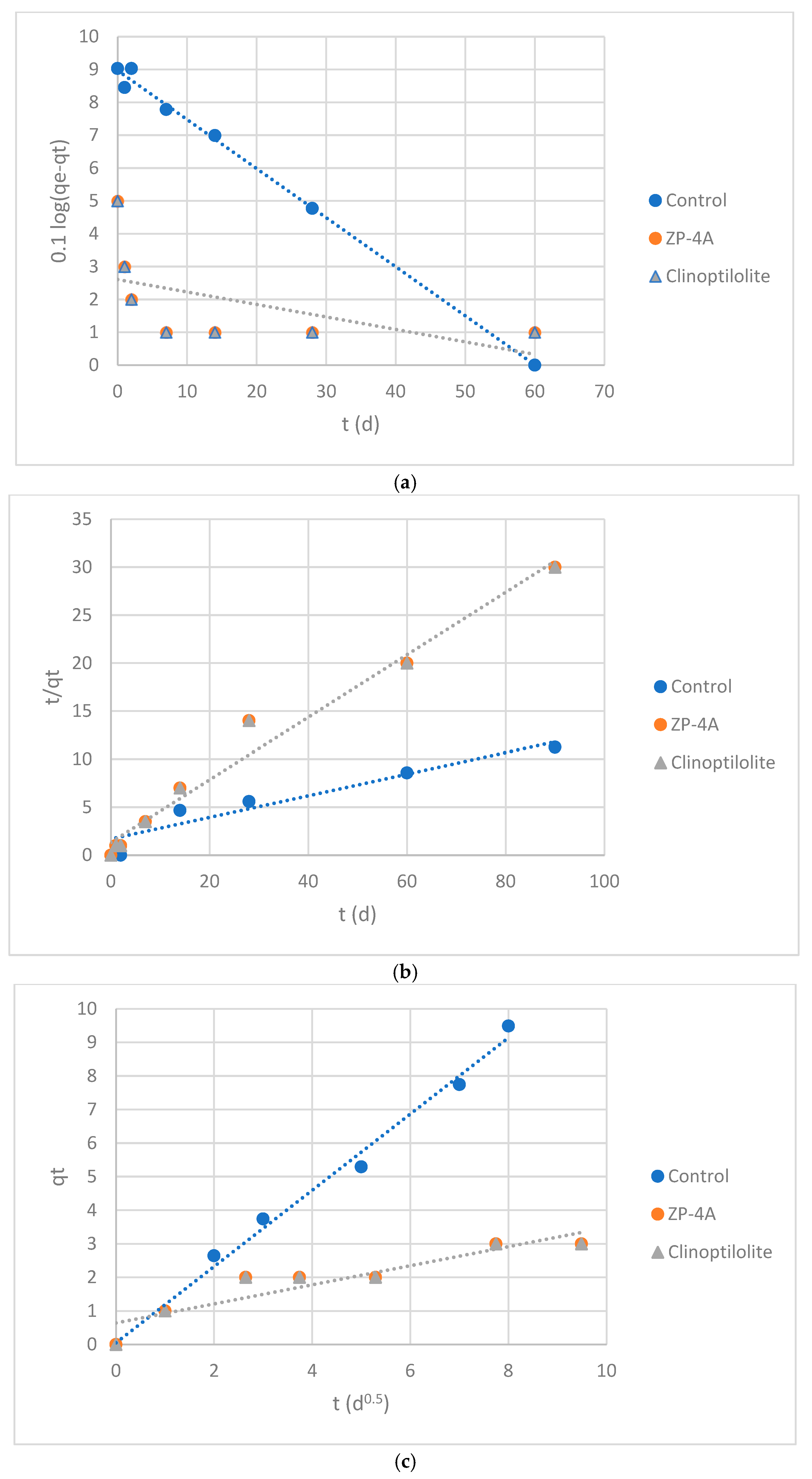
- qt is the sorption capacity at time t (mg/g);
- qe is the sorption capacity at equilibrium (mg/g);
- k1 is the PFO reaction rate constant (d−1).
3. Results
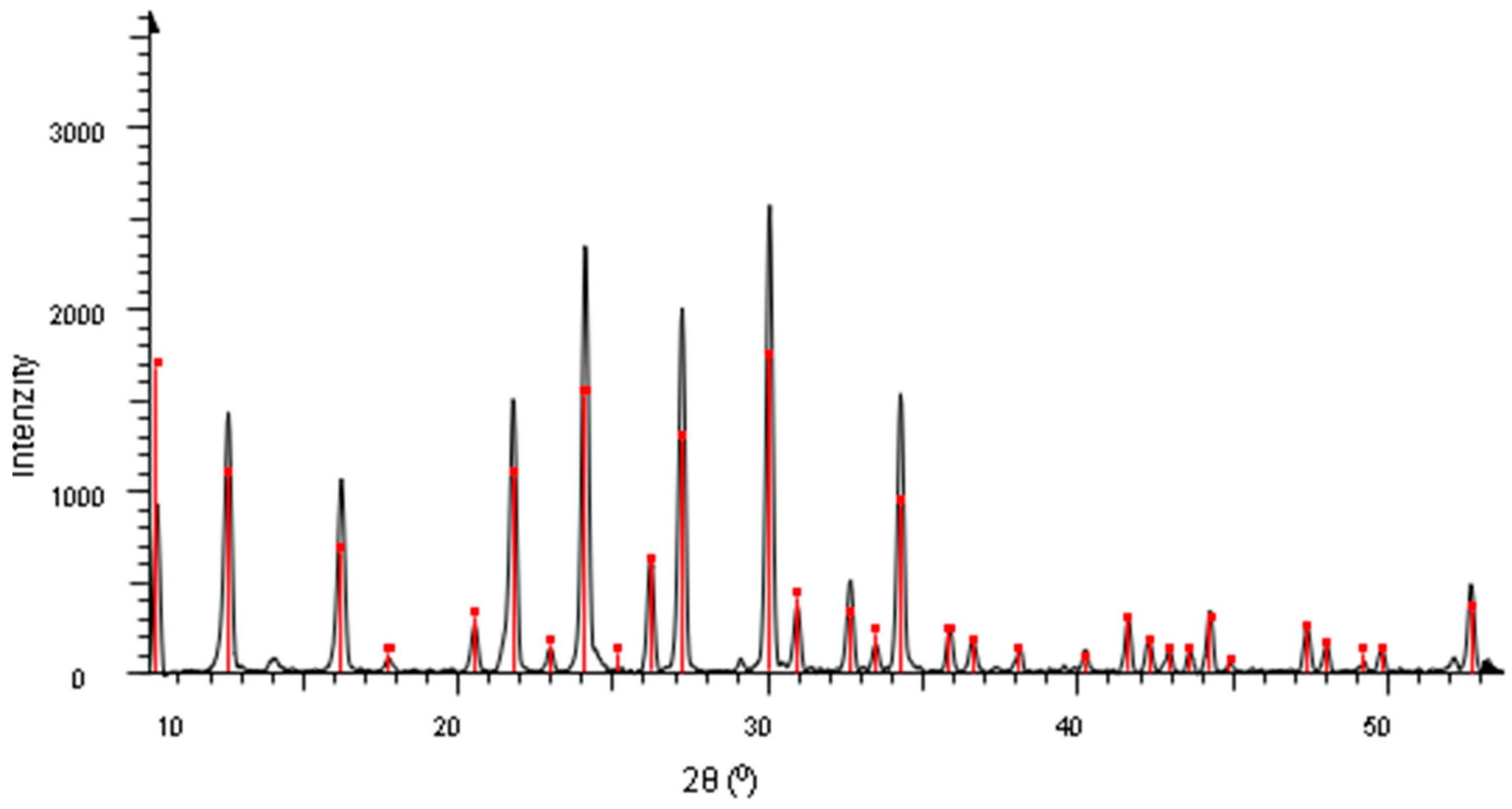
3.1. Characterization of Zeolites
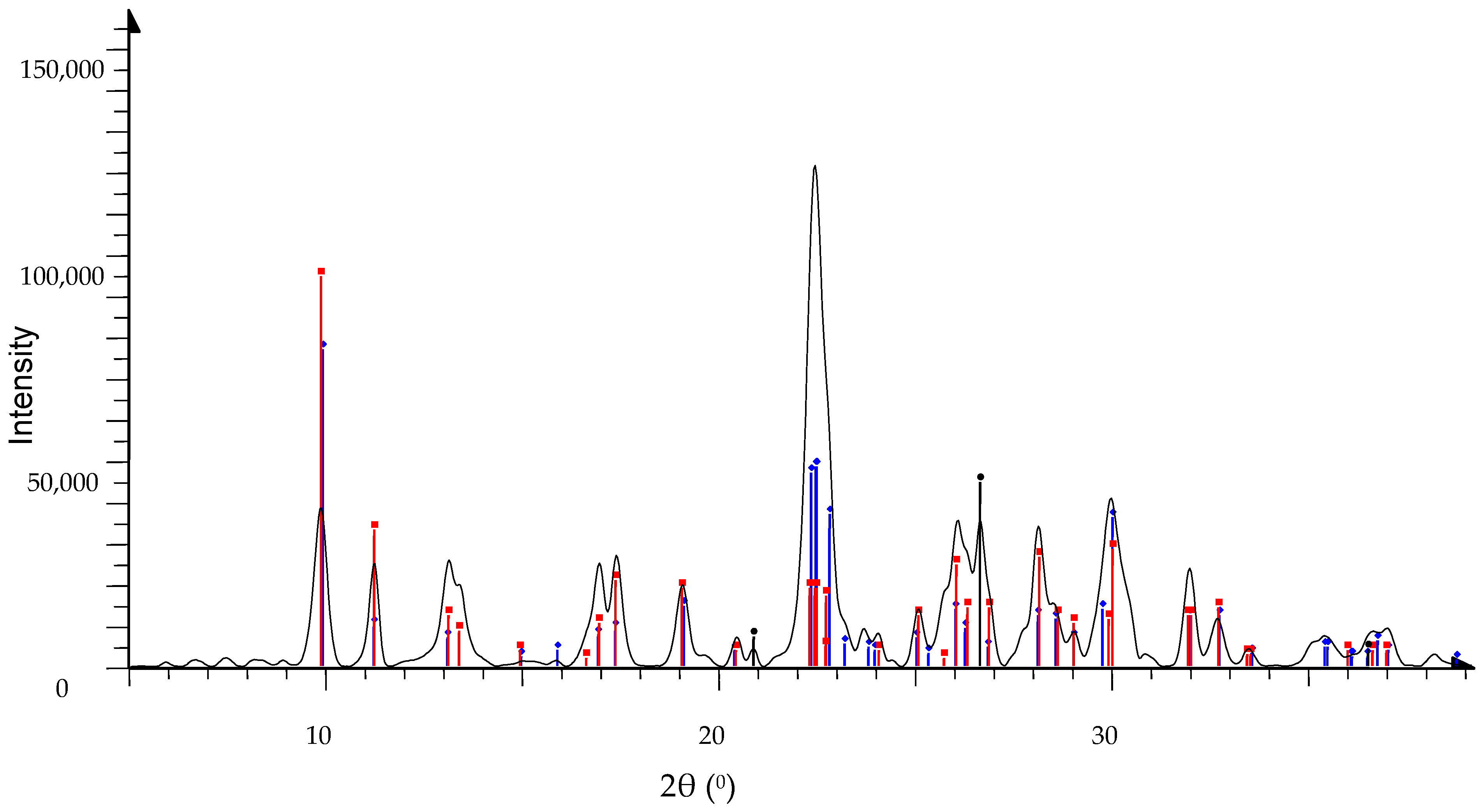
3.2. Characterization of Sediment
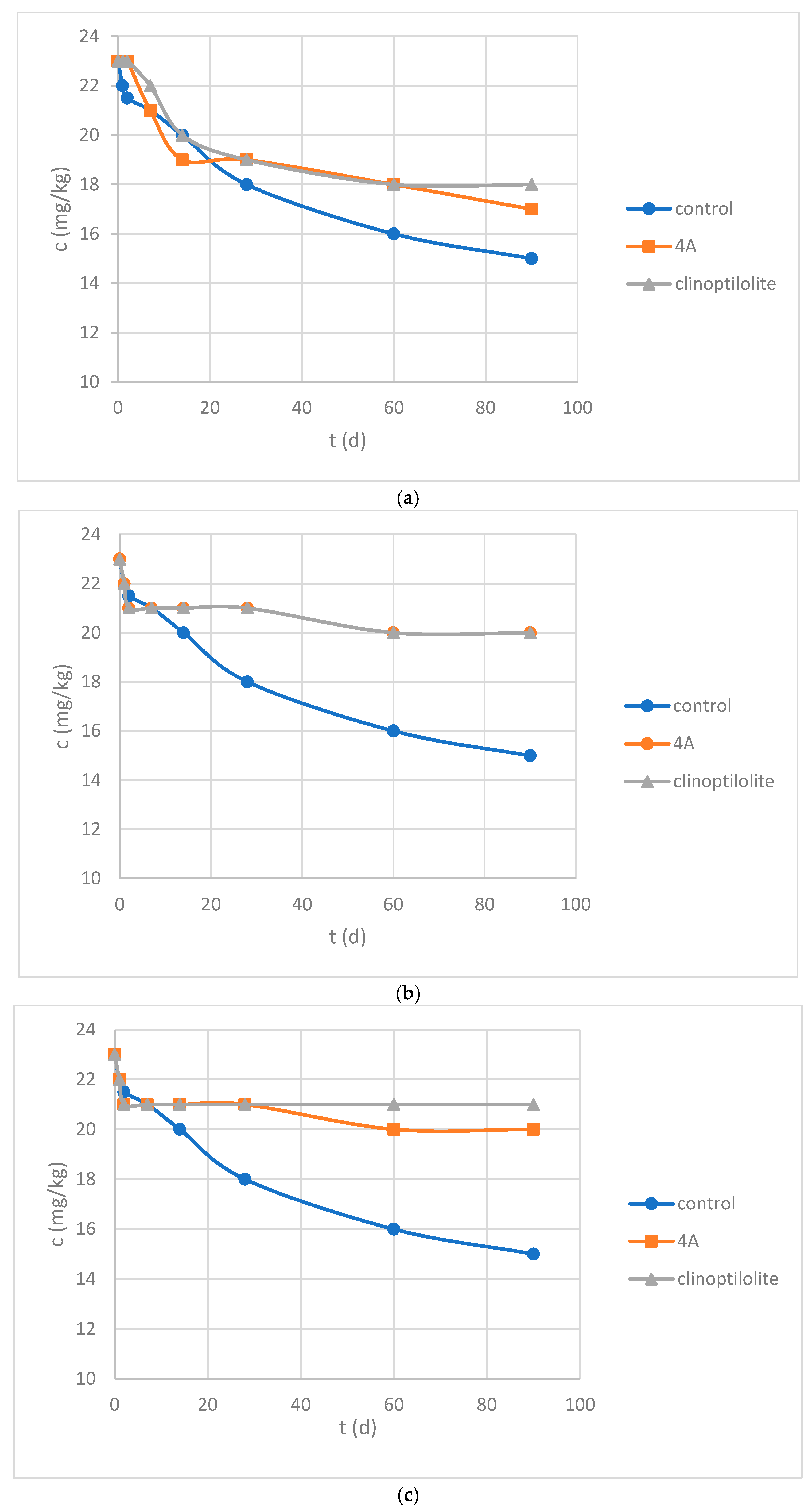
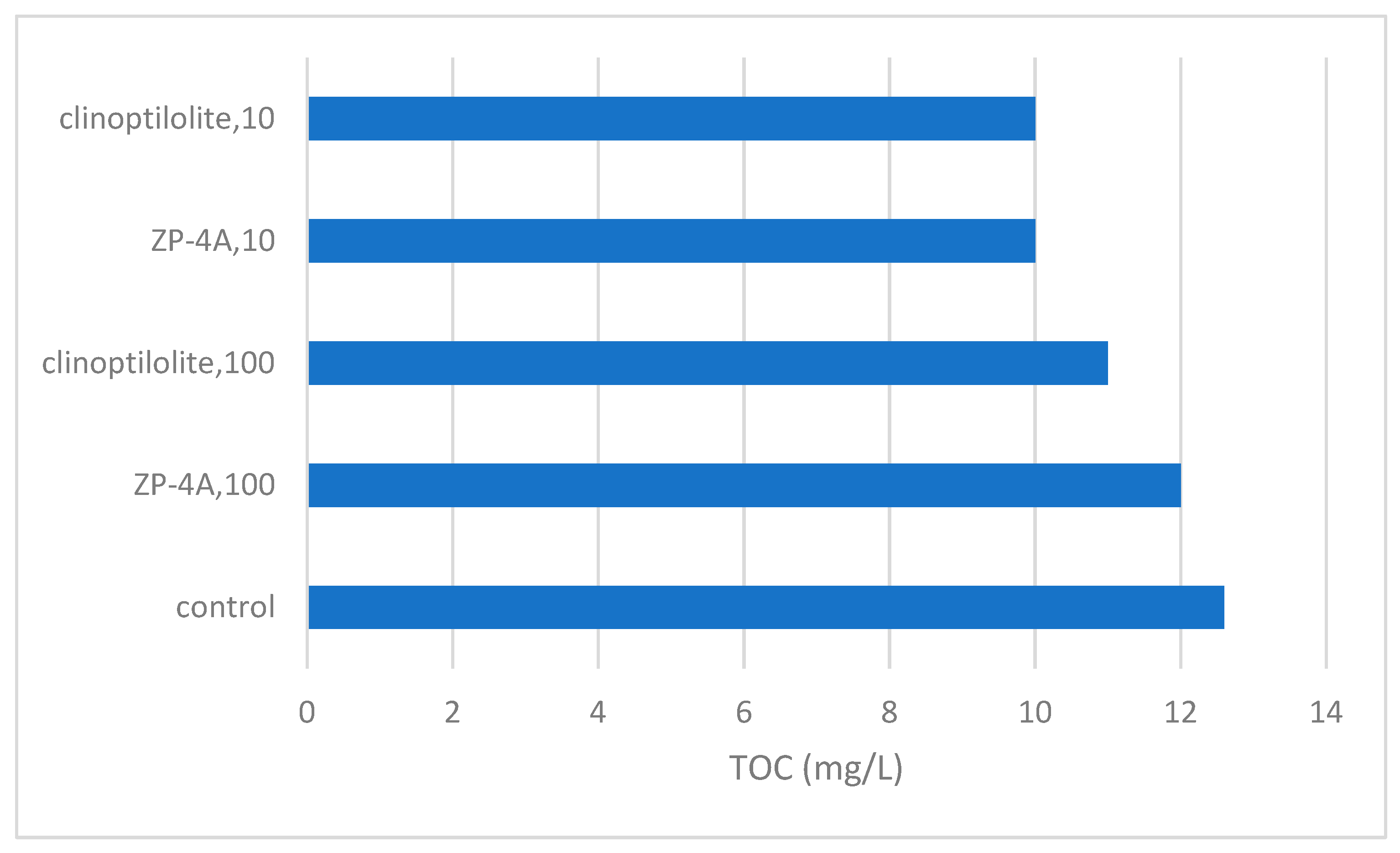
3.3. Leaching Experiments
3.4. Results of Kinetics Study
3.5. Future Research
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IPD | Intra-particle diffusion model |
| PFO | Pseudo-first kinetic model |
| PSO | Pseudo-second kinetic model |
| TOC | Total organic carbon |
| ZP-4A | Zeolite 4A |
References
- Wen, J.; Yi, Y.; Zeng, G. Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J. Environ. Manag. 2016, 178, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zeng, G. Chemical and biological assessment of Cd-polluted sediment for land use: The effect of stabilization using chitosan-coated zeolite. J. Environ. Manag. 2018, 212, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Szerement, J.; Szatanik-Kloc, A.; Jarosz, R.; Bajda, T.; Mierzwa-Hersztek, M. Contemporary applications of natural and synthetic zeolites from fly ash in agriculture and environmental protection. J. Clean. Prod. 2021, 311, 127461. [Google Scholar] [CrossRef]
- Masoom, H.; Courtier-Murias, D.; Farooq, H.; Soong, R.; Kelleher, B.P.; Zhang, C.; Maas, W.E.; Fey, M.; Kumar, R.; Monette, M.; et al. Soil Organic Matter in Its Native State: Unravelling the Most Complex Biomaterial on Earth. Environ. Sci. Technol. 2016, 50, 1670–1680. [Google Scholar] [CrossRef]
- Tang, W.; Sun, L.; Zhu, Y.; Ng, J.C.; Huang, J.; Xu, Z.; Zhang, H. Difference analysis of organic matter-mediated heavy metal pollution in the sediments of urban water bodies. Sci. Total Environ. 2025, 968, 178747. [Google Scholar] [CrossRef]
- Fan, T.; Yao, X.; Sun, Z.; Sang, D.; Liu, L.; Deng, H.; Zhang, Y. Properties and metal binding behaviors of sediment dissolved organic matter (SDOM) in lakes with different trophic states along the Yangtze River Basin: A comparison and summary. Water Res. 2023, 231, 119605. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Syono, Y.; Hemley, R.J. Physics Meets Mineralogy. Geol. Mag. 2002, 139, 719–723. [Google Scholar]
- Calvo, B.; Canoira, L.; Morante, F.; Martínez-Bedia, J.M.; Vinagre, C.; García-González, J.; Elsen, J.; Alcantara, R. Continuous elimination of Pb2+, Cu2+, Zn2+, H+ and NH4+ from acidic waters by ionic exchange on natural zeolites. J. Hazard. Mater. 2009, 166, 619–627. [Google Scholar] [CrossRef]
- Yi, Y.; Wen, J.; Zeng, G.; Zhang, T.; Huang, F.; Haiyan, Q.; Tian, S. A comparative study for the stabilisation of heavy metal contaminated sediment by limestone, MnO2 and natural zeolite. Environ. Sci. Pollut. Res. 2017, 24, 795–804. [Google Scholar] [CrossRef]
- Napia, C.; Sinsiri, T.; Jaturapitakkul, C.; Chindaprasirt, P. Leaching of heavy metals from solidified waste using Portland cement and zeolite as a binder. Waste Manag. 2012, 32, 1459–1467. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Zheng, R.; Feng, X.; Zou, W.; Wang, R.; Yang, D.; Wei, W.; Li, S.; Chen, H. Converting loess into zeolite for heavy metal polluted soil remediation based on ‘soil for soil-remediation’ strategy. J. Hazard. Mater. 2021, 412, 125199. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.M.; Guerron, D.B.; Calderon, C.M. Natural zeolite as a chromium VI removal agent in tannery effluents. Heliyon 2021, 7, e07974. [Google Scholar] [CrossRef]
- ISO 22036:2024; Environmental Solid Matrices—Determination of Elements Using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2024.
- Rahman, N.; Raheem, A. Adsorption of Cd(II) ions on magnetic graphene oxide/cellulose modified with β-cyclodextrin: Analytical interpretation via statistical physics modeling and fractal like kinetic approach. Environ. Res. 2024, 243, 117868. [Google Scholar] [CrossRef]
- Simonič, M.; Kristl, M. Učinkovitost Zeolitov pri Odstranjevanju Kromovih (VI) Ionov iz Modelnih Vod; Kemija v šoli in družbi; Biteks Publisher: Ljubljana, Slovenia, 2013. [Google Scholar]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, S.; Dai, Z.; Zhu, L.; Xiao, T.; Zhang, X.; Yin, S.; Soltanian, M.R. Adsorption model identification for chromium (VI) transport in unconsolidated sediments. J. Hydrol. 2021, 598, 126228. [Google Scholar] [CrossRef]
- Hu, C.; Wang, X.; Li, J.; Luo, L.; Liu, F.; Wu, W.; Xu, Y.; Li, H.; Tan, B.; Zhang, G. Trends in the research on soil nitrogen leaching from farmland: A bibliometric analysis (2014–2023). Clim. Smart Agric. 2024, 1, 100026. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, Y.; Li, Y.; Zha, J.; Tian, G.; Wang, F.; Zhang, H.; Liang, J. Synthesis of zeolites by in-situ conversion of geopolymers and their performance of heavy metal ion removal in wastewater: A review. J. Clean. Prod. 2022, 349, 131441. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Adsorption of Chromium(VI) from Water by Clays. Ind. Eng. Chem. Res. 2006, 45, 7232–7240. [Google Scholar] [CrossRef]
- Hommaid, O.; Hamdo, J.Y. Adsotprion of chromium(VI) from an aqueous solution on a Syrian surfactant-modified zeolite. ChemTech Res. 2014, 6, 3753–3761. [Google Scholar]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, M.; Yu, X.; Niu, N.; Chen, L. Application of lignin adsorbent in wastewater Treatment: A review. Sep. Purif. Technol. 2022, 302, 122116. [Google Scholar] [CrossRef]
- Rahman, N.; Raheem, A. Graphene oxide/Mg-Zn-Al layered double hydroxide for efficient removal of doxycycline from water: Taguchi approach for optimization. J. Mol. Liq. 2022, 354, 118899. [Google Scholar] [CrossRef]
- Chang, J.; Wang, H.; Qiao, K.; Zhu, J.; Chen, Y.; Zhang, J.; Xue, Q.; Zhang, H.; Chen, H.; Xi, B. Quantitative insight into Cr(VI) adsorption and reduction by ferrihydrite-humic acid co-precipitates: Development of multistep kinetic model integrating synergistic/antagonistic factors. Chem. Geol. 2025, 678, 122659. [Google Scholar] [CrossRef]
- Shi, Z.; Peng, S.; Lin, X.; Liang, Y.; Lee, S.Z.; Allen, H.E. Predicting Cr(VI) adsorption on soils: The role of the competition of soil organic matter. Environ. Sci. Process. Impact 2020, 22, 95–104. [Google Scholar] [CrossRef]
- Possinger, A.R.; Zachman, M.J.; Enders, A.; Levin, B.D.A.; Muller, D.A.; Kourkoutis, L.F.; Lehmann, J. Organo–organic and organo–mineral interfaces in soil at the nanometer scale. Nat. Commun. 2020, 11, 6103. [Google Scholar] [CrossRef]
- Gu, Y.; Yin, M.; Zhang, H.; Wang, Y.; Shi, J. Study on the binding interaction of chromium(VI) with humic acid using UV–vis, fluorescence spectroscopy and molecular modeling. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1702–1709. [Google Scholar] [CrossRef]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B.B. EUR Report; CEC: Brussels, Belgium, 1992; Volume 85, p. 14763. [Google Scholar]
| Compound | Clinoptilolite wt [%] | ZP-4A wt [%] |
|---|---|---|
| SiO2 | 64.9 | 34 |
| Al2O3 | 13.4 | 30 |
| Fe2O3 | 2.0 | 0 |
| MgO | 1.1 | 0 |
| CaO | 2.0 | 0 |
| Na2O | 2.4 | 19 |
| K2O | 1.3 | 0 |
| Sample | d [nm] | w [%] | ζ [mV] |
|---|---|---|---|
| Control | 878.5 | 100.0 | −15.1 |
| Sediment:4A = 100:1 | 140.5 722.1 | 86.4 13.6 | −37.8 |
| Sediment:4A = 10:1 | 267.3 876.9 | 58.2 41.8 | −47.5 |
| Sediment: clinop = 100:1 | 185.5 841.1 | 91.4 8.6 | −42.9 |
| Sediment: clinop = 10:1 | 168.3 963.5 | 90.9 9.1 | −49.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonič, M. The Application of Zeolites for Fixation of Cr(VI) Ions in Sediments. Physchem 2025, 5, 19. https://doi.org/10.3390/physchem5020019
Simonič M. The Application of Zeolites for Fixation of Cr(VI) Ions in Sediments. Physchem. 2025; 5(2):19. https://doi.org/10.3390/physchem5020019
Chicago/Turabian StyleSimonič, Marjana. 2025. "The Application of Zeolites for Fixation of Cr(VI) Ions in Sediments" Physchem 5, no. 2: 19. https://doi.org/10.3390/physchem5020019
APA StyleSimonič, M. (2025). The Application of Zeolites for Fixation of Cr(VI) Ions in Sediments. Physchem, 5(2), 19. https://doi.org/10.3390/physchem5020019






