Unravelling the Potentials of Managing Metabolic Diabetes and Related Oxidative Stresses with Extracts from Five South African Hypoxis Species
Abstract
1. Introduction
2. Results
2.1. Phytochemical Screening
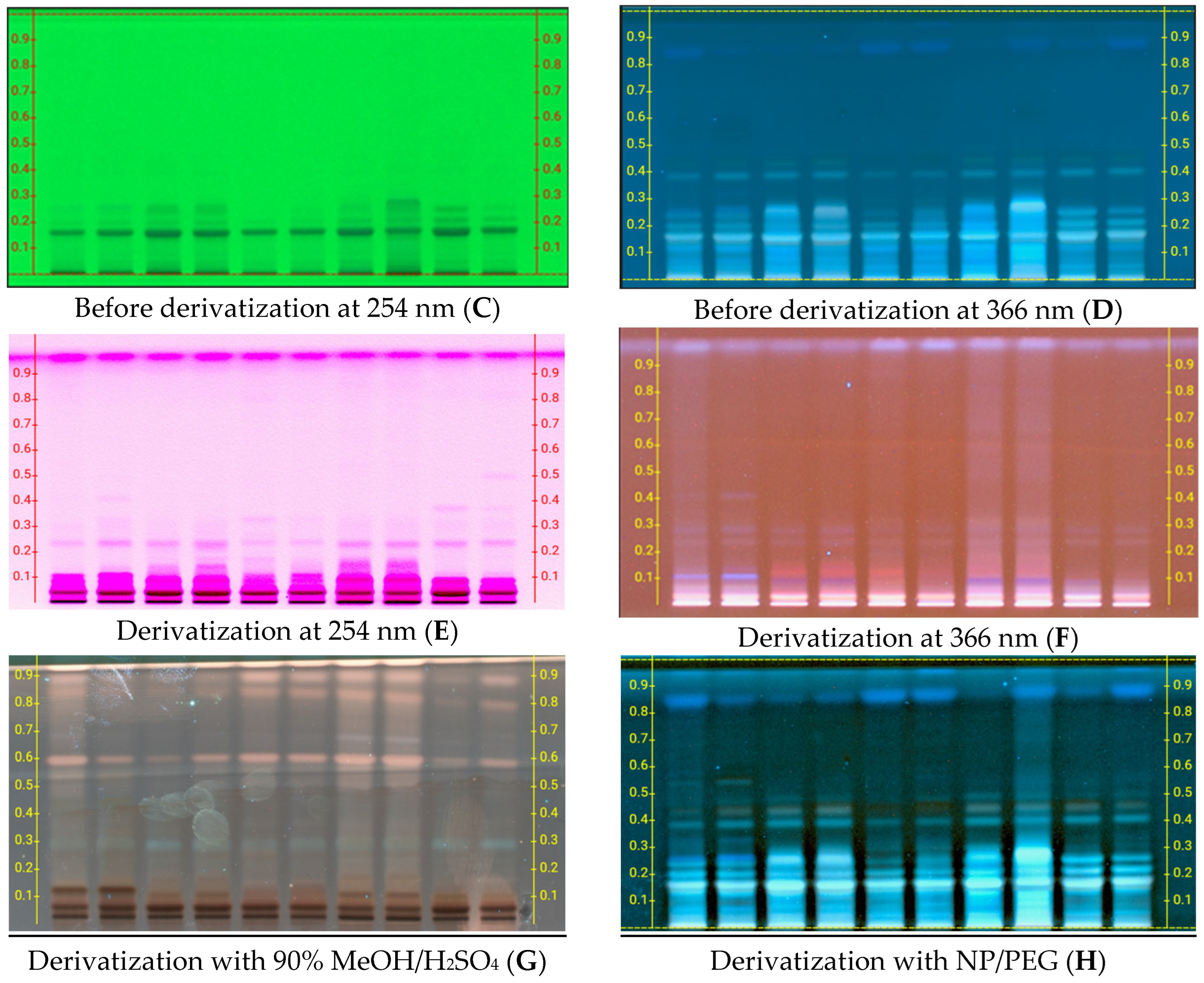
2.2. High-Performance Thin-Layer Chromatography (HPTLC) Analysis
2.3. Quantitative Antioxidant Potential by DPPH Assay of the Five Hypoxis Species
2.3.1. The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.3.2. Hydrogen Hydroxide (H2O2) Free Radical Scavenging Assay
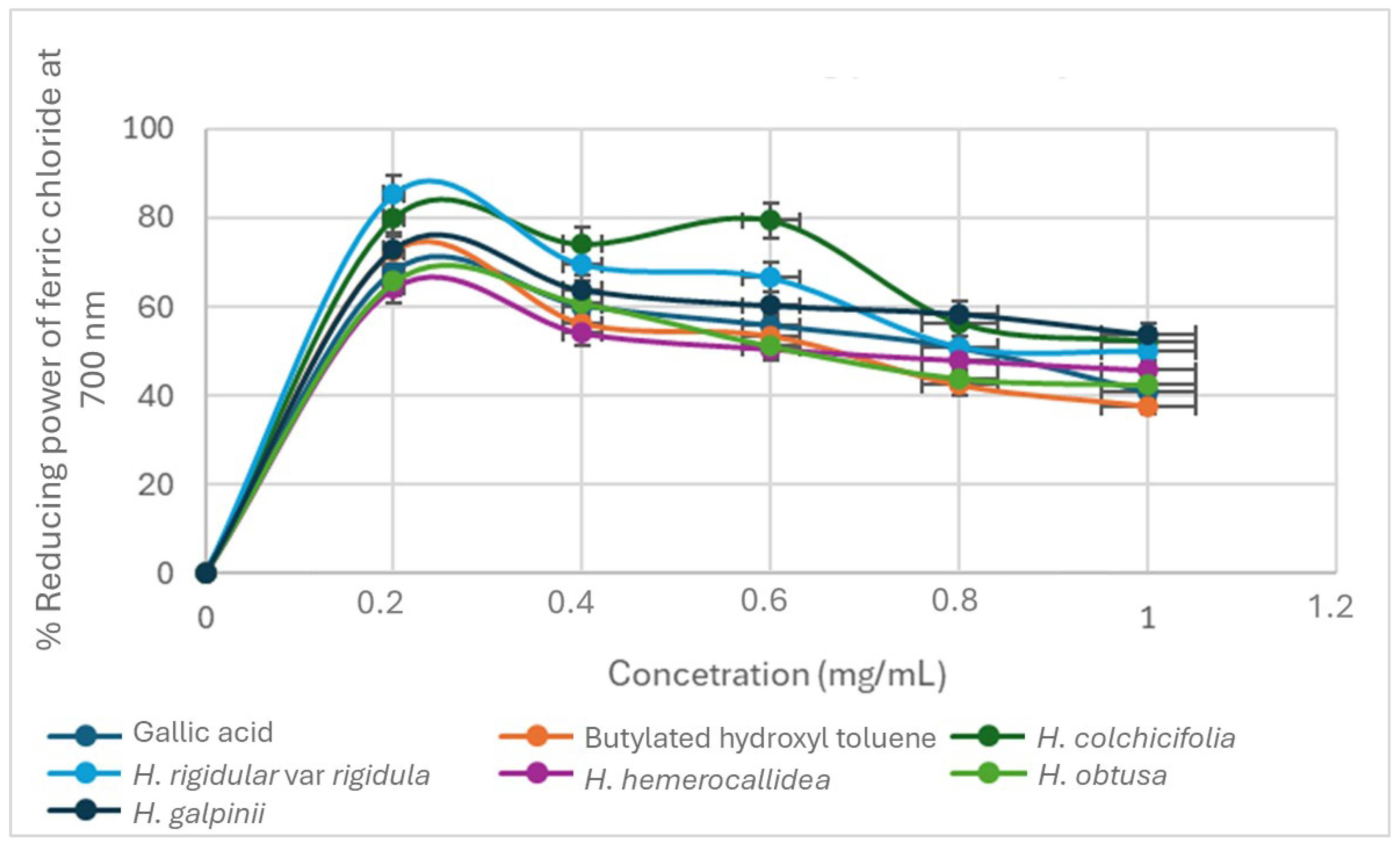
2.3.3. Ferric Reducing Antioxidant Assay
2.4. Total Phenolic Content (TPC) Determination of the Five Hypoxis Species
2.5. Determination of the Antidiabetic Potentials of the Five Hypoxis Species
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Collection, Comminution, and Extraction
4.3. Phytochemical Analysis
4.3.1. Alkaloids
4.3.2. Tannins
4.3.3. Phlobatannins
4.3.4. Terpenoids
4.3.5. Deoxy Sugar of Cardenolides
4.3.6. Saponins
4.4. High-Performance Thin-Layer Chromatography (HPTLC) Analysis
4.5. Determination of Antioxidant Potential of the Five Hypoxis Species
4.5.1. DPPH Radical Scavenging Activity
4.5.2. Hydrogen Peroxide Free Radical Scavenging Activity
4.5.3. Ferric Chloride Reducing Power Method
4.6. The Determination of Total Phenolic Content
4.7. Determination of the Antidiabetic Activity
4.7.1. α-Amylase Inhibition Assay
4.7.2. β-Glucosidase Inhibition Assay
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants, and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Engwa, G.A.; Nweke, F.N.; Nkeh-Chungag, B.N. Free radicals, oxidative stress-related diseases and antioxidant supplementation. Altern. Ther. Health Med. 2022, 28, 114. [Google Scholar] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef]
- Preiser, J.C. Oxidative stress. J. Parenter. Enter. Nutr. 2012, 36, 147–154. [Google Scholar] [CrossRef]
- Sharma, V.; Mehdi, M.M. Oxidative stress, inflammation and hormesis: The role of dietary and lifestyle modifications on aging. Neurochem. Int. 2023, 164, 105490. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. BioMed Res. Int. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Vaseghi, G.; Pourfarzam, M.; Abdollahi, A. Are antioxidants helpful for disease prevention? Res. Pharm. Sci. 2010, 5, 1. [Google Scholar] [PubMed]
- Viana da Silva, M.; Santos, M.R.C.; Alves Silva, I.R.; Macedo Viana, E.B.; Dos Anjos, D.A.; Santos, I.A.; Barbosa de Lima, N.G.; Wobeto, C.; Jorge, N.; Lannes, S.C.D.S. Synthetic and natural antioxidants used in the oxidative stability of edible oils: An overview. Food Rev. Int. 2022, 38, 349–372. [Google Scholar] [CrossRef]
- Ayza, M.A.; Zewdie, K.A.; Yigzaw, E.F.; Ayele, S.G.; Tesfaye, B.A.; Tafere, G.G.; Abrha, M.G. Potential protective effects of antioxidants against cyclophosphamide-induced nephrotoxicity. Int. J. Nephrol. 2022, 2022, e5096825. [Google Scholar] [CrossRef] [PubMed]
- Caleja, C.; Barros, L.; Antonio, A.L.; Carocho, M.; Oliveira, M.B.P.; Ferreira, I.C. Fortification of yogurts with different antioxidant preservatives: A comparative study between natural and synthetic additives. Food Chem. 2016, 210, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kumar, P.; Shukla, K.S.; Maheshwari, S. AGE RAGE Pathways: Cardiovascular disease and oxidative stress. Drug Res. 2023, 73, 408–411. [Google Scholar] [CrossRef]
- Uzombah, T.A. The implications of replacing synthetic antioxidants with natural ones in the food systems. In Natural Food Additives; IntechOpen: London, UK, 2022. [Google Scholar]
- Thakur, M.; Singh, K.; Khedkar, R. Phytochemicals: Extraction process, safety assessment, toxicological evaluations, and regulatory issues. In Functional and Preservative Properties of Phytochemicals; Academic Press: Cambridge, MA, USA, 2020; pp. 341–361. [Google Scholar]
- Singh, Y. Systematics of Hypoxis (Hypoxidaceae) in Southern Africa. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2006. [Google Scholar]
- Bassey, K.; Viljoen, A.; Combrinck, S.; Choi, Y.H. New phytochemicals from the corms of medicinally important South African Hypoxis species. Phytochem. Lett. 2014, 10, lxix–lxxv. [Google Scholar] [CrossRef]
- Oguntibeju, O.O.; Meyer, S.; Aboua, Y.G.; Goboza, M. Hypoxis hemerocallidea Significantly Reduced Hyperglycaemia and Hyperglycaemic-Induced Oxidative Stress in the Liver and Kidney Tissues of Streptozotocin-Induced Diabetic Male Wistar Rats. Evid. Based Complement. Altern. Med. 2016, 2016, 8934362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pereus, D.; Otieno, J.; Ghorbani, A.; Kocyan, A.; Hilonga, S.; de Boer, H. Diversity of Hypoxis species used in ethnomedicine in Tanzania. S. Afr. J. Bot. 2019, 122, 336–341. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Zimudzi, C. African Potato (Hypoxis Spp.): Diversity and Comparison of the Phytochemical Profiles and Cytotoxicity Evaluation of four Zimbabwean Species. J. Appl. Pharm. Sci. 2014, 4, 79–83. [Google Scholar] [CrossRef]
- Kumari, M.; Jain, S. Tannins: An Antinutrient with Positive Effect to Manage Diabetes. Res. J. Recent Sci. 2012, 2277, 2502. [Google Scholar]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- McLoone, P.; Oladejo, T.O.; Kassym, L.; McDougall, G.J. Honey Phytochemicals: Bioactive Agents with Therapeutic Potential for Dermatological Disorders. Phytother. Res. 2024, 38, 5741–5764. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.D.; Dairam, A.; Agbonon, A.; Arnason, J.T.; Foster, B.C.; Kanfer, I. Investigation of the antioxidant activity of African potato (Hypoxis hemerocallidea). J. Agri. Food Chem. 2007, 55, 1707–1711. [Google Scholar] [CrossRef]
- Kumar, V.; Okem, A.; Moyo, M.; Gruz, J.; Doležal, K.; Van Staden, J. Effect of zinc on the production of phenolic acids and hypoxoside in micropropagated Hypoxis hemerocallidea. Plant Growth Regul. 2019, 89, 19–24. [Google Scholar] [CrossRef]
- Poovitha, S.; Parani, M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complement. Altern. Med. 2016, 16, 185. [Google Scholar] [CrossRef]
- Chokki, M.; Cudălbeanu, M.; Zongo, C.; Dah-Nouvlessounon, D.; Ghinea, I.O.; Furdui, B.; Raclea, R.; Savadogo, A.; Baba-Moussa, L.; Avamescu, S.M.; et al. Exploring Antioxidant and Enzymes (A-Amylase and B-Glucosidase) Inhibitory Activity of Morinda lucida and Momordica charantia Leaves from Benin. Foods 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verma, N.; Behera, B.C.; Sharma, B.O. Glucosidase inhibitory radical scavenging properties of lichen metabolites salazinic acid sekikaic acid usnic acid Hacettepe. J. Biol. Chem. 2012, 40, 7–21. [Google Scholar]
- Mahlo, S.J.; More, G.K.; Oladipo, A.O.; Lebelo, S.L. In vitro α-amylase/α-glucosidase, cytotoxicity and radical scavenging potential of Hypoxis hemerocallidea synthesized magnesium oxide nanoparticles. Discov. Appl. Sci. 2024, 6, 62. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative stress and antioxidants—A critical review on in vitro antioxidant assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- de Torre, M.P.; Cavero, R.Y.; Calvo, M.I.; Vizmanos, J.L. A simple and a reliable method to quantify antioxidant activity in vivo. Antioxidants 2019, 8, 142. [Google Scholar] [CrossRef]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed. Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harborne, J.B. Phenolic compounds. In Phytochemical Methods; Springer: Dordrecht, The Netherlands, 1973; pp. 33–88. [Google Scholar]
- Evans, W.C. Trease and Evans Pharmacognosy, 15th ed.; Sanders Co., Ltd.: Singapore, 2002. [Google Scholar]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Salkowski, E. Ueber cholesterin. Z. Phys. Chem. 1871, 1, 1–8. [Google Scholar]
- DagerAlbalawi, M.A. Chemistry spectroscopic characteristics biological activity of natural occurring cardiac glycosides. IOSR J. Biotechnol. Biochem. 2016, 2, 20–35. [Google Scholar]
- Mapfumari, S.; Matseke, B.; Bassey, K. Isolation of a Marker Olean-12-en-28-butanol Derivative from Viscum continuum E. Mey. Ex Sprague and the Evaluation of Its Antioxidant and Antimicrobial Potentials. Plants 2024, 13, 1382. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]





| Phytochemical Tests | Observation for Different Extracts | ||||
|---|---|---|---|---|---|
| H. colchifolia | H. galpinii | H. rigidular var. rigidula | H. hemerocallidea | H. obtusa | |
| Alkaloids | − | − | − | − | − |
| Flavonoids | + | + | + | + | + |
| Tannins | − | − | − | − | − |
| Phlobatannins | − | − | − | − | − |
| Saponins | + | + | + | + | + |
| Terpenoids | + | + | + | + | + |
| Deoxy sugar | + | + | + | + | + |
| IC50 (mg/mL) | ||||
|---|---|---|---|---|
| Hypoxis Species | Regression Equation | DPPH | H2O2 | FRAP |
| H. rigidular var. rigidula | y = 70.344x + 41.294 y = 63.502x + 47.305 y = 20.652x + 43.39 | 0.124 | 0.0424 | 0.320 |
| H. obtusa | y = 68.866x + 46.353 y = 60.489x + 46.881 y = 19.647x + 34.164 | 0.053 | 0.052 | 0.806 |
| H. colchicifolia | y = 66.23x + 34.71 y = 65.496x + 47.228 y = 2797x + 43.003 | 0.230 | 0.0423 | 0.2500 |
| H. hemerocallidea | y = 68.838x + 45.862 y = 57.806x + 47.49 y = 25.214x + 31.167 | 0.060 | 0.043 | 0.745 |
| H. galpinii | y = 63.686x + 39.92 y = 64.085x + 47.271 y = 31.671x + 35.72 | 0.158 | 0.043 | 0.451 |
| Gallic acid | y = 66.23x + 34.715 y = 70.26x + 47.358 y = 21.091x + 35.31 | 0.087 | 0.037 | 0.695 |
| BHT | y = 74.866x + 40.384 y = 70.271x + 47.498 y = 13.598x + 36.859 | 0.128 | 0.035 | 0.966 |
| H. Species | H. rigidula var. reigidula | H. obtusa | H. colchicifolia | H. hemerocallidea | H. galpinii | Gallic Acid | BHT |
|---|---|---|---|---|---|---|---|
| TPC (mg (GAE/g) | 188.52 ± 2.6 | 335.61 ± 1.8 | 12.201 ± 0.13 | 251.34 ± 0.27 | 27.43 ± 0.14 | - | - |
| %DPPH | 89.6 ± 3.27 | 97.17 ± 0.21 | 74.06 ± 4.55 | 96.34 ± 0.42 | 83.06 ± 1.79 | 97.78 ± 3.95 | 87.85 ± 4.55 |
| H2O2 | 97.08 ± 2.36 | 98.42 ± 3.12 | 96.58 ± 1.78 | 97.95 ± 4.25 | 97.85 ± 2.16 | 67.92 ± 0.03 | 72.41 ± 0.02 |
| FRAP | 85.28 ± 0.01 | 65.64 ± 0.01 | 79.90 ± 0.03 | 64.23 ± 0.08 | 73.01 ± 0.02 | 67.92 ± 0.01 | 72.41 ± 0.02 |
| Antioxidant IC50 | 0.083 ± 0.057 | 0.052 ± 0.001 | 0.1366 ± 0.133 | 0.065 ± 0.031 | 0.100 ± 0.081 | 0.097 ± 0.085 | 0.082 ± 0.066 |
| H. Species | H. rigidular var. rigidula | H. obtusa | H. colchifolia | H. hemerocallidea | H. galpinii | Acarbose |
|---|---|---|---|---|---|---|
| α-amylase IC50 (mg/mL) | 0.346 | 0.395 | 0.375 | 0.404 | 0.370 | 0.209 |
| β-glucosidase IC50 (mg/mL) | 0.553 | 0.210 | 0.384 | 0.241 | 0.425 | 0.209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matseke, B.; Poka, M.; Demana, P.; Bassey, K. Unravelling the Potentials of Managing Metabolic Diabetes and Related Oxidative Stresses with Extracts from Five South African Hypoxis Species. Stresses 2025, 5, 53. https://doi.org/10.3390/stresses5030053
Matseke B, Poka M, Demana P, Bassey K. Unravelling the Potentials of Managing Metabolic Diabetes and Related Oxidative Stresses with Extracts from Five South African Hypoxis Species. Stresses. 2025; 5(3):53. https://doi.org/10.3390/stresses5030053
Chicago/Turabian StyleMatseke, Buang, Madan Poka, Patrick Demana, and Kokoette Bassey. 2025. "Unravelling the Potentials of Managing Metabolic Diabetes and Related Oxidative Stresses with Extracts from Five South African Hypoxis Species" Stresses 5, no. 3: 53. https://doi.org/10.3390/stresses5030053
APA StyleMatseke, B., Poka, M., Demana, P., & Bassey, K. (2025). Unravelling the Potentials of Managing Metabolic Diabetes and Related Oxidative Stresses with Extracts from Five South African Hypoxis Species. Stresses, 5(3), 53. https://doi.org/10.3390/stresses5030053








