Biochemical and Perceptual Markers of Physiological Stress During Acute Exercise Overload in U20 Elite Basketball Players
Abstract
1. Introduction
2. Results
2.1. Participants
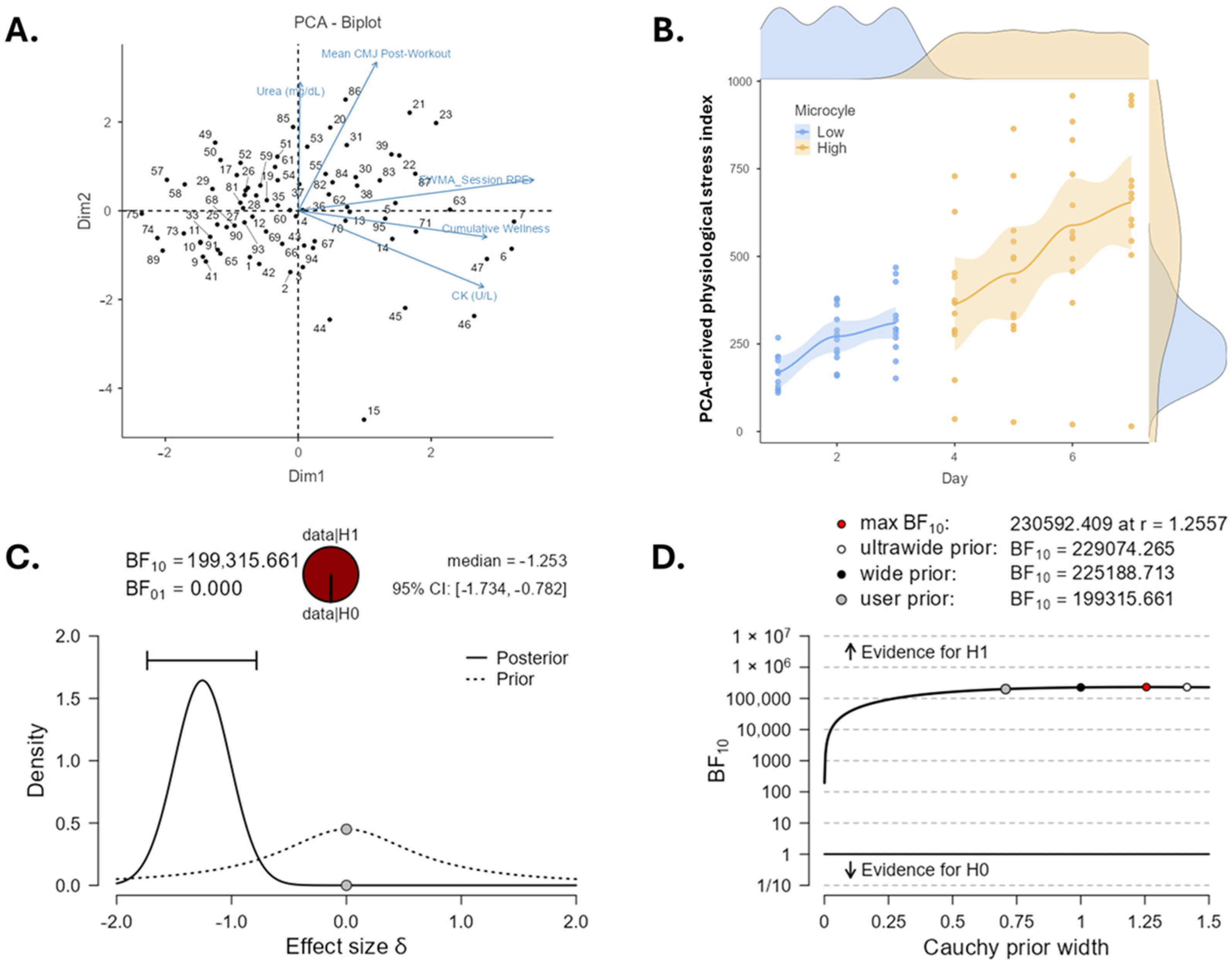
2.2. Outcome Data
3. Discussion
Limitations and Future Directions
4. Methods
4.1. Study Design
4.2. Setting
4.3. Participants
4.4. Variables
4.5. Data Sources/Measurement
4.5.1. Biochemical Analysis
4.5.2. Psychometric Variables
4.5.3. Countermovement Jump (CMJ)
4.6. Bias
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALindex | Allostatic load index |
| CK | Creatine kinase |
| CMJ | Countermovement jump |
| EWMA | Exponentially weighted moving average |
| PCA | Principal component analysis |
| RPE | Rating of perceived exertion |
References
- Bonilla, D.A.; Stout, J.R.; Gleeson, M.; Campbell, B.I.; Escalante, G.; Rojas-Valverde, D.; Petro, J.L.; Kreider, R.B.; Odriozola-Martínez, A. The 4Rs Framework of Sports Nutrition: An Update with Recommendations to Evaluate Allostatic Load in Athletes. Life 2025, 15, 867. [Google Scholar] [CrossRef]
- Bettinger, J.S.; Friston, K.J. Conceptual foundations of physiological regulation incorporating the free energy principle and self-organized criticality. Neurosci. Biobehav. Rev. 2023, 155, 105459. [Google Scholar] [CrossRef] [PubMed]
- Bobba-Alves, N.; Juster, R.P.; Picard, M. The energetic cost of allostasis and allostatic load. Psychoneuroendocrinology 2022, 146, 105951. [Google Scholar] [CrossRef] [PubMed]
- Schuth, G.; Szigeti, G.; Dobreff, G.; Revisnyei, P.; Pasic, A.; Toka, L.; Gabbett, T.; Pavlik, G. Factors Influencing Creatine Kinase Response in Youth National Team Soccer Players. Sports Health 2021, 13, 332–340. [Google Scholar] [CrossRef]
- Doeven, S.H.; Brink, M.S.; Kosse, S.J.; Lemmink, K. Postmatch recovery of physical performance and biochemical markers in team ball sports: A systematic review. BMJ Open Sport Exerc. Med. 2018, 4, e000264. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Manzi, V.; Iellamo, F.; Impellizzeri, F.; D’Ottavio, S.; Castagna, C. Relation between individualized training impulses and performance in distance runners. Med. Sci. Sports Exerc. 2009, 41, 2090–2096. [Google Scholar] [CrossRef]
- Becker, M.; Sperlich, B.; Zinner, C.; Achtzehn, S. Intra-Individual and Seasonal Variation of Selected Biomarkers for Internal Load Monitoring in U-19 Soccer Players. Front. Physiol. 2020, 11, 838. [Google Scholar] [CrossRef]
- Simmons, R.; Doma, K.; Sinclair, W.; Connor, J.; Leicht, A. Acute Effects of Training Loads on Muscle Damage Markers and Performance in Semi-elite and Elite Athletes: A Systematic Review and Meta-analysis. Sports Med. 2021, 51, 2181–2207. [Google Scholar] [CrossRef]
- Greenham, G.; Buckley, J.D.; Garrett, J.; Eston, R.; Norton, K. Biomarkers of Physiological Responses to Periods of Intensified, Non-Resistance-Based Exercise Training in Well-Trained Male Athletes: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 2517–2548. [Google Scholar] [CrossRef]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef] [PubMed]
- Lupo, C.; Tessitore, A.; Gasperi, L.; Gomez, M. Session-RPE for quantifying the load of different youth basketball training sessions. Biol. Sport 2017, 34, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Chaouachi, A.; Wong, D.P.; Castagna, C.; Hambli, M.; Hue, O.; Chamari, K. Influence of fatigue, stress, muscle soreness and sleep on perceived exertion during submaximal effort. Physiol. Behav. 2013, 119, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Saw, A.E.; Main, L.C.; Gastin, P.B. Monitoring the athlete training response: Subjective self-reported measures trump commonly used objective measures: A systematic review. Br. J. Sports Med. 2016, 50, 281–291. [Google Scholar] [CrossRef]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A new approach to monitoring exercise training. J. Strength Cond. Res. 2001, 15, 109–115. [Google Scholar]
- Liu, H.; Yang, W.; Liu, H.; Bao, D.; Cui, Y.; Ho, I.M.K.; Li, Q. A meta-analysis of the criterion-related validity of Session-RPE scales in adolescent athletes. BMC Sports Sci. Med. Rehabil. 2023, 15, 101. [Google Scholar] [CrossRef]
- Berriel, G.P.; Costa, R.R.; da Silva, E.S.; Schons, P.; de Vargas, G.D.; Peyre-Tartaruga, L.A.; Kruel, L.F.M. Stress and recovery perception, creatine kinase levels, and performance parameters of male volleyball athletes in a preseason for a championship. Sports Med. Open 2020, 6, 26. [Google Scholar] [CrossRef]
- Inoue, A.; Dos Santos Bunn, P.; do Carmo, E.C.; Lattari, E.; da Silva, E.B. Internal Training Load Perceived by Athletes and Planned by Coaches: A Systematic Review and Meta-Analysis. Sports Med. Open 2022, 8, 35. [Google Scholar] [CrossRef]
- Pillitteri, G.; Petrigna, L.; Ficarra, S.; Giustino, V.; Thomas, E.; Rossi, A.; Clemente, F.M.; Paoli, A.; Petrucci, M.; Bellafiore, M.; et al. Relationship between external and internal load indicators and injury using machine learning in professional soccer: A systematic review and meta-analysis. Res. Sports Med. 2024, 32, 902–938. [Google Scholar] [CrossRef]
- Halson, S.L. Monitoring training load to understand fatigue in athletes. Sports Med. 2014, 44 (Suppl. S2), S139–S147. [Google Scholar] [CrossRef]
- Gathercole, R.; Sporer, B.; Stellingwerff, T. Countermovement Jump Performance with Increased Training Loads in Elite Female Rugby Athletes. Int. J. Sports Med. 2015, 36, 722–728. [Google Scholar] [CrossRef]
- Hagstrom, A.D.; Shorter, K.A. Creatine kinase, neuromuscular fatigue, and the contact codes of football: A systematic review and meta-analysis of pre- and post-match differences. Eur. J. Sport Sci. 2018, 18, 1234–1244. [Google Scholar] [CrossRef]
- Heishman, A.D.; Daub, B.D.; Miller, R.M.; Freitas, E.D.S.; Bemben, M.G. Monitoring External Training Loads and Neuromuscular Performance for Division I Basketball Players over the Preseason. J. Sports Sci. Med. 2020, 19, 204–212. [Google Scholar]
- Sansone, P.; Tschan, H.; Foster, C.; Tessitore, A. Monitoring Training Load and Perceived Recovery in Female Basketball: Implications for Training Design. J. Strength Cond. Res. 2020, 34, 2929–2936. [Google Scholar] [CrossRef]
- Edwards, T.; Spiteri, T.; Piggott, B.; Bonhotal, J.; Haff, G.G.; Joyce, C. Monitoring and Managing Fatigue in Basketball. Sports 2018, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Carbone, J.T.; Clift, J.; Alexander, N. Measuring allostatic load: Approaches and limitations to algorithm creation. J. Psychosom. Res. 2022, 163, 111050. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, S.; Kenny, R.A.; McCrory, C. Does the choice of Allostatic Load scoring algorithm matter for predicting age-related health outcomes? Psychoneuroendocrinology 2020, 120, 104789. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.; McLoughlin, S.; Layte, R.; NiCheallaigh, C.; O’Halloran, A.M.; Barros, H.; Berkman, L.F.; Bochud, M.; Crimmins, E.M.; Farrell, M.T.; et al. Towards a consensus definition of allostatic load: A multi-cohort, multi-system, multi-biomarker individual participant data (IPD) meta-analysis. Psychoneuroendocrinology 2023, 153, 106117. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Perez-Idarraga, A.; Odriozola-Martinez, A.; Kreider, R.B. The 4R’s Framework of Nutritional Strategies for Post-Exercise Recovery: A Review with Emphasis on New Generation of Carbohydrates. Int. J. Environ. Res. Public Health 2020, 18, 103. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Herrera-Gonzalez, E.; Bonilla, D.A. Sports injuries as reversible involution: A novel approach to rehabilitation and readaptation. Front. Sports Act. Living 2025, 7, 1519404. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Cardozo, L.A.; Velez-Gutierrez, J.M.; Arevalo-Rodriguez, A.; Vargas-Molina, S.; Stout, J.R.; Kreider, R.B.; Petro, J.L. Exercise Selection and Common Injuries in Fitness Centers: A Systematic Integrative Review and Practical Recommendations. Int. J. Environ. Res. Public Health 2022, 19, 2710. [Google Scholar] [CrossRef]
- Philipp, N.M.; Cabarkapa, D.; Nijem, R.M.; Fry, A.C. Changes in countermovement jump force-time characteristic in elite male basketball players: A season-long analyses. PLoS ONE 2023, 18, e0286581. [Google Scholar] [CrossRef] [PubMed]
- Cruz, I.F.; Pereira, L.A.; Kobal, R.; Kitamura, K.; Cedra, C.; Loturco, I.; Cal Abad, C.C. Perceived training load and jumping responses following nine weeks of a competitive period in young female basketball players. PeerJ 2018, 6, e5225. [Google Scholar] [CrossRef] [PubMed]
- Sansone, P.; Rago, V.; Kellmann, M.; Alcaraz, P.E. Relationship Between Athlete-Reported Outcome Measures and Subsequent Match Performance in Team Sports: A Systematic Review. J. Strength Cond. Res. 2023, 37, 2302–2313. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.F.; Hicks, K.M.; Russell, M.; Hayes, P.R. The Reliability of Potential Fatigue-Monitoring Measures in Elite Youth Soccer Players. J. Strength Cond. Res. 2021, 35, 3448–3452. [Google Scholar] [CrossRef]
- Andersen, T.R.; Kastner, B.; Arvig, M.; Larsen, C.H.; Madsen, E.E. Monitoring load, wellness, and psychological variables in female and male youth national team football players during international and domestic playing periods. Front. Sports Act. Living 2023, 5, 1197766. [Google Scholar] [CrossRef]
- Chatzinikolaou, A.; Draganidis, D.; Avloniti, A.; Karipidis, A.; Jamurtas, A.Z.; Skevaki, C.L.; Tsoukas, D.; Sovatzidis, A.; Theodorou, A.; Kambas, A.; et al. The microcycle of inflammation and performance changes after a basketball match. J. Sports Sci. 2014, 32, 870–882. [Google Scholar] [CrossRef]
- Rabbani, A.; Clemente, F.M.; Kargarfard, M.; Chamari, K. Match Fatigue Time-Course Assessment over Four Days: Usefulness of the Hooper Index and Heart Rate Variability in Professional Soccer Players. Front. Physiol. 2019, 10, 109. [Google Scholar] [CrossRef]
- Campos, F.; Molina Correa, J.C.; Canevari, V.C.M.; Branco, B.H.M.; Andreato, L.V.; de Paula Ramos, S. Monitoring Internal Training Load, Stress-Recovery Responses, and Immune-Endocrine Parameters in Brazilian Jiu-Jitsu Training. J. Strength Cond. Res. 2022, 36, 723–731. [Google Scholar] [CrossRef]
- Tate, T.; Roberts, S.; Main, L.C.; Bruce, L. The influence of training load and schedule on youth athletes’ sleep. J. Sleep Res. 2025, e70013. [Google Scholar] [CrossRef]
- Clemente, F.M.; Mendes, B.; Bredt, S.; Praca, G.M.; Silverio, A.; Carrico, S.; Duarte, E. Perceived Training Load, Muscle Soreness, Stress, Fatigue, and Sleep Quality in Professional Basketball: A Full Season Study. J. Hum. Kinet. 2019, 67, 199–207. [Google Scholar] [CrossRef]
- Sawczuk, T.; Jones, B.; Scantlebury, S.; Till, K. The influence of training load, exposure to match play and sleep duration on daily wellbeing measures in youth athletes. J. Sports Sci. 2018, 36, 2431–2437. [Google Scholar] [CrossRef]
- Clemente, F.M.; Afonso, J.; Costa, J.; Oliveira, R.; Pino-Ortega, J.; Rico-Gonzalez, M. Relationships between Sleep, Athletic and Match Performance, Training Load, and Injuries: A Systematic Review of Soccer Players. Healthcare 2021, 9, 808. [Google Scholar] [CrossRef]
- Andersson, H.; Raastad, T.; Nilsson, J.; Paulsen, G.; Garthe, I.; Kadi, F. Neuromuscular fatigue and recovery in elite female soccer: Effects of active recovery. Med. Sci. Sports Exerc. 2008, 40, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Obminski, Z.; Crewther, B.; Cook, C.J. Disentangling the dynamic interplay between muscle damage and energetics in male boxers during a short training block. Biol. Sport 2024, 41, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Manzi, V.; D’ottavio, S.; Impellizzeri, F.; Chaouachi, A.; Chamari, K.; Castagna, C. Profile of weekly training load in elite male professional basketball players. J. Strength Cond. Res. 2010, 24, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.Y.; Pereira, L.A.; Rabelo, F.N.; Ramirez-Campillo, R.; Loturco, I. Faster Futsal Players Perceive Higher Training Loads and Present Greater Decreases in Sprinting Speed During the Preseason. J. Strength Cond. Res. 2016, 30, 1553–1562. [Google Scholar] [CrossRef]
- Pereira, L.A.; Freitas, T.T.; Zanetti, V.; Loturco, I. Variations in Internal and External Training Load Measures and Neuromuscular Performance of Professional Soccer Players During a Preseason Training Period. J. Hum. Kinet. 2022, 81, 149–162. [Google Scholar] [CrossRef]
- Gabbett, T.J. The training-injury prevention paradox: Should athletes be training smarter and harder? Br. J. Sports Med. 2016, 50, 273–280. [Google Scholar] [CrossRef]
- Marrier, B.; Le Meur, Y.; Robineau, J.; Lacome, M.; Couderc, A.; Hausswirth, C.; Piscione, J.; Morin, J.B. Quantifying Neuromuscular Fatigue Induced by an Intense Training Session in Rugby Sevens. Int. J. Sports Physiol. Perform. 2017, 12, 218–223. [Google Scholar] [CrossRef]
- Claudino, J.G.; Cronin, J.; Mezencio, B.; McMaster, D.T.; McGuigan, M.; Tricoli, V.; Amadio, A.C.; Serrao, J.C. The countermovement jump to monitor neuromuscular status: A meta-analysis. J. Sci. Med. Sport 2017, 20, 397–402. [Google Scholar] [CrossRef]
- Alba-Jimenez, C.; Moreno-Doutres, D.; Pena, J. Trends Assessing Neuromuscular Fatigue in Team Sports: A Narrative Review. Sports 2022, 10, 33. [Google Scholar] [CrossRef]
- Gamonales, J.M.; Hernandez-Beltran, V.; Escudero-Tena, A.; Ibanez, S.J. Analysis of the External and Internal Load in Professional Basketball Players. Sports 2023, 11, 195. [Google Scholar] [CrossRef]
- Reina, M.; García-Rubio, J.; Esteves, P.T.; Ibáñez, S.J. How external load of youth basketball players varies according to playing position, game period and playing time. Int. J. Perform. Anal. Sport. 2020, 20, 917–930. [Google Scholar] [CrossRef]
- Yang, K. Quarterly fluctuations in external and internal loads among professional basketball players. Front. Physiol. 2024, 15, 1419097. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, M.F.; Mountjoy, M.; Armstrong, N.; Chia, M.; Cote, J.; Emery, C.A.; Faigenbaum, A.; Hall, G., Jr.; Kriemler, S.; Leglise, M.; et al. International Olympic Committee consensus statement on youth athletic development. Br. J. Sports Med. 2015, 49, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Henze, A.S.; Voit, T.; Latzel, R.; Moser, O. Athlete Monitoring Systems in Elite Men’s Basketball: Challenges, Recommendations, and Future Perspectives. Transl. Sports Med. 2024, 2024, 6326566. [Google Scholar] [CrossRef] [PubMed]
- Sarlis, V.; Tjortjis, C. Sports Analytics: Data Mining to Uncover NBA Player Position, Age, and Injury Impact on Performance and Economics. Information 2024, 15, 242. [Google Scholar] [CrossRef]
- Cardozo, L.A.; Peña-Ibagón, J.C.; Florez-Escobar, W.; Castillo-Daza, C.A.; Bonilla-Ocampo, D.A.; Reina-Monroy, J.L. Autoconcepto físico en estudiantes universitarios: Generación de perfiles por clasificación jerárquica sobre componentes principales (Physical self-concept in university students: Generating profiles with hierarchical classification on principal components). Retos 2023, 48, 167–177. [Google Scholar] [CrossRef]
- Miaoulis, D.; Stivaros, I.; Koubias, S. Developing a Novel Muscle Fatigue Index for Wireless sEMG Sensors: Metrics and Regression Models for Real-Time Monitoring. Electronics 2025, 14, 2097. [Google Scholar] [CrossRef]
- Jeffries, A.C.; Wallace, L.; Coutts, A.J.; McLaren, S.J.; McCall, A.; Impellizzeri, F.M. Athlete-Reported Outcome Measures for Monitoring Training Responses: A Systematic Review of Risk of Bias and Measurement Property Quality According to the COSMIN Guidelines. Int. J. Sports Physiol. Perform. 2020, 15, 1203–1215. [Google Scholar] [CrossRef]
- Wellm, D.; Willberg, C.; Zentgraf, K. Differences in Player Load of Professional Basketball Players as A Function of Distance to the Game Day During a Competitive Season. Int. J. Strength Cond. 2023, 3. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, H.; Quan, W.; Jiang, X.; Liang, M.; Li, S.; Ugbolue, U.C.; Baker, J.S.; Gusztav, F.; Ma, X.; et al. A new method proposed for realizing human gait pattern recognition: Inspirations for the application of sports and clinical gait analysis. Gait Posture 2024, 107, 293–305. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bowling, J.L.; Katayev, A. An Evaluation of the Roche Cobas c 111. Lab. Med. 2010, 41, 398–402. [Google Scholar] [CrossRef]
- Hooper, S.L.; Mackinnon, L.T. Monitoring overtraining in athletes. Recommendations. Sports Med. 1995, 20, 321–327. [Google Scholar] [CrossRef]
- Murray, N.B.; Gabbett, T.J.; Townshend, A.D.; Blanch, P. Calculating acute:chronic workload ratios using exponentially weighted moving averages provides a more sensitive indicator of injury likelihood than rolling averages. Br. J. Sports Med. 2017, 51, 749–754. [Google Scholar] [CrossRef]
- Nobari, H.; Arslan, E.; Martins, A.D.; Oliveira, R. Are acute:chronic workload ratios of perceived exertion and running based variables sensible to detect variations between player positions over the season? A soccer team study. BMC Sports Sci. Med. Rehabil. 2022, 14, 51. [Google Scholar] [CrossRef]
- Rojas-Jaramillo, A.; Leon-Sanchez, G.; Calvo-Lluch, A.; Gonzalez-Badillo, J.J.; Rodriguez-Rosell, D. Comparison of 10% vs. 30% Velocity Loss during Squat Training with Low Loads on Strength and Sport-Specific Performance in Young Soccer Players. Sports 2024, 12, 43. [Google Scholar] [CrossRef]
- Bernards, J.R.; Sato, K.; Haff, G.G.; Bazyler, C.D. Current Research and Statistical Practices in Sport Science and a Need for Change. Sports 2017, 5, 87. [Google Scholar] [CrossRef]
- Vaccaro, M.G.; Innocenti, B.; Cione, E.; Gallelli, L.; De Sarro, G.; Bonilla, D.A.; Cannataro, R. Acute effects of a chewable beetroot-based supplement on cognitive performance: A double-blind randomized placebo-controlled crossover clinical trial. Eur. J. Nutr. 2024, 63, 303–321. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Duque-Zuluaga, L.T.; Munoz-Urrego, L.P.; Franco-Hoyos, K.; Agudelo-Martinez, A.; Kammerer-Lopez, M.; Petro, J.L.; Kreider, R.B. Development and Validation of a Novel Waist Girth-Based Equation to Estimate Fat Mass in Young Colombian Elite Athletes (F20(CA) Equation): A STROSA-Based Study. Nutrients 2022, 14, 59. [Google Scholar] [CrossRef]
- Algina, J.; Keselman, H.J.; Penfield, R.D. An alternative to Cohen’s standardized mean difference effect size: A robust parameter and confidence interval in the two independent groups case. Psychol. Methods 2005, 10, 317–328. [Google Scholar] [CrossRef]
- Wilcox, R.R.; Tian, T.S. Measuring effect size: A robust heteroscedastic approach for two or more groups. J. Appl. Stat. 2011, 38, 1359–1368. [Google Scholar] [CrossRef]
- Nathoo, F.S.; Masson, M.E.J. Bayesian alternatives to null-hypothesis significance testing for repeated-measures designs. J. Math. Psychol. 2016, 72, 144–157. [Google Scholar] [CrossRef]
- The jamovi Project. jamovi (Version 2.6) [Computer Software]. Available online: https://www.jamovi.org/about.html (accessed on 28 October 2024).



| 95% Confidence Interval | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Microcycle | N | Mean | SD | Inferior | Superior | BF10 | p Value | ξ | |
| CK (U/L) | Deload | 36 | 221.6 | 105.6 | 185.85 | 257.31 | 608.8 | <0.001 | 0.653 |
| Overload | 54 | 438.9 | 284.08 | 361.41 | 516.48 | ||||
| Urea (mg/dL) | Deload | 36 | 5.48 | 1.062 | 5.12 | 5.84 | 0.42 | 0.479 | 0.094 |
| Overload | 54 | 5.81 | 1.407 | 5.43 | 6.2 | ||||
| CMJ post-Blood | Deload | 24 | 33.65 | 6.854 | 30.75 | 36.54 | 1.12 | 0.117 | 0.353 |
| Overload | 44 | 36.19 | 4.31 | 34.88 | 37.5 | ||||
| CMJ Pre-Workout | Deload | 36 | 38.31 | 5.136 | 36.57 | 40.05 | 0.70 | 0.005 | 0.455 |
| Overload | 43 | 40.8 | 8.082 | 38.31 | 43.29 | ||||
| CMJ Post-Workout | Deload | 36 | 40.76 | 5.729 | 38.82 | 42.7 | 0.745 | 0.029 | 0.407 |
| Overload | 43 | 43.52 | 8.621 | 40.87 | 46.17 | ||||
| Session-RPE | Deload | 36 | 269.7 | 268.68 | 178.81 | 360.63 | 1.23 × 105 | <0.001 | 0.833 |
| Overload | 45 | 732.7 | 405.81 | 610.75 | 854.58 | ||||
| EWMA of Session-RPE | Deload | 36 | 158.3 | 79.823 | 131.31 | 185.32 | 1.11 × 108 | <0.001 | 0.841 |
| Overload | 48 | 418.1 | 195.32 | 361.39 | 474.82 | ||||
| Sleep | Deload | 36 | 3.36 | 1.222 | 2.95 | 3.77 | 1.29 | 0.053 | 0.329 |
| Overload | 54 | 3.85 | 1.071 | 3.56 | 4.14 | ||||
| Stress | Deload | 36 | 2.72 | 0.944 | 2.4 | 3.04 | 0.25 | 0.833 | 0.050 |
| Overload | 54 | 2.59 | 1.221 | 2.26 | 2.93 | ||||
| Fatigue | Deload | 36 | 2.58 | 1.251 | 2.16 | 3.01 | 0.70 | 0.08 | 0.265 |
| Overload | 54 | 3.04 | 1.331 | 2.67 | 3.4 | ||||
| Muscle soreness | Deload | 36 | 2.25 | 0.967 | 1.92 | 2.58 | 58.92 | 0.003 | 0.496 |
| Overload | 54 | 3.15 | 1.25 | 2.81 | 3.49 | ||||
| Mood | Deload | 36 | 2.69 | 1.064 | 2.33 | 3.05 | 0.43 | 0.172 | 0.233 |
| Overload | 54 | 2.98 | 1.09 | 2.68 | 3.28 | ||||
| Wellness Score | Deload | 36 | 13.61 | 3.871 | 12.3 | 14.92 | 0.52 | 0.118 | 0.231 |
| Overload | 56 | 15.05 | 5.358 | 13.62 | 16.49 | ||||
| Variable | PC1 | PC2 | Uniqueness | KMO |
|---|---|---|---|---|
| CK (U/L) | 0.823 | 0.296 | 0.557 | |
| Cumulative Wellness | 0.540 | 0.693 | 0.558 | |
| Mean CMJ Post-Workout | 0.809 | 0.274 | 0.545 | |
| Session-RPE | 0.500 | 0.722 | 0.228 | 0.694 |
| EWMA of session-RPE | 0.652 | 0.662 | 0.135 | 0.625 |
| Microcycle | Strength Training | On-Court Training | Competition Load |
|---|---|---|---|
| Deload |
|
| None |
| Overload | Four high intensity matches during the week |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cuervo, J.M.; Rojas-Jaramillo, A.; García-Caro, A.; González-Santamaria, J.; Humeres, G.; Stout, J.R.; Odriozola-Martínez, A.; Bonilla, D.A. Biochemical and Perceptual Markers of Physiological Stress During Acute Exercise Overload in U20 Elite Basketball Players. Stresses 2025, 5, 52. https://doi.org/10.3390/stresses5030052
López-Cuervo JM, Rojas-Jaramillo A, García-Caro A, González-Santamaria J, Humeres G, Stout JR, Odriozola-Martínez A, Bonilla DA. Biochemical and Perceptual Markers of Physiological Stress During Acute Exercise Overload in U20 Elite Basketball Players. Stresses. 2025; 5(3):52. https://doi.org/10.3390/stresses5030052
Chicago/Turabian StyleLópez-Cuervo, Juan M., Andrés Rojas-Jaramillo, Andrés García-Caro, Jhonatan González-Santamaria, Gustavo Humeres, Jeffrey R. Stout, Adrián Odriozola-Martínez, and Diego A. Bonilla. 2025. "Biochemical and Perceptual Markers of Physiological Stress During Acute Exercise Overload in U20 Elite Basketball Players" Stresses 5, no. 3: 52. https://doi.org/10.3390/stresses5030052
APA StyleLópez-Cuervo, J. M., Rojas-Jaramillo, A., García-Caro, A., González-Santamaria, J., Humeres, G., Stout, J. R., Odriozola-Martínez, A., & Bonilla, D. A. (2025). Biochemical and Perceptual Markers of Physiological Stress During Acute Exercise Overload in U20 Elite Basketball Players. Stresses, 5(3), 52. https://doi.org/10.3390/stresses5030052










