Fish Diversity in Relation to Salinity Gradient in the Meghna River Estuary, Bangladesh
Abstract
:1. Introduction
2. Materials and Methods
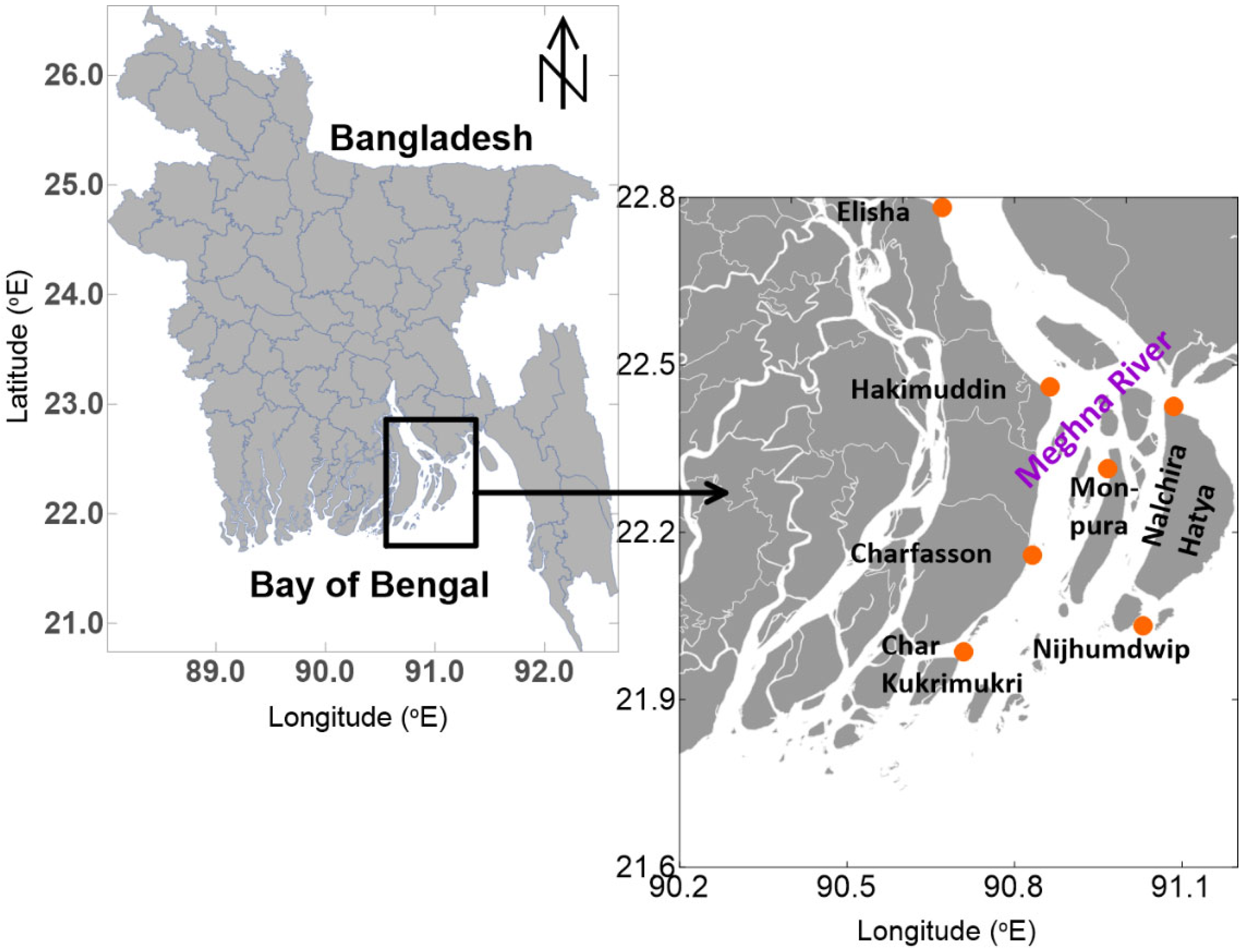
2.1. Study Area
2.2. Spatio-Temporal Salinity Observation
2.3. Fish Sampling
2.4. Laboratory Analysis
2.5. Fish Diversity Indices
2.6. Site Suitability Index
2.7. Statistical Analysis
3. Results
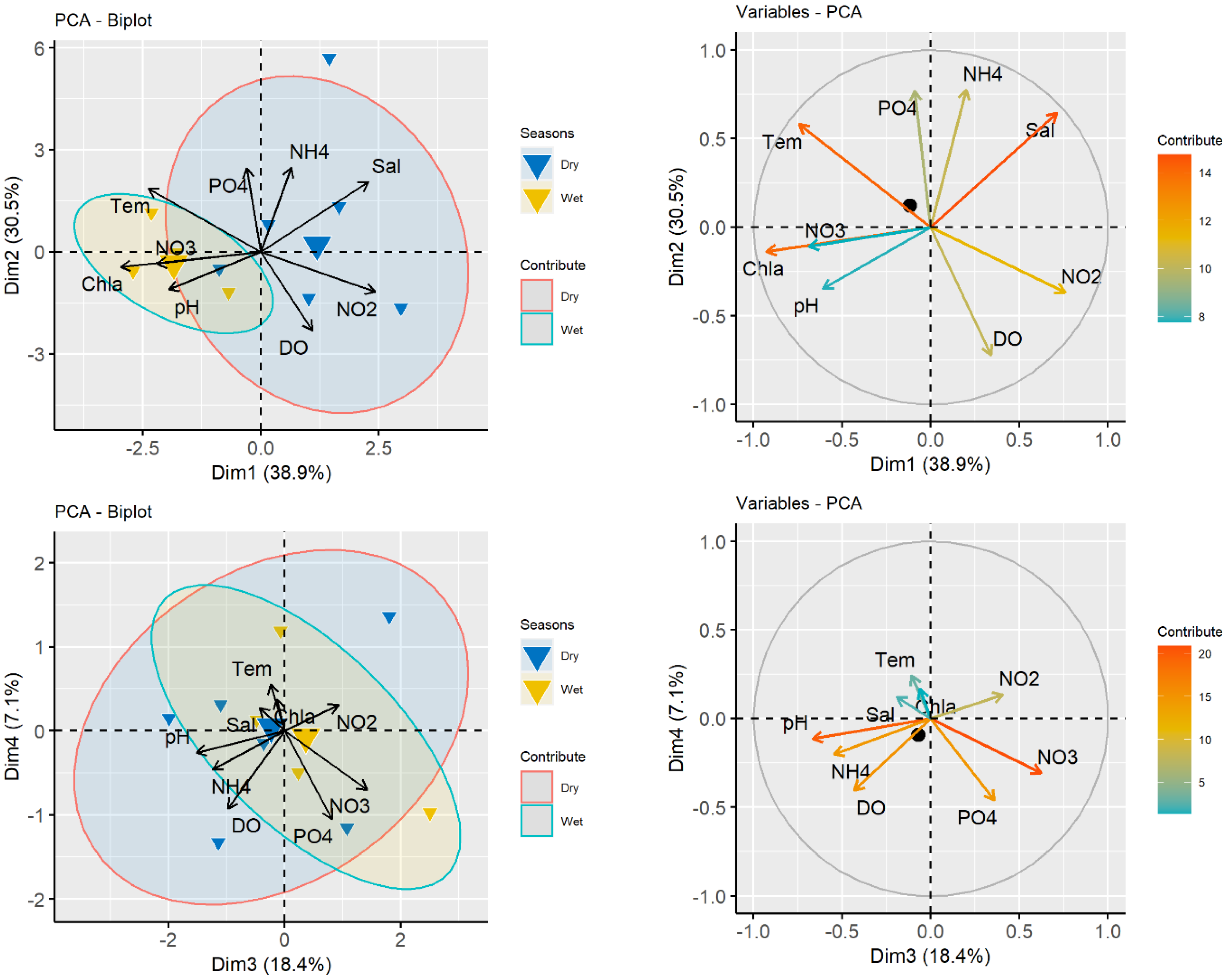
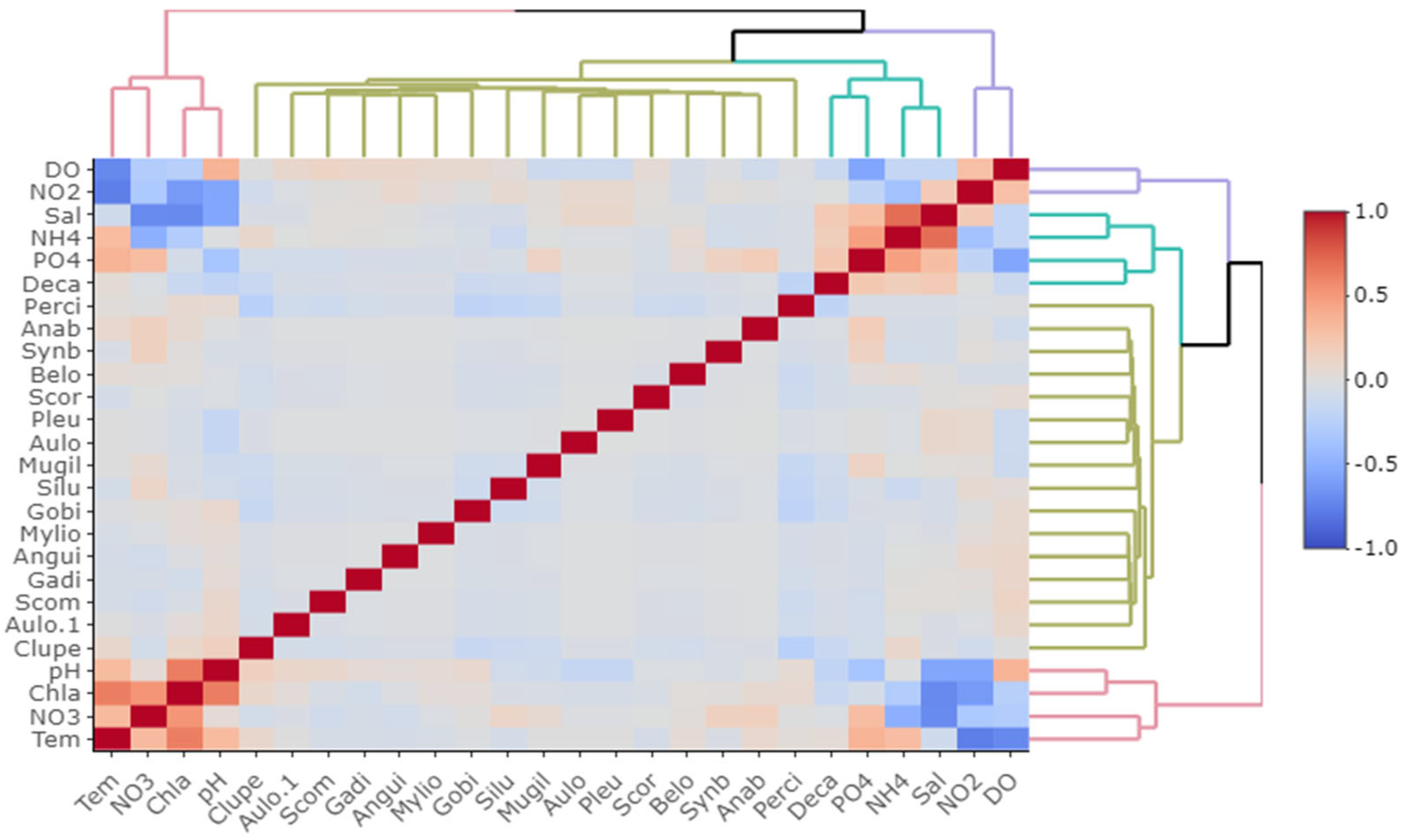
3.1. Water Quality Parameter
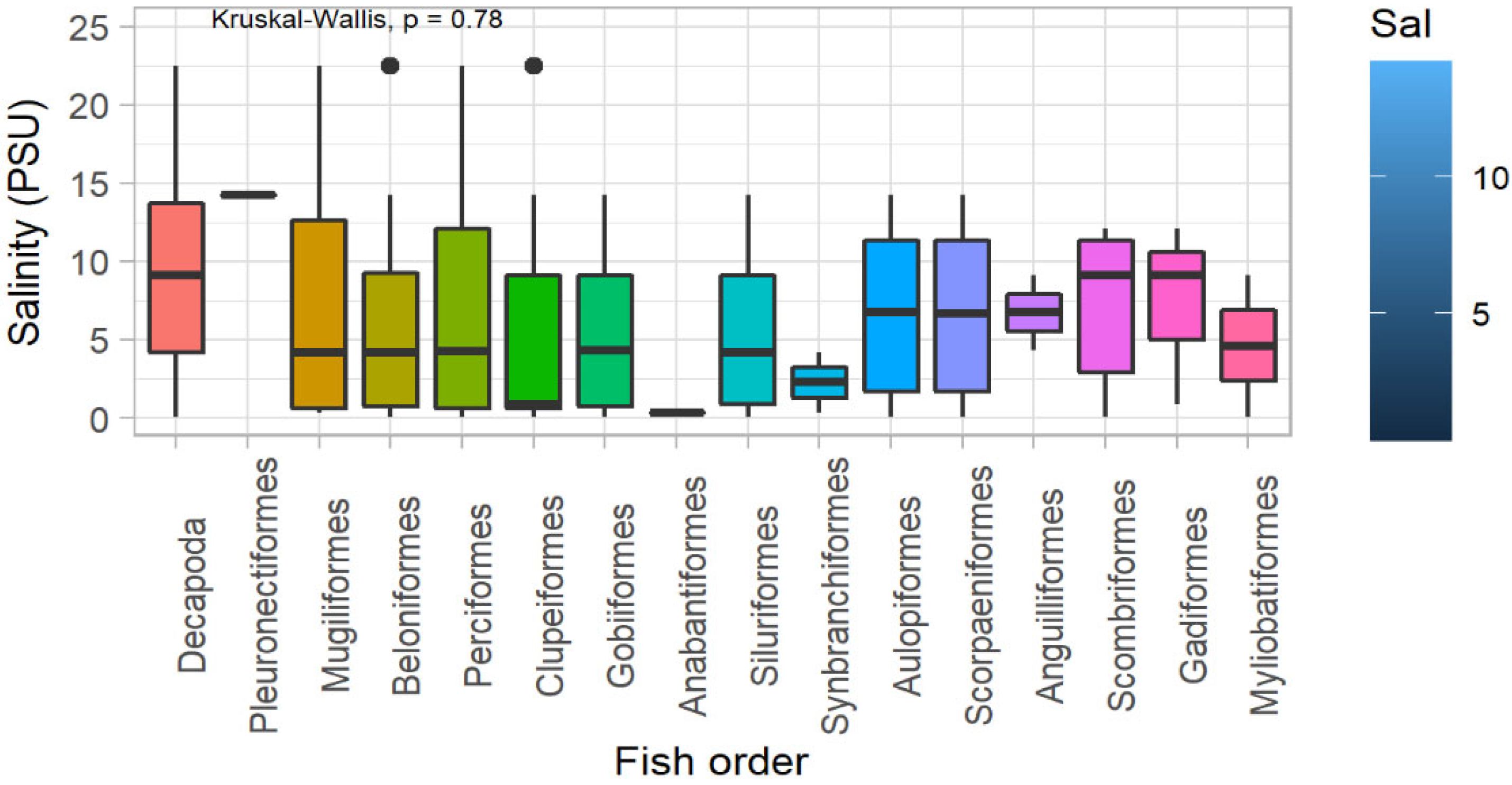
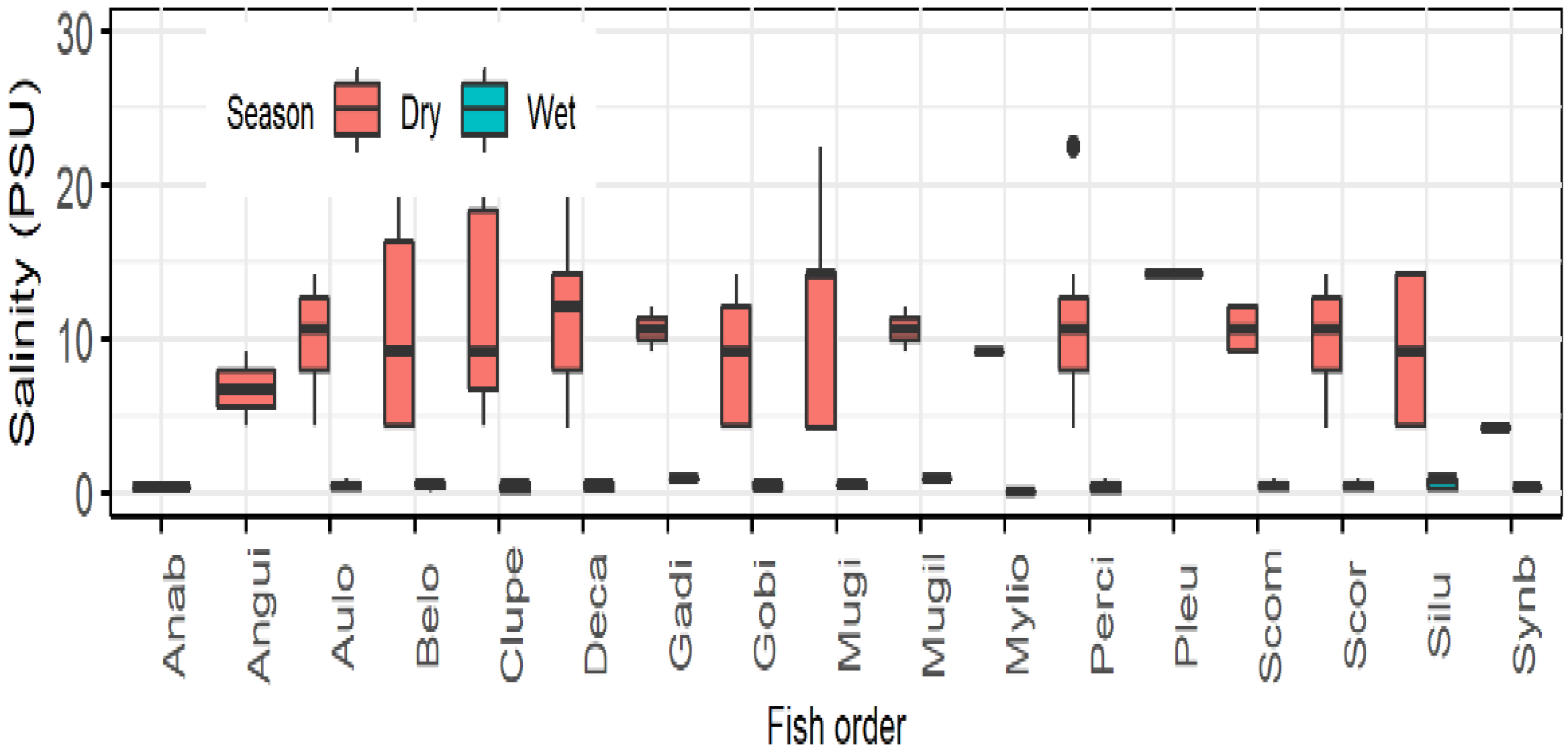
3.2. Fish Diversity and Distribution in Relation to Salinity in the Meghna River Estuary
3.3. Suitability Index for Mariculture of the Commercially Important Fisheries Species
3.4. Fish Species Diversity Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valle-Levinson, A. Contemporary Issues in Estuarine Physics; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Shaha, D.C.; Cho, Y.K. Salt plug formation caused by decreased river discharge in a multi-channel estuary. Sci. Rep. 2016, 6, 27176. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hassan, M.Q.; Islam, M.S.; Shamsad, S.Z.K.M. Environmental impact assessment on water quality deterioration caused by the decreased Ganges outflow and saline water intrusion in south-western Bangladesh. Environ. Geol. 2000, 40, 31–40. [Google Scholar] [CrossRef]
- Akhter, S.; Hasan, M.; Khan, Z.H. Impact of climate change on saltwater intrusion in the coastal area of Bangladesh. In Proceedings of the Eighth International Conference on Coastal and Port Engineering in Developing Countries, Chennai, India, 20–24 February 2012; pp. 20–24. [Google Scholar]
- Montagna, P.; Palmer, P.; Pollack, J. Hydrological Changes and Estuarine Dynamics. Springer Briefs Environ. Sci. 2013, 8, 94. [Google Scholar]
- Dasgupta, S.; Huq, M.; Mustafa, M.G.; Sobhan, M.I.; Wheeler, D. The impact of aquatic salinization on fish habitats and poor communities in a changing climate: Evidence from southwest coastal Bangladesh. Ecol. Econ. 2017, 139, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Venancio, C.; Castro, B.B.; Ribeiro, R.; Antunes, S.C.; Abrantes, N.; Soares, A.M.V.M.; Lopes, I. Sensitivity of freshwater species under single and multigenerational exposure to seawater intrusion. Philos. Trans. R. Soc. B 2019, 374, 20180252. [Google Scholar] [CrossRef] [Green Version]
- Canedo-Arguelles, M.; Hawkins, C.P.; Kefford, B.J.; Schäfer, R.B.; Dyack, B.J.; Brucet, S.; Timpano, A.J. Saving freshwater from salts. Science 2016, 351, 914–916. [Google Scholar] [CrossRef]
- Nicholls, R.J.; Cazenave, A. Sea-level rise and its impact on coastal zones. Science 2010, 328, 1517–1520. [Google Scholar] [CrossRef]
- Farnandes, J.A.; Kay, S.H.; Mostafa, A.R.; Ahmed, M.; Cheung, W.W.L.; Lazar, A.N.; Barange, M. Projecting marine fish production and catch potential in Bangladesh in the 21st century under long-term environmental change and management scenario. ICES J. Mar. Sci. 2015, 73, 1357–1369. [Google Scholar] [CrossRef] [Green Version]
- Rabbi, M.F.; Ahmed, E. Environmental degradation of the southwest region of Bangladesh and need for a barrage on the Ganges. In Proceedings of the International Conference on Large Scale Water Resources Development in Developing Countries: New Dimensions of Prospects and Problems, Kathmandu, Nepal, 20–23 October 1997. [Google Scholar]
- Hossain, M.S.; Das, N.G.; Chowdhury, M.S.N. Fisheries Management of the Naaf River; Chittagong, Coastal and Ocean Research Group of Bangladesh: Chittagong, Bangladesh, 2007; p. 257. [Google Scholar]
- Nabi, M.R.U.; Mamun, M.A.A.; Ullah, M.H.; Mustafa, M.G. Temporal and spatial distribution of fish and shrimp assemblage in the Bakkhali river estuary of Bangladesh in relation to some waterquality parameters. Mar. Biol. Res. 2011, 7, 436452. [Google Scholar]
- Hossain, M.S.; Das, N.G.; Sarker, S.; Rahaman, M.Z. Fish diversity and habitat relationship with environmental variables at Meghna river estuary, Bangladesh. Egypt. J. Aquat. Res. 2012, 38, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.N.; Gnauck, A. Water shortage in the Gorai river basin and damage of mangrove wetland ecosystems in Sundarbans, Bangladesh. In Proceedings of the 3rd International Conference on Water & Food Management (ICWFM-2011), Dhaka, Bangladesh, 8–10 January 2011; pp. 8–10. [Google Scholar]
- Mirza, M.M.Q. The Ganges water diversion: Environmental effects and implications an introduction. J. Hydrol. 2004, 44, 214–223. [Google Scholar]
- Otero, P.; Ruiz-Villarreal, M.; Peliz, A.; Cabanas, J.M. Climatology and reconstruction of runoff time series in northwest Iberia: Influence in the shelf buoyancy budget off Ria de Vigo. Sci. Mar. 2010, 74, 247–266. [Google Scholar] [CrossRef]
- Ahsan, M.E. Coastal Zone of Bangladesh: Fisheries Resources and Its Potentials; Lap LAMBERT Academic Publishing: Chisinau, Republic of Moldova, 2013. [Google Scholar]
- Hanif, M.A.; Siddik, M.A.B.; Chaklader, M.R.; Nahar, A.; Mahmud, S. Fish diversity in the southern coastal waters of Bangladesh: Present status, threats and conservation perspectives. Croat. J. Fish. Ribar. 2015, 73, 148–161. [Google Scholar] [CrossRef]
- Hach Company. Water Analysis Handbook Hach Company, 7th ed.; Hach Company: Loveland, CO, USA, 2012. [Google Scholar]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; p. 173. [Google Scholar]
- Hu, M.; Wang, C.; Liu, Y.; Zhang, X.; Jian, S. Fish species composition, distribution and community structure in the lower reaches of Ganjiang River, Jiangxi, China. Sci. Rep. 2019, 9, 10100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, A.; Das, R.; Vimexen, V. Seasonal phytoplankton succession in Netravathi–Gurupura estuary, Karnataka, India: Study on a three tier hydrographic platform. Estuar. Coast. Shelf Sci. 2020, 242, 106830. [Google Scholar]
- Brown, A.R.; Daniels, C.; Jeffery, K.; Tyler, C.R.; Brown, A.R.; Daniels, C.; Tyler, C.R. Developing general rules to facilitate evidence-based policy for mariculture development in and around Marine Protected Areas (MPAs) in England. In Final Report to Research England (Strategic Priorities Fund); Strategic Priorities Fund: London, UK, 2020. [Google Scholar]
- Pinheiro, J.; Bates, D.; Deb, R.S.; Sarkar, D.; R Core Team. Linear and Nonlinear Mixed Effects Models, R Package Version; Springer: Berlin/Heidelberg, Germany, 2007; Volume 3, pp. 1–89. [Google Scholar]
- Galili, T.; Callaghan, A.O.; Sidi, J.; Sievert, C. Heatmaply: An R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 2018, 34, 1600–1602. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhou, F.; Huang, J.; Ma, Z.; Jiang, S.; Qiu, L.; Qin, J.G. Ammonia and salinity tolerance of P. monodon across eight breeding families. Springer Plus 2016, 5, 171. [Google Scholar] [CrossRef] [Green Version]
- Tuwo, A.; Tresnati, J. Sea Cucumber Farming in Southeast Asia (Malaysia, Philippines, Indonesia, Vietnam). In Echinoderm Aquaculture; John Wiley & Sons, Inc.: New York, NY, USA, 2015; pp. 331–352. [Google Scholar]
- Laing, I. Effect of salinity on growth and survival of king scallop spat (Pecten maximus). Aquaculture 2002, 205, 171–181. [Google Scholar] [CrossRef]
- Morse, D.L.; Cowperthwaite, H.S.; Perry, N.; Britsch, M. Methods and Materials for Aquaculture Production of Sea Scallops (Placopecten magellanicus). National Oceanic and Atmospheric Administration, 2020, 1–12. Available online: https://repository.library.noaa.gov/view/noaa/38586 (accessed on 20 May 2022).
- Ellis, S. Nursery and Grow-Out Techniques for Giant Clams (Bivalvia: Tridacnidae); Center for Tropical and Subtropical Aquaculture: Waimanalo, HI, USA, 2000; p. 99. [Google Scholar]
- Hadley, N.H.; Whetstone, J.M. Hard clam hatchery and nursery production. South. Reg. Aquac. Cent. Publ. 2007, 4301, 1–8. [Google Scholar]
- Narasimham, K.A.; Laxmilatha, P. Clam culture. CMFRI Bull.-Artif. Reefs Seafar. Technol. 1996, 48, 76–87. [Google Scholar]
- Monwar, M.M.; Sarker, A.R.A.; Das, N.G. Polyculture of seabass with tilapia for the utilization of brown fields in the coastal areas of Cox’s Bazar, Bangladesh. Int. J. Fish. Aquac. 2013, 6, 104–109. [Google Scholar]
- Haque, M.A.; Hossain, M.I.; Uddin, S.A.; Dey, P.K. Review on distribution, culture practices, food and feeding, brood development and artificial breeding of Seabass, Lates calcarifer (BLOCH 1790): Bangladesh perspective. Res. Agric. Livest. Fish. 2019, 6, 405–414. [Google Scholar] [CrossRef]
- Ahmed, A.; Akter, N.; Hasan, S.; Ataullah, M. Spatio-temporal variations of water quality and phytoplankton diversity of the different rivers flowing within the Sundarbans mangrove wetland ecosystem of Bangladesh. J. Biodivers. Conserv. Bio-Resour. Manag. 2019, 5, 61–76. [Google Scholar] [CrossRef]
- Hoque, N.; Shakil, A.; Sultana, F.; Wahab, M.; Rahman, M.; Nahidujjaman, M.; Asaduzzaman, M. Feasibility study of green mussel Perna viridis farming in the south-east Bangladesh coast of the Bay of Bengal. J. Indian Soc. Coast. Agric. Res. 2021, 39, 195–205. [Google Scholar] [CrossRef]
- Prema, D. Site selection and water quality in mariculture. In CMFRI Manual Customized Training Book. Karala; Central Marine Fisheries Research Institute: Kochi, India, 2013; pp. 36–39. [Google Scholar]
- Wilson, C.; Scotto, L.; Scarpa, J.; Volety, A.; Laramore, S.; Haunert, D. Survey of water quality, oyster reproduction and oyster health status in the St. Lucie Estuary. J. Shellfish. Res. 2005, 24, 157–165. [Google Scholar]
- Rybovich, M.; La Peyre, M.K.; Hall, S.G.; La Peyre, J.F. Increased temperatures combined with lowered salinities differentially impact oyster size class growth and mortality. J. Shellfish. Res. 2016, 35, 101–113. [Google Scholar] [CrossRef]
- Aypa, S.M. Mussel culture. Food and Agriculture Organization of the United Nations (FAO) 1990, Chapter 4. Available online: http://www.fao.org/3/ab737e/AB737E04.htm (accessed on 11 January 2019).
- Mandal, S.K. A Note on Salinity Tolerance of L. parsia HAM. J. Ichthyol. 1987, 46, 534–544. [Google Scholar]
- Joseph, S. Different species used for coastal pond farming in India. Aquaculture 2015, 30, 178–285. [Google Scholar]
- Siddiqui, A.A.M.; Kashem, M.A.; Mondal, M.A.I.; Shafiuddin, M. Commercially important seaweed cultivation and its potentials for the coastal areas of Cox’s Bazar, Bangladesh. Int. J. Fish Aquac. Study 2019, 7, 463–470. [Google Scholar]
- Shelley, C.; Lovatelli, A. Mud Crab Aquaculture: A Practical Manual. In FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2011; Volume 567, p. I. [Google Scholar]
- Davenport, J.; Wong, T.M. Responses of adult mud crabs (Scylla serrata) (Forskal) to salinity and low oxygen tension. Comp. Biochem. Physiol. Part A Physiol. 1987, 86, 43–47. [Google Scholar] [CrossRef]
- Tran, N.H.; Nguyen, T.P.; Le, Q.V.; Huynh, K.H.; Do, T.T.H. Giant freshwater prawn (M. rosenbergii de Man, 1879) farming in brackish water areas of the Mekong Delta, Vietnam. Can Tho University. J. Sci. 2017, 7, 82–90. [Google Scholar]
- Chand, B.K.; Trivedi, R.K.; Dubey, S.K.; Rout, S.K.; Beg, M.M.; Das, U.K. Effect of salinity on survival and growth of giant freshwater prawn M. rosenbergii (de Man). Aquac. Rep. 2015, 2, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Cardona, L. Effects of salinity on the habitat selection and growth performance of Mediterranean flathead grey mullet M. cephalus (Osteichthyes, Mugilidae). Estuar. Coast. Shelf Sci. 2000, 50, 727–737. [Google Scholar] [CrossRef]
- Karim, E.; Haque, M.A.; Shahabuddin, M.; Rahman, M.J.; Rahman, M.M. Effects of supplementary feeds on growth and survival of stripe mullets (Mugil cephalus) in outdoor cistern ponds of MFTS, Cox’s Bazar. Int. J. Anim. Fish. Sci. 2012, 5, 428–432. [Google Scholar]
- Shailender, M.; Suresh Babu, C.H.; Srikanth, B.; Kishor, B.; Silambarasan, D.; Jayagopal, P. Sustainable culture method of giant black tiger shrimp, Penaeus monodon (Fabricius) in Andhra Pradesh, India. J. Agric. Vet. Sci. 2012, 1, 12–16. [Google Scholar]
- Choudhury, H.A. Polyculture System of Thai pangus at Alpha Fisheries, Trishal, Mymensingh. In Unpublished Master’s Thesis; Bangladesh Agricultural University: Mymensingh, Bangladesh, 2000. [Google Scholar]
- Sayeed, M.A.B.; Hossain, G.S.; Mistry, S.K.; Huq, K.A. Growth performance of Thai pangus (Pangasius hypophthalmus) in polyculture system using different supplementary feeds. Univ. J. Zool. Rajshahi Univ. 2008, 27, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Ruby, M.; Michael, M.G.; Mario, V. Pangasius juveniles tolerate moderate salinity in test. Glob. Aquac. Alliance 2016, 67, 218–223. [Google Scholar]
- Lalramchhani, C.; Balasubramanian, C.P.; Panigrahi, A.; Ghoshal, T.K.; Das, S.; Shyne Anand, P.S.; Vijayan, K.K. Polyculture of Indian white shrimp (P. indicus) with milkfish (Chanos chanos) and its effect on growth performances, water quality and microbial load in brackishwater pond. J. Coast. Res. 2019, 86, 43–48. [Google Scholar] [CrossRef]
- Kumlu, M.; Jones, D.A. Salinity tolerance of hatchery-reared post larvae of P. indicus H. Milne Edwards originating from India. Aquaculture 1995, 130, 287–296. [Google Scholar] [CrossRef]
- Mondal, A.; Chakravartty, D.; Zaman, S. Feeding Ecology with Prey Electivity and Growth Performance of Indigenous Asian Striped Dwarf Catfish, M. vittatus (Bloch, 1794) in Low Saline Earthen Ponds of Indian Sundarbans. Ann. Mar. Sci. 2017, 1, 032–038. [Google Scholar] [CrossRef]
- Arunachalam, S.; Reddy, S.R. Food intake, growth, food conversion, and body composition of catfish exposed to different salinities. Aquaculture 1979, 16, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Ahamed, S.; Mostafiz, M.; Akter, T.; Hassan, M.M.; Islam, M.A.; Islam, M.M. Polyculture of M. gulio (Hamilton 1822) in salinity intrusion prone areas of Bangladesh. Bangladesh J. Fish. 2019, 31, 91–99. [Google Scholar]
- Bhaumik, U.; Sharma, A.P. The fishery of Indian Shad (T. ilisha) in the Bhagirathi-Hooghly river system. Fish. Chimes 2011, 31, 21–27. [Google Scholar]
- Jorgensen, S.E.; Xu, F.L.; Salas, F.; Marques, J.C. Application of indicators for the assessment of ecosystem health. Handb. Ecol. Indic. Assess. Ecosyst. Health 2005, 2, 5–65. [Google Scholar]
- Smith, B.; Wilson, J.B. A consumer’s guide to evenness indices. Oikos 1996, 76, 70–82. [Google Scholar] [CrossRef]
- Li, R.H.; Liu, S.M.; Li, Y.W.; Zhang, G.L.; Ren, J.L.; Zhang, J. Nutrient dynamics in tropical rivers, lagoons, and coastal ecosystems of eastern Hainan Island, South China Sea. Biogeosciences 2014, 11, 481–506. [Google Scholar] [CrossRef] [Green Version]
- Saifullah, A.S.M.; Kamal, A.H.M.; Idris, M.H.; Rajaee, A.H.; Bhuiyan, M.K.A.; Hoque, M.N. Inter-linkage among some physico-chemical and biological factors in the tropical mangrove estuary. Zool. Ecol. 2016, 26, 141–149. [Google Scholar] [CrossRef]
- Sridhar, R.T.; Thangaradjou, S.; Kumar, S.; Kannan, L. Water Quality and the Phytoplankton Characteristics in the Palk Bay, South East Coast of India. J. Environ. Biol. 2006, 27, 561–566. [Google Scholar]
- Rajasekar, K.T.; Rajkumar, M.; Jun, S.; Prabu, A.; Perumal, P. Seasonal Variations of Phytoplankton Diversity in the Coleroon Coastal Waters, Southeast Coast of India. Acta Oceanol. Sin. 2010, 29, 97–108. [Google Scholar]
- Macedo, M.; Duarte, P.; Mendes, P.; Ferreira, J.G. Annual variation of environmental variables, phytoplankton species composition and photosynthetic parameters in a coastal lagoon. J. Plankton Res. 2001, 23, 719–732. [Google Scholar] [CrossRef]
- Yin, K. Monsoonal influence on seasonal variations in nutrients and phytoplankton biomass in coastal waters of Hong Kong in the vicinity of the Pearl River estuary. Mar. Ecol. Prog. Ser. 2002, 245, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Verity, P.G.; Blanton, J.O.; Amft, J.; Barans, C.; Knott, D.; Stender, B.; Wenner, E. Influences of physical oceanographic processes on chlorophyll distributions in coastal and estuarine waters of the South Atlantic Bight. J. Mar. Res. 1998, 56, 681–711. [Google Scholar] [CrossRef]
- Dyer, K. Coastal and Estuarine Sediment Dynamics; John Wiley Sons: Chichester, UK, 1986; p. 358. [Google Scholar]
- Flynn, K.J.; Butler, I. Nitrogen sources for the growth of marine microalgae: Role of dissolved free amino acids. Mar. Ecol. Prog. Ser. 1986, 34, 281–304. [Google Scholar] [CrossRef]
- Boney, A.D. Phytoplanktons; Edward, Arnold Company: London, UK, 1983. [Google Scholar]
- Guo, Q.; Liu, X.; Ao, X.; Qin, J.; Wu, X.; Ouyang, S. Fish diversity in the middle and lower reaches of the Ganjiang River of China: Threats and conservation. PLoS ONE 2018, 13, e0205116. [Google Scholar] [CrossRef] [Green Version]
- Matthews, W.J.; Hill, L.G. Influence of physico-chemical factors on habitat selection by red shiners, Notropis lutrensis (Pisces: Cyprinidae). Copeia 1979, 1, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Siddique, M.A.B.; Hussain, M.A.; Flowra, F.A.; Alam, M.M. Assessment of fish fauna in relation to biodiversity indices of Chalan Beel, Bangladesh. Int. J. Aquat. Biol. 2016, 4, 345–352. [Google Scholar]

| Parameter | Dry Season | Wet Season | p Value |
|---|---|---|---|
| Temperature °C | 24.67 ± 5.34 | 28.39 ± 1.24 | p < 0.05 |
| Salinity (psu) | 10.59 ± 6.37 | 0.46 ± 0.12 | p < 0.05 |
| DO (mg/L) | 7.94 ± 1.23 | 7.32 ± 0.26 | p < 0.05 |
| pH | 7.87 ± 0.23 | 7.98 ± 0.17 | p > 0.05 |
| Chlorophyll-a (µg/L) | 3.81 ± 1.08 | 7.57 ± 3.27 | p < 0.05 |
| Nitrate (mg/L) | 0.03 ± 0.002 | 0.14 ± 0.001 | p < 0.05 |
| Nitrite (mg/L) | 0.01 ± 0.001 | 0.01 ± 0.003 | p > 0.05 |
| Ammonia (mg/L) | 0.52 ± 0.20 | 0.20 ± 0.04 | p < 0.05 |
| DIN (mg/L) | 0.55 ± 0.23 | 0.35 ± 0.05 | p < 0.05 |
| DIP (mg/L) | 0.43 ± 0.05 | 0.36 ± 1.24 | p > 0.05 |
| SL No | Scientific Name | Local Name | Salinity (psu) of Observed Months and Seasons | Salinity (psu) for Individual Species | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| February 21 | March 21 | June 21 | September 21 | November 21 | Dry | Wet | ||||
| 0.35–14.2 | 0.6–18.07 | 0.06–9.8 | 0.06–0.11 | 0.14–3.2 | 0.35~18.1 | 0.06~3.2 | ||||
| Trichiuridae Family | ||||||||||
| 01 | Trichiurus lepturus | Churi | + | + | + | + | + | 0.06~18.1 | ||
| Anguillidae Family | ||||||||||
| 02 | A. japonica | Kuchia | + | + | + | 0.35~14.2 | ||||
| Ariidae Family | ||||||||||
| 03 | A. gagora | Gagra tengra | + | + | 0.35~14.2 | |||||
| Palaemonidae Family | ||||||||||
| 04 | Macrobrachium dolichodactylus | Goda chingri | + | + | + | + | 0.35~18.1 | |||
| 05 | M. rosenbergii | Golda chingri | + | + | 0.6~18.1 | |||||
| 06 | Macrobrachium nobilii | Lal icha | + | + | + | + | 0.06~9.8 | |||
| Bagridae Family | ||||||||||
| 07 | M. gulio | Gulia | + | + | + | + | + | 0.06~14.2 | ||
| Clupeidae Family | ||||||||||
| 08 | Tenualosa ilisha | Ilish | + | + | + | + | + | 0.06~14.2 | ||
| 09 | Escualosa thoracata | Boccha | + | + | + | 0.06~14.2 | ||||
| Belonidae Family | ||||||||||
| 10 | Xenentodon cancila | Kakila | + | + | + | + | + | 0.06~9.8 | ||
| Schilbeidae Family | ||||||||||
| 11 | Silonia silondia | Shillong | + | + | + | 0.06~3.2 | ||||
| Lactariidae Family | ||||||||||
| 12 | Lactarius lactarius | Parava | + | + | + | 0.06~9.8 | ||||
| Platycephalidae Family | ||||||||||
| 13 | Platycephalus indicus | Chota bele | + | + | + | + | + | + | 0.06~18.1 | |
| Penaeidae Family | ||||||||||
| 14 | Trachysalambria curvirostris | Kharkharia chingri | + | + | + | + | + | 0.06~18.1 | ||
| 15 | Metapenaeus spinulatus | Lalia/chama chingri | + | + | + | + | + | 0.06~18.1 | ||
| 16 | P. monodon | Bagda | + | + | + | + | + | 0.06~18.1 | ||
| Synodontidae Family | ||||||||||
| 17 | H. nehereus | Loitta | + | + | + | + | + | + | + | 0.06~18.1 |
| Sciaenidae Family | ||||||||||
| 18 | Otolithoides pama | Bhola/Poa | + | + | + | + | + | 0.06~18.1 | ||
| Polynemidae Family | ||||||||||
| 19 | Polynemus paradiseus | Ricksha | + | + | + | 0.35~18.1 | ||||
| 20 | Eleutheronema tetradactylum | Tarail | + | + | + | + | + | 0.06~18.1 | ||
| Mugilidae Family | ||||||||||
| 21 | M. cephalus | Bata | + | + | + | + | + | 0.14~18.1 | ||
| Latidae Family | ||||||||||
| 22 | L. calcarifer | Coral | + | + | + | + | + | 0.06~18.1 | ||
| Sparidae Family | ||||||||||
| 23 | Acanthopagrus latus | Datina/Java bhola | + | + | 0.06~0.11 | |||||
| Scombridae Family | ||||||||||
| 24 | Katsuwonus pelamis | Rupsha | + | + | + | + | + | 0.14~18.1 | ||
| 25 | Scomberomorus guttatus | Surmai | + | + | 0.6~18.1 | |||||
| Sillaginidae Family | ||||||||||
| 26 | Sillaginopsis panijus | Tular dandi | + | + | + | + | + | + | 0.06~18.1 | |
| Gadidae Family | ||||||||||
| 27 | Gadus morhua | Tara fish | + | + | + | + | + | 0.14~18.1 | ||
| Engraulidae Family | ||||||||||
| 28 | S. phasa | Phasa | + | + | 0.06~0.11 | |||||
| 29 | Colia ramacarati | Oula | + | + | + | + | + | 0.06~9.8 | ||
| Plotosidae Family | ||||||||||
| 30 | Pethia canius | Kain magur | + | + | 0.14~3.2 | |||||
| Pangasiidae Family | ||||||||||
| 31 | Pangasianodon hypophthalmus | Samudrik pangas | + | + | + | + | 0.06~9.8 | |||
| Sactophagidae Family | ||||||||||
| 32 | Scatophagus argus | Chitra | + | + | + | + | + | 0.06~18.1 | ||
| Gobiidae Family | ||||||||||
| 33 | Glossogobius giuris | Bailla/Bele | + | + | + | 0.06~3.2 | ||||
| Oxudercidae Family | ||||||||||
| 34 | O. rubicundus | Lal chewa | + | + | + | + | + | + | + | 0.06~18.1 |
| 35 | Apocryptes bato | Chiring | + | + | + | + | 0.06~18.1 | |||
| 36 | P. elongatus | Chewa/chemu | + | + | + | + | + | + | + | 0.06~18.1 |
| Stromateidae Family | ||||||||||
| 37 | Pampus argenteus | Foli chanda | + | + | + | + | 0.06~14.2 | |||
| Dasyatidae Family | ||||||||||
| 38 | Brevitrygon walga | Shapla pata | + | + | + | + | 0.06~14.2 | |||
| Order | No. of Family | No. of Species | % Family | % Species | |||||
|---|---|---|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet |
| Anguilliformes | Aulopiformes | 1 | 1 | 1 | 1 | 4 | 4 | 3 | 3 |
| Aulopiformes | Beloniformes | 1 | 1 | 1 | 1 | 4 | 4 | 3 | 3 |
| Beloniformes | Clupeiformes | 1 | 2 | 1 | 3 | 4 | 7 | 3 | 10 |
| Clupeiformes | Decapoda | 2 | 2 | 3 | 4 | 9 | 7 | 9 | 14 |
| Decapoda | Gadiformes | 2 | 1 | 7 | 1 | 9 | 4 | 20 | 3 |
| Gadiformes | Gobiiformes | 1 | 1 | 1 | 2 | 4 | 4 | 3 | 7 |
| Gobiiformes | Mugiliformes | 1 | 1 | 3 | 1 | 4 | 4 | 9 | 3 |
| Mugiliformes | Myliobatiformes | 1 | 1 | 1 | 1 | 4 | 4 | 3 | 3 |
| Myliobatiformes | Perciformes | 1 | 8 | 1 | 8 | 4 | 31 | 3 | 27 |
| Perciformes | Scombriformes | 7 | 2 | 8 | 2 | 29 | 8 | 23 | 7 |
| Scombriformes | Scorpaeniformes | 2 | 1 | 3 | 1 | 8 | 4 | 9 | 3 |
| Scorpaeniformes | Siluriformes | 1 | 4 | 1 | 4 | 4 | 15 | 3 | 14 |
| Siluriformes | 3 | 3 | 13 | 9 | |||||
| Total = 13 | 11 | 26 | 24 | 33 | 29 | 100 | 100 | 100 | 100 |
| Season | Temp. (°C) | Salinity (psu) | pH | DO (mg/L) | Ammonia (mg/L) | Nitrate (mg/L) | Nitrite (mg/L) | Inorganic Phosphorus (mg/L) | Suitability of Species for Mariculture | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry | 19.68–29.89 | 0.12–18.07 | 7.30–9.09 | 6.72–9.99 | 0.01–2.84 | 0.01–0.18 | 0.00–0.09 | 0.02–2.08 | Value | S * /NS ** | This study value | |
| Wet | 26.24–31.16 | 0.06–3.16 | 7.10–8.79 | 6.64–7.94 | 0.09–0.37 | 0.10–0.23 | 0.00–0.01 | 0.09–1.77 | ||||
| Sea cucumber | 10–30 | 28–37 | 4.6–8.6 | >6 | 0.4–0.7 | <0.01 | [28] [29] | |||||
| Dry | S * | NS ** | S * | S * | NS ** | NS ** | 0.42 | NS ** | ||||
| Wet | S * | NS ** | NS ** | S * | NS ** | NS ** | 0.33 | NS ** | ||||
| Scallop | 10–18 | 23–35 | 7.5–8.2 | >4.5 | 0.01–0.02 | [30] [31] | ||||||
| Dry | NS ** | NS ** | S * | S * | NS ** | 0.40 | NS ** | |||||
| Wet | NS ** | NS ** | S * | S * | NS ** | 0.40 | NS ** | |||||
| Clam | 18–26 | 20–30 | 9 | 4.5–6.5 | [32] [33] [34] | |||||||
| Dry | S * | S * | S * | S * | 1.00 | S * | ||||||
| Wet | NS ** | NS ** | NS ** | S * | 0.25 | NS ** | ||||||
| L. calcarifer (Coral/Sea bass) | 15–40 | 10–30 | 7.5–8.5 | 4–9 | <1 | <0.02 | [35] [36] | |||||
| Dry | S * | S * | S * | S * | S * | S * | 1.00 | S * | ||||
| Wet | S * | NS ** | NS ** | S * | S * | NS ** | 0.42 | NS ** | ||||
| Mussel | 26–32 | 27–35 | 7.9–8.2 | >8 | <0.4 | [37] [38] [39] | ||||||
| Dry | S * | NS ** | S * | NS ** | NS ** | 0.40 | NS ** | |||||
| Wet | S * | NS ** | NS ** | S * | NS ** | 0.40 | NS ** | |||||
| Oyster | 17–33 | 10–28 | 6.3 | 2–5 | <1.2 | [40] [41] [42] | ||||||
| Dry | S * | S * | NS ** | NS ** | S * | 0.60 | S * | |||||
| Wet | S * | NS ** | NS ** | NS ** | S * | 0.40 | NS ** | |||||
| L. parsia (Grey mullet) | 3–35 | 0–38 | 6.5–9 | >4 | 0–0.5 | 0.1–4.5 | 0.03–0.26 | >0.06 | [43] [44] | |||
| Dry | S * | S * | S * | S * | S * | S * | S * | S * | 1.00 | S * | ||
| Wet | S * | S * | S * | S * | S * | S * | NS ** | S * | 0.87 | S * | ||
| Sea weed | 24–32 | 27–35 | 7.5–9.13 | 4–8.5 | 0.03–0.05 | [45] [39] | ||||||
| Dry | NS ** | NS ** | NS ** | S * | S * | 0.40 | NS ** | |||||
| Wet | S * | NS ** | S * | NS ** | NS ** | 0.40 | NS ** | |||||
| S. serrata (Mud crab) | 21–35 | 0–30 | 5–9 | 4–9 | 0.31–0.57 | 0–0.007 | [46] [47] [39] | |||||
| Dry | S * | S * | S * | S * | NS ** | S * | 0.83 | S * | ||||
| Wet | S * | S * | S * | S * | NS ** | S * | 0.83 | S * | ||||
| M. rosenbergii | 25–29 | 0–15 | 7–8.5 | 4.4–7.1 | [48] [49] [39] | |||||||
| Dry | S * | NS ** | NS ** | S * | 0.40 | NS ** | ||||||
| Wet | S * | S * | S * | S * | 1.00 | S * | ||||||
| M. cephalus | 26–29.3 | <30 | 7.8–8.2 | 5.7–6.1 | [50] [51] | |||||||
| Dry | S * | S * | S * | NS ** | 0.75 | S * | ||||||
| Wet | S * | NS ** | S * | NS ** | 0.40 | NS ** | ||||||
| P. monodon | 26–30.8 | 10–55 | 7–8.9 | 4.5–7.2 | 0.1–0.3 | 0.09–0.2 | 0.01–0.05 | 0.31 | [28] [52] [39] | |||
| Dry | S * | S * | S * | S * | S * | S * | S * | S * | 1.00 | S * | ||
| Wet | S * | NS ** | S * | S * | NS ** | S * | NS ** | NS ** | 0.44 | NS ** | ||
| P. hypophthalmus | 20–35 | <20 | 6.7–8.6 | 5–8 | [53] [54] [55] | |||||||
| Dry | NS ** | NS ** | S * | NS ** | 0.25 | NS ** | ||||||
| Wet | S * | S * | NS ** | S * | 0.75 | S * | ||||||
| P. indicus | 30.3–31.6 | 10.25–30 | 7.8–7.9 | 5.6–5.9 | [56] [57] [39] | |||||||
| Dry | S * | S * | S * | NS ** | 0.75 | S * | ||||||
| Wet | S * | NS ** | S * | NS ** | 0.40 | NS ** | ||||||
| M. vittatus | 26.1–28 | <10 | 7.1–8.2 | 5.9–6.5 | [58] [59] | |||||||
| Dry | S * | NS ** | S * | NS ** | 0.40 | NS ** | ||||||
| Wet | S * | S * | S * | NS ** | 0.75 | S * | ||||||
| M. gulio | 25.8–29 | 1.73–3 | 7.1–7.6 | 4.9–5.6 | 0.34–0.36 | 0.05–0.08 | 0.02–0.05 | 0.42 | [60] | |||
| Dry | S * | NS ** | S * | NS ** | S * | S * | NS ** | NS ** | 0.45 | NS ** | ||
| Wet | S * | S * | S * | NS ** | S * | NS ** | NS ** | S * | 0.73 | S * | ||
| T. ilisha | 29.3–30.8 | <0.1 | 7.7–8.4 | 4.8–6.8 | [61] | |||||||
| Dry | S * | NS ** | S * | NS ** | 0.40 | NS ** | ||||||
| Wet | NS ** | S * | S * | S * | 0.75 | S * | ||||||
| Average | Dry Season | Wet Season | Standard | References | |
|---|---|---|---|---|---|
| Total number of species (S) | 38 | 33 | 29 | ||
| Total number of individuals (N) | 102 | 62 | 40 | ||
| Shannon-Weaver diversity index (H′) | 0.37 | 0.34 | 0.25 | 0–5 | [62] |
| Pielou’s species evenness (J’) | 0.11 | 0.09 | 0.07 | 0–1 | [63] |
| Margalef species richness (d) | 8.00 | 7.90 | 7.30 | >5 | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaha, D.C.; Ahmed, S.; Hasan, J.; Kundu, S.R.; Haque, F.; Rahman, M.J.; Nahiduzzaman, M.; Wahab, M.A. Fish Diversity in Relation to Salinity Gradient in the Meghna River Estuary, Bangladesh. Conservation 2022, 2, 414-434. https://doi.org/10.3390/conservation2030028
Shaha DC, Ahmed S, Hasan J, Kundu SR, Haque F, Rahman MJ, Nahiduzzaman M, Wahab MA. Fish Diversity in Relation to Salinity Gradient in the Meghna River Estuary, Bangladesh. Conservation. 2022; 2(3):414-434. https://doi.org/10.3390/conservation2030028
Chicago/Turabian StyleShaha, Dinesh Chandra, Salman Ahmed, Jahid Hasan, Sampa Rani Kundu, Farhana Haque, Mohammad Jalilur Rahman, Md. Nahiduzzaman, and Md. Abdul Wahab. 2022. "Fish Diversity in Relation to Salinity Gradient in the Meghna River Estuary, Bangladesh" Conservation 2, no. 3: 414-434. https://doi.org/10.3390/conservation2030028
APA StyleShaha, D. C., Ahmed, S., Hasan, J., Kundu, S. R., Haque, F., Rahman, M. J., Nahiduzzaman, M., & Wahab, M. A. (2022). Fish Diversity in Relation to Salinity Gradient in the Meghna River Estuary, Bangladesh. Conservation, 2(3), 414-434. https://doi.org/10.3390/conservation2030028







