Abstract
Native bee communities in Arkansas remain poorly documented, particularly within fire-managed prairie ecosystems that provide critical habitat for pollinators. This study surveyed bee assemblages at two native prairie remnants in the Arkansas River Valley, one large (Cherokee Prairie Natural Area, CPNA) and one small urban fragment (Jewel Moore Nature Reserve, JMNR), both managed using prescribed fire. Using pan trapping, we recorded 599 individuals representing 96 species across 25 genera, including 49% singletons. Despite differences in size and landscape context, both prairies supported similarly rich bee communities per sample day, with JMNR and CPNA averaging 16.1 and 13.75 species, respectively. However, species composition diverged notably, with only 34.5% similarity, suggesting distinct community structure driven by site-specific habitat conditions and management histories. CPNA was dominated by large-bodied ground-nesting and cavity-nesting solitary bees, while JMNR supported smaller eusocial halictids and cavity nesters. Results highlight the value of prescribed fire in maintaining nesting substrates and floral resources. Even small, urban prairie remnants like JMNR can support high pollinator richness, emphasizing their role as conservation assets. Our findings contribute to a foundational baseline for native bee diversity in Arkansas and highlight the importance of both large and small fire-managed prairies in regional pollinator conservation planning.
1. Introduction
Pollinators are essential to both wild ecosystems and agricultural systems, contributing to the reproduction of over 85% of flowering plants and the productivity of a wide array of crops [1,2,3]. Among these, bees (Hymenoptera: Apoidea) are considered the most effective and abundant pollinators, owing to their specialized morphology, behavior, and dependence on pollen as a larval food source [4,5]. While vertebrate pollinators such as birds and bats contribute to specific mutualisms, insect taxa, including bees, flies, butterflies, and beetles, are responsible for the vast majority of pollination events globally [6].
Native bees, in particular, exhibit considerable functional and taxonomic diversity. With over 20,000 described species across more than 400 genera [5,7], they encompass a wide range of nesting strategies (e.g., ground, wood, stem cavities), social structures (solitary to eusocial), phenological patterns, and degrees of floral specialization. This diversity allows native bee assemblages to respond variably to local habitat structure, floral availability, and broader landscape context [8,9,10,11]. Despite their ecological significance, much of this diversity remains poorly documented, especially in under-sampled regions of North America.
Bee populations worldwide face multiple interacting threats, including habitat loss, lack of diverse floral food resources, pesticide exposure, invasive species and natural enemies, pathogens, and climate change [12,13,14,15]. These pressures are particularly acute in temperate grassland ecosystems, such as North America’s tallgrass and mixed-grass prairies, which have experienced widespread conversion to agriculture, urban development, and fire suppression [16]. Today, less than 4% of historic prairie landscapes remain, and these remnants often occur as small, isolated patches [17]. As a result, efforts to conserve pollinator diversity in prairie systems must focus not only on protection, but also on ecologically informed management.
Prescribed fire is a key management tool in prairie ecosystem conservation, helping to limit the spread of woody plants, enhance the variety of native wildflowers, and preserve the open landscape typical of these habitats [18,19]. Fire influences bee populations indirectly through changes in floral composition and bloom phenology [20], and directly through effects on nesting habitat availability, soil exposure, and overwintering success [21,22]. Although our understanding remains incomplete, fire generally appears to have a positive effect on bee communities. However, some species respond negatively, and frequent burning can be detrimental to their populations in general [23]. While many ground-nesting species benefit from post-burn soil conditions, the impacts of fire are highly context-dependent, shaped by burn frequency, timing, intensity, and the spatial scale of disturbance [24,25]. Patch-mosaic fire regimes that preserve unburned refugia may enhance beta-diversity and reduce the exclusion of fire-sensitive taxa [26].
Despite growing interest in fire–pollinator dynamics, few studies have evaluated native bee communities in the southeastern United States, and baseline data from Arkansas remain especially limited. The state lies at the confluence of major ecoregions, including the Ozark Highlands, Ouachita Mountains, and Mississippi Alluvial Plain, supporting a rich biotic assemblage and diverse floral resources [27]. However, comprehensive inventories of native bees in Arkansas are lacking, with only approximately 150 species formally reported [28,29,30,31,32,33]. Most available studies are taxonomically narrow or focused on agricultural systems and conspicuous genera such as Apis, Bombus, Xylocopa, and Melissodes [34,35,36]. In contrast, many states in the U.S.A. with more systematic surveys typically report 400–600 native bee species [7], suggesting that Arkansas remains substantially under-sampled.
In this study, we examined native bee assemblages in two prairie remnants in Arkansas: a large, fire-managed tallgrass prairie (CPNA) and a smaller suburban prairie (JMNR) managed under prescribed fire regime. The main objectives were to: (1) quantify species richness, abundance, and community composition of native bees; (2) assess nesting guild structure in relation to habitat and fire management; and (3) evaluate the contribution of small prairie remnants to regional bee diversity. By linking bee assemblage structure to prescribed fire and habitat context, our work provides critical baseline data and contributes to evidence-based strategies for pollinator conservation in fire-adapted landscapes of the southeastern United States.
2. Materials and Methods
2.1. Study Site Description
This study was conducted at two prairie remnants located within the Arkansas River Valley ecoregion, selected to represent contrasting habitat sizes and surrounding landscape contexts: the Cherokee Prairie Natural Area (CPNA) in Franklin County and the Jewel Moore Nature Reserve (JMNR) in Faulkner County, Arkansas (Figure 1). Both sites fall within the central portion of the state, characterized by transitional grassland–woodland ecotones and formerly extensive tallgrass prairie. Cherokee Prairie Natural Area (CPNA) is a 236-hectare high-quality tallgrass prairie managed by the Arkansas Natural Heritage Commission. It is considered one of the most intact prairie remnants in the Arkansas River Valley and serves as a regional ecological reference for pre-settlement prairie conditions [37]. The site is actively managed through prescribed fire and invasive species control to maintain its native species richness and structural heterogeneity. In contrast, Jewel Moore Nature Reserve (JMNR) comprises approximately 4 hectares of native prairie located on the campus of the University of Central Arkansas in the city of Conway [38]. While the reserve retains characteristic prairie vegetation, it is embedded in a suburban matrix, bordered by residential housing, commercial infrastructure, and fragmented woodland (approximately 4 ha) to the north. Despite its small size and isolation, JMNR is periodically managed with fire and mowing to preserve native plant communities. Both sites are subjected to prescribed fire management regimes aimed at promoting native flora and maintaining prairie structure. CPNA undergoes rotational burning on multi-year cycles, while JMNR is burned less regularly due to its location within an urbanized area.
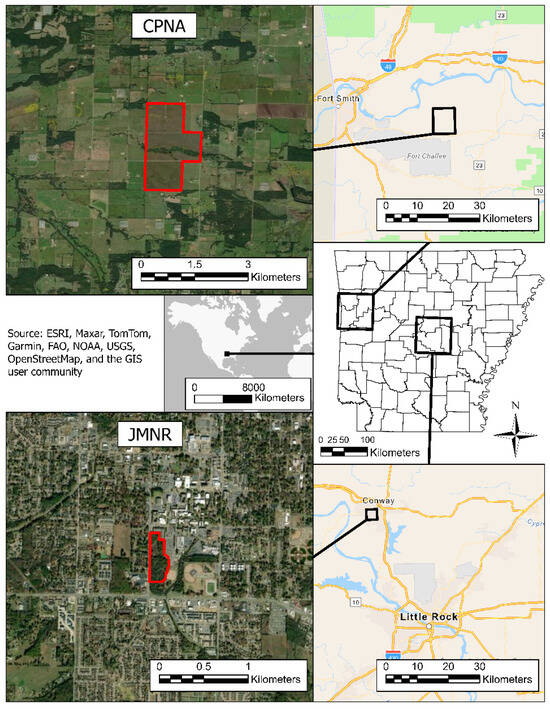
Figure 1.
Sampling site locations, highlighted in red. Map generated via ArcGIS Pro 3.5.
2.2. Bee Sampling
To characterize native bee assemblages at each site, a standardized pan trapping method was used. This combination allowed for sampling across a broad range of bee taxa, including both cryptic ground-nesting species and visually conspicuous foragers. The pan trapping technique that was used was adapted from Sam Droege of the USGS. Pan trapping was conducted using SOLOTM 3.25 oz (approx. 96 mL) colored plastic bowls (Krylon® fluorescent yellow spray paint, Krylon® fluorescent blue spray paint, and unpainted white plastic) (Figure 2), each filled with a water solution containing a small amount of unscented dish soap to reduce surface tension. These fluorescent colors were chosen because of their ultraviolet reflectance. Bowls were arranged in linear transects of 15 traps (5 of each color), spaced 3 m apart, and centered in areas representative of prairie vegetation (Figure 3). Pan trapping is now understood to have biases and does not produce standardized samples; therefore, specific taxa were likely missed and comparisons for significance were not conducted [39,40,41]. Early in the season, bowls were mounted on ~1 m wooden stakes to raise them above dense vegetation. However, due to dry, compacted soil later in the season, traps were placed directly on the ground from July onward. Each site contained three pan trap transects, and transect placement was rotated between sampling periods to capture spatial heterogeneity. Traps were deployed for 24 h during early sampling events, then reduced to 12 and later 8 h in late summer and fall due to logistical constraints and trap saturation in high bee activity periods. Sampling in 2011 occurred across ten sampling days at JMNR between May 28 and October 2, while CPNA was sampled on four occasions (approximately monthly) between July 3 and October 8. The difference in sampling frequency reflects site accessibility and scheduling constraints. Therefore, the two sites were not sampled equally and JMNR was sampled across a wider range of active flight periods, increasing the chances of collecting a higher richness.
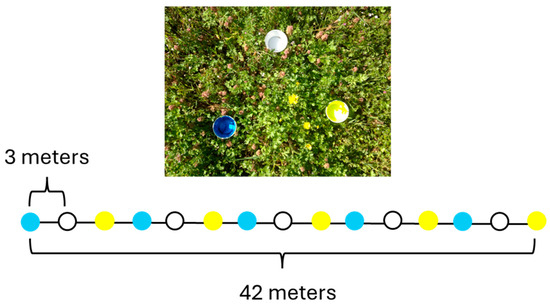
Figure 2.
Pan trapping design, traps themselves are shown in the image while below demonstrates layout of a transect. The order of colors was randomly decided before each day of sampling, but colors always alternated in sets of 3.
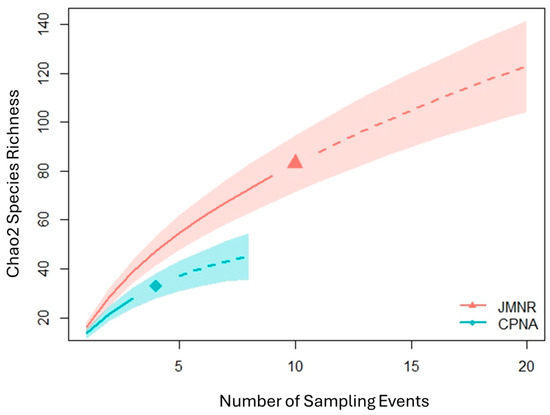
Figure 3.
Incidence-based (Chao2) species accumulation (rarefaction) curves comparing the two study sites (JMNR and CPNA). Solid lines indicate rarified observed values, dashed lines represent extrapolation.
2.3. Sample Processing and Identification
All collected specimens were initially preserved in 70% ethanol in the field, then transferred to the laboratory for processing. Bees were later dried, pinned, and labeled following standard entomological protocols [42]. Each individual was assigned a unique identification number and entered into a relational database with metadata including date, site, trap type, and collector. Specimens were identified to species using current keys, regional field guides, and published taxonomic resources. All identifications were subsequently verified by Mike Arduser, a taxonomic specialist in North American bees, to ensure accuracy and consistency across the dataset. Voucher specimens are retained in the University of Central Arkansas entomological collection for reference. After identification, species were classified by nesting habit (e.g., ground, wood, cavity, stem/pith, or external) and seasonal activity (spring–winter) based on a synthesis of ecological literature, regional floras, and natural history accounts [7,43,44,45,46,47].
2.4. Data Analysis
To evaluate bee community structure across the two prairie sites, several standard ecological metrics were calculated. Species richness was defined as the total number of unique species recorded per transect per sampling day, while abundance referred to the total number of individual bees collected within the same unit of effort. To capture both richness and evenness in community composition, Chao1 estimates of Shannon’s Index were generated using the iNext package in R using species and abundance data collected for each sample day [48], providing a composite measure of diversity sensitive to both common and rare species. To account for sampling effort, species accumulation curves were generated in R using vegan and iNext packages [49,50]. Additionally, Sorensen Percent Similarity Index (PSI) was calculated to quantify the degree of species overlap between the two sites, based on relative abundance data. Together, these metrics enabled a comprehensive comparison of native bee diversity, abundance, and assemblage similarity between the extensively fire-managed tallgrass prairie at CPNA and the smaller, suburban remnant at JMNR, offering insights into how site size and management context influence pollinator communities.
3. Results
In this study, bee sampling yielded 599 individuals, representing 96 species across 25 genera. Of these, 49% were singletons (species represented by a single individual; Table 1). Species richness was greater at JMNR (83 species) than at CPNA (33 species); however, this difference reflects unequal sampling effort. Species accumulation curves indicate if sampling were even between sites, their overall species richness would have been similar (Figure 3). When standardized per sample day, both sites exhibited comparable metrics: mean species richness was 16.1 ± 2.4 species/sampling day at JMNR and 13.75 ± 2.3 at CPNA (Figure 4c); mean abundance was 40.7 ± 8.6 and 48.0 ± 14.9 individuals/sampling day, respectively (Figure 4b); and estimated Shannon’s diversity index averaged 2.93 ± 0.25 and 2.71 ± 0.09 (Figure 4a). The Percent Similarity Index (PSI) between sites indicated only 34.5% species similarity, suggesting distinct community composition.

Table 1.
Abundance of bee species collected from study sites. For nest substrate: S = soil, W = wood, H = hive, P = pith, C = cavity. Plant specificity: P = polylectic, O = oligolectic. Behavior: S = solitary, E = eusocial, B = semisocial, P = parasitic. * indicates introduced species.
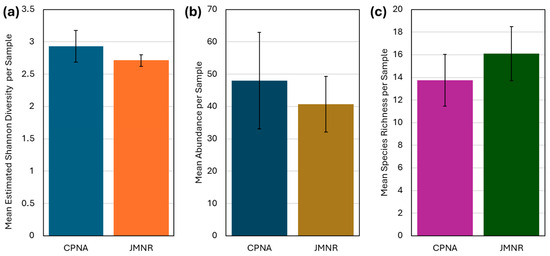
Figure 4.
Community indices for study sites (CPNA and JMNR) with error bars representing ± standard error (SE). (a) Mean estimated Shannon entropy/diversity for each prairie site, Chao1 estimates for each sample day. Both locations displayed similar levels of bee diversity. (b) Mean number of bees collected per transect per day. (c) Mean species richness per sampling day at study sites.
Dominant genera at JMNR included Lasioglossum (19.1%), Megachile (15.7%), Augochlorella (12.8%), Bombus (10.3%), and Melissodes (7.4%). In contrast, CPNA was dominated by Megachile (27.2%), Bombus (23.0%), Melissodes (16.8%), Xylocopa virginica (10.5%), and Epimelissodes (5.8%). Species-level data reflected these trends: CPNA was characterized by large-bodied soil or cavity/pith nesters and social apines, including Melissodes communis (30), Bombus griseocollis (38), Megachile brevis (28), and X. virginica (20). JMNR was dominated by eusocial halictines and bumble bees, as well as pith-nesting and reed-specialist bees, including Augochlorella persimilis (31), Ceratina (29), B. griseocollis (26), Augochlorella aurata (21), Halictus ligatus (21), Lasioglossum coreopsis (20), B. impatiens (12). JMNR also had cavity- and soil-nesting species present, including Ptilothrix bombiformis (20), Epimelissodes petulca (19), M. texana (19), M. brevis (13), and M. mendica (12), but were of lower proportions.
Nesting guild analysis revealed that ground-nesting bees were the most diverse and abundant functional group at both sites (e.g., Andrena, Calliopsis, Halictus, Lasioglossum, Melissodes, Epimelissodes), consistent with widespread use of exposed mineral soils (Figure 5a). Cavity- and pith-nesting species (e.g., Megachile, Ceratina, Hylaeus rudbeckiae) formed a secondary component, while wood- and hive-nesting species such as X. virginica and Bombus spp. were also present. Cleptoparasitic bees were infrequent but detected at both sites (e.g., Coelioxys mexicanus, Coelioxys octodentatus, Coelioxys sayi, Epeolus lectoides, Nomada erigeronis, Triepeolus helianthi), aligning with the presence of their hosts (Figure 5b). Notably, two introduced cavity-nesting species, M. rotundata and M. sculpturalis, were recorded at low abundance.
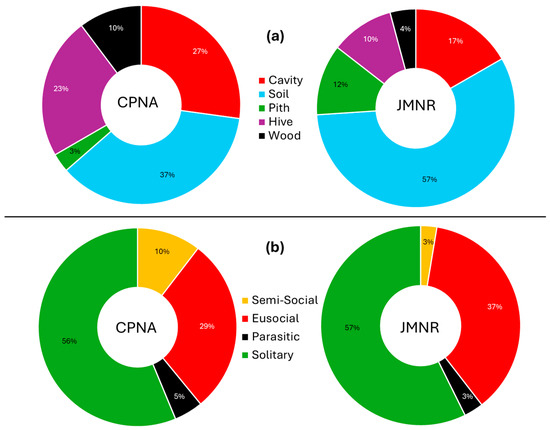
Figure 5.
Composition of individual caught at both sites for (a) nesting guild and (b) social behavior.
4. Discussion
Our survey of two prairie remnants in Arkansas revealed a diverse bee assemblage, with 96 species representing 25 genera. Nearly half (49%) were singletons, a pattern commonly reported in pollinator inventories [4,5] and indicative of high species turnover, rarity, or under-sampling. These findings support the view that Arkansas’s bee fauna remains under-documented and that prairie habitats may serve as important reservoirs of pollinator biodiversity [31].
When controlling sampling effort, the small urban prairie (JMNR) supported bee assemblages comparable to the larger remnant (CPNA) in terms of species richness, abundance, and diversity. This underscores the conservation potential of small, well-managed sites in maintaining functionally rich pollinator communities, even within fragmented or developed landscapes [6,51]. The unexpectedly high richness at JMNR likely reflects a combination of continuous bloom availability, structural complexity, and proximity to ornamental or semi-natural habitats that can act as sources of colonists or floral subsidies [52].
Despite their differences in size and setting, both the large prairie remnant (CPNA) and the small suburban prairie (JMNR) supported similarly diverse bee assemblages on a per-sample-day basis. These results demonstrate that even small patches of high-quality prairie can sustain diverse bee communities, provided they maintain sufficient floral and nesting resources [52,53]. The presence of continuous bloom and a structurally complex habitat matrix at JMNR likely explains its unexpectedly high richness, despite its small (~4 ha) area. These findings support broader work indicating that even modest green spaces can serve as critical refugia for pollinators within developed landscapes [53].
The community compositions, however, diverged markedly between sites. The Percent Similarity Index revealed only 34.5% similarity, with each site harboring a largely distinct assemblage. CPNA was dominated by large-bodied, ground- or cavity-nesting solitary bees and corbiculate social taxa, such as Melissodes communis, Epimelissodes petulcus, Megachile brevis, Xylocopa virginica, and Bombus griseocollis. These species often require extensive habitat for nesting and foraging, and many are pollen specialists on Asteraceae that rely on late-season bloom. In contrast, JMNR’s assemblage skewed toward smaller, generalist halictid bees, including Lasioglossum, Augochlorella aurata, and Halictus ligatus, as well as cavity nesters such as Megachile texana, M. brevis, and M. mendica. The relatively high abundance of Bombus impatiens at JMNR, a common generalist bumble bee in the eastern U.S., further supports the idea that suburban sites may receive input from broader urban landscapes, including gardens and ornamental plantings.
Additionally, two non-native cavity-nesting bees, Megachile rotundata and M. sculpturalis, were detected at JMNR in low numbers, likely reflecting human-mediated introduction pathways and the higher propagule pressure typical of urban settings. Their low abundance suggests minimal current impact, but continued monitoring is warranted to assess potential competition with native species.
Nesting guild structure was broadly consistent across sites, with ground-nesting bees dominating both numerically and taxonomically. These included Andrena, Halictus, Lasioglossum, Melissodes, and Epimelissodes, taxa that require access to exposed mineral soil. Cavity- and pith-nesting taxa, such as Megachile, Ceratina, and Hylaeus, formed a substantial minority, and wood-nesting and hive-nesting bees, such as Xylocopa and Bombus, were also present. Cleptoparasitic bees, e.g., Coelioxys mexicanus, Coelioxys octodentatus, Coelioxys sayi, Epeolus lectoides, and Triepeolus helianthi, occurred at low frequency but in sufficient numbers to reflect healthy host populations [5]. The presence of Ptilothrix bombiformis, a specialist on Hibiscus, at JMNR is notable and suggests that even small remnants may sustain specialist bees if their host plants persist. Although P. bombiformis also gathers pollen from Ipomoea [54], both plant genera inhabit similar environments that support the persistence of this bee species.
The composition of each site reflects its management context. CPNA, as a large and fire-managed remnant, maintains prairie plant assemblages and structure approximating pre-settlement conditions [37], which are critical for sustaining late-season floral specialists and large-bodied solitary bees. JMNR, despite its suburban location, likely benefits from edge effects and colonization from surrounding habitats, including ornamental landscapes. Urban-adapted taxa may also exploit diverse nesting substrates such as retaining walls, garden edges, and woody debris. The high diversity observed at JMNR may be influenced by the combined effects of management practices both within and around its boundaries. Urban landscaping and prescribed fire appear to complement one another by providing a wider variety of nesting substrates and floral resources essential for sustaining bee communities. At JMNR, patch-level differences in fire and mowing regimes may help explain internal variation in community composition, suggesting that even small sites benefit from heterogeneity in disturbance. Such variability supports a wider range of bee species by providing both nesting substrates and floral continuity, as well as refugia for fire-sensitive taxa [21,22].
Bee communities can benefit from wildfire, particularly through the increased habitat heterogeneity that fire introduces. Previous studies have shown that variation in fire regimes enhances bee abundance and diversity by creating a mosaic of conditions that support a range of species and functional traits [55]. At the same time, responses to fire may not be uniform across different invertebrate taxa. Some groups may experience population declines; others show little change; and certain taxa, including Hymenoptera, often increase in abundance following fire events [56]. Prescribed fire has also been associated with higher abundances of several bee taxa [57] and may help explain the elevated species richness observed at JMNR. However, without direct comparisons to ecologically similar but unburned or differently managed sites, the interpretation of JMNR’s diversity patterns remains tentative. Furthermore, long-term monitoring and expanded sampling across additional prairie remnants in other regions will be essential to further elucidate regional bee community dynamics and inform evidence-based conservation planning. Our study contributes an important baseline, emphasizing that even small, well-managed remnants can play a key role in supporting native bee diversity within fire-adapted landscapes.
5. Conclusions
This study provides a critical baseline of native bee diversity in fire-managed prairies of Arkansas, documenting 599 individuals across 96 species. Both the large and small suburban prairies sampled in this study were found to support similarly rich bee assemblages per sampling day. Ground-nesting bees were dominant at both sites, while community composition differed. These findings highlight the value of even small, well-managed prairie remnants for pollinator conservation. Prescribed fire, when applied with ecological sensitivity, fosters floral and nesting resources essential to sustaining diverse bee communities across fragmented landscapes.
Author Contributions
Conceptualization, C.Z.L. and N.K.J.; methodology, C.Z.L.; software, C.Z.L.; validation, C.Z.L. and N.K.J.; formal analysis, C.Z.L.; investigation, C.Z.L.; resources, C.Z.L. and N.K.J.; data curation, C.Z.L. and N.K.J.; writing—original draft preparation, C.Z.L.; writing—review and editing, C.Z.L. and N.K.J.; visualization, C.Z.L. and N.K.J.; supervision, N.K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank the University of Central Arkansas (C.L.) and the University of Arkansas Division of Agriculture (N.J.) for institutional support. C.L. extends special thanks to K.C. Larson (retired, University of Central Arkansas) for advising and assistance with plant identification, and to David Dussourd for guidance on insect specimen pinning and curation. We are grateful to Mike Arduser for his expert verification and correction of bee specimen identifications. C.L. also acknowledges the technical training and taxonomic support received from Sam Droege, Alana Taylor, and Rob Jean, which significantly contributed to bee identification efforts. We also thank Jenn Wagner for helping with sample processing and pinning, and Kyle Hurley for valuable assistance with the fieldwork.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Winfree, R.; Tarrant, S. How Many Flowering Plants Are Pollinated by Animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop Production in the USA Is Frequently Limited by a Lack of Pollinators. Proc. R. Soc. B 2020, 287, 20200922. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. Effects of Introduced Bees on Native Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 1–26. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Rader, R.; Bartomeus, I.; Garibaldi, L.A.; Garratt, M.P.D.; Howlett, B.G.; Winfree, R.; Cunningham, S.A.; Mayfield, M.M.; Arthur, A.D.; Andersson, G.K.S.; et al. Non-Bee Insects Are Important Contributors to Global Crop Pollination. Proc. Natl. Acad. Sci. USA 2016, 113, 146–151. [Google Scholar] [CrossRef]
- Ascher, J.S.; Pickering, J. Discover Life Bee Species Guide and World Checklist. 2024. Available online: https://www.discoverlife.org/mp/20q?guide=Apoidea_species (accessed on 28 August 2025).
- Williams, N.M.; Crone, E.E.; Roulston, T.H.; Minckley, R.L.; Packer, L.; Potts, S.G. Ecological and Life-History Traits Predict Bee Species Responses to Environmental Disturbances. Biol. Conserv. 2010, 143, 2280–2291. [Google Scholar] [CrossRef]
- Winfree, R.; Aguilar, R.; Vázquez, D.P.; Lebuhn, G.; Aizen, M.A. A Meta-Analysis of Bees’ Responses to Anthropogenic Disturbance. Ecology 2009, 90, 2068–2076. [Google Scholar] [CrossRef]
- Joshi, N.K.; Otieno, M.; Rajotte, E.G.; Fleischer, S.J.; Biddinger, D.J. Proximity to Woodland and Landscape Structure Drives Pollinator Visitation in Apple Orchard Ecosystem. Front. Ecol. Evol. 2016, 4, 38. [Google Scholar] [CrossRef]
- Kline, O.; Joshi, N.K. Mitigating the Effects of Habitat Loss on Solitary Bees in Agricultural Ecosystems. Agriculture 2020, 10, 115. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Belsky, J.; Joshi, N.K. Impact of Biotic and Abiotic Stressors on Managed and Feral Bees. Insects 2019, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Belsky, J.; Joshi, N.K. Effects of Fungicide and Herbicide Chemical Exposure on Apis and Non-Apis Bees in Agricultural Landscape. Front. Environ. Sci. 2020, 8, 81. [Google Scholar] [CrossRef]
- Samson, F.B.; Knopf, F.L. Prairie Conservation in North America. BioScience 1994, 44, 418–421. [Google Scholar] [CrossRef]
- Hopwood, J.L. The Contribution of Roadside Grassland Restorations to Native Bee Conservation. Biol. Conserv. 2008, 141, 2632–2640. [Google Scholar] [CrossRef]
- Collins, S.L.; Calabrese, L.B. Effects of Fire, Grazing and Topographic Variation on Vegetation Structure in Tallgrass Prairie. J. Veg. Sci. 2012, 23, 563–575. [Google Scholar] [CrossRef]
- Towne, E.G.; Craine, J.M. Ecological Consequences of Shifting the Timing of Burning Tallgrass Prairie. PLoS ONE 2014, 9, e103423. [Google Scholar] [CrossRef]
- Mola, J.M.; Williams, N.M. Fire-Induced Change in Floral Abundance, Density, and Phenology Benefits Bumble Bee Foragers. Ecosphere 2018, 9, e02056. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Wilson, A.C.; Ohlson, G.C.; Pokswinksi, S.M.; Hiers, J.K. Frequent Prescribed Fires Favour Ground-Nesting Bees in Southeastern US Forests. Insect Conserv. Divers. 2021, 14, 527–534. [Google Scholar] [CrossRef]
- Fulton, L.J.; Ulyshen, M.D.; Mallinger, R.E. Frequent Fire Supports Ground-Nesting Bees and Wasps in Florida Longleaf Pine Flatwoods. J. For. 2025, 1–22. [Google Scholar] [CrossRef]
- Carbone, L.M.; Tavella, J.; Marquez, V.; Ashworth, L.; Pausas, J.G.; Aguilar, R. Fire Effects on Pollination and Plant Reproduction: A Quantitative Review. Ann. Bot. 2025, 135, 43–56. [Google Scholar] [CrossRef]
- Campbell, J.W.; Hanula, J.L.; Waldrop, T.A. Effects of Prescribed Fire and Fire Surrogates on Floral Visiting Insects of the Blue Ridge Province in North Carolina. Biol. Conserv. 2007, 134, 393–404. [Google Scholar] [CrossRef]
- Kimoto, C.; DeBano, S.J.; Thorp, R.W.; Taylor, R.V.; Schmalz, H.; DelCurto, T.; Johnson, T.; Kennedy, P.L.; Rao, S. Short-Term Responses of Native Bees to Livestock and Implications for Managing Ecosystem Services in Grasslands. Ecosphere 2012, 3, 1–19. [Google Scholar] [CrossRef]
- Hartley, M.K.; Rogers, W.E.; Siemann, E. Responses of Prairie Arthropod Communities to Fire and Fertilizer: Balancing Plant and Arthropod Conservation. Am. Midl. Nat. 2007, 157, 92–105. [Google Scholar] [CrossRef]
- Foti, T.L. Blackland prairies of southwestern Arkansas. Proc. Ark. Acad. Sci. 1989, 43, 23–28. [Google Scholar]
- Warriner, M.D. Arkansas Native Bee Checklist; Arkansas Natural Heritage Commission: Little Rock, AR, USA, 2011. [Google Scholar]
- Tripodi, A.D.; Szalanski, A.L. Further Range Extension of Xylocopa micans Lepeletier (Hymenoptera: Apidae). J. Kans. Entomol. Soc. 2011, 84, 163–164. [Google Scholar] [CrossRef]
- Stephenson, P.L.; Griswold, T.L.; Arduser, M.S.; Dowling, A.; Krementz, D. Checklist of Bees (Hymenoptera: Apoidea) from Managed Emergent Wetlands in the Lower Mississippi Alluvial Valley of Arkansas. Biodivers. Data J. 2018, 6, e24071. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, P.L.; Dowling, A.P.; Krementz, D.G. Bee Communities of Emergent Wetlands under Restoration in the Lower Mississippi Alluvial Valley of Arkansas. Southeast. Nat. 2020, 19, 472–490. [Google Scholar] [CrossRef]
- Acharya, R.S.; Burke, J.M.; Leslie, T.; Loftin, K.; Joshi, N.K. Wild Bees Respond Differently to Sampling Traps with Vanes of Different Colors and Light Reflectivity in a Livestock Pasture Ecosystem. Sci. Rep. 2022, 12, 9783. [Google Scholar] [CrossRef]
- Acharya, R.S.; Leslie, T.; Burke, J.; Naithani, K.; Fitting, E.; Loftin, K.; Joshi, N.K. Sheep Grazing Influences the Abundance, Diversity, and Community Composition of Wild Bees and Other Insects in Livestock Pastures. Ecol. Indic. 2024, 162, 111839. [Google Scholar] [CrossRef]
- Chandler, L.; McCoy, C. Observations on Arkansas Bees. Proc. Ark. Acad. Sci. 1965, 19, 20–23. [Google Scholar]
- Cameron, S.A.; Hines, H.M.; Williams, P.H. A Comprehensive Phylogeny of the Bumble Bees (Bombus). Biol. J. Linn. Soc. 2007, 91, 161–188. [Google Scholar] [CrossRef]
- Posey, M.H.; Cameron, A.E.; Lambert, B. Pollination Biology of Oilseed Sunflower in Arkansas. J. Ark. Acad. Sci. 1986, 40, 53–57. [Google Scholar]
- Baker, B.T. The Vascular Flora of Scott County and Yell County, Arkansas. Master’s Thesis, University of Central Arkansas, Conway, AR, USA, 2007. [Google Scholar]
- Wright, R.D.; Culwell, D.E. Early Stages of Prairie Restoration on a 1.5 Hectare Field in Faulkner County, Arkansas. J. Ark. Acad. Sci. 1982, 36, 80–81. [Google Scholar]
- Krahner, A.; Dietzsch, A.C.; Jütte, T.; Pistorius, J.; Everaars, J. Standardising Bee Sampling: A Systematic Review of Pan Trapping and Associated Floral Surveys. Ecol. Evol. 2024, 14, e11157. [Google Scholar] [CrossRef]
- Hutchinson, L.A.; Oliver, T.H.; Breeze, T.D.; O’Connor, R.S.; Potts, S.G.; Roberts, S.P.; Garratt, M.P. Inventorying and Monitoring Crop Pollinating Bees: Evaluating the Effectiveness of Common Sampling Methods. Insect Conserv. Divers. 2022, 15, 299–311. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Osborn, A.L.; Brown, E.R.; Pavlick, C.R.; Enríquez, E.; Tscheulin, T.; Petanidou, T.; Hranitz, J.M.; Barthell, J.F. Effect of Pan Trap Size on the Diversity of Sampled Bees and Abundance of Bycatch. J. Insect Conserv. 2020, 24, 409–420. [Google Scholar] [CrossRef]
- Droege, S. The Very Handy Manual: How to Catch and Identify Bees and Manage a Collection; U.S. Geological Survey, Native Bee Inventory and Monitoring Program: Beltsville, MD, USA, 2010; p. 71. [Google Scholar]
- Mitchell, T.B. Bees of the Eastern United States; North Carolina Agricultural Experiment Station: Raleigh, NC, USA, 1960; Volume I. [Google Scholar]
- Michener, C.D. Interaction among Workers from Different Colonies of Sweat Bees (Hymenoptera: Halictidae). Anim. Behav. 1966, 14, 126–129. [Google Scholar] [CrossRef]
- Brothers, D.J.; Michener, C.D. Interactions in Colonies of Primitively Social Bees: III. Ethometry of Division of Labor in Lasioglossum zephyrum (Hymenoptera: Halictidae). J. Comp. Physiol. 1974, 90, 129–168. [Google Scholar] [CrossRef]
- Lerman, S.B.; Milam, J. Bee Fauna and Floral Abundance within Lawn-Dominated Suburban Yards. Ann. Entomol. Soc. Am. 2016, 109, 713–723. [Google Scholar] [CrossRef]
- Wilson, J.S.; Carril, O.M. The Bees in Your Backyard; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Jost, L. Entropy and Diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Chao, A.; Henderson, P.A.; Chiu, C.H.; Moyes, F.; Hu, K.H.; Dornelas, M.; Magurran, A.E. Measuring Temporal Change in Alpha Diversity: A Framework Integrating Taxonomic, Phylogenetic and Functional Diversity and the iNEXT.3D Standardization. Methods Ecol. Evol. 2021, 12, 1926–1940. [Google Scholar] [CrossRef]
- Roswell, M.; Dushoff, J.; Winfree, R. A Conceptual Guide to Measuring Species Diversity. Oikos 2021, 130, 321–338. [Google Scholar] [CrossRef]
- Lerman, S.B.; Contosta, A.R.; Milam, J.; Bang, C. To Mow or to Mow Less: Lawn Mowing Frequency Affects Bee Abundance and Diversity in Suburban Yards. Biol. Conserv. 2018, 221, 160–174. [Google Scholar] [CrossRef]
- Tonietto, R.; Fant, J.; Ascher, J.; Ellis, K.; Larkin, D. A Comparison of Bee Communities of Chicago Green Roofs, Parks and Prairies. Landsc. Urban Plan. 2011, 103, 102–108. [Google Scholar] [CrossRef]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; Roberts, S.; O’Toole, C.; Ne’Eman, G.; Willmer, P. Role of Nesting Resources in Organizing Diverse Bee Communities in a Mediterranean Landscape. Ecol. Entomol. 2005, 30, 78–85. [Google Scholar] [CrossRef]
- Sharkey, J.K.; Pindar, A.; Raine, N.E. First Canadian Record of the Specialist Hibiscus Bee, Ptilothrix bombiformis (Cresson) (Hymenoptera: Apidae). J. Entomol. Soc. Ont. 2020, 151, 41–48. [Google Scholar] [CrossRef]
- Burkle, L.A.; Simanonok, M.P.; Durney, J.S.; Myers, J.A.; Belote, R.T. Wildfires Influence Abundance, Diversity, and Intraspecific and Interspecific Trait Variation of Native Bees and Flowering Plants across Burned and Unburned Landscapes. Front. Ecol. Evol. 2019, 7, 252. [Google Scholar] [CrossRef]
- Zumr, V.; Nakládal, O.; Remeš, J. Two-Year Post-Fire Abundance of Arthropod Groups Across Different Types of Forest in Temperate Central Europe. Fire 2025, 8, 305. [Google Scholar] [CrossRef]
- Frank, G.S.; Rivers, J.W.; Verschuyl, J.; Best, L.R.; Betts, M.G.; Kroll, A.J.; Swanson, M.E.; Krawchuk, M.A. Comparison of Early Seral Forest Bee Communities Following Clearcutting or Wildfire Depends on Stand Age and Nesting Guild. J. For. 2025, 1–45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).