Methyl Jasmonate Acts as a Crucial Player in Abiotic Stress Responses in Grape
Abstract
1. Introduction
2. Discovery of MeJA/JA
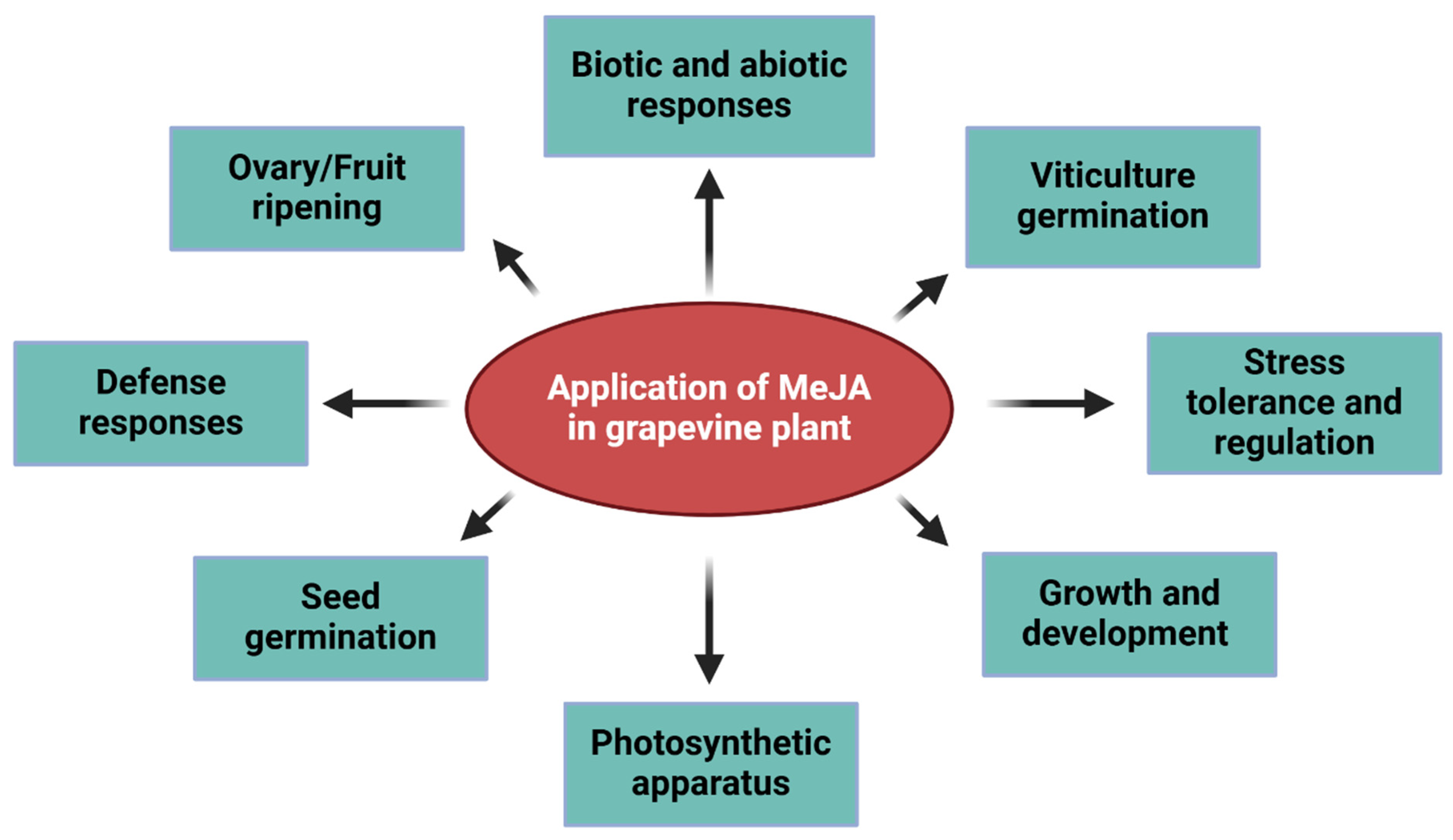
3. Applications
4. Importance
5. Occurrence
6. MeJA in the Grape Growth and Development
7. Role of MeJA Against Different Stresses in Grape
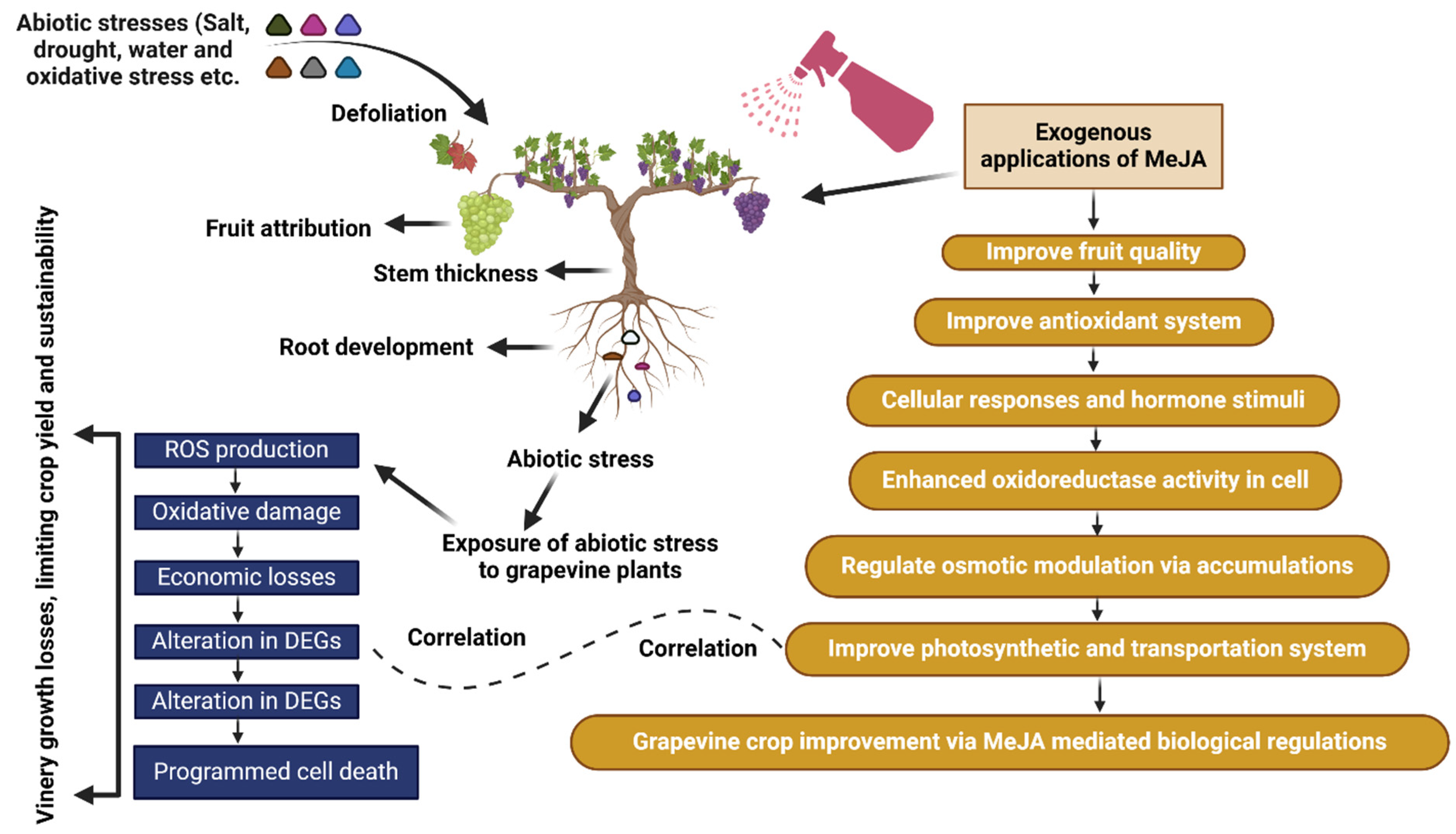
8. Interaction Between Grape and MeJA Under Abiotic Stresses
9. MeJA Regulation of Gene Expression with Transcription Factors and Disease Resistance in Grape Under Abiotic Stresses
10. MeJA Signaling Mechanisms
11. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armijo, G.; Espinoza, C.; Loyola, R.; Restovic, F.; Santibáñez, C.; Schlechter, R.; Agurto, M.; Arce-Johnson, P.J. Grapevine biotechnology: Molecular approaches underlying abiotic and biotic stress responses. In Grape and Wine Biotechnology; IntechOpen: London, UK, 2016; pp. 3–42. [Google Scholar]
- Vezzulli, S.; Gramaje, D.; Tello, J.; Gambino, G.; Bettinelli, P.; Pirrello, C.; Schwandner, A.; Barba, P.; Angelini, E.; Anfora, G. Genomic designing for biotic stress resistant grapevine. In Genomic Designing for Biotic Stress Resistant Fruit Crops; Springer: Cham, Switzerland, 2022; pp. 87–255. [Google Scholar]
- Martin, D.M.; Aubourg, S.; Schouwey, M.B.; Daviet, L.; Schalk, M.; Toub, O.; Lund, S.T.; Bohlmann, J. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol. 2010, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, A.; Torregrosa, L.; Iocco, P.; Thomas, M.R. Grapevine (Vitis vinifera L.). In Agrobacterium Protocols Volume 2; Humana Press: Totowa, NJ, USA, 2006; pp. 273–285. [Google Scholar]
- Vivier, M.A.; Pretorius, I.S. Genetically tailored grapevines for the wine industry. Trends Biotechnol. 2002, 20, 472–478. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, P.; Teixeira, G.; Lopes, C.; Monteiro, A. The role of grapevine leaf morphoanatomical traits in determining capacity for coping with abiotic stresses: A review. Ciência e Técnica Vitivinícola 2021, 36, 75–88. [Google Scholar] [CrossRef]
- Louime, C.; Vasanthaiah, H.K.; Basha, S.M.; Lu, J. Perspective of biotic and abiotic stress research in grapevines (Vitis sp.). Int. J. Fruit Sci. 2010, 10, 79–86. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.-T.; Machado, N.; Moutinho Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef]
- Murcia, G.; Fontana, A.; Pontin, M.; Baraldi, R.; Bertazza, G.; Piccoli, P.N. ABA and GA3 regulate the synthesis of primary and secondary metabolites related to alleviation from biotic and abiotic stresses in grapevine. Phytochemistry 2017, 135, 34–52. [Google Scholar] [CrossRef]
- Shehata, R.S. The Role of Environmental Factors and Plant Growth Regulators on Grapes Coloration. Vitic. Stud. (VIS) 2024, 4, 9–20. [Google Scholar] [CrossRef]
- Khandani, Y.; Sarikhani, H.; Gholami, M.; Rad, A.C.; Yousefi, S.; Sodini, M.; Sivilotti, P.J.J.o.S.S.; Nutrition, P. Exogenous auxin improves the growth of grapevine (Vitis vinifera L.) under drought stress by mediating physiological, biochemical and hormonal modifications. J. Soil Sci. Plant Nutr. 2024, 24, 3422–3440. [Google Scholar] [CrossRef]
- Lovisolo, C.; Lavoie-Lamoureux, A.; Tramontini, S.; Ferrandino, A. Grapevine adaptations to water stress: New perspectives about soil/plant interactions. Theor. Exp. Plant Physiol. 2016, 28, 53–66. [Google Scholar] [CrossRef]
- Toups, H.; Cochetel, N.; Galdamez, K.; Deluc, L.; Cramer, G. Abscisic Acid Metabolism in Leaves and Roots of Four Vitis Species in Response to Water Deficit. Preprint 2021. [Google Scholar] [CrossRef]
- Iqbal, S.; Elatafi, E.; Shaonan, L.; Ali, S.; Hakeem, A.; Badar Aziz, R.; Mauligen, E.; Tariq, K.; Elhendawy, B.; Shangguan, L.; et al. Drought Stress and the Modulation of Physiochemical Parameters and Antioxidant Enzymes in Grapevine Rootstocks: Insights into the Protective Role of Methyl Jasmonate. Horticulturae 2025, 11, 164. [Google Scholar] [CrossRef]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Pei, D.; Ren, Y.; Yu, W.; Zhang, P.; Dong, T.; Jia, H.; Fang, J. The roles of Brassinosteroids and Methyl Jasmonate on postharvest grape by regulating the interaction between VvDWF4 and VvTIFY 5 A. Plant Sci. 2023, 336, 111830. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Marusig, D.; Tombesi, S. Abscisic Acid Mediates Drought and Salt Stress Responses in Vitis vinifera—A Review. Int. J. Mol. Sci. 2020, 21, 8648. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Sabbatini, P.; Squeri, C.; Lavado Rodas, N.; Palliotti, A.; Poni, S. Kaolin Reduces ABA Biosynthesis through the Inhibition of Neoxanthin Synthesis in Grapevines under Water Deficit. Int. J. Mol. Sci. 2020, 21, 4950. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Markowska, A.J.M. Jasmonate compounds and their derivatives in the regulation of the neoplastic processes. Molecules 2021, 26, 2901. [Google Scholar] [CrossRef]
- Ellis, C.; Turner, J. The Arabidopsis Mutant cev1 Has Constitutively Active Jasmonate and Ethylene Signal Pathways and Enhanced Resistance to Pathogens. The Plant Cell 2001, 13, 1025–1033. [Google Scholar] [CrossRef]
- Lv, J.; Dong, T.; Zhang, Y.; Ku, Y.; Zheng, T.; Jia, H.; Fang, J. Metabolomics Profiling of Brassinolide and Abscisic Acid in Re-sponse to High Temperature Stress. Plant Cell Rep. 2022, 41, 935–946. [Google Scholar] [CrossRef]
- Ju, Y.; Liu, M.; Zhao, H.; Meng, J.-F.; Fang, Y. Effect of Exogenous Abscisic Acid and Methyl Jasmonate on Anthocyanin Composition, Fatty Acids, and Volatile Compounds of Cabernet Sauvignon (Vitis vinifera L.) Grape Berries. Molecules 2016, 21, 1354. [Google Scholar] [CrossRef]
- Aldridge, D.; Galt, S.; Giles, D.; Turner, W.B. Metabolites of Lasiodiplodia theobromae. J. Chem. Soc. C Org. 1971, 1623–1627. [Google Scholar] [CrossRef]
- Hamberg, M. Mechanism of corn hydroperoxide isomerase: Detection of 12,13(S)-oxido-9(Z),11-octadecadienoic acid. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1987, 920, 76–84. [Google Scholar] [CrossRef]
- Hamberg, M.; Gardner, H.W. Oxylipin pathway to jasmonates: Biochemistry and biological significance. Biochim. Biophys. Acta 1992, 1165, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.C.; Meldau, S.; Gaquerel, E.; Diezel, C.; McGale, E.; Greenfield, S.; Baldwin, I.T. The active jasmonate JA-Ile regulates a specific subset of plant jasmonate-mediated resistance to herbivores in nature. Front. Plant Sci. 2018, 9, 787. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Sharma, A.; Yuan, H.; Kumar, V.; Ramakrishnan, M.; Kohli, S.K.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Zheng, B.J.E.; Safety, E. Castasterone attenuates insecticide induced phytotoxicity in mustard. Ecotoxicol. Environ. Saf. 2019, 179, 50–61. [Google Scholar] [CrossRef]
- Mueller, M.J. Enzymes involved in jasmonic acid biosynthesis. Physiol. Plant. 1997, 100, 653–663. [Google Scholar] [CrossRef]
- Vezzulli, S.; Civardi, S.; Ferrari, F.; Bavaresco, L. Methyl jasmonate treatment as a trigger of resveratrol synthesis in cultivated grapevine. Am. J. Enol. Vitic. 2007, 58, 530–533. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Portu, J.; López, R.; Santamaría, P. Effect of methyl jasmonate application to grapevine leaves on grape amino acid content. Food Chem. 2016, 203, 536–539. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef]
- Kondo, S.; Fukuda, K. Changes of jasmonates in grape berries and their possible roles in fruit development. Sci. Hortic. 2001, 91, 275–288. [Google Scholar] [CrossRef]
- Wang, J.; VanderWeide, J.; Yan, Y.; Tindjau, R.; Pico, J.; Deluc, L.; Zandberg, W.F.; Castellarin, S.D. Impact of hormone applications on ripening-related metabolites in Gewürztraminer grapes (Vitis vinifera L.): The key role of jasmonates in terpene modulation. Food Chem. 2022, 388, 132948. [Google Scholar] [CrossRef] [PubMed]
- Großkinsky, D.K.; van der Graaff, E.; Roitsch, T. Regulation of abiotic and biotic stress responses by plant hormones. In Plant Pathogen Resistance Biotechnology; Wiley: Hoboken, NJ, USA, 2016; pp. 131–154. [Google Scholar]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Methyl jasmonate effects on table grape ripening, vine yield, berry quality and bioactive compounds depend on applied concentration. Sci. Hortic. 2019, 247, 380–389. [Google Scholar] [CrossRef]
- Belhadj, A.; Saigne, C.; Telef, N.; Cluzet, S.; Bouscaut, J.; Corio-Costet, M.-F.; Mérillon, J.-M. Methyl jasmonate induces defense responses in grapevine and triggers protection against Erysiphe necator. J. Agric. Food Chem. 2006, 54, 9119–9125. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Mateluna-Cuadra, R.; Díaz-Gálvez, I.; Mejía, N.; Verdugo-Vásquez, N. Methyl jasmonate applications in viticulture: A tool to increase the content of flavonoids and stilbenes in grapes and wines. Horticulturae 2021, 7, 133. [Google Scholar] [CrossRef]
- Li, W.; Li, W.-F.; Yang, S.; Ma, Z.; Zhou, Q.; Mao, J.; Han, S.; Chen, B. Transcriptome and Metabolite Conjoint Analysis Reveals that Exogenous Methyl Jasmonate Regulates Monoterpene Synthesis in Grape Berry Skin. J. Agric. Food Chem. 2020, 68. [Google Scholar] [CrossRef]
- Bidabadi, S.S.; Mehri, H.; Ghobadi, C.; Baninasab, B.; Afazel, M. Morphological, physiological and antioxidant responses of some Iranian grapevine cultivars to methyl jasmonate application. J. Crop Sci. Biotechnol. 2013, 16, 277–283. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Effect of methyl jasmonate on the aroma of Sangiovese grapes and wines. Food Chem. 2018, 242, 352–361. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of benzothiadiazole and methyl jasmonate on the volatile compound composition of Vitis vinifera L. Monastrell grapes and wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- An, C.; Lu, L.; Yao, Y.; Liu, R.; Cheng, Y.; Lin, Y.; Qin, Y.; Zheng, P.J. Selection and Validation of Reference Genes in Clinacanthus nutans Under Abiotic Stresses, MeJA Treatment, and in Different Tissues. Int. J. Mol. Sci. 2025, 26, 2483. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Sajjadi, S.E.; Parang, K. A review (research and patents) on jasmonic acid and its derivatives. Arch. Der. Pharm. 2014, 347, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Miersch, O.; Büttner, C.; Dathe, W.; Sembdner, G. Occurrence of the plant growth regulator jasmonic acid in plants. J. Plant Growth Regul. 1984, 3, 1–8. [Google Scholar] [CrossRef]
- Jiang, L.; Jin, P.; Wang, L.; Yu, X.; Wang, H.; Zheng, Y. Methyl jasmonate primes defense responses against Botrytis cinerea and reduces disease development in harvested table grapes. Sci. Hortic. 2015, 192, 218–223. [Google Scholar] [CrossRef]
- Wu, Y.; Sexton, W.K.; Zhang, Q.; Bloodgood, D.; Wu, Y.; Hooks, C.; Coker, F.; Vasquez, A.; Wei, C.-I.; Xiao, S. Leaf abaxial immunity to powdery mildew in Arabidopsis is conferred by multiple defense mechanisms. J. Exp. Bot. 2024, 75, 1465–1478. [Google Scholar] [CrossRef]
- Wang, P.; Meng, F.; Yang, Y.; Ding, T.; Liu, H.; Wang, F.; Li, A.; Zhang, Q.; Li, K.; Fan, S.J.H.R. De novo assembling a high-quality genome sequence of Amur grape (Vitis amurensis Rupr.) gives insight into Vitis divergence and sex determination. Hortic. Res. 2024, 11, uhae117. [Google Scholar] [CrossRef]
- Guo, Y.; Li, S.; García-Caparros, P.; Wang, L.; Liang, Z.J. Grapevine adaptation to cold and heat stress. J. Exp. Bot. 2025, eraf158. [Google Scholar] [CrossRef]
- Loyola, R.; Herrera, D.; Mas, A.; Wong, D.C.J.; Höll, J.; Cavallini, E.; Amato, A.; Azuma, A.; Ziegler, T.; Aquea, F. The photomorphogenic factors UV-B RECEPTOR 1, ELONGATED HYPOCOTYL 5, and HY5 HOMOLOGUE are part of the UV-B signalling pathway in grapevine and mediate flavonol accumulation in response to the environment. J. Exp. Bot. 2016, 67, 5429–5445. [Google Scholar] [CrossRef]
- Andi, S.A.; Gholami, M.; Ford, C.M.; Maskani, F. The effect of light, phenylalanine and methyl jasmonate, alone or in combination, on growth and secondary metabolism in cell suspension cultures of Vitis vinifera. J. Photochem. Photobiol. B Biol. 2019, 199, 111625. [Google Scholar] [CrossRef]
- Hampel, D.; Mosandl, A.; Wüst, M. Induction of de novo volatile terpene biosynthesis via cytosolic and plastidial pathways by methyl jasmonate in foliage of Vitis vinifera L. J. Agric. Food Chem. 2005, 53, 2652–2657. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Donati, L.; Bramosanti, M.; Rosciani, R.; Palocci, C.; Pasqua, G.; Valletta, A. Microfluidic synthesis of methyl jasmonate-loaded PLGA nanocarriers as a new strategy to improve natural defenses in Vitis vinifera. Sci. Rep. 2019, 9, 18322. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Liu, F.-Q.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, A.R.; Lakra, N.; Saharan, B.S.; Luhach, A.; Kumar, R.; Seth, C.S.; Duhan, J.S.J. Enhancing plant disease resistance: Insights from biocontrol agent strategies. J. Plant Growth Regul. 2025, 44, 436–459. [Google Scholar] [CrossRef]
- Xu, A.; Zhan, J.-C.; Huang, W.-D. Effects of ultraviolet C, methyl jasmonate and salicylic acid, alone or in combination, on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet Sauvignon. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 122, 197–211. [Google Scholar] [CrossRef]
- Belchí-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru, R.; Pedreño, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep. 2012, 31, 81–89. [Google Scholar] [CrossRef]
- Yin, X.; Gao, Y.; Song, S.; Hassani, D.; Lu, J. Identification, characterization and functional analysis of grape (Vitis vinifera L.) mitochondrial transcription termination factor (mTERF) genes in responding to biotic stress and exogenous phytohormone. BMC Genom. 2021, 22, 136. [Google Scholar] [CrossRef]
- Faurie, B.; Cluzet, S.; Marie-France, C.-c.; Merillon, J.-M. Methyl jasmonates/Ethephon synergistically induces stilbene production in Vitis vinifera cell suspensions but fails to trigger resistance to Erysiphe necator. J. Int. Des Sci. De La Vigne et du Vin 2009, 43, 99–110. [Google Scholar] [CrossRef]
- Karimi, R.; Gavili-Kilaneh, K.; Khadivi, A. Methyl jasmonate promotes salinity adaptation responses in two grapevine (Vitis vinifera L.) cultivars differing in salt tolerance. Food Chem. 2022, 375, 131667. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Y.; Gu, B.; Cui, X.; Zhang, J. VaMYB44 transcription factor from Chinese wild Vitis amurensis negatively regulates cold tolerance in transgenic Arabidopsis thaliana and V. vinifera. Plant Cell Rep. 2022, 41, 1673–1691. [Google Scholar] [CrossRef]
- Giraud, E.; Ivanova, A.; Gordon, C.; Whelan, J.; Considine, M. Sulphur dioxide evokes a large scale reprogramming of the grape berry transcriptome associated with oxidative signalling and biotic defence responses. Plant Cell Environ. 2011, 35, 405–417. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Liu, X.; Liang, Z. Hormone biosynthesis and metabolism members of 2OGD superfamily are involved in berry development and respond to MeJA and ABA treatment of Vitis vinifera L. BMC Plant Biol. 2022, 22, 427. [Google Scholar] [CrossRef]
- Luo, D.; Huang, T.; Kou, X.; Zhang, Y.; Ba, L.; Wang, X.; Cao, S. MeJA enhances antioxidant activity and reduces membrane lipid degradation by maintaining energy charge levels in crystal grapes. Postharvest Biol. Technol. 2024, 216, 113078. [Google Scholar] [CrossRef]
- Seif, S.N.; Tafazzoli, E.; Talaii, A.-R.; Aboutalebi, A.; Abdosi, V. Evaluation of two grape cultivars (Vitis vinifera L.) against salinity stress and surveying the effect of methyl jasmonate and epibrassinolide on alleviation the salinity stress. Int. J. Biosci. (IJB) 2014, 5, 116–125. [Google Scholar]
- Huang, T.; Liu, G.; Zhu, L.; Liu, J.; Xiang, Y.; Xu, X.; Zhang, Z. Mitigation of chilling injury in mango fruit by methyl jasmonate is associated with regulation of antioxidant capacity and energy homeostasis. Postharvest Biol. Technol. 2024, 211, 112801. [Google Scholar] [CrossRef]
- Ahmadi, F.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, R.; Liu, S.; Li, R.; Wang, Y.; Chen, Y.; Hou, H.; Dai, Q. Methyl jasmonate alleviates the deleterious effects of salinity stress by augmenting antioxidant enzyme activity and ion homeostasis in rice (Oryza sativa L.). Agronomy 2022, 12, 2343. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, C.; Pervaiz, T.; Zhao, P.; Zhongjie, L.; Wang, B.; Wang, C.; Zhang, L.; Fang, J.; Qian, J. Jasmonic acid involves in grape fruit ripening and resistant against Botrytis cinerea. Funct. Integr. Genom. 2016, 16, 79–94. [Google Scholar] [CrossRef]
- Muganu, M.; Paolocci, M. Adaptation of Local Grapevine Germplasm: Exploitation of Natural Defence Mechanisms to Biotic Stress. In The Mediterranean Genetic Code-Grapevine and Olive; IntechOpen: London, UK, 2013; pp. 221–246. [Google Scholar]
- Kennelly, M.M.; Gadoury, D.M.; Wilcox, W.F.; Magarey, P.A.; Seem, R.C. Seasonal development of ontogenic resistance to downy mildew in grape berries and rachises. Phytopathology 2005, 95, 1445–1452. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, T.; Liu, J.; Xu, X.; Zhang, Z. Transcriptome analysis reveals the potential mechanism of methyl jasmonate alleviated ripening disorder in mango fruit at low temperature. Food Chem. 2025, 463, 141093. [Google Scholar] [CrossRef]
- Lu, S.; Che, L.; Gou, H.; Li, M.; Zeng, B.; Yang, J.; Chen, B.; Mao, J. Integrated Transcriptomic and Proteomic Analyses Demonstrated That MeJA-Regulated VvPAL10 Enhances Cold Tolerance in Grapevines. Physiol. Plant. 2025, 177, e70299. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Mirian, G.; Pérez-Álvarez, E.; Urturi, I.; Marín San Román, S.; Murillo-Peña, R.; Garde-Cerdan, T. Influence of methyl jasmonate and methyl jasmonate plus urea foliar applications on amino acids composition throughout ‘Tempranillo’ grape ripening over two seasons. Eur. Food Res. Technol. 2024, 250, 1823–1836. [Google Scholar] [CrossRef]
- Sarabandi, M.; Farokhzad, A.; Mandoulakani, B.A.; Ghasemzadeh, R. Biochemical and gene expression responses of two Iranian grape cultivars to foliar application of methyl jasmonate under boron toxicity conditions. Sci. Hortic. 2019, 249, 355–363. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.; Lachhab, N.; Sanzani, S.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Ali, S.; Mir, R.A.; Haque, M.A.; Danishuddin; Almalki, M.A.; Alfredan, M.; Khalifa, A.; Mahmoudi, H.; Shahid, M.; Tyagi, A. Exploring physiological and molecular dynamics of drought stress responses in plants: Challenges and future directions. Front. Plant Sci. 2025, 16, 1565635. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.-l.; Yue, X.-f.; Zhao, X.-f.; Zhao, H.; Fang, Y.-l. Physiological, micro-morphological and metabolomic analysis of grapevine (Vitis vinifera L.) leaf of plants under water stress. Plant Physiol. Biochem. 2018, 130, 501–510. [Google Scholar] [CrossRef]
- Wang, Z.; Wong, D.C.J.; Wang, Y.; Xu, G.; Ren, C.; Liu, Y.; Kuang, Y.; Fan, P.; Li, S.; Xin, H. GRAS-domain transcription factor PAT1 regulates jasmonic acid biosynthesis in grape cold stress response. Plant Physiol. 2021, 186, 1660–1678. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Romanazzi, G.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. A review of the use of biostimulants in the vineyard for improved grape and wine quality: Effects on prevention of grapevine diseases. J. Sci. Food Agric. 2019, 99, 1001–1009. [Google Scholar] [CrossRef]
- Shah, A.T.; Baba, A.I. Plant Root Responses and Acclimation to Flooding Stress. In Plant Flooding: Sensitivity and Tolerance Mechanisms; Springer: Cham, Switzerland, 2025; pp. 29–54. [Google Scholar]
- Li, J.; Li, D.; Liu, B.; Wang, R.; Yan, Y.; Li, G.; Wang, L.; Ma, C.; Xu, W.; Zhao, L. Effects of root restriction on phytohormone levels in different growth stages and grapevine organs. Sci. Rep. 2022, 12, 1323. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, X.; Chen, M.; Wang, L.; Xia, J.; Wang, Z.; Fang, J.; Tran, L.-S.; Shangguan, L. Copper stress in grapevine: Consequences, responses, and a novel mitigation strategy using 5-aminolevulinic acid. Environ. Pollut. 2022, 307, 119561. [Google Scholar] [CrossRef]
- Belchí-Navarro, S.; Almagro, L.; Sabater-Jara, A.B.; Fernández-Pérez, F.; Bru, R.; Pedreño, M.A. Early signaling events in grapevine cells elicited with cyclodextrins and methyl jasmonate. Plant Physiol. Biochem. 2013, 62, 107–110. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, M.; Dang, S.; Zhou, J.; Zhang, Y. Comparative transcriptomic analysis of transcription factors and hormones during flower bud differentiation in ‘Red Globe’ grape under red-blue light. Sci. Rep. 2023, 13, 8932. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.U.; Hanif, M.; Wan, R.; Hou, X.; Ahmad, B.; Wang, X. Screening Vitis genotypes for responses to Botrytis cinerea and evaluation of antioxidant enzymes, reactive oxygen species and jasmonic acid in resistant and susceptible hosts. Molecules 2018, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Fang, L.; Zhu, Z.; Zhang, L.; Sun, X.; Wang, Y.; Wang, Q.; Li, S.; Xin, H. The transcription factor VaNAC17 from grapevine (Vitis amurensis) enhances drought tolerance by modulating jasmonic acid biosynthesis in transgenic Arabidopsis. Plant cell reports 2020, 39, 621–634. [Google Scholar] [CrossRef] [PubMed]
- González-Herranz, R.; Cathline, K.A.; Fidelibus, M.W.; Burns, J.K. Potential of methyl jasmonate as a harvest aid for ‘Thompson Seedless’ grapes: Concentration and time needed for consistent berry loosening. HortScience 2009, 44, 1330–1333. [Google Scholar] [CrossRef]
- Ahmad, W. Abiotic stresses and their effects, responses, and adaptations in grapevines (Vitis vinifera): Overview of modern research: A review. Rev. Artic. Am. J. Agric. Res. 2019, 4, 33. [Google Scholar]
- Vera-Guzmán, A.M.; Aispuro-Hernández, E.; Vargas-Arispuro, I.; Islas-Osuna, M.A.; Martínez-Téllez, M.Á. Expression of antioxidant-related genes in flavedo of cold-stored grapefruit (Citrus paradisi Macfad cv. Rio Red) treated with pectic oligosaccharides. Sci. Hortic. 2019, 243, 274–280. [Google Scholar] [CrossRef]
- Rahman, F.U.; Zhang, Y.; Khan, I.A.; Liu, R.; Sun, L.; Wu, Y.; Jiang, J.; Fan, X.; Liu, C. The promoter analysis of VvPR1 gene: A candidate gene identified through transcriptional profiling of methyl jasmonate treated grapevine (Vitis vinifera L.). Plants 2022, 11, 1540. [Google Scholar] [CrossRef]
- Uçarlı, C. Drought Stress and the Role of NAC Transcription Factors in Drought Response. In Drought Stress: Review and Recommendations; Springer: Cham, Switzerland, 2025; pp. 295–320. [Google Scholar]
- Dubrovina, A.S.; Kiselev, K.V.; Khristenko, V.S.; Aleynova, O.A. VaCPK20, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., mediates cold and drought stress tolerance. J. Plant Physiol. 2015, 185, 1–12. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V.; Khristenko, V.S.; Aleynova, O.A. VaCPK21, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., is involved in grape response to salt stress. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 124, 137–150. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.-T.; Zhao, F.-L.; Hu, Y.; Gao, Y.-R.; Ma, Y.-F.; Zheng, Y.; Wang, Y.-J.; Wen, Y.-Q. Genome-wide identification and expression analysis of the CDPK gene family in grape, Vitis spp. BMC Plant Biol. 2015, 15, 164. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, J.; Xu, W.; Li, H.; He, M.; Xu, Y.; Xu, T.; Yang, Y.; Cao, J.; Wang, Y. Three ERF transcription factors from Chinese wild grapevine Vitis pseudoreticulata participate in different biotic and abiotic stress-responsive pathways. J. Plant Physiol. 2013, 170, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Cavallini, E.; Zenoni, S.; Finezzo, L.; Begheldo, M.; Ruperti, B.; Tornielli, G.B. A grapevine TTG2-like WRKY transcription factor is involved in regulating vacuolar transport and flavonoid biosynthesis. Front. Plant Sci. 2017, 7, 1979. [Google Scholar] [CrossRef] [PubMed]
- Paolacci, A.R.; Catarcione, G.; Ederli, L.; Zadra, C.; Pasqualini, S.; Badiani, M.; Musetti, R.; Santi, S.; Ciaffi, M. Jasmonate-mediated defence responses, unlike salicylate-mediated responses, are involved in the recovery of grapevine from bois noir disease. BMC Plant Biol. 2017, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Hou, L.; Xiao, P.; Guo, Y.; Deyholos, M.K.; Liu, X. VvWRKY30, a grape WRKY transcription factor, plays a positive regulatory role under salinity stress. Plant Sci. 2019, 280, 132–142. [Google Scholar] [CrossRef]
- Zhao, N.; Lin, H.; Lan, S.; Jia, Q.; Chen, X.; Guo, H.; Chen, F. VvMJE1 of the grapevine (Vitis vinifera) VvMES methylesterase family encodes for methyl jasmonate esterase and has a role in stress response. Plant Physiol. Biochem. 2016, 102, 125–132. [Google Scholar] [CrossRef]
- Shangguan, L.; Fang, X.; Chen, L.; Cui, L.; Fang, J. Genome-wide analysis of autophagy-related genes (ARGs) in grapevine and plant tolerance to copper stress. Planta 2018, 247, 1449–1463. [Google Scholar] [CrossRef]
- Leng, X.; Wang, P.; Zhu, X.; Li, X.; Zheng, T.; Shangguan, L.; Fang, J. Ectopic expression of CSD1 and CSD2 targeting genes of miR398 in grapevine is associated with oxidative stress tolerance. Funct. Integr. Genom. 2017, 17, 697–710. [Google Scholar] [CrossRef]
- Armijo, G.; Schlechter, R.; Agurto, M.; Muñoz, D.; Nuñez, C.; Arce-Johnson, P. Grapevine Pathogenic Microorganisms: Understanding Infection Strategies and Host Response Scenarios. Front. Plant Sci. 2016, 7, 382. [Google Scholar] [CrossRef]
- Agurto, M.; Schlechter, R.; Armijo, G.; Carrera Solano, E.I.; Serrano, C.; Contreras, R.; Zuñiga, G.; Arce-Johnson, P. RUN1 and REN1 Pyramiding in Grapevine (Vitis vinifera cv. Crimson Seedless) Displays an Improved Defense Response Leading to Enhanced Resistance to Powdery Mildew (Erysiphe necator). Front. Plant Sci. 2017, 8, 758. [Google Scholar] [CrossRef]
- Katula-Debreceni, D.; Lencsés, A.; Szőke, A.; Veres, A.; Hoffmann, S.; Kozma, P.; Kovács, L.; Heszky, L.; Kiss, E. Marker-assisted selection for two dominant powdery mildew resistance genes introgressed into a hybrid grape population. Sci. Hortic. 2010, 126, 448–453. [Google Scholar] [CrossRef]
- Lin, H.; Leng, H.; Guo, Y.; Kondo, S.; Zhao, Y.; Shi, G.; Guo, X. QTLs and candidate genes for downy mildew resistance conferred by interspecific grape (V. vinifera L.× V. amurensis Rupr.) crossing. Sci. Hortic. 2019, 244, 200–207. [Google Scholar] [CrossRef]
- Le Floch, G.; Vallance, J.; Benhamou, N.; Rey, P. Combining the oomycete Pythium oligandrum with two other antagonistic fungi: Root relationships and tomato grey mold biocontrol. Biol. Control 2009, 50, 288–298. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Pessina, S.; Lenzi, L.; Perazzolli, M.; Campa, M.; Dalla Costa, L.; Urso, S.; Valè, G.; Salamini, F.; Velasco, R.; Malnoy, M. Knockdown of MLO genes reduces susceptibility to powdery mildew in grapevine. Hortic. Res. 2016, 3, 16016. [Google Scholar] [CrossRef]
- Pap, D.; Riaz, S.; Dry, I.B.; Jermakow, A.; Tenscher, A.C.; Cantu, D.; Oláh, R.; Walker, M.A. Identification of two novel powdery mildew resistance loci, Ren6 and Ren7, from the wild Chinese grape species Vitis piasezkii. BMC Plant Biol. 2016, 16, 170. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Li, X.-Z.; Wu, Z.-J.; Chen, D.-X.; Li, G.-R.; Li, X.-Q.; Zhang, G.-H. VvZFP11, a Cys2His2-type zinc finger transcription factor, is involved in defense responses in Vitis vinifera. Biol. Plant. 2016, 60, 292–298. [Google Scholar] [CrossRef]
- Böttcher, C.; Burbidge, C.A.; di Rienzo, V.; Boss, P.K.; Davies, C. Jasmonic acid-isoleucine formation in grapevine (Vitis vinifera L.) by two enzymes with distinct transcription profiles. J. Integr. Plant Biol. 2015, 57, 618–627. [Google Scholar] [CrossRef]
- Boss, P.K.; Davies, C.; Robinson, S.P. Anthocyanin composition and anthocyanin pathway gene expression in grapevine sports differing in berry skin colour. Aust. J. Grape Wine Res. 1996, 2, 163–170. [Google Scholar] [CrossRef]
- Boss, P.K.; Davies, C.; Robinson, S.P. Expression of anthocyanin biosynthesis pathway genes in red and white grapes. Plant Mol. Biol. 1996, 32, 565–569. [Google Scholar] [CrossRef]
- Goto-Yamamoto, N.; Wan, G.H.; Masaki, K.; Kobayashi, S. Structure and transcription of three chalcone synthase genes of grapevine (Vitis vinifera). Plant Sci. 2002, 162, 867–872. [Google Scholar] [CrossRef]
- Pérez-Castro, R.; Kasai, K.; Gainza-Cortés, F.; Ruiz-Lara, S.; Casaretto, J.A.; Peña-Cortés, H.; Tapia, J.; Fujiwara, T.; González, E. VvBOR1, the grapevine ortholog of AtBOR1, encodes an efflux boron transporter that is differentially expressed throughout reproductive development of Vitis vinifera L. Plant Cell Physiol. 2012, 53, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.-l.; Liu, B.-c.; Xu, X.-l.; Wu, J.-r.; Sun, W.; Fang, Y.-l. Targeted metabolomic and transcript level analysis reveals the effects of exogenous strigolactone and methyl jasmonate on grape quality. Sci. Hortic. 2022, 299, 111009. [Google Scholar] [CrossRef]
- Francini, A.; Giro, A.; Ferrante, A. Biochemical and molecular regulation of phenylpropanoids pathway under abiotic stresses. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 183–192. [Google Scholar]
- Qu, J.-G.; Yu, X.-J.; Zhang, W.; Jin, M.-F. Significant improved anthocyanins biosynthesis in suspension cultures of Vitis vinifera by process intensification. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2006, 22, 299–305. [Google Scholar]
- Larronde, F.; Gaudillère, J.P.; Krisa, S.; Decendit, A.; Deffieux, G.; Mérillon, J.M. Airborne methyl jasmonate induces stilbene accumulation in leaves and berries of grapevine plants. Am. J. Enol. Vitic. 2003, 54, 63–66. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Bahmani, R.; Rathor, P.; More, P.; Prithiviraj, B. Synergistic activation of grapevine defense mechanisms against downy mildew by Ascophyllum nodosum extract and Pseudomonas fluorescens CHA0. Front. Plant Sci. 2025, 16, 1568426. [Google Scholar] [CrossRef]
- Yang, Y.; He, M.; Zhu, Z.; Li, S.; Xu, Y.; Zhang, C.; Singer, S.D.; Wang, Y. Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biol. 2012, 12, 140. [Google Scholar] [CrossRef]
- Sandroni, M.; Liljeroth, E.; Mulugeta, T.; Alexandersson, E. Plant resistance inducers (PRIs): Perspectives for future disease management in the field. CABI Rev. 2020, 1–10. [Google Scholar] [CrossRef]
- Qi, J.; Wang, H.; Wu, X.; Noman, M.; Wen, Y.; Li, D.; Song, F. Genome-wide characterization of the PLATZ gene family in watermelon (Citrullus lanatus L.) with putative functions in biotic and abiotic stress response. Plant Physiol. Biochem. 2023, 201, 107854. [Google Scholar] [CrossRef]
- Ponti, L.; Gutierrez, A.P.; Boggia, A.; Neteler, M. Analysis of grape production in the face of climate change. Climate 2018, 6, 20. [Google Scholar] [CrossRef]
- Lv, N.; Li, L.; Wang, N.; Guo, C.-j.; Zhang, H.-y. Methyl Jasmonate Treatment Relieves Chilling Injury and Improves the Postharvest Quality of Snap Bean by Regulating Antioxidant Metabolism. Agric. Res. 2024, 13, 198–203. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.; Gil-Muñoz, R. Metabolic Response Induced by Methyl Jasmonate and Benzothiadiazole in Vitis vinifera cv. Monastrell Seedlings. Horticulturae 2025, 11, 277. [Google Scholar] [CrossRef]
- Rachappanavar, V.; Padiyal, A.; Sharma, J.K.; Gupta, S.K. Plant hormone-mediated stress regulation responses in fruit crops-a review. Sci. Hortic. 2022, 304, 111302. [Google Scholar] [CrossRef]
- Han, J.; Dai, J.; Chen, Z.; Li, W.; Li, X.; Zhang, L.; Yao, A.; Zhang, B.; Han, D. Overexpression of a ‘Beta’ MYB Factor Gene, VhMYB15, Increases Salinity and Drought Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 1534. [Google Scholar] [CrossRef]
- Eitle, M.W.; Griesser, M.; Vankova, R.; Dobrev, P.; Aberer, S.; Forneck, A. Grape phylloxera (D. vitifoliae) manipulates SA/JA concentrations and signalling pathways in root galls of Vitis spp. Plant Physiol Biochem. 2019, 144, 85–91. [Google Scholar]
- Wang, P.; Su, L.; Gao, H.; Jiang, X.; Wu, X.; Li, Y.; Zhang, Q.; Wang, Y.; Ren, F. Genome-Wide Characterization of bHLH Genes in Grape and Analysis of their Potential Relevance to Abiotic Stress Tolerance and Secondary Metabolite Biosynthesis. Front. Plant Sci. 2018, 9, 64. [Google Scholar] [CrossRef]
- Gong, P.; Kang, J.; Sadeghnezhad, E.; Bao, R.; Ge, M.; Zhuge, Y.; Shangguan, L.; Fang, J. Transcriptional profiling of resistant and susceptible cultivars of grapevine (Vitis L.) reveals hypersensitive responses to Plasmopara viticola. Front. Microbiol. 2022, 13, 846504. [Google Scholar] [CrossRef]




| Genes/MeJA | Gene Functions | Plant/Cultivar | Stress Levels | References |
|---|---|---|---|---|
| VvMJEA1 | Involved in the homology modeling and demethylation reaction mechanisms | Vitis vinifera | Heat, cold, and UV-B | [102] |
| VaNAC17 | Enhancement of abscisic acid and no apical meristem modulation. | Vitis amurensis | Drought | [89] |
| VvARGs | Autophagy-related genes (ARGs) were involved in embryonic development, protein degradation pathways, and seed abortion. | Vitis vinifera | Copper | [103] |
| VvCSDs | Sex determination system, protein initiation, regulation of cell death, and ROS scavenging systems | Vitis vinifera | Oxidative stress | [104] |
| VaCPK20 | Involved in calcium-dependent protein kinase, increased the accumulation of proteins, crop resistant, and regulated hormone relief. | Vitis amurensis Rupr | Cold and drought | [95] |
| VaCPK21 | Involved in calcium-dependent protein kinase, contribution of hormones to stress tolerance, and accumulation of compatible solutes | Vitis amurensis Rupr | Salt | [96] |
| VvT4SS | Involved in hormonal regulation, favoring hyphal growth, and virulence to the pathogens | Vitis vinifera | Pathogenic microorganism | [105] |
| VvRUN1 and VvREN1 | Involved in the defense responses, fungal tolerance, disease management, and resistance to vinery disease | Crimson seedless | Powdery mildew | [106] |
| Genes | Elucidation of Gene | Crop/Variety | Functions | References |
|---|---|---|---|---|
| VvGH3-7 and VvGH3-9 | Gretchen Hagen3 (GH3) proteins synthetases | Vitis vinifera | Play positive role in Jasmonic acid-isoleucine formation | [124] |
| DHNs | The Dehydrins (DHNs) gene superfamily desiccation harm over environmental stress | Vitis vinifera | Involved in the resistance to numerous pathogens | [125] |
| VvPR1 | Pathogenesis-related gene 1 is a marker gene | Vitis vinifera | Carries an essential role in hormonal protection mechanism | [126] |
| VvSTS | The stilbene synthase for pathway | Vitis vinifera | Probably enclosed in controlling cell-dropping. | [127] |
| VviGT | The gene encoding for glycosyltransferases | Gewürztraminer grapes | Synthesis of terpene and glycosidic linkage | [35] |
| VvChs1, VvChs2 and VvChs3 | Play a significant role in transcription regulation | Cabernet Sauvignon | They are probably responsible for promoting coloration | [117] |
| UFGT | Genes encoding with the berry skin coloration | Shiraz grapes | Enhanced expression level of anthocyanin biosynthesis | [116] |
| UFGT | UDP glucose-flavonoid 3-o-glucosyl transferase (UFGT) | Colored grapes | Involved in increasing anthocyanin synthesis | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakeem, A.; Li, S.; Nasiru, M.M.; Mustafa, G.; Elatafi, E.; Shangguan, L.; Fang, J. Methyl Jasmonate Acts as a Crucial Player in Abiotic Stress Responses in Grape. Stresses 2025, 5, 40. https://doi.org/10.3390/stresses5020040
Hakeem A, Li S, Nasiru MM, Mustafa G, Elatafi E, Shangguan L, Fang J. Methyl Jasmonate Acts as a Crucial Player in Abiotic Stress Responses in Grape. Stresses. 2025; 5(2):40. https://doi.org/10.3390/stresses5020040
Chicago/Turabian StyleHakeem, Abdul, Shaonan Li, Mustapha Muhammad Nasiru, Ghulam Mustafa, Essam Elatafi, Lingfei Shangguan, and Jinggui Fang. 2025. "Methyl Jasmonate Acts as a Crucial Player in Abiotic Stress Responses in Grape" Stresses 5, no. 2: 40. https://doi.org/10.3390/stresses5020040
APA StyleHakeem, A., Li, S., Nasiru, M. M., Mustafa, G., Elatafi, E., Shangguan, L., & Fang, J. (2025). Methyl Jasmonate Acts as a Crucial Player in Abiotic Stress Responses in Grape. Stresses, 5(2), 40. https://doi.org/10.3390/stresses5020040









