Bacterial Biofilm Development and Its Relationship with Catheter-Associated Urinary Tract Infection
Abstract
1. Introduction
2. Urinary Catheter
3. Formation of Biofilm
3.1. Steps of Biofilm Formation
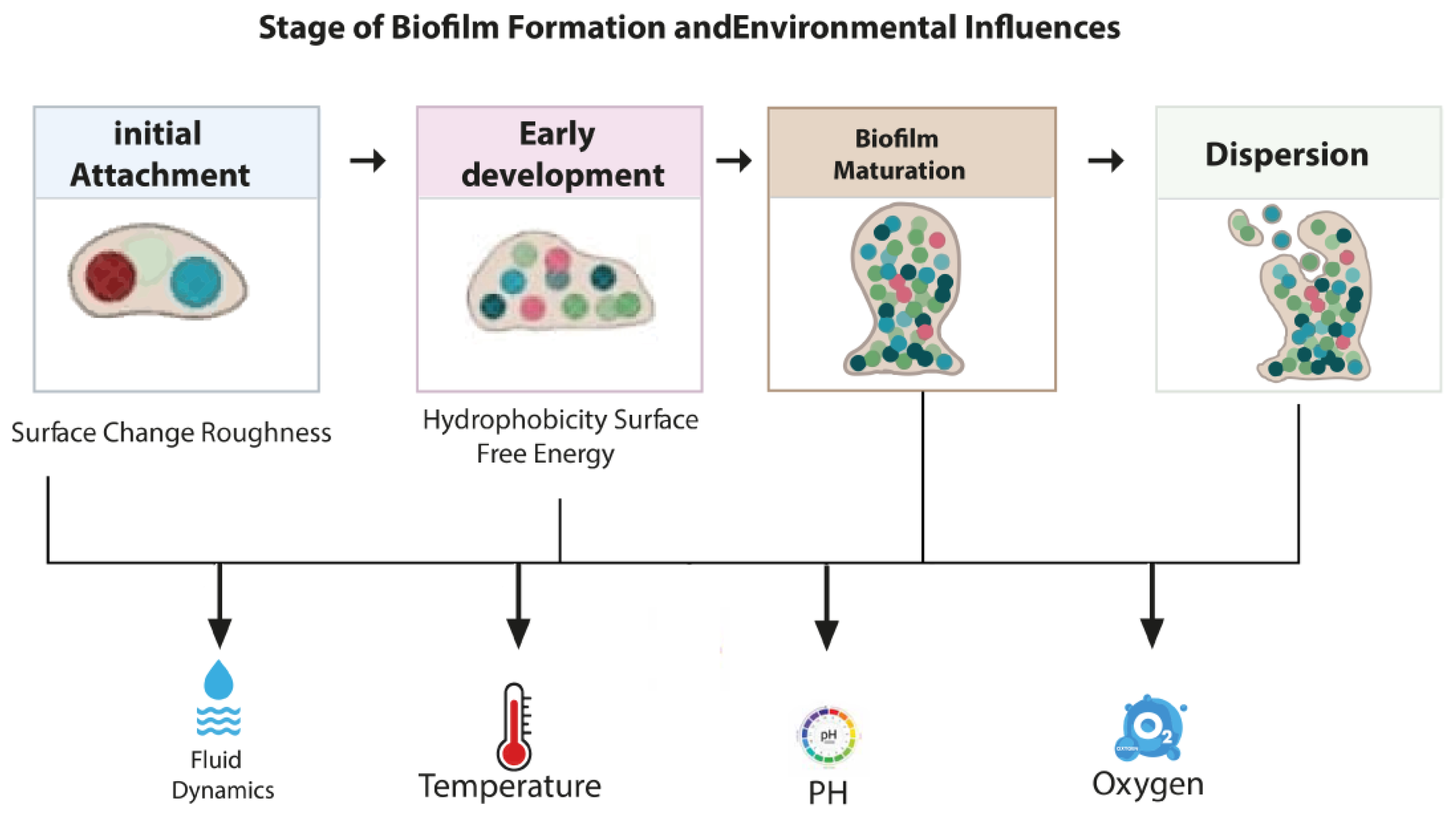
3.2. Environmental Factors Affecting Biofilm Formation
3.3. Biofilm Architecture (Structure)
3.4. Components in the Biofilm Matrix
3.5. Role of Quorum Sensing in Biofilm Formation
3.6. Diseases Related to Biofilm Formation
4. Mechanisms of Catheter-Associated Urinary Tract Infection
5. Biofilm Formation in Urinary Catheters and Its Pathogenesis
6. Catheter Insertion Techniques and Their Role in Reducing Biofilm Formation
6.1. Aseptic Technique
6.2. Catheter Design
7. Bacterial Biofilm Mechanism of Antibiotic Resistance
7.1. Reduced Antibiotic Penetration
7.2. Layered Metabolic Activity in Biofilms
7.3. Quorum Sensing and Population Density
7.4. Enhanced Horizontal Gene Transfer
7.5. Persister Cells
7.6. Adaptive Gene Expression
7.7. Efflux Pumps
8. Factors Affecting the Biofilm Development of Catheter-Associated Urinary Tract Infections
8.1. Gender and Age
8.2. Catheter Types
8.3. Duration of Catheterization
9. Treatments and Challenges in the Future
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilks, S.A.; Koerfer, V.V.; Prieto, J.A.; Fader, M.; Keevil, C.W. Biofilm Development on Urinary Catheters Promotes the Appearance of Viable but Nonculturable Bacteria. MBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Goda, R.M.; El-Baz, A.M.; Khalaf, E.M.; Alharbi, N.K.; Elkhooly, T.A.; Shohayeb, M.M. Combating Bacterial Biofilm Formation in Urinary Catheter by Green Silver Nanoparticle. Antibiotics 2022, 11, 495. [Google Scholar] [CrossRef]
- Oumer, Y.; Regasa Dadi, B.; Seid, M.; Biresaw, G.; Manilal, A. Catheter-Associated Urinary Tract Infection: Incidence, Associated Factors and Drug Resistance Patterns of Bacterial Isolates in Southern Ethiopia. Infect. Drug Resist. 2021, 14, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Vivehananthan, K.; Thevashayinath, S.; Abeygunawardena, I. Extraction and Screening of Biofilm Producing Bacterial Isolated in Short and Long-Term Catheters. Adv. Technol. 2021, 1, 329–345. [Google Scholar] [CrossRef]
- Verma, A.; Bhani, D.; Tomar, V.; Bachhiwal, R.; Yadav, S. Differences in Bacterial Colonization and Biofilm Formation Property of Uropathogens between the Two Most Commonly Used Indwelling Urinary Catheters. J. Clin. Diagn. Res. JCDR 2016, 10, PC01. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Al-Wrafy, F.A.; Al-Gheethi, A.A.; Ponnusamy, S.K.; Noman, E.A.; Fattah, S.A. Nanoparticles Approach to Eradicate Bacterial Biofilm-Related Infections: A Critical Review. Chemosphere 2022, 288, 132603. [Google Scholar] [CrossRef]
- Flemming, H.C. EPS—Then and Now. Microorganisms 2016, 4, 41. [Google Scholar] [CrossRef]
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three Faces of Biofilms: A Microbial Lifestyle, a Nascent Multicellular Organism, and an Incubator for Diversity. npj Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef]
- Ramírez-Larrota, J.S.; Eckhard, U. An Introduction to Bacterial Biofilms and Their Proteases, and Their Roles in Host Infection and Immune Evasion. Biomolecules 2022, 12, 306. [Google Scholar] [CrossRef]
- Werneburg, G.T.; Nguyen, A.; Henderson, N.S.; Rackley, R.R.; Shoskes, D.A.; Le Sueur, A.L.; Corcoran, A.T.; Katz, A.E.; Kim, J.; Rohan, A.J. The Natural History and Composition of Urinary Catheter Biofilms: Early Uropathogen Colonization with Intraluminal and Distal Predominance. J. Urol. 2020, 203, 357–364. [Google Scholar] [CrossRef]
- El-sayed, H.; Fahmy, Y. Correlation between Biofilm Formation and Multidrug Resistance in Clinical Isolates of Pseudomonas Aeruginosa. Microbes Infect. Dis. 2021, 2, 541–549. [Google Scholar] [CrossRef]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative Study of the Antimicrobial Activity of Selenium Nanoparticles with Different Surface Chemistry and Structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by Bacterial Human Pathogens: Clinical Relevance-Development, Composition and Regulation-Therapeutical Strategies. Microb. Cell 2021, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Awoke, N.; Kassa, T.; Teshager, L. Magnitude of Biofilm Formation and Antimicrobial Resistance Pattern of Bacteria Isolated from Urinary Catheterized Inpatients of Jimma University Medical Center, Southwest Ethiopia. Int. J. Microbiol. 2019, 2019, 5729568. [Google Scholar] [CrossRef] [PubMed]
- Nouri, S.; Sharif, M.R.; Hosseinpour, M.; Farokhi, S.; Sharif, M.H. A Comparison between Foley and Nelatone Urinary Catheters in Causing Urinary Tract Infection in Animal Models. Nurs. Midwifery Stud. 2015, 4, e24363. [Google Scholar] [CrossRef] [PubMed]
- Gayani, B.; Dilhari, A.; Kottegoda, N.; Ratnaweera, D.R.; Weerasekera, M.M. Reduced Crystalline Biofilm Formation on Superhydrophobic Silicone Urinary Catheter Materials. ACS Omega 2021, 6, 11488–11496. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A Review of the Recent Advances in Antimicrobial Coatings for Urinary Catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef]
- Pelling, H.; Nzakizwanayo, J.; Milo, S.; Denham, E.L.; MacFarlane, W.M.; Bock, L.J.; Sutton, J.M.; Jones, B.V. Bacterial Biofilm Formation on Indwelling Urethral Catheters. Lett. Appl. Microbiol. 2019, 68, 277–293. [Google Scholar] [CrossRef]
- Dias, L.D.; Duarte, L.S.; Naves, P.L.F.; Napolitano, H.B.; Bagnato, V.S. Self-Disinfecting Urethral Catheter to Overcome Urinary Infections: From Antimicrobial Photodynamic Action to Antibacterial Biochemical Entities. Microorganisms 2022, 10, 2484. [Google Scholar] [CrossRef]
- Murphy, C. Innovating Urinary Catheter Design: An Introduction to the Engineering Challenge. Proc. Inst. Mech. Eng. Part H. J. Eng. Med. 2019, 233, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.-R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic Hydrogels Implanted in Mice Resist the Foreign-Body Reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef]
- Bryers, J.D. Medical Biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Berhe, N.; Tefera, Y.; Tintagu, T. Review on Biofilm Formation and Its Control Options. Int. J. Adv. Res. Biol. Sci. 2017, 8, 122–133. [Google Scholar]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T. Biofilms: Formation, Drug Resistance and Alternatives to Conventional Approaches. AIMS Microbiol. 2022, 8, 239. [Google Scholar] [CrossRef]
- Dewasthale, S.; Mani, I.; Vasdev, K. Microbial Biofilm: Current Challenges in Health Care Industry. J. Appl. Biotechnol. Bioeng. 2018, 5, 160–164. [Google Scholar] [CrossRef][Green Version]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Cortese, Y.J.; Wagner, V.E.; Tierney, M.; Devine, D.; Fogarty, A. Review of Catheter-associated Urinary Tract Infections and in Vitro Urinary Tract Models. J. Healthc. Eng. 2018, 2018, 2986742. [Google Scholar] [CrossRef]
- Karaguler, T.; Kahraman, H.; Tuter, M. Analyzing Effects of ELF Electromagnetic Fields on Removing Bacterial Biofilm. Biocybern. Biomed. Eng. 2017, 37, 336–340. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Gao, F.; Li, J.; Zheng, L.; Sun, C.; He, C.; Wang, Z.; Qu, L. Marine Microplastic-Associated Bacterial Community Succession in Response to Geography, Exposure Time, and Plastic Type in China’s Coastal Seawaters. Mar. Pollut. Bull. 2019, 145, 278–286. [Google Scholar] [CrossRef]
- Azeem, K.; Fatima, S.; Ali, A.; Ubaid, A.; Husain, F.M.; Abid, M. Biochemistry of Bacterial Biofilm: Insights into Antibiotic Resistance Mechanisms and Therapeutic Intervention. Life 2025, 15, 49. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614, Erratum in Microorganisms 2024, 12, 1961. [Google Scholar] [CrossRef]
- Silva, V.; Pereira, J.E.; Maltez, L.; Poeta, P.; Igrejas, G. Influence of Environmental Factors on Biofilm Formation of Staphylococci Isolated from Wastewater and Surface Water. Pathogens 2022, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review on the Recent Use of Phage-Based Strategies with Other Antibiofilm Agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory Mechanisms and Promising Applications of Quorum Sensing-Inhibiting Agents in Control of Bacterial Biofilm Formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef]
- Sahreen, S.; Mukhtar, H.; Imre, K.; Morar, A.; Herman, V.; Sharif, S. Exploring the Function of Quorum Sensing Regulated Biofilms in Biological Wastewater Treatment: A Review. Int. J. Mol. Sci. 2022, 23, 9751. [Google Scholar] [CrossRef]
- Jabir, D.M. Evaluation of the Ability of Some Bacterial Species Isolated from UTI to Form Biofilm. Al-Qadisiyah J. Pure Sci. 2022, 27, 8–14. [Google Scholar] [CrossRef]
- Murugan, K.; Selvanayaki, K.; Al-Sohaibani, S. Urinary Catheter Indwelling Clinical Pathogen Biofilm Formation, Exopolysaccharide Characterization and Their Growth Influencing Parameters. Saudi J. Biol. Sci. 2016, 23, 150–159. [Google Scholar] [CrossRef]
- Patel, F.M.; Goswami, P.N.; Khara, R. Detection of Biofilm Formation in Device Associated Clinical Bacterial Isolates in Cancer Patients. Sri Lankan J. Infect. Dis. 2016, 6, 43–50. [Google Scholar] [CrossRef]
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Rep. Urol. 2022, 14, 109–133. [Google Scholar] [CrossRef] [PubMed]
- Mota, É.C.; Oliveira, A.C. Catheter-Associated Urinary Tract Infection: Why Do Not We Control This Adverse Event? Rev. Da Esc. Enferm. Da USP 2019, 53, e03452. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.A.; Ghnawate, N. Detection of Biofilm Forming Bacterial Communities from Urinary Catheter of Patients with Change in Its Antibiotic Susceptibility Pattern and Triclosan Effect from Different Hospitals of Amravati City Maharashtra, India. Open J. Med. Microbiol. 2017, 7, 51–66. [Google Scholar] [CrossRef][Green Version]
- Freeman, D.J.; Falkiner, F.R.; Keane, C.T. New Method for Detecting Slime Production by Coagulase Negative Staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef]
- Sultan, A.M.; Nabiel, Y. Tube Method and Congo Red Agar versus Tissue Culture Plate Method for Detection of Biofilm Production by Uropathogens Isolated from Midstream Urine: Which One Could Be Better? Afr. J. Clin. Exp. Microbiol. 2019, 20, 60–66. [Google Scholar] [CrossRef]
- Basnet, A.; Tamang, B.; Shrestha, M.R.; Shrestha, L.B.; Rai, J.R.; Maharjan, R.; Dahal, S.; Shrestha, P.; Rai, S.K. Assessment of Four in Vitro Phenotypic Biofilm Detection Methods in Relation to Antimicrobial Resistance in Aerobic Clinical Bacterial Isolates. PLoS ONE 2023, 18, e0294646. [Google Scholar] [CrossRef]
- Mori, S.; Yamada, A.; Kawai, K. Evaluation of the Biofilm Detection Capacity of the Congo Red Agar Method for Bovine Mastitis-Causing Bacteria. Jpn. J. Vet. Res. 2024, 71, 109–116. [Google Scholar]
- Navarro, S.; Sherman, E.; Colmer-Hamood, J.A.; Nelius, T.; Myntti, M.; Hamood, A.N. Urinary Catheters Coated with a Novel Biofilm Preventative Agent Inhibit Biofilm Development by Diverse Bacterial Uropathogens. Antibiotics 2022, 11, 1514. [Google Scholar] [CrossRef]
- Gunardi, W.D.; Karuniawati, A.; Umbas, R.; Bardosono, S.; Lydia, A.; Soebandrio, A.; Safari, D. Biofilm-producing Bacteria and Risk Factors (Gender and Duration of Catheterization) Characterized as Catheter-associated Biofilm Formation. Int. J. Microbiol. 2021, 2021, 8869275. [Google Scholar] [CrossRef]
- Ramadan, R.; Omar, N.; Dawaba, M.; Moemen, D. Bacterial Biofilm Dependent Catheter Associated Urinary Tract Infections: Characterization, Antibiotic Resistance Pattern and Risk Factors. Egypt. J. Basic. Appl. Sci. 2021, 8, 64–74. [Google Scholar] [CrossRef]
- Zarmouh, H.M.; Karayem, K.J.; Swieb, S.M.; Alhadad, O.S.; Rhouma, N.R. Biofilm Formation of Pathogenic Bacterial Species Isolated From Urinary Catheters. J. Med. Sci. 2021, 16, 16–19. [Google Scholar]
- Marschall, J.; Mermel, L.A.; Fakih, M.; Hadaway, L.; Kallen, A.; O’Grady, N.P.; Pettis, A.M.; Rupp, M.E.; Sandora, T.; Maragakis, L.L. Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Infect. Control Hosp. Epidemiol. 2014, 35, S89–S107. [Google Scholar] [CrossRef]
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Health Care-Associated Infections–an Overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Price-Whelan, A.; Dietrich, L.E.P. Gradients and Consequences of Heterogeneity in Biofilms. Nat. Rev. Microbiol. 2022, 20, 593–607. [Google Scholar] [CrossRef] [PubMed]
- AlQurashi, D.M.; AlQurashi, T.F.; Alam, R.I.; Shaikh, S.; Tarkistani, M.A.M. Advanced Nanoparticles in Combating Antibiotic Resistance: Current Innovations and Future Directions. J. Nanotheranostics 2025, 6, 9. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef]
- Shan, Y.; Brown Gandt, A.; Rowe, S.E.; Deisinger, J.P.; Conlon, B.P.; Lewis, K. ATP-Dependent Persister Formation in Escherichia coli. MBio 2017, 8, 10–1128. [Google Scholar] [CrossRef]
- Niu, H.; Gu, J.; Zhang, Y. Bacterial Persisters: Molecular Mechanisms and Therapeutic Development. Signal Transduct. Target. Ther. 2024, 9, 174. [Google Scholar] [CrossRef]
- Hajiagha, M.N.; Kafil, H.S. Efflux Pumps and Microbial Biofilm Formation. Infect. Genet. Evol. 2023, 112, 105459. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of Bacterial Efflux Pumps in Antibiotic Resistance, Virulence, and Strategies to Discover Novel Efflux Pump Inhibitors. Microbiology 2023, 169, 1333. [Google Scholar] [CrossRef]
- Anggi, A.; Wijaya, D.W.; Ramayani, O.R. Risk Factors for Catheter-Associated Urinary Tract Infection and Uropathogen Bacterial Profile in the Intensive Care Unit in Hospitals in Medan, Indonesia. Open Access Maced. J. Med. Sci. 2019, 7, 3488. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liang, X.; Vorstius, J.; Keatch, R.; Corner, G.; Nabi, G.; Davidson, F.; Gadd, G.M.; Zhao, Q. Enhanced Antibacterial and Antiadhesive Activities of Silver-PTFE Nanocomposite Coating for Urinary Catheters. ACS Biomater. Sci. Eng. 2019, 5, 2804–2814. [Google Scholar] [CrossRef]
- Qindeel, M.; Barani, M.; Rahdar, A.; Arshad, R.; Cucchiarini, M. Nanomaterials for the Diagnosis and Treatment of Urinary Tract Infections. Nanomaterials 2021, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
| Biofilm Bacteria | Source of Sample | References |
|---|---|---|
| Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, Citrobacter Proteus, Staphylococcus aureus | urine samples | [3,39,48] |
| E. coli, E. faecalis, Klebsiella pneumoniae and P. aeruginosa | Catheter and Urine samples | [49] |
| K. pneumoniae, E. coli, S. aureus, S. epidermidis, E. faecalis, Streptococcus spp., S. epidermidis, P. aeruginosa, and Acinetobacter | Catheter | [43,50,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaml, N.L.; Hafez, R.M.; Khalil, M.S.; Moussa, T.A.A. Bacterial Biofilm Development and Its Relationship with Catheter-Associated Urinary Tract Infection. Stresses 2025, 5, 58. https://doi.org/10.3390/stresses5030058
Jaml NL, Hafez RM, Khalil MS, Moussa TAA. Bacterial Biofilm Development and Its Relationship with Catheter-Associated Urinary Tract Infection. Stresses. 2025; 5(3):58. https://doi.org/10.3390/stresses5030058
Chicago/Turabian StyleJaml, Nousiba L., Rehab M. Hafez, Mary S. Khalil, and Tarek A. A. Moussa. 2025. "Bacterial Biofilm Development and Its Relationship with Catheter-Associated Urinary Tract Infection" Stresses 5, no. 3: 58. https://doi.org/10.3390/stresses5030058
APA StyleJaml, N. L., Hafez, R. M., Khalil, M. S., & Moussa, T. A. A. (2025). Bacterial Biofilm Development and Its Relationship with Catheter-Associated Urinary Tract Infection. Stresses, 5(3), 58. https://doi.org/10.3390/stresses5030058






