Forecasting Divergence: Climate-Driven Habitat Shifts in North American Odonates Depend on Functional Groups
Abstract
1. Introduction
2. Methods
2.1. Defining the Scope
2.2. Retrieving and Cleaning Data
2.3. Selecting Background Points and Environmental Variables
2.4. Constructing Final Species Distribution Models
2.5. Analysis
3. Results
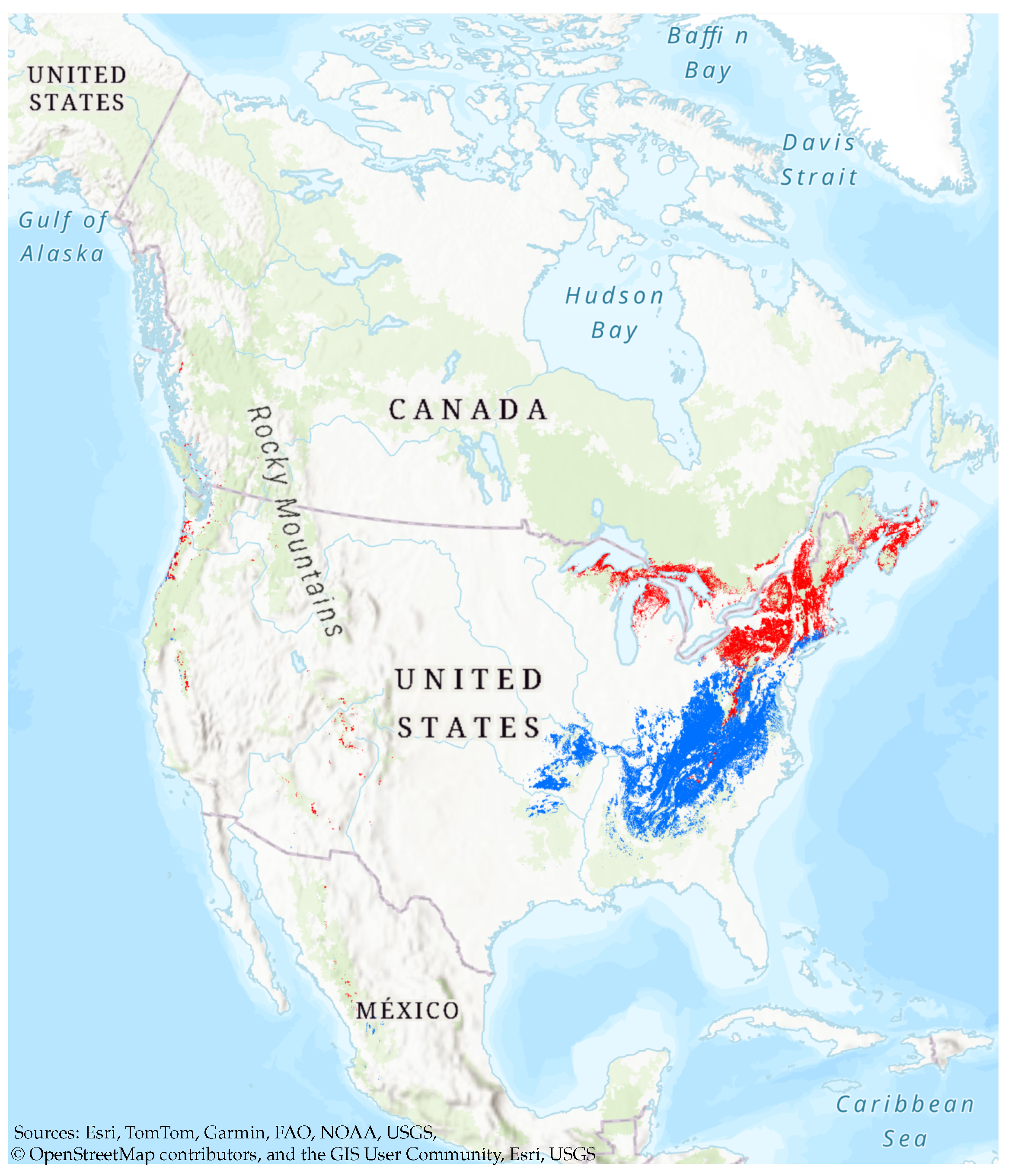
3.1. Overall Habitat Suitability Changes
3.2. Range Size Changes
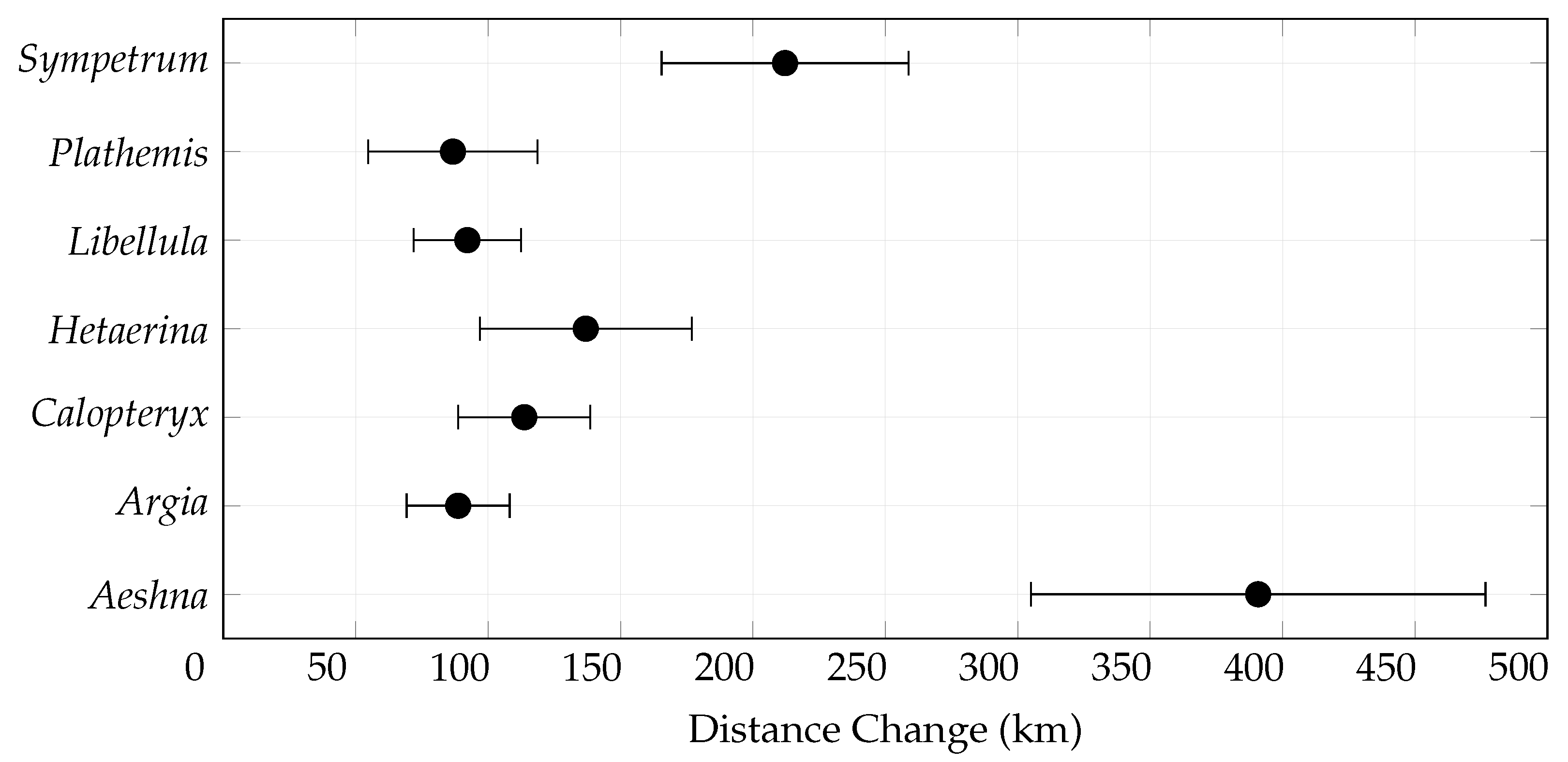
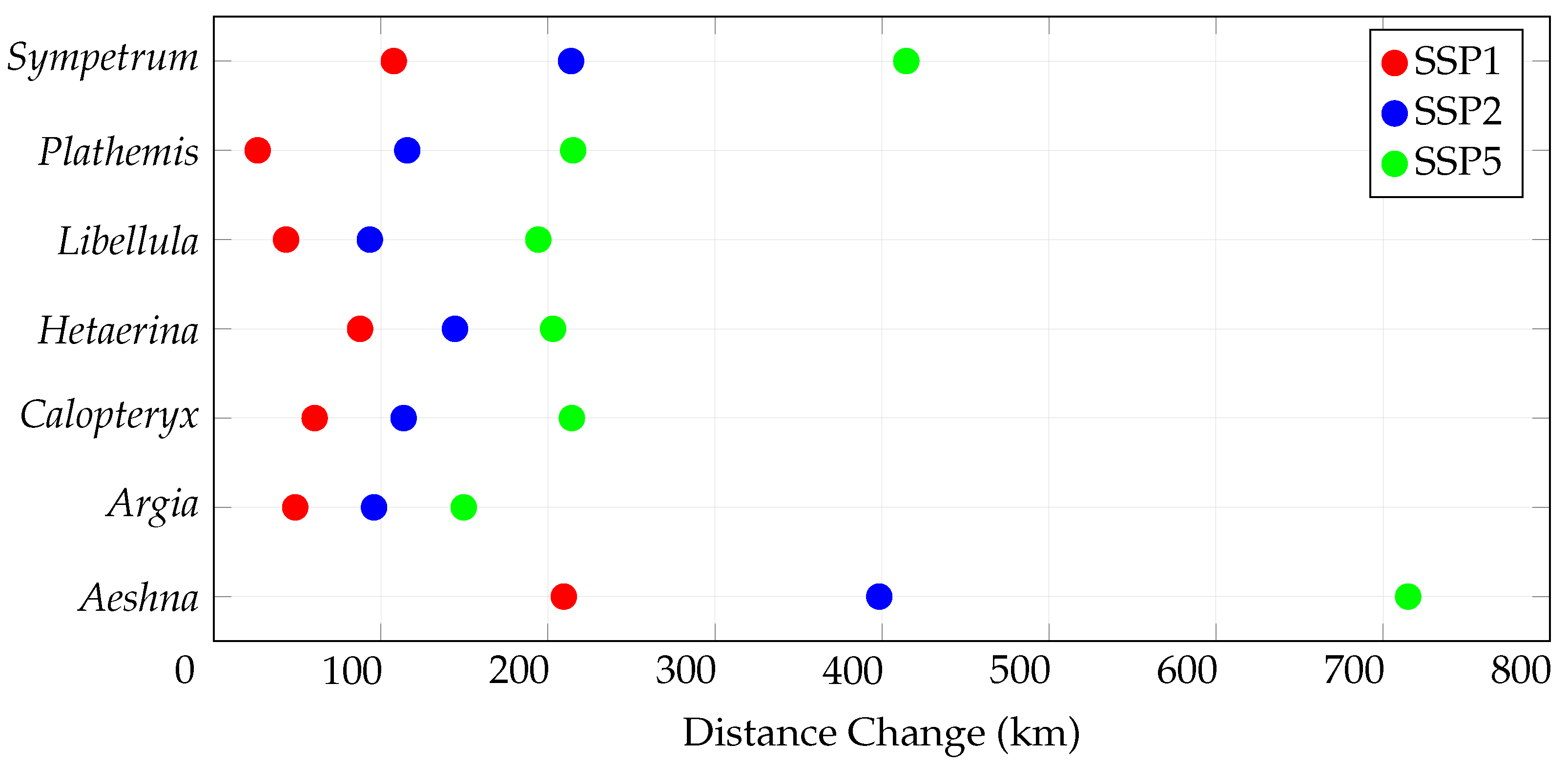
3.3. Centroid Change
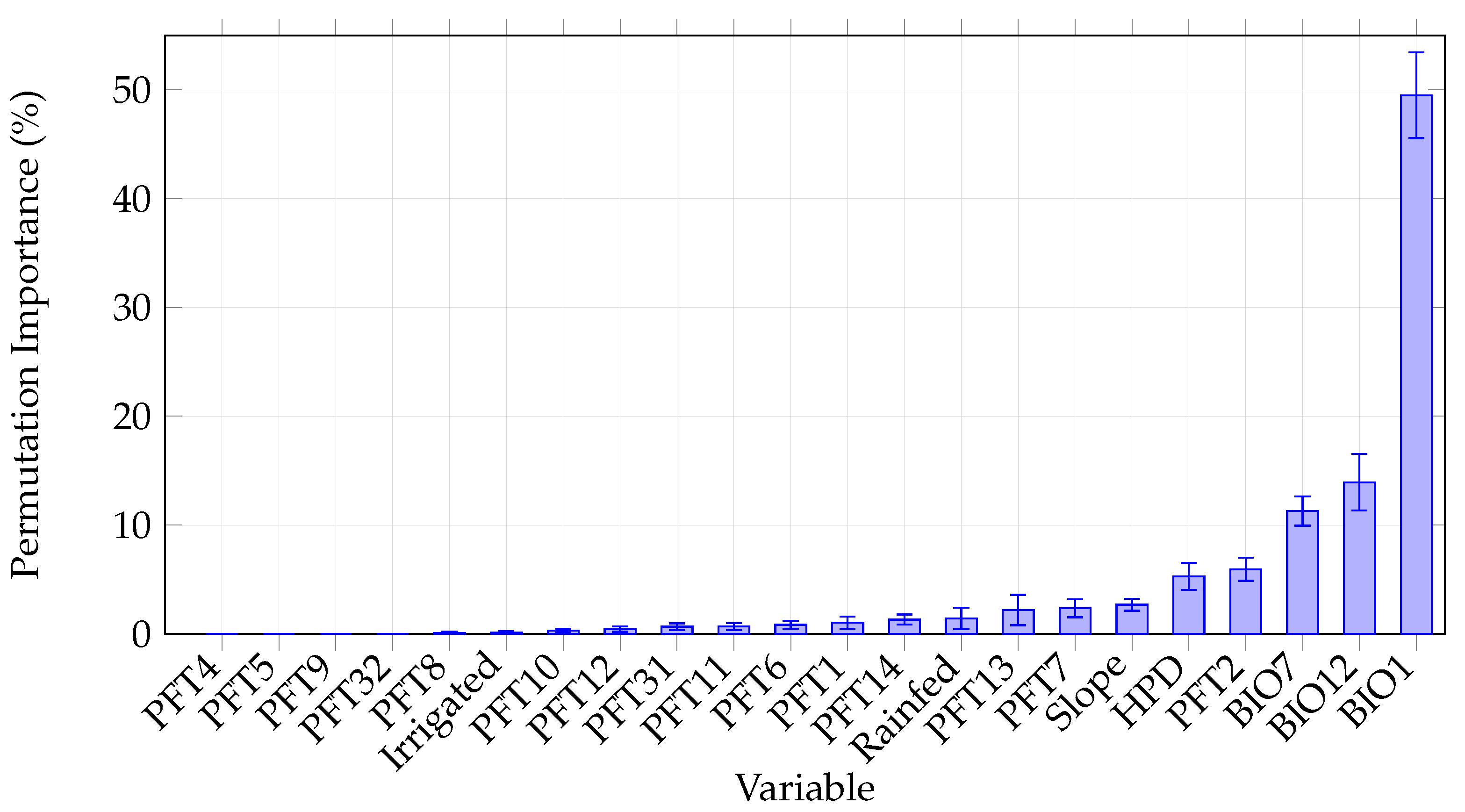
3.4. Environmental Variable Permutation Importance
4. Discussion
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2001: The Scientific Basis; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Pereira, H.M.; Leadley, P.W.; Proença, V.; Alkemade, R.; Scharlemann, J.P.W.; Fernandez-Manjarrés, J.F.; Araújo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.L.; et al. Scenarios for Global Biodiversity in the 21st Century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Collins, S.D.; McIntyre, N.E. Modeling the distribution of odonates: A review. Freshw. Sci. 2015, 34, 1144–1158. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Change 2017, 42, 153–168. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Ebi, K.L.; Kemp-Benedict, E.; Riahi, K.; Rothman, D.S.; Van Ruijven, B.J.; Van Vuuren, D.P.; Birkmann, J.; Kok, K.; et al. The roads ahead: Narratives for shared socioeconomic pathways describing world futures in the 21st century. Glob. Environ. Change 2017, 42, 169–180. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Riahi, K.; Calvin, K.; Dellink, R.; Emmerling, J.; Fujimori, S.; Kc, S.; Kriegler, E.; O’Neill, B. The Shared Socio-economic Pathways: Trajectories for human development and global environmental change. Glob. Environ. Change 2017, 42, 148–152. [Google Scholar] [CrossRef]
- Kok, M.T.J.; Kok, K.; Peterson, G.D.; Hill, R.; Agard, J.; Carpenter, S.R. Biodiversity and ecosystem services require IPBES to take novel approach to scenarios. Sustain. Sci. 2017, 12, 177–181. [Google Scholar] [CrossRef]
- Price, P.W.; Denno, R.F.; Eubanks, M.D.; Finke, D.L.; Kaplan, I. Insect Ecology: Behavior, Populations and Communities, 1st ed.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Thomas, J.A.; Rose, R.J.; Clarke, R.T.; Thomas, C.D.; Webb, N.R. Intraspecific variation in habitat availability among ectothermic animals near their climatic limits and their centres of range. Funct. Ecol. 1999, 13, 55–64. [Google Scholar] [CrossRef]
- Hassall, C.; Thompson, D.J. The effects of environmental warming on Odonata: A review. Int. J. Odonatol. 2008, 11, 131–153. [Google Scholar] [CrossRef]
- Caissie, D. The thermal regime of rivers: A review. Freshw. Biol. 2006, 51, 1389–1406. [Google Scholar] [CrossRef]
- Buisson, L.; Thuiller, W.; Lek, S.; Lim, P.; Grenouillet, G. Climate change hastens the turnover of stream fish assemblages. Glob. Change Biol. 2008, 14, 2232–2248. [Google Scholar] [CrossRef]
- Oertli, B.; Joye, D.A.; Castella, E.; Juge, R.; Cambin, D.; Lachavanne, J.B. Does size matter? The relationship between pond area and biodiversity. Biol. Conserv. 2002, 104, 59–70. [Google Scholar] [CrossRef]
- May, M.L. Odonata: Who They Are and What They Have Done for Us Lately: Classification and Ecosystem Services of Dragonflies. Insects 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Nock, C.A.; Vogt, R.J.; Beisner, B.E. Functional Traits. In Encyclopedia of Life Sciences, 1st ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 1–8. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Renault, D.; Leclerc, C.; Colleu, M.; Boutet, A.; Hotte, H.; Colinet, H.; Chown, S.L.; Convey, P. The rising threat of climate change for arthropods from Earth’s cold regions: Taxonomic rather than native status drives species sensitivity. Glob. Change Biol. 2022, 28, 5914–5927. [Google Scholar] [CrossRef]
- Rathore, M.K.; Sharma, L.K. Efficacy of species distribution models (SDMs) for ecological realms to ascertain biological conservation and practices. Biodivers. Conserv. 2023, 32, 3053–3087. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive performance of presence-only species distribution models: A benchmark study with reproducible code. Ecol. Monogr. 2022, 92, e01486. [Google Scholar] [CrossRef]
- Høye, T.T.; Loboda, S.; Koltz, A.M.; Gillespie, M.A.K.; Bowden, J.J.; Schmidt, N.M. Nonlinear trends in abundance and diversity and complex responses to climate change in Arctic arthropods. Proc. Natl. Acad. Sci. USA 2021, 118, e2002557117. [Google Scholar] [CrossRef] [PubMed]
- Woods, T.; McGarvey, D.J. Drivers of Odonata flight timing revealed by natural history collection data. J. Anim. Ecol. 2023, 92, 310–323. [Google Scholar] [CrossRef]
- Abbott, J.C. Odonata Central. 2025. Available online: https://www.gbif.org/dataset/3ab13bbb-8efa-4f6a-838b-b6a8eaa3f4df (accessed on 26 April 2025).
- GBIF.org. Occurrence Download. 2025. Available online: https://www.gbif.org/occurrence/download/0000380-250426092105405 (accessed on 26 April 2025).
- The Pandas Development Team. pandas-dev/pandas: Pandas. 2020. Available online: https://zenodo.org/records/17229934 (accessed on 14 April 2025).
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J. terra: Spatial Data Analysis. 2024. Available online: https://rspatial.github.io/terra/ (accessed on 31 March 2025).
- Wang, X.; Meng, X.; Long, Y. Projecting 1 km-grid population distributions from 2020 to 2100 globally under shared socioeconomic pathways. Sci. Data 2022, 9, 563. [Google Scholar] [CrossRef]
- Chen, M.; Vernon, C.R.; Graham, N.T.; Hejazi, M.; Huang, M.; Cheng, Y.; Calvin, K. Global land use for 2015–2100 at 0.05° resolution under diverse socioeconomic and climate scenarios. Sci. Data 2020, 7, 320. [Google Scholar] [CrossRef]
- Hoyer, S.; Joseph, H. xarray: N-D labeled Arrays and Datasets in Python. J. Open Res. Softw. 2017, 5, 10. [Google Scholar] [CrossRef]
- Amatulli, G.; Domisch, S.; Tuanmu, M.N.; Parmentier, B.; Ranipeta, A.; Malczyk, J.; Jetz, W. A suite of global, cross-scale topographic variables for environmental and biodiversity modeling. Sci. Data 2018, 5, 180040. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.L.; Boyce, M.S. Modelling distribution and abundance with presence-only data. J. Appl. Ecol. 2006, 43, 405–412. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling. Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- O’Brien, R.M. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Qual. Quant. 2007, 41, 673–690. [Google Scholar] [CrossRef]
- Schnase, J.L.; Carroll, M.L. Automatic variable selection in ecological niche modeling: A case study using Cassin’s Sparrow (Peucaea cassinii). PLoS ONE 2022, 17, e0257502. [Google Scholar] [CrossRef]
- Ab Lah, N.Z.; Yusop, Z.; Hashim, M.; Mohd Salim, J.; Numata, S. Predicting the Habitat Suitability of Melaleuca cajuputi Based on the MaxEnt Species Distribution Model. Forests 2021, 12, 1449. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. dismo: Species Distribution Modeling. 2024. Available online: https://cran.r-project.org/web/packages/dismo/index.html (accessed on 31 March 2025).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Hijmans, R.J. predicts: Spatial Prediction Tools. 2024. Available online: https://cran.r-project.org/package=predicts (accessed on 31 March 2025).
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecologica 2007, 31, 361–369. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Davison, A.C.; Hinkley, D.V. Bootstrap Methods and Their Applications; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. 2025. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 31 March 2025).
- O’Brien, S.F.; Yi, Q.L. How do I interpret a confidence interval? Transfusion 2016, 56, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J. A Brief Tutorial on Maxent. Available online: https://web.archive.org/web/20060504165624/https://www.cs.princeton.edu/~schapire/maxent/tutorial/tutorial.doc (accessed on 4 May 2006).
- Wallingford, P.D.; Morelli, T.L.; Allen, J.M.; Beaury, E.M.; Blumenthal, D.M.; Bradley, B.A.; Dukes, J.S.; Early, R.; Fusco, E.J.; Goldberg, D.E.; et al. Adjusting the lens of invasion biology to focus on the impacts of climate-driven range shifts. Nat. Clim. Change 2020, 10, 398–405. [Google Scholar] [CrossRef]
- Randalls, S. History of the 2 °C climate target. WIREs Clim. Change 2010, 1, 598–605. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Esri. Topographic. Available online: https://www.arcgis.com/home/item.html?id=67372ff42cd145319639a99152b15bc3 (accessed on 2 June 2025).
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Muirhead-Thompson, R.C. Laboratory evaluation of pesticide impact on stream invertebrates. Freshw. Biol. 1973, 3, 479–498. [Google Scholar] [CrossRef]
- Mabry, C.; Dettman, C. Odonata Richness and Abundance in Relation to Vegetation Structure in Restored and Native Wetlands of the Prairie Pothole Region, USA. Ecol. Restor. 2010, 28, 475–484. [Google Scholar] [CrossRef]
- Van Schalkwyk, J.; Pryke, J.S.; Samways, M.J.; Gaigher, R. Congruence between arthropod and plant diversity in a biodiversity hotspot largely driven by underlying abiotic factors. Ecol. Appl. 2019, 29, e01883. [Google Scholar] [CrossRef]
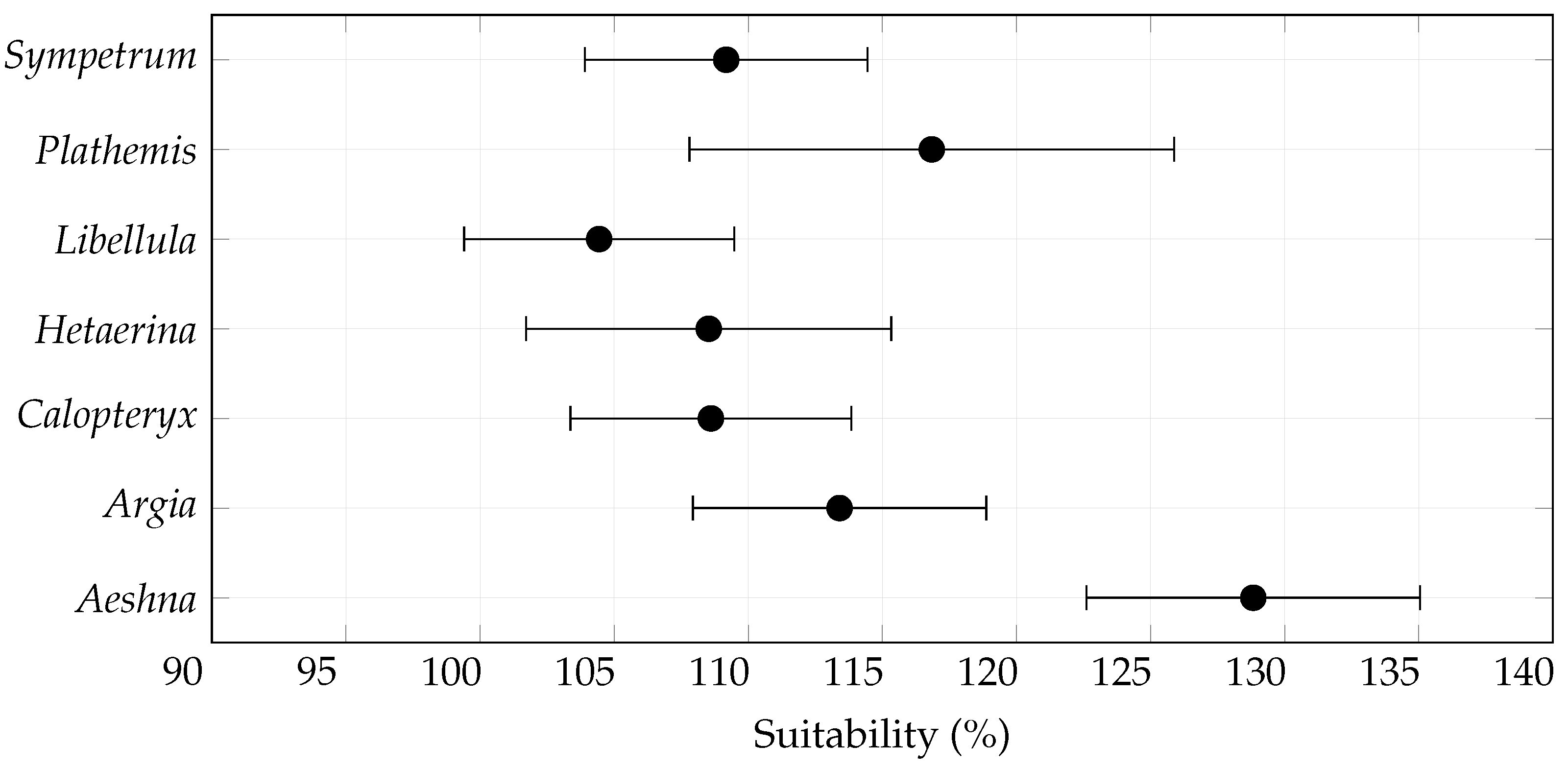
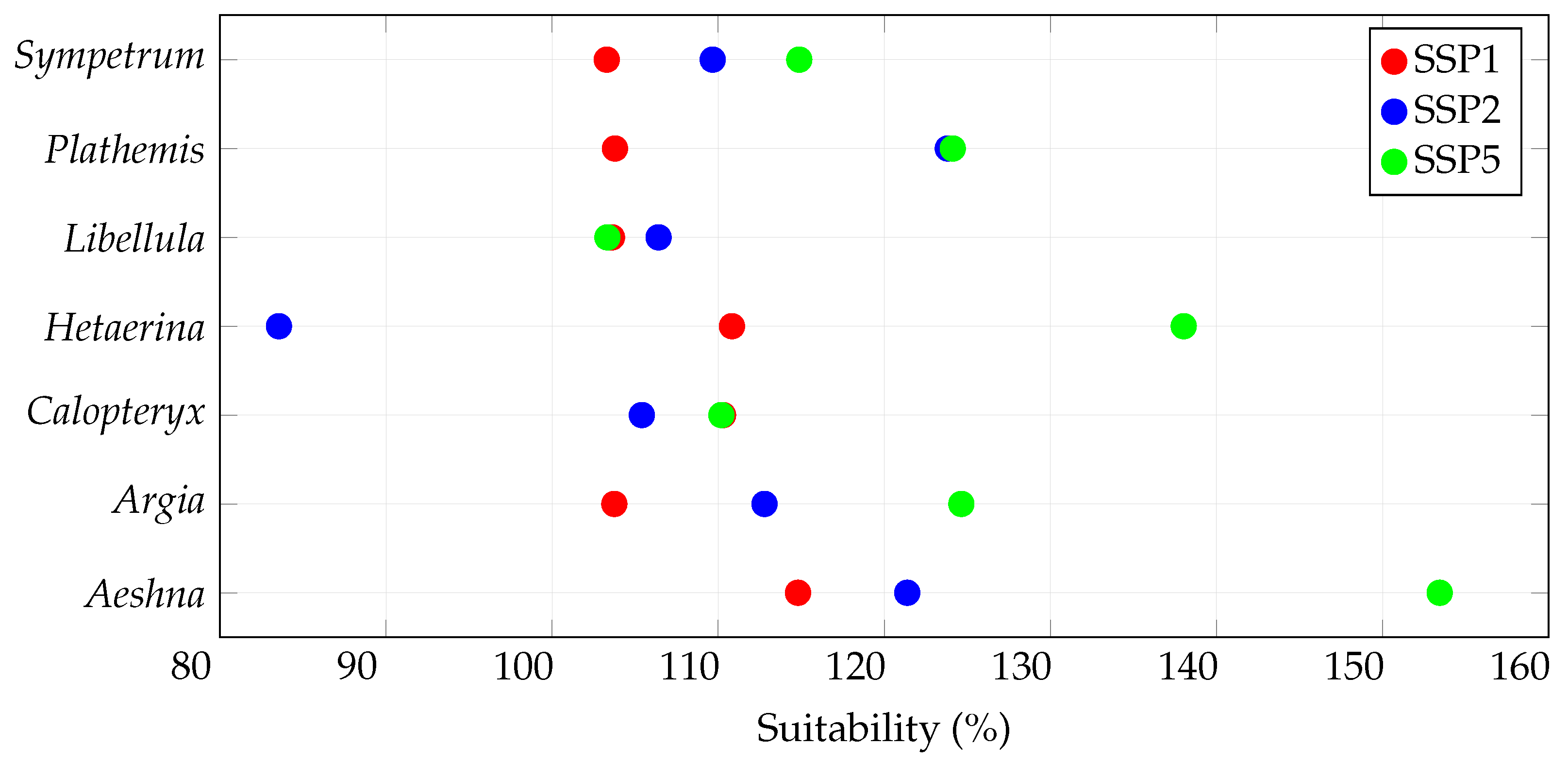
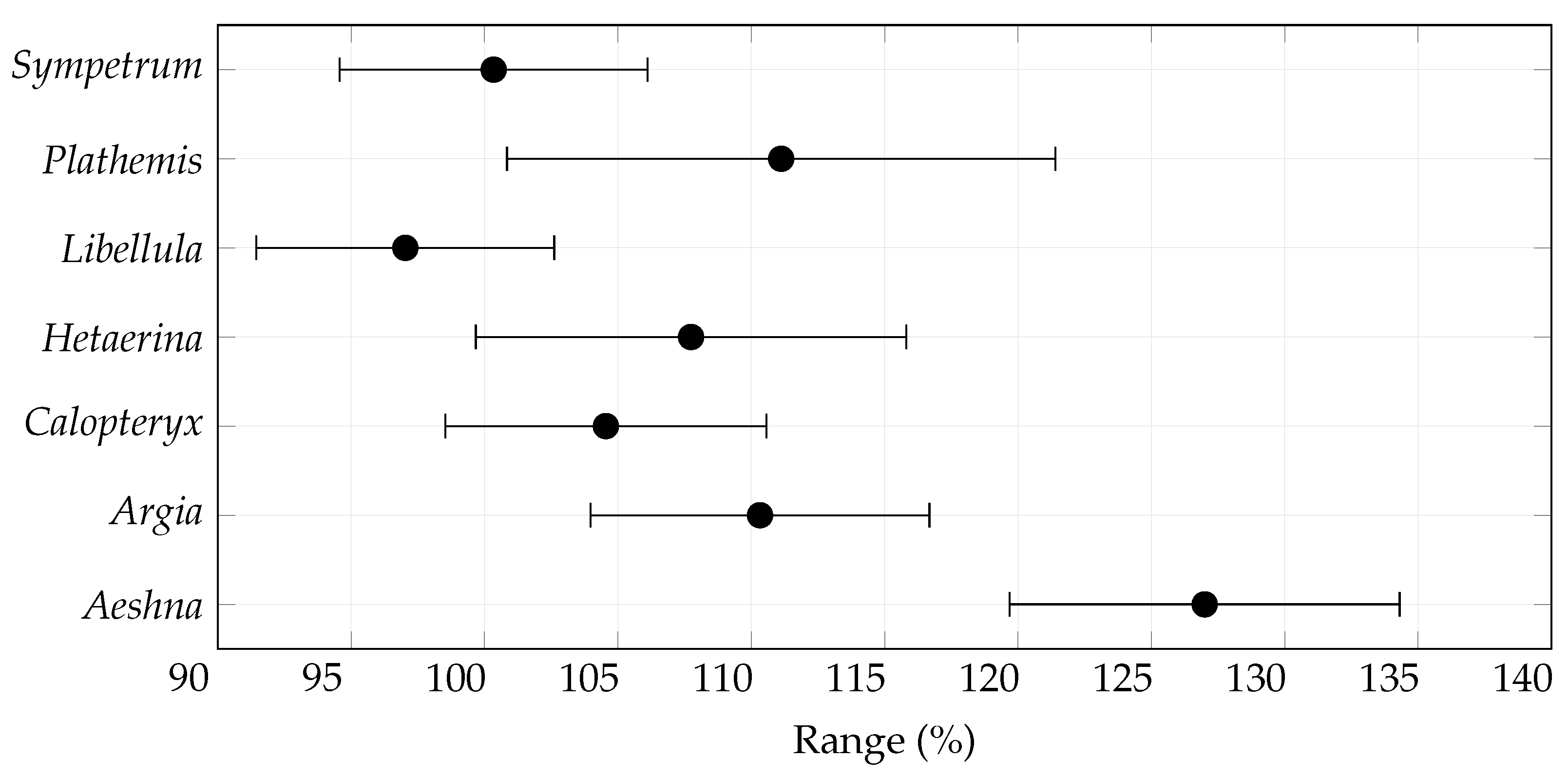
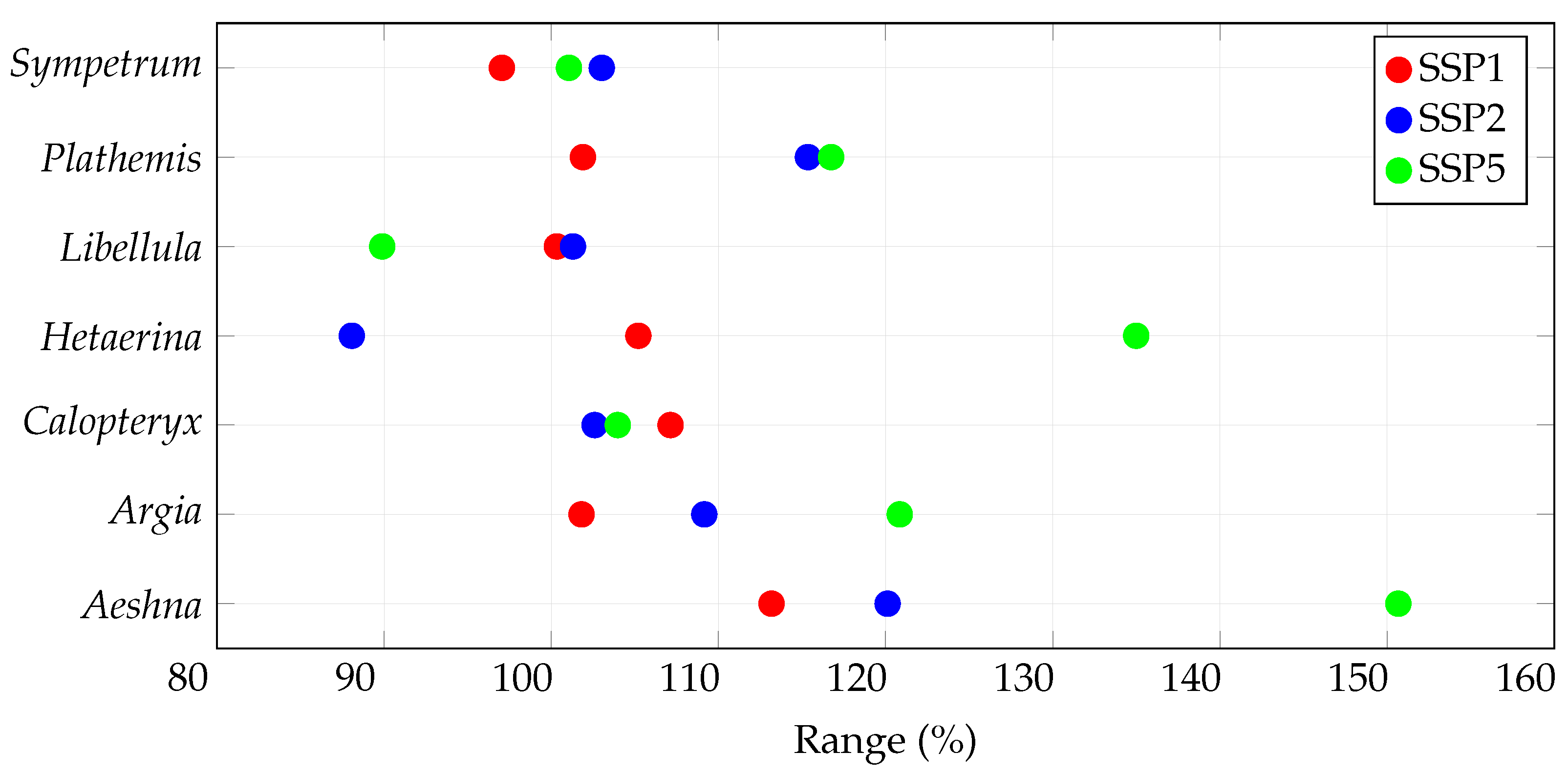
| Species | Records | Species | Records |
|---|---|---|---|
| Aeshna umbrosa | 1010 | Hetaerina americana | 2509 |
| Aeshna palmata | 578 | Hetaerina titia | 540 |
| Aeshna canadensis | 496 | Hetaerina vulnerata | 179 |
| Aeshna interrupta | 428 | Libellula luctuosa | 6393 |
| Aeshna constricta | 291 | Libellula incesta | 5135 |
| Argia fumipennis | 2922 | Libellula pulchella | 3959 |
| Argia apicalis | 2858 | Libellula vibrans | 2656 |
| Argia moesta | 2678 | Libellula cyanea | 1691 |
| Argia sedula | 1866 | Plathemis lydia | 8991 |
| Argia tibialis | 1614 | Plathemis subornata | 127 |
| Calopteryx maculata | 3669 | Sympetrum vicinum | 2880 |
| Calopteryx aequabilis | 514 | Sympetrum corruptum | 2157 |
| Calopteryx dimidiata | 307 | Sympetrum semicinctum | 1073 |
| Calopteryx angustipennis | 89 | Sympetrum obtrusum | 876 |
| Calopteryx amata | 72 | Sympetrum ambiguum | 749 |
| Variables | VIF | Variables | VIF |
|---|---|---|---|
| BIO1 (Annual Mean Temperature) | 11.350 | PFT10 (Bdlf Dcds Shrub Temperate) | 4.303 |
| BIO7 (Temperature Annual Range) | 5.164 | PFT11 (Bdlf Dcds Shrub Boreal) | 7.013 |
| BIO12 (Annual Precipitation) | 3.031 | PFT12 (C3 Arctic) | 3.776 |
| PFT1 (Ndlf Evgr Tree Temperate) | 5.136 | PFT13 (C3 Grass) | 11.423 |
| PFT2 (Ndlf Evgr Tree Boreal) | 20.126 | PFT14 (C4 Grass) | 6.675 |
| PFT4 (Bdlf Evgr Tree Tropical) | 3.705 | PFT31 (Urban) | 1.352 |
| PFT5 (Bdlf Evgr Tree Temperate) | 1.389 | PFT32 (Barren) | 5.640 |
| PFT6 (Bdlf Dcds Tree Tropical) | 2.528 | PFT Rainfed Crops Cumulative | 5.035 |
| PFT7 (Bdlf Dcds Tree Temperate) | 3.566 | PFT Irrigated Crops Cumulative | 1.497 |
| PFT8 (Bdlf Dcds Tree Boreal) | 1.798 | HPD (Human Population Density) | 1.028 |
| PFT9 (Bdlf Evgr Shrub Temperate) | 1.011 | Slope | 1.377 |
| Genus | General Suitability (%) | Range Size (%) | Centroid Distance (km) | |||
|---|---|---|---|---|---|---|
| Aeshna | ||||||
| Argia | ||||||
| Calopteryx | ||||||
| Hetaerina | ||||||
| Libellula | ||||||
| Plathemis | ||||||
| Sympetrum | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y. Forecasting Divergence: Climate-Driven Habitat Shifts in North American Odonates Depend on Functional Groups. Conservation 2025, 5, 76. https://doi.org/10.3390/conservation5040076
Tan Y. Forecasting Divergence: Climate-Driven Habitat Shifts in North American Odonates Depend on Functional Groups. Conservation. 2025; 5(4):76. https://doi.org/10.3390/conservation5040076
Chicago/Turabian StyleTan, Yunchao. 2025. "Forecasting Divergence: Climate-Driven Habitat Shifts in North American Odonates Depend on Functional Groups" Conservation 5, no. 4: 76. https://doi.org/10.3390/conservation5040076
APA StyleTan, Y. (2025). Forecasting Divergence: Climate-Driven Habitat Shifts in North American Odonates Depend on Functional Groups. Conservation, 5(4), 76. https://doi.org/10.3390/conservation5040076






