Abstract
The relationship between various central nervous system (CNS) disorders linked to pesticide exposure highlights a growing concern worldwide, as the extensive use of these compounds causes toxic effects on the CNS of non-target organisms. Reports indicate that exposure to pesticides, including carbamates, organophosphates, and pyrethroids, produces various adverse impacts on neurological function in humans, ranging from acute symptoms such as headaches and dizziness to long-term conditions leading to developmental delays in children, cognitive impairment, and neurodegenerative diseases, such as Parkinson’s and Alzheimer’s being among the most important. The scientific evidence suggests that pesticide exposure induces oxidative stress and disruptions in neurotransmission, resulting in neuronal damage and alterations in brain development. The review discusses scientific evidence of neurodegenerative disease development related to pesticide exposure, as well as alternatives to chemical pesticides used in agriculture, emphasizing Agroecological Crop Protection (ACP), which combines biological control, crop rotation, and natural predators and is presented as a practical approach to reducing reliance on pesticides. Organic farming methods, which employ natural substances and minimal input of chemicals, also offer safer alternatives. In addition, advances in biopesticides, which target specific pests without harming non-target organisms, provide promising solutions that protect the environment and human health. Pesticides are well-known environmental stressors that menace biodiversity and pose important threats to human health. Reducing pesticide use and remediating pesticide-polluted sites are urgent tasks to avoid adverse effects of pesticide exposure in non-target organisms.
1. Introduction
Pesticides are a diverse group of chemicals that play a crucial role in pest control for agricultural practices. They help ensure crop productivity and improve the quality of food and raw materials throughout the value chain [1,2]. Additionally, pesticides are essential for managing pests in industrial and domestic settings as well as for controlling disease vectors in public health contexts [3]. Important social and economic benefits are generated from their use [4].
The use of pesticides in agriculture is highly generalized throughout the world [5]. According to data from the Food and Agriculture Organization (FAO), in 2022, pesticides employed in the agricultural sector reached the amount of 3.69 million tons (Mt). Herbicides were the most used pesticides, accounting for approximately 1.94 Mt (52.6%) of total usage. This was followed by fungicides and bactericides, which comprised 0.79 Mt (21.5%), and insecticides at 0.77 Mt (20.9%). Lastly, rodenticides were used in a small proportion, totaling 0.017 Mt (0.5%). On average, the application of pesticides in crops worldwide was 2.37 kg/ha, while the global per capita consumption of pesticides was 0.46 kg [6].
Pesticides are well-known environmental stressors. The widespread employment of pesticides worldwide has generated severe environmental pollution events, and pesticide residues have been identified in air, soil, and water, generating great environmental concern globally [7,8]. The occurrence of pesticides in the environment promotes exposure of non-target organisms at both the acute and chronic levels, generating severe ecotoxicological impacts that endanger biodiversity and the structure of aquatic and terrestrial ecosystems [9,10,11,12]. Excessive pesticide use has been connected to multiple harmful impacts on human health, such as poisoning, respiratory, reproductive, and neurological diseases. Likewise, exposure to pesticides has been linked to the onset of chronic degenerative diseases, including diabetes, cancer, Alzheimer’s, and Parkinson’s, among others [13,14,15].
Pesticide exposure has neurotoxic effects on different organisms, such as insects, aquatic organisms, birds, and mammals. Pesticide exposure-induced neurotoxicity is caused by oxidative stress, neuroinflammation, and alterations in neurotransmitters and their receptor activity, leading to neuronal dysfunction, developmental delays in the central nervous system (CNS), behavioral modifications, and reductions in cognitive functions, such as spatial orientation, memory, learning, among others [16,17,18]. This review explores the key neurotoxic impacts of exposure to different types of pesticides on non-target species, such as humans and other mammals, and evaluates innovative strategies aimed at reducing chemical pesticide use in agricultural practices.
2. Pesticides Definition and Classification
Pesticides are substances or a mixture of substances of chemical or biological origin used to prevent, eliminate, or control pests and diseases that affect agricultural crops, livestock, or even human health [19,20]. These pests can be bacteria, fungi, insects, weeds, rodents, as well as other organisms that compete with humans for food or pose a risk to human and environmental health [21]. The classification of pesticides varies according to the study approach; the main classifications are based on (1) their use, (2) the type of pest they control, (3) their formulation, (4) chemical composition, (5) mode of action, and (6) hazardous or toxicity degree [22].
For example, according to their use, the following types of pesticides stand out: agricultural pesticides, used in agricultural production systems and in products or by-products of plant origin; forestry pesticides, employed in forests and wood production; urban pesticides, utilized in urban and industrial areas; gardening pesticides that are applied in garden maintenance and pest control in ornamental plants; livestock pesticides that are used on animals or animal production facilities for human consumption or industrial uses; domestic pesticides, commonly used inside homes; and public health pesticides that are used to control insect vectors of diseases for humans [23].
Pesticides are also categorized based on the type of pest they target. Herbicides are used to inhibit weed development, and fungicides are used to hinder or halt fungal growth; insecticides are employed to manage insect populations; acaricides eliminate mites that feed on animals and plants; molluscicides eliminate mollusks; nematicides combat nematodes; rodenticides control mice and other rodents; and bactericides act against pathogenic bacteria that affect plants, animals, or humans [20,24].
According to their formulation, pesticides are mixtures of active and inert ingredients [24,25]. Active ingredients are specific chemicals designed to target and control pests, while inert ingredients include water, petroleum-derived solvents, wetting agents, spreaders, adhesives, and extenders. Additionally, pesticides can be categorized by their physical form into three main types: solids (which include powders, granules, tablets, pellets, gels, and baits), liquids (such as suspensions and solutions), and gases (like aerosols and fumes).
Pesticides are categorized based on how they work, falling into the following groups: contact, ingestion, systemic, fumigants, defoliants, and repellents [24]. Each one has a specific mode of action to determine their efficacy and use in different pest management contexts. A breakdown of each category is described below:
- Contact: Their action is performed upon direct contact with the target organism, disrupting essential biological processes or causing physical harm to the pests.
- Ingestion: When ingested, they are absorbed by the pest, affecting its digestive or metabolic system and leading to death.
- Systemic: These pesticides are taken up by plants and animals. For plants, once applied, they are absorbed and transported through the vascular system to the whole plant. Depending on their nature, some move to the top of the plant, others to the bottom, and some can move in both directions. These pesticides enter animals through the ingestion of plants that contain them.
- Fumigants: These are gases or vapors that, once inhaled by pests, interfere with their biological processes and cause their elimination.
- Defoliants: They eliminate plant leaves, affecting photosynthetic capacity and weakening the organism.
- Repellents: They do not cause pest death but act as a deterrent, preventing target organisms from approaching treated plants or surfaces.
On the other hand, under the Globally Harmonized System (GHS) for the classification and labeling of chemicals, pesticides are categorized based on their level of hazard or toxicity, ranging from “extremely hazardous” to “unlikely to present acute risk”, according to their potential for causing harm through oral or dermal exposure [26].
The GHS defines Class Ia as “extremely hazardous”, with an oral LD50 of less than 5 mg/kg and a dermal LD50 of less than 50 mg/kg. Class Ib, “highly hazardous”, includes substances with oral LD50 values between 5–50 mg/kg and dermal values between 50–200 mg/kg. Class II, “moderately hazardous”, refers to substances with oral LD50 values between 50–2000 mg/kg and dermal LD50 values between 200–2000 mg/kg. Class III, “slightly hazardous”, includes substances with oral and dermal LD50 values greater than 2000 mg/kg. Finally, Class U, “unlikely to present acute hazard”, applies to substances with both oral and dermal LD50 values exceeding 5000 mg/kg. The LD50 (lethal dose, 50%) is a statistical estimate representing the dose of a substance, expressed in milligrams per kilogram of body weight, required to cause death in 50% of a test population of animals [26].
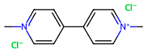
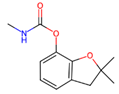
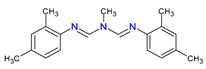
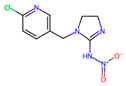
Depending on their chemical composition, pesticides are also classified into several categories, including bipyridyls, carbamates, neonicotinoids, organochlorines, organophosphates pyrethrins, and pyrethroids, as well as a special class of pesticides known as biopesticides (Table 1). Each category is defined by the chemical structure of its active compounds and their functional mechanisms targeting pests. Organochlorines are distinguished by their prolonged environmental persistence. In contrast, organophosphates and carbamates work by inhibiting essential enzymes in the CNS of pest organisms. Pyrethroids are man-made compounds derived from natural pyrethrins that mimic the effects of these natural insecticides. Bipyridyls are primarily used for weed control. Biological pesticides are those derived from living organisms or their products, such as microbial agents, bacteria, fungi, and viruses, that are designed to specifically target pests [27].

Table 1.
Chemical classification of pesticides.
3. Pesticides Mode of Action
Pesticides have different modes of action depending on the chemical group they belong to. They act on various physiological pathways in pests, including neurotransmission alterations, parasitism, and even increasing heavy metal toxicity. Understanding the action mechanisms of pesticides is essential to finding solutions to adverse consequences caused by these substances, such as environmental pollution, toxicity, and chronic disease induction, and improving public health [31].
3.1. Bipyridyls
Bipyridyl pesticides are a class of herbicides chemically characterized by their structure consisting of two pyridine rings that are aromatic, where one of the carbon atoms is replaced by one of nitrogen and are linked by an ethylene group. These compounds are used to control the growth of weeds and grasses in farming areas. They are known for their ability to disrupt plant cell processes, resulting in rapid damage and the eventual death of the target plants. A commonly known bipyridyl pesticide is paraquat (1,10-dimethyl-4,40-bipyridylium dichloride), which produces reactive oxygen species (ROS) in plant cells, resulting in oxidative damage that ultimately causes plant death. Another example is diquat (1,10-ethylene-2,20-bipyridylium dibromide), which operates through a similar mechanism [32,33,34,35,36]. Bipyridyl pesticides are extremely hazardous to both humans and animals [37]; they cause seizures, depression, and lack of coordination. Additionally, they can lead to gastroenteritis, respiratory difficulties due to pulmonary edema, and the development of alveolar fibrosis [34,36]. The use of paraquat has been restricted or regulated in numerous countries; for example, paraquat use is now banned in the European community [38].
3.2. Carbamates
Carbamate pesticides are organic compounds that originate from carbamic acid (NH2COOH), which are commonly used in homes, gardens, agriculture, aquaculture, and vector control due to their low environmental persistence and effectiveness in controlling insects on crops. Similar to organophosphates, carbamate pesticides like carbofuran (2,3-dihydro-2,2-dimethyl-7-benzofuranyl methylcarbamate), bendiocarb (2,2-dimethyl-2H-1,3-benzodioxol-4-yl methylcarbamate), carbaryl (1-naphthyl-N-methylcarbamate), and propoxur (2-[(propan-2-yl)oxy]phenyl methylcarbamate), among others, inhibit acetylcholinesterase (AchE) in a reversible manner, a key enzyme in the CNS. The accumulation of acetylcholine (Ach) at nerve synapses leads to continuous muscle stimulation, affecting smooth muscles like those in the iris, ciliary muscle, gut, bladder, and heart, and glands such as salivary and gastric glands, resulting in toxic symptoms such as muscle twitching, seizures, and respiratory failure [39,40].
3.3. Formamidines
Formamidine pesticides, such as amitraz (N,N-[(methylamino)dimethylidyne] di-2,4-xylidine) and chlordimeform (N′-(4-chloro-o-tolyl)-N,N-dimethylformamidine) [41], are used as acaricides, insecticides, and antiparasitic in the pharmaceutical, veterinary, and agricultural sectors. These compounds primarily target alpha-2 adrenergic receptors in the CNS [42,43,44], inhibiting the release of neurotransmitters like norepinephrine. Unique among insecticides, formamidines trigger toxic effects in invertebrates by activating an adenylate cyclase that is dependent on octopamine. This leads to sedation, hypotension, and, in some cases, a coma-like state due to the disruption of sympathetic nervous system function. Additionally, they affect other physiological processes, such as the heart rate, contributing to bradycardia [42,45].
3.4. Neonicotinoids
Neonicotinoids are a class of synthetic neurotoxic insecticides that are used mainly on crops, lawns, and gardens. Neonicotinoids act on the CNS by binding to nicotinic acetylcholine receptors. This group includes pesticides like acetamiprid, clothianidin, dinotefuran, imidacloprid, and thiamethoxam, which are often used to treat seeds, and in crops such as cotton, corn, grains, oilseed rape, and sugar beet [46,47]. They bind to these receptors and stimulate them, mimicking the action of ACh. However, unlike ACh, neonicotinoids do not break down quickly, leading to prolonged receptor activation, and therefore, nerves are hyperactivated. Such toxicity exerted by this type of pesticide on insects causes paralysis and, finally, death [47].
3.5. Organochlorines
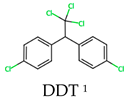
Organochlorine pesticides were developed during the 1930s and 1940s, and they are classified as persistent organic pollutants (POPs) and commonly employed in agriculture, industry, and household applications [48]. These compounds are mainly composed of chlorine, hydrogen, carbon, and sometimes oxygen atoms. Organochlorine pesticides are lipophilic compounds, providing them with the ability to accumulate in fatty tissues, leading to bioaccumulation in both humans and other species, which may result in toxicity and adverse health effects, including carcinogenesis, as well as immunological and reproductive disorders, which have been observed in living organisms, including humans and wildlife [49,50]. A representative example of an organochlorine pesticide is Dichlorodiphenyltrichloroethane, commonly known as DDT [31,51]. DDT was widely used to combat pests such as the Anopheles mosquito, which transmits the Plasmodium parasite that causes malaria; lice, transmitters of bacteria such as Rickettsia prowazekii, are known to cause typhus; and fleas are carriers of the zoonotic bacteria Yersinia pestis, responsible for bubonic plague [27], but there are also others, which are also known as endosulfan, lindane, endrin, dieldrin, and aldrin [50].
Organochlorine pesticides act by prolonging the sodium channels’ opening in nerve cells, which causes persistent depolarization and disrupts normal neural signal transmission. Additionally, they can block the inhibitory neurotransmitter GABA (gamma-aminobutyric acid) receptors and chloride channels regulated by voltage, which impairs neurotransmission, resulting in neurotoxic effects, convulsions, and death in the target organism. Inhibitory synapse disruption leads to continuous nervous excitation, resulting in hyperactivity, seizures, and, finally, paralysis [27,51,52].
3.6. Organophosphates
Since organophosphate pesticides are less persistent in the environment, biodegradable, and have lower toxicity, they have replaced organochlorine pesticides [27]. These pesticides are phosphoric acid esters primarily used as insecticides and herbicides. Malathion, parathion, and methyl-parathion are some examples. Organophosphate pesticides exert their effect by inhibiting AChE, the enzyme that normally degrades ACh into acetate and choline within the synaptic cleft. This inhibition causes ACh to accumulate at synapses, leading to excessive stimulation of muscles, glands, and the CNS [53].
3.7. Pyrethrins and Pyrethroids
Pyrethrins, which are derived from the Chrysanthemum cinerariaefolium and Chrysanthemum cineum, were historically among the most economically important natural neurotoxic pesticides used to manage a broad range of insect pests and vector transmitters of malaria, dengue, or yellow fever [27,54,55]. The development of pyrethroids was based on the structural framework of natural pyrethrins. These synthetic insecticides share a common chemical architecture composed of an acid moiety, an ester bond at the center, and an alcohol moiety. The acid segment contains two chiral centers, which typically results in the formation of cis and trans stereoisomers. Additionally, some pyrethroids contain a third chiral center located within the alcohol moiety, resulting in a total of eight possible stereo enantiomers. These structural characteristics are crucial, as pyrethroids target voltage-gated sodium channels [54,56] and both their insecticidal efficacy and toxicity to mammals are influenced by stereochemistry. Pyrethroid compounds like permethrin act by inducing the prolonged activation of these sodium channels. This disrupts normal neuronal signaling, leading to continuous nerve firing and ultimately causing paralysis. The neurotoxic effects include hyperexcitability, muscle tremors, paralysis, paresthesias in the mouth, extremities, and tongue, and even the organism’s death [51,57,58].
3.8. Triazines
Triazine pesticides represent a class of chemical compounds predominantly utilized in agriculture for weed control [59]. Their herbicidal action stems from their capacity to inhibit photosynthesis in plants, ultimately leading to plant death [60]. The triazine family contains various herbicides commonly used to control weeds, both broadleaf and grassy, in crops such as corn, sorghum, and other related plants [61]. These herbicides are particularly effective during both pre-emergence and post-emergence stages. A key triazine herbicide is atrazine [2-chloro-4-(ethylamino)-6-(isopropylamino)-s-triazine], one of the most commonly employed herbicides, especially in corn and sorghum fields, and in turf management. Atrazine disrupts plant photosynthesis by binding to D1 protein in Photosystem II [62]. Another common triazine herbicide is Simazine (6-chloro-N,N’-diethyl-1,3,5-triazine-2,4-diamine), utilized to regulate broadleaf and grassy weeds in different crops and non-cultivated areas, also acting through photosynthesis inhibition [63], reducing energy production and leading to plant cell death [64,65].
Certain triazine herbicides, especially atrazine, are known to persist in the environment and potentially contaminate ground and surface water, raising concerns about drinking water quality [66,67]. These chemicals may also harm non-target organisms, such as those of aquatic life. Evidence has shown that exposure to triazines produces negative effects on amphibians, fish, crabs, algae, and other wildlife [68]. Additionally, long-term exposure to triazine pesticides is associated with several health problems, including endocrine disruption, reproductive issues, and cancer [69,70].
4. Pesticide Environmental Stressor
Pollution caused by the indiscriminate use of pesticides in agriculture is recognized as an important environmental stressor factor that threatens non-target organisms and could disrupt ecosystems [8,71]. The high-scale use of pesticides worldwide causes pollution in soil, water, and air [72]. Pesticides reache the environment due to their high-scale industrial production and extensive application in agriculture; spills, leaks, wastewater, and pesticide containers’ inappropriate disposal during manufacturing and agricultural practices have been identified among the most important environmental pollution sources [73,74,75].
Pesticides are toxic and highly persistent molecules; they can also bioaccumulate and biomagnify in organisms’ tissues, disrupting food webs. Due to these characteristics, pesticides persist in the environment for extended periods, favoring their interaction with non-target organisms [76]. The interaction of wildlife with pesticides in the environment includes acute and chronic exposure [77,78]. Several negative impacts on non-target organisms (including animals, microorganisms, and plants) derived from pesticide exposure have been broadly documented in different environments worldwide [79].
Pesticide pollution in soils induces adverse effects in microbial communities, reducing beneficial soil microorganisms’ diversity due to direct toxicity reduction in microbial growth and metabolic activity [80]. Pesticides in the soil also affect important soil organisms, such as nematodes, acari, micro- and macro-arthropods, collembola, earthworms [81], as well as advantageous insects [82] like pollinators such as honeybees, or inclusively, birds [83], menacing soil fertility and sustainability of agroecosystems [84].
In aquatic environments, pesticide presence is considered an important environmental risk [85,86]. Pesticides have been detected in surface and underground water bodies [87], and their occurrence of pesticides of different chemical nature and biological activity in aquatic environments represents a risk to microbial, plant, and animal biodiversity [78,88]. Some examples of pesticide pollution’s impact on aquatic environments are described below; exposure to pesticide mixtures (insecticide esfenvalerate and fungicide prochloraz) induces synergistic adverse effects on the model organism Daphnia magna (crustacean), especially in stressing environmental conditions, such as food limitation [89]. Pesticides present in commercial formulations are bioaccumulated in fish tissues (Mystus keletius; catfish), causing hematological disorders, such as decreases in total erythrocyte, hemoglobin, and hematocrit and interfering in the correct activity of the respiratory enzymes glyceraldehyde dehydrogenase, malate dehydrogenase, and succinate dehydrogenase [90], while exposure to organophosphate and triazine pesticides causes cellular damage, hepatoxicity, endocrine disruption, and immunological and neurological disorders in different fish species [76]. Moreover, bioaccumulation and biomagnification of pesticides compromise the integrity of food webs in aquatic environments [91,92].
The environmental presence of pesticides favors human exposure to these toxic chemicals, generating both acute and chronic effects (Figure 1). Among the more representative acute effects include abdominal pain, breathing difficulty, convulsions, cough, diarrhea, dizziness, headache, nausea, skin irritation, vomiting, and weakness, while chronic exposure to pesticides has been associated with the manifestation of different illnesses, such as cancer, diabetes, respiratory, reproductive, and neurological disorders [13,93,94,95]. The presence of pesticides in the environment induces severe negative impacts on ecosystems and human health. These negative impacts include the deterioration of soil, water, and air quality, threats to wildlife biodiversity, and acute and chronic toxic effects on humans that are derived from pesticide exposure [9,94,96,97].
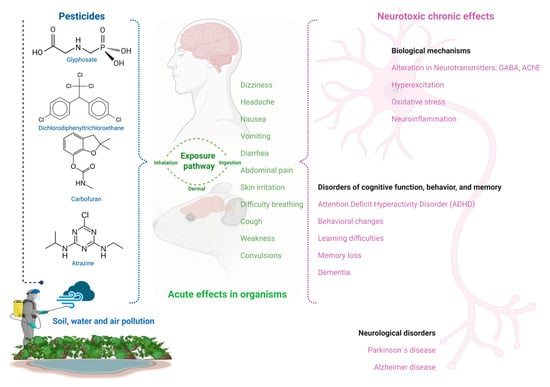
Figure 1.
Pesticide exposure’s acute effects and neurotoxicity in mice and humans.
5. Environmental Stress-Induced Neurotoxicity
The rise in the proportion of the aging population has led to an increase in age-related neurodegenerative disorders, which are attributed to genetic or environmental causes. Among the environmental factors, there are many environmental pollutants (heavy metals, solvents, and pesticides, among others) that can contribute to environmental stress-induced neurotoxicity, as they trigger transduction responses that lead to the onset of neurodegenerative disorders. Acute or chronic exposure, as well as the administration of these environmental stressor molecules, have been shown to alter neurotransmission in vitro and in vivo within different assays and animal models [98,99]. These environmental stressor molecules play an essential role in the characterization of adverse effects on differentiation and development, as well as in the cellular response to stress, as they cause a wide variety of toxic effects, including cell death, the promotion of several metabolic pathways, such as that of the β-oxidation of fatty acids, as well as oxidative stress, mitochondrial dysfunction, and tryptophan and glutathione metabolism, among others, which aggravate neurodegenerative diseases by directly altering several neurotransmission mechanisms, such as the inhibition of enzymes that are in charge of synthesizing or degrading neurotransmitters or those involved in their subsequent release and uptake, as well as enzymes that activate postsynaptic receptors. In addition, it has been reported that neurotoxic agents can disturb neurotransmission via energy metabolism by interfering with the adequate operation of sodium channels and ATPases [100,101,102]. Among neurotoxic pollutants, pesticides are of special concern; chronic exposure to different pesticides has been related to several neurologic disorders and the development of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis [103,104]. The neurotoxic effects of different classes of pesticides are described below.
5.1. Formamidines
Formamidines may cause neurotoxicity in mammals through the inhibition of the enzyme monoamine oxidase, as has been evaluated in vitro and in vivo; formamidines’ poisoning symptoms are sympathomimetic. Moreover, it has been reported that formamidines can inhibit oxidative phosphorylation and prostaglandin synthesis, produce a local anesthetic-like effect, and block neuromuscular transmission [42,100].
Furthermore, alpha2-adrenoreceptors are adrenergic receptors in the central nervous system and presynaptic peripheral neuron terminals. They mediate responses to endogenous catecholamines, noradrenaline, and adrenaline. These receptors are classified as alpha-adrenergic receptors, which regulate most of the excitatory actions (vasoconstriction, contraction of the uterine musculature, contraction of the ureter, pupillary dilation, etc.) and an important inhibitory function (intestinal relaxation). Additionally, this class of compounds induces seizures, among other effects. Amitraz acts as an agonist of alpha2-adrenergic receptors in the CNS. Since excitatory and inhibitory neurotransmission are mediated mainly by glutamate and GABA, respectively, their alteration could be responsible for seizure onset. In this sense, the agonist action of amitraz has been suggested as probably responsible for this effect since it could alter these neurotransmitter systems and lead to convulsion occurrence. In contrast, beta-adrenergic receptors modulate most of the inhibitory effects (vasodilatation, relaxation of the uterine musculature, bronchodilatation, etc.) as well as important excitatory actions (e.g., cardiac stimulation) [105].
These receptors are indeed a significant target for the toxic effects of formamidine pesticides. Yohimbine, an alpha2-adrenergic antagonist, has been shown to counteract the effects of amitraz on the heart rate, intestinal movement, pupil size, and blood pressure in mice, rats, and dogs. Similarly, yohimbine has also been found to reverse the effects of chlordimeform on visual function and pain sensitivity in rats.
These formamidine pesticides inhibit the 3H-clonidine and 3H-yohimbine binding with alpha2-adrenoreceptors in vitro. The in vivo assays have also shown that chlordimeform and amitraz inhibit the binding of 3H-clonidine to those receptors in a dose-dependent manner in rat forebrain [106].
The activation of CNS alpha2-adrenoceptors by amitraz is responsible for altering the mental state (sedation and unconsciousness); as it is an adrenergic antagonist, it results in a potent neurotoxic compound that induces symptoms such as loss of the right-turn reflex, motor incoordination, appetite alteration, hyper-reactivity to external stimuli, aggressiveness, among other effects, through the alteration of different neurotransmitter systems [42]. The administration of this pesticide at 20, 50, and 80 mg/kg body weight increases the levels of serotonin (5-HT), norepinephrine (NE), and dopamine (DA), decreases those of their metabolites and the turnover rates in the CNS of male rats, with the striatum, the prefrontal cortex, and the hippocampus being the most affected. This pesticide also alters the sex steroid hormones that regulate the activity and expression of important enzymes for the synthesis and metabolism of neurotransmitters, such as aldehyde dehydrogenase (AD), catechol-O-methyltransferase (COMT), dopamine-B-hydroxylase (DBH), MAO, tyrosine hydroxylase (TH), and tryptophan hydroxylase (TRH). Considering that amitraz alters the hepatic metabolism of 17b-estradiol (E2) and testosterone (T) in rats and increases serum T in male rats at doses of 25 and 50 mg/kg, its effect on the monoaminergic neurotransmitter in male rats could be via changes in T or its E2 metabolites. This idea is supported by the evidence that E2 regulates the expression of enzymes involved in the synthesis and metabolism of 5-HT, DA, and NE [107]. Thus, affective, cognitive, motor, and behavioral functions are largely controlled by the 5-HT, NE, and DA systems. Therefore, dysregulation of these neurotransmitters by amitraz could lead to the impairment of these functions since E2 modulates neural functions, such as affect, anxiety, mood, fear, and cognitive function, as well as learning and memory [108].
5.2. Neonicotinoids
Neonicotinoids are among the most employed insecticides today [109]. However, because of their molecular mechanism and toxicity, neonicotinoids have undesirable harmful effects that can damage non-target species, including humans [110]. These pesticides can translocate to all plant parts, making them toxic to herbivorous insects. They also make the whole plant a potential vector for transfering these harmful chemicals to non-target species. Moreover, neonicotinoids are more persistent than other pesticides, as they cannot be easily removed for safe food consumption. Neonicotinoids are insecticidal molecules with a similar structure to nicotine, acting as agonists of insect nicotinic acetylcholine receptors (nAChRs). However, some metabolites derived from these pesticides have also demonstrated comparable or higher affinity than nicotine in mammalian nAChRs [111,112].
Abou-Donia et al. [113] investigated the effects of imidacloprid administered at a dose of 337 mg/kg body weight (single dose) to pregnant Sprague–Dawley rats on the ninth day of gestation. Inclined plane, beam-walking, and forepaw grip performance were evaluated 30 days after birth, with the motor cortex, septal hippocampus, and cerebellum examined for histopathology. The results of these evaluations evidenced deficits in beam-walk time, in the inclined plane test, and grip time, along with sensory-motor alterations, increased brain AChE activity, a significant rise in ligand binding for the muscarinic acetylcholine receptors m2mAChR, and glial fibrillary acidic protein (GFAP) expression in the motor cortex and hippocampus of adolescent offspring. In another study, the electrophysiological properties of stellate cells (known to possess both muscarinic and nicotinic AChl receptors) of the ventral cochlear nucleus (VCN) in mice exposed to imidacloprid at concentrations ≥10 μM for <1 min was studied using the patch clamp technique, and it was concluded that this pesticide can change the membrane properties of neurons possessing nAChRs and, as a consequence, their function [114].
Furthermore, it was reported that clothianidin exposure in the diets (0, 20, 60, or 180 ppm) of pregnant mice females throughout gestation and lactation or from gestation to offspring adulthood (at constant dietary levels of 0, 30, 60, or 120 ppm; from five weeks after birth, up to week 11) produced some effects in a few variables of exploratory behavior for a novel environment since it showed a slight tendency to be active in treatment groups in male offspring and showed a significant depression in the middle-dose group of adult females. Spontaneous behavior was significantly active in the middle-dose group in adult male mice of the F1 generation. Therefore, it seems that sensitivity to clothianidin is dependent on sex in mice. Clothianidin also decreases motor activity and habituation responses to acoustic stimuli during lactation. Females (F0) that consumed this same pesticide with 0% (control), 0.003%, 0.006%, and 0.012% of the diet during the period of preconception (from 5 weeks of age to mating), mating (five days), gestation (14 days), and lactation (from birth to weaning), produced offspring (F1) that also consumed the same doses of Clothianidin in their diet, from 4 to 11 weeks of age. One female and a male of the F0 and F1 generations were randomly selected from each group; those of F1 were still lactating and were subjected to different tests to evaluate their behavioral development. Clothianidin in F0 adults increased exploratory behavior and motor activity in a dose-dependent manner in both males and females. In F1 males, the dose of 0.006% delayed the development of swimming head angle and increased olfactory orientation. Moreover, clothianidin in F1 females accelerated surface righting and the development of swimming head angle, negative geotaxis at a low dose (0.003%), and retarded olfactory orientation (0.006%). Tests performed in these studies included surface righting, negative geotaxis, cliff avoidance, swimming behavior, olfactory orientation, cognitive function (in a multiple T de Biel water maze), exploratory behavior (3 and 8 weeks old), and spontaneous behavior (9–10 weeks old) [115,116].
In another study, once-daily administration of clothianidin by gavage at 0, 2, 8, and 24 mg/kg was reported in juvenile male Wistar rats, and the possible effect of this pesticide on memory and learning was evaluated. Tests to assess cognitive function included the Morris water maze and measurement of the mRNA expression levels of N-methyl D-aspartate 1 (GRIN 1), muscarinic receptor M1, synaptophysin (SYP), and growth-associated protein 43 (gap-43). The results showed that clothianidin did not affect the acquisition (learning phase) of the Morris water maze or gene expression at any dose, but there was a significant difference in the performance between infants and the control group after 24 mg/kg was applied. The study concluded that exposure to high doses of clothianidin causes deterioration of cognitive functions in infant rats [117].
Analyzing the results of this study, the effects that clothianidin exerts on exploratory behavior, motor activity, memory, and learning depend on the time of exposure, the dose employed, the administration pathway, and possibly the animal model chosen. However, in general, the effects are more evident in the offspring, especially when exposure to this pesticide begins during embryonic development. This could lead to an increased impairment of structures such as the blood–brain barrier, promoting activation of microglia and astrocytes, resulting in neuroinflammation and/or neurodegeneration. In addition, administration by gavage means that this chemical reaches the stomach directly, avoiding previous stages of degradation for its absorption.
5.3. Organochlorine
Overall, pesticide exposure exerts several neurotoxic effects on different organisms, including humans. Among organochlorine pesticides, DDT is without doubt the most widely known. Acute exposure to DDT at high doses induces several neurotoxic effects, such as motor disturbances, increased frequency of spontaneous movements, abnormal susceptibility to fear, and hypersensitivity to external stimuli (light, touch, sound), followed by fine tremors that subsequently evolve into gross tremors and tonic-clonic convulsions [16,98].
Many commercially developed insecticides act on the sodium channel and the GABA inhibitory neurotransmission system. Voltage-dependent sodium channels are critical for the generation and propagation of action potentials in neurons [118]. They are the primary target of several classes of insecticides, such as DDT, an organochlorine insecticide; pyrethroids, plant extract compounds and derivatives; and sodium channel-blocking insecticides (SCBIs), such as the synthetic pesticides indoxacarb and metaflumizone [119,120,121]. It has been demonstrated that once the sodium channels open, DDT acts on them to prolong their opening since DDT does not affect this ion channel when it is closed. DDT also inhibits the enzyme Ca2+-ATPase, located on the outer surface of the cell membrane, which is responsible for maintaining elevated calcium levels outside the neuron. Therefore, when inhibited by DDT, this compound reduces the extracellular concentration of calcium, promoting membrane instability and repeated firing [51].
In accordance with the aforementioned, this class of pesticides causes the sodium channels to remain open much longer than normal, generating a prolonged sodium current in the cell, characterized by a strong sodium tail current that slowly decreases once depolarization is reached; thus, the membrane remains depolarized for a longer period of time, favoring repetitive discharges and synaptic alterations that lead to hyperexcitatory symptoms of poisoning in animals. In addition, they can also induce a shift in voltage activation towards hyperpolarization [122].
5.4. Pyrethroids
Pyrethroids can drive the shift of voltage-dependent sodium channel activation to inactivation, resulting in hyperpolarized potential, leading the membrane voltage towards more negative potential. Such fluctuations in sensory neuron membrane potential increase electrical activity, neurotransmitter release, as well as the frequency of postsynaptic potentials [123]. These changes may be responsible for the different symptoms associated with the stress-induced response caused by exposure to these environmental chemicals, such as hyperexcitability, hypersensitivity, convulsions, and tremors [124]. In contrast, SCBIs block sodium channels by binding to the inactivated state. Additionally, synthetic pyrethroids (structural derivatives of pyrethrins), as well as DDT, also primarily target these channels [125].
5.5. Organophosphates
Organophosphate pesticide toxicity is commonly related to acute exposure events, but chronic exposure to organophosphates has been suggested as a potential cause for the development of chronic neurodegenerative diseases. Toxicity caused by organophosphate exposure is also associated with four primary neurotoxic disorders in humans, including cholinergic syndrome, intermediate syndrome, organophosphate-induced delayed polyneuropathy, and chronic organophosphate-induced neuropsychiatric disorder [103,104,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146]. Organophosphate pesticides exert their insecticidal effects primarily by inhibiting the enzyme AChE in an irreversible manner [147,148].
ACh was the first neurotransmitter characterized [149]. ACh is used as a neurotransmitter in all cholinergic neurons, playing a crucial excitatory role in neurons along the cholinergic pathway within the central nervous system (CNS) and the peripheral nervous system (PNS) [150]. In cholinergic synapses, ACh is released by exocytosis to reach the synaptic cleft and bind to specific receptors, including muscarinic and nicotinic receptors, or it can also be quickly hydrolyzed by the enzyme AChE, producing acetate and choline [151].
In the brain, nicotinic receptors allow Na+ and K+ influx. When ACh binds to these receptors, Na+ influx into the cell predominates over that of K+, depolarizing the membrane and reducing permeability to K+ so that ACh-sensitive neurons are more susceptible to other excitatory influences. In the human cerebral cortex, the cholinergic network is extraordinarily dense, where ACh binds to both muscarinic and nicotinic receptors to mediate various critical physiological processes, such as the stress response, wakefulness and sleep, sensory information, and attention, learning, and memory [152]. Thus, cholinergic activity is essential for maintaining the hippocampal rhythm. On the other hand, promoting cholinergic transmission through the use of cholinesterase inhibitors can improve attention in humans. The cholinergic system is involved in the learning process, and published data indicate that ACh is involved in memory. Additional studies have shown that endogenous ACh is important in modulating the acquisition, encoding, consolidation, and retrieval of memory [153]. Indeed, the effect that ACh exerts on memory is very important since degeneration of these specific neurons, common in Alzheimer’s disease (AD), contributes to the distinctive memory loss observed in this disorder [154].
In vertebrates, ACh activates two classes of receptors that promote stimulation or inhibition, which differ in their chemical structure and physiological properties. Nicotinic receptors (nAChR; ionotropic) are ion receptor channels that act selectively for cations (K+, Na+, and Ca2+) to promote the opening of ion channels. They are expressed in both the CNS and PNS [155].
In the CNS, these receptors are expressed on the presynaptic neuronal membrane to regulate the release of neurotransmitters (such as glutamate, GABA, dopamine, serotonin, norepinephrine, and ACh), whereas, in the PNS, they are mainly expressed postsynaptically, promoting rapid synaptic transmission. Moreover, within the CNS, these molecules mainly modulate responses beyond excitatory or inhibitory ones as they are highly permeable to Ca2+, a cation promoter of the long-lasting modulatory effects of nicotinic receptors that influence the strength and precision of synapses [156].
Muscarinic receptors (mAChR; metabotropic) are G protein-coupled receptors, which able to modulate the aperture or closure of various ion channels such as Ca2+, K+, or Cl− [157], leading to depolarization or hyperpolarization, depending on the cell type where they express [158,159]. When activated, they inhibit the resting K+ channels’ opening to reduce the flux of this cation [160]. They are found in several organs and tissues of the periphery (cardiac tissue, smooth muscle, and exocrine glands), whereas in the CNS, they are located in synaptic terminals, controlling neurotransmitter autoreceptor and heteroreceptor release [161].
Organophosphate pesticide’s (OP) mode of action is based on inhibiting AChE, a serine hydrolase that cleaves choline esters (its main substrate is the neurotransmitter ACh) into acetate and choline. Therefore, the main objective of AChE is to interrupt neuronal transmission and signaling between synapses to avoid the dispersion of ACh and receptor activation [162,163]. OP’s mechanism of action involves the binding and phosphorylation of a nucleophilic serine at the enzyme’s catalytic site, blocking ACh hydrolysis and causing excessive ACh elevation at the cholinergic synapses [53]. Acute toxicity of insecticides occurs as a result of AChE inhibition and accumulation of ACh in cholinergic receptors. Chronic exposure to these compounds leads to tolerance to their toxicity, which is associated with a decreased density of muscarinic and nicotinic receptors in the central and peripheral nervous systems [164]. A central cholinergic crisis is induced along with peripheral symptoms of lacrimation, salivation, and miosis, as well as neuromuscular and respiratory difficulties, followed by death due to asphyxia [165]. Insects whose CNS utilizes ACh as the major excitatory neurotransmitter are killed instantly by OP, primarily due to the hyperstimulation of nicotinic cholinergic receptors, which are the most abundant in their CNS [166].
6. CNS Disorders Associated with Stress Responses Induced by Pesticide Exposure in Humans and Mice
Pesticides are not highly selective and are generally toxic to many non-target species, including humans [167]. Adverse health effects of pesticides in humans cover a variety of domains; some compounds may only exert some mild irritant effects on the skin, while others may affect liver or lung functions. Additionally, several pesticides, such as paraquat, dieldrin, pyrethroids, and OPs, are neurotoxic since various stress-induced physiological responses are triggered by acute exposure at high doses, even after chronic exposure to low doses. In fact, it has been suggested that exposure to these environmental pollutants elicits a number of physiological responses upon induced damage, including oxidative stress, lipid peroxidation, impaired transporters of some neurotransmitters, mitochondrial dysfunction, α-synuclein (αSyn) fibrillation, apoptosis, inflammation, and neuroinflammation [104].
Neurotoxicity is defined as any adverse effect on the CNS or PNS that occurs when diverse stress responses are triggered after exposure to various chemical, biological, or physical agents [127,128,129,168,169]. Neurotoxicity caused by pesticides can manifest in various types of neurological disorders, including neurodevelopmental conditions (such as ADHD, autism spectrum disorders, developmental delays, and intellectual disabilities), neurobehavioral issues, neuropsychiatric disorders (like depression, suicide attempts, anxiety, insomnia, and cognitive decline), and neurodegenerative diseases (such as Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis, and multiple sclerosis), many of which represent some of the most severe and disabling health challenges in humans [103,104,130].
6.1. Alzheimer’s Disease (AD)
Exposure to environmental pollutants is recognized as one of the risk factors contributing to the development and progression of Alzheimer’s disease, with research linking such exposure to cognitive decline [103,109,114,162,165,166]. Pesticides tend to accumulate in the body’s fatty tissues, and since the brain and central nervous system are rich in lipids, such as sphingolipids, cholesterol, glycerophospholipids, and omega-3 and omega-6 polyunsaturated fatty acids, and are particularly susceptible to pesticide accumulation. This lipid-rich composition makes these regions especially vulnerable, allowing pesticides to interfere with neurogenesis and contribute to cognitive deficits [170].
AD is characterized by severe abnormalities in behavior, as well as cognitive and functional impairments. It is closely associated with the deposition of amyloid-β (Aβ) plaques and tau protein in the brain. Tau protein, which is found at the distal ends of axons, plays a crucial role in maintaining microtubule stability [171]. When tau protein becomes hyperphosphorylated, it leads to the disassociation of microtubules, disrupts axonal extension, and promotes the aggregation of insoluble tau protein. This process results in the formation of neurofibrillary tangles and neurotrophic neurites, ultimately causing synaptic alterations and contributing to tauopathy [172].
Additionally, the pathophysiology of AD includes impairment of the cholinergic system, as levels of ACh significantly decrease due to increased synthesis and activity of the extracellular enzyme AChE. The reduced availability of ACh in the brain contributes to the development of neurodegenerative symptoms associated with AD. Currently, anti-AChE drugs remain the first-line treatment used to alleviate these symptoms. These therapeutic inhibitors, such as Rivastigmine and Donepezil, are administered in controlled doses under medical supervision, ensuring partial inhibition of AChE to enhance cholinergic neurotransmission in people with AD [173].
6.1.1. Pesticides and AChE Inhibition
OPs and carbamate pesticides inhibit AChE through the phosphorylation of catalytic serine residue, leading to ACh accumulation at brain synapses and neuromuscular junctions, causing cholinergic crisis and, ultimately, death [131]. Since pesticides vary in their molecular structure, differences in their interactions on AChE-active site residues can be observed with different OP compounds such as methamidophos, fenamiphos, phosalone, and ethoprophos [132].
Loss of white matter, neurons, synapses, and reactive microgliosis are also frequently observed in AD patients. The etiology and pathogenesis of AD are significantly influenced by CNS cells, namely, glial cells and neurons, which are directly engaged in transmitting electrical signals and processing information. Emerging evidence suggests that exposure to OPs can trigger inflammatory responses in glial cells, leading to various signaling responses triggered by stressor factors that contribute to neuroinflammation, like neuronal damage, oxidative stress, dysregulation of microRNA, accumulation of toxic protein aggregates, such as Aβ and, ultimately, AD pathogenesis [133].
6.1.2. Pesticides Promote the Characteristics of AD Tauopathy and Neuroinflammation
The results of multiple studies indicate that exposure to pesticides modifies the mechanisms involved in tau protein phosphorylation. For example, postmortem human brains with or without organochlorine exposure (0–10 years) showed increased tau protein phosphorylation, altered mitochondrial genes encoding microtubule-associated with protein tau (MAPT), and the microtubule-associated protein 1B (MAP1B) gene, both of which are related to tau protein aggregate generation [134]. DDT exposure (3 µM) for two hours on Caenorhabditis elegans (N2 BR5270) strains exacerbated tau protein toxicity, reduced mitochondrial respiration, and induced apoptosis [135]. Exposure to 2,3,7,8 tetrachlorodibenzo-p-dioxin induced overexpression of glycogen synthase kinase 3 beta (GSK-3β), and hence tau phosphorylation [136]. Therefore, organochlorine has been associated with the development of tauopathy, which leads to axonal instability, mitochondrial dysfunction, and neuroinflammation [134].
Exposure to OPs (chlorpyrifos and malathion) increases the level of hyperphosphorylated tau protein, overstimulates glial cells, increases the levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-β, chemokines, NADPH oxidase, and COX-2, thereby inducing neuroinflammation. Intensified inflammation accelerates pathologies associated with neurodegenerative processes [137]. Exposure to malathion in Wistar rats at doses of 100 mg/kg for 14 days increased the level of hyperphosphorylated tau protein in Thr205 and Ser404, suggesting a correlation between phosphatase inactivation and increased GSK-3β activity. In addition, a decrease in mRNA expression of protein phosphatase-2A (PP2A) was reported due to exposure to malathion [138].
Moreover, mice exposure to the herbicide paraquat (10 mg/kg) for six weeks showed a 67% increase in tau protein hyperphosphorylation in the striatal region, suggesting that paraquat may inhibit the proteasome 20S as tau protein overexpression occurs [139]. Meanwhile, acetamiprid or imidacloprid (both neonicotinoid insecticides) exposure (1–100 μM), in primary cultures of cerebellar neurons from neonatal Sprague–Dawley rats increased the intracellular Ca2+ influx in cerebellar neurons by activating calcium/calmodulin-dependent kinases, thereby over-stimulating tau protein phosphorylation [174]. Moreover, imidacloprid and thiamethoxam’s accidental intake activates the flow of intracellular Ca2+, thereby altering the response of kinase enzymes and causing an excitatory neurological phase [175].
As AD pathophysiology is so complex, there is an increasing interest in developing treatments aimed at combatting the main pathophysiological stages to slow down neurodegeneration progression, thus delaying the onset of mild cognitive impairment or avoiding its transition to clinically diagnosed AD dementia. The AChE inhibitors, originally introduced as symptomatic treatments for AD dementia, were intended to amplify the impact of remaining endogenous ACh in the neocortex and hippocampus to improve cognition. However, they had no expectations about their impact on the AD progression rhythm. Nevertheless, multiple retrospective studies provided evidence that patients undergoing prolonged treatment with AChE inhibitors experienced slower progression through more severe AD clinical stages [131,132,133,170,171,172,173]. It should be noted that there are currently several research lines focused on the development of efficient alternative treatments, such as those based on medicinal plants, natural products, or phytomedicines with useful pharmacological potential to combat AD pathophysiological aspects.
6.2. Parkinson’s Disease (PD)
Parkinson’s disease (PD) is a movement disorder primarily caused by the degeneration of dopaminergic (DA) neurons in the basal ganglia. A hallmark feature of PD is the presence of Lewy bodies—intracytoplasmic inclusions within DA neurons—composed mainly of aggregated α-synuclein (αSyn) protein [176]. PD is considered idiopathic and typically presents with symptoms such as resting tremors, muscle rigidity, slowed movement (bradykinesia), and a sustained response to L-DOPA treatment [177]. Research indicates that both age and sex influence the risk of developing PD, and familial studies point to genetic factors in cases of early onset. Environmental influences are also significant, with epidemiological studies linking exposures to substances such as pesticides, metals, polychlorinated biphenyls, and solvents to the development of neurodegenerative conditions like PD [178].
Several studies have supported the idea that pesticide exposure may contribute to the development of Parkinson’s disease (PD). For instance, paraquat—a herbicide with effects similar to the neurotoxin MPTP—has been shown to trigger Parkinson-like behaviors and dopamine depletion in northern leopard frogs (Rana pipiens) [140]. In male Wistar rats, direct injection of paraquat into the brain (1–5 micrograms) led to the loss of dopaminergic neurons in the substantia nigra (SN), reduced dopamine levels in that region, and an increased response to apomorphine-induced rotational behavior [141]. Similarly, systemic exposure to paraquat in C57BL/6 mice caused degeneration of dopaminergic neurons in the SN, damage to dopaminergic fibers in the striatum, and decreased mobility [142]. Notably, paraquat has also been found to accumulate in the ventral midbrain of mice, with a half-life of approximately 28 days [143].
Individuals exposed to paraquat have a 1.3 to 3.6 times greater risk of developing Parkinson’s disease (PD) compared to the general population [144]. Research has shown that paraquat-induced Parkinsonism is driven by several molecular mechanisms, including excessive production of reactive oxygen species, mitochondrial dysfunction, disrupted autophagic processes, and excitotoxicity [16]. When administered intraperitoneally at a dose of 10 mg/kg in male mice, paraquat alters lipid metabolism, increases the production of pro-inflammatory lipids, triggers neuroinflammation in the midbrain, and leads to motor impairments. These neurotoxic effects are closely associated with the underlying mechanisms of paraquat-related PD development [145].
6.3. Dementia
Although Alzheimer’s disease is considered one of the leading causes of dementia, it is not the only one. Pesticide exposure can be linked to other neurological effects that can lead to other types of dementia. It is known that according to the pesticide class, the specific properties of each product may cause several adverse effects in humans, especially in case of acute intoxication. Cognitive impairment and dementia heavily affect a person’s quality of life, and scientific data have been hinting towards an association between them and antecedent chronic pesticide exposure.
In particular, both organophosphates and carbamates inhibit the enzyme AChE, resulting in ACh accumulation in synapses. Although their acute toxicity symptoms are similar, poisoning caused by carbamates is usually rapidly reversible, unlike organophosphates [179]. In addition, organophosphates have been associated with more chronic adverse effects compared to carbamates, such as organophosphate-induced delayed polyneuropathy (OPIDP), neuropsychological sequelae, and developmental neurotoxicity [146]. Adult Wistar rats (20 males and 20 females) were exposed to pyrethroids at sublethal doses (25, 50, and 75%) once a day for 45 days. The possible effect of this compound on cognitive behavioral changes (spatial learning and memory) was then evaluated through the Y maze and object recognition tests. Pyrethroid exposure was found to significantly (p ≤ 0.05) induce cognitive alterations (spatial memory) in rats through a dose-dependent pattern. Histomorphology showed that astrocytes exhibited nuclear fragmentation, excessive proliferation, as well as increased expression of neuroinflammatory molecules and Glial Fibrillary Acidic Protein (GFAP) immunoreactivity in the hippocampal region after exposure to pyrethroids. In addition, aggregates of amyloid beta protein stained with Congo Red were detected [180]. Regarding neonicotinoids, there are no human studies reported; however, acute and subchronic imidacloprid exposure induces a reduction in learning performance, explored by conditioned proboscis extension reflex (PER) in the honeybee Apis mellifera L. [181].
Studies on OPs, in particular chlorpyrifos (CP), on typical neuronal development have shown that besides the inhibition of AChE, other pathways involve the deregulation of transcription factors critical for neurite outgrowth and enzymatic biomarkers for CNS cell differentiation [182]. Other mechanisms, such as the induction of a xanthine oxidase triggered by chlorpyrifos-ethyl (CE), may also play a role in cognitive impairment, both in a high (0, 0.4, 2, 10, 50, and 100 g/L) and low (0, 0.01, and 0.1 g/L) concentration range in red blood cells packs collected from six volunteers [183]. The versatile activity of OPs does not seem to stop at enzymes but extends to the genetic level as well since genome-wide DNA methylation analysis of DNA samples obtained from the human hematopoietic cell line K562 exposed to ethanol (control) and various OP pesticides (fonofos, parathion, terbufos, chlorpyrifos, diazinon, malathion, and phorate) have been shown to alter DNA methylation in several genes [184] as well as on DNA samples obtained from human hematopoietic K562 cell exposed to diazinon and ethanol using the Illumina Infinium HumanMethylation27 BeadChip [185].
6.4. Attention Deficit Hyperactivity Disorder (ADHD)
ADHD is a prevalent and debilitating disorder diagnosed based on persistent and developmentally inappropriate levels of hyperactivity, inattention, and impulsiveness. It is one of the most frequently diagnosed neurodevelopmental disorders in children [186]. Treatment for ADHD includes both pharmacological and non-pharmacological approaches [187]. However, the effectiveness of pharmacological treatment and the hereditary nature of the disorder have led many researchers to explore a potential underlying neurobiological cause [188].
According to existing epidemiological and experimental studies, a close relationship has been observed between pesticide exposure and the incidence of various health disorders in humans, especially ADHD [189,190,191]—there is now evidence of the role of environmental risk factors, such as exposure to pesticides, on the incidence of ADHD in children. Analyses performed on urine and blood samples showed elevated odds ratios (OR) of 1.5 and 5.1 for ADHD related to organophosphate exposure [192].
ADHD cases related to organophosphate exposure indicated that organophosphate biomarkers, such as dialkylphosphate metabolites, were 2–3 times more detectable in the urine of cases than in that of controls used in that trial [193]. Additionally, another cohort study, where organophosphate biomarkers were measured in maternal urine samples, revealed an OR of 1.3 for ADHD risk in association with maternal exposure to OPs [194].
6.5. Neuroinflammation
Neuroinflammation of the CNS is a process that involves the blood–brain barrier (BBB), glial cells, and neurons. It plays a crucial role in the development of various neurodegenerative disorders. Neuroinflammation can be triggered by several biological mechanisms, including infection, trauma, ischemia, exposure to toxins, oxidative stress, glial cell reactions [146,176,191,195], and pesticide toxicity [145,180,190,196]. In addition, pesticides can affect the brain by damaging the BBB, as they can alter tight junctions between its cellular constituents, including astrocytes, resulting in increased permeability, which leads to neuroinflammation that promotes the onset of cognitive impairment or other neurological problems [196].
6.5.1. Astrocytes
Astrocytes are crucial CNS cells, important for BBB stability and neuroinflammation regulation. When these cells are activated, they contribute directly to BBB damage [137,173,195,197,198,199,200,201,202,203]. The cellular toxicity of the OPs malathion and malaoxon was evaluated using in vitro BBB models constructed with bovine brain microvascular endothelial cells (BMECs) or human RBE4 endothelial cells, both cocultured with rat astrocytes and neuroblastoma cells (SH-SY5Y). In this study, it was found that the pesticides tested (1 mM) altered astrocyte viability by 92% and 100% in BMECs and RBE4 cells. With respect to SH-SY5Y, malaoxon (1 mM) reduced cell viability by 97%. In BMECs and RBE4 in vitro systems showed a malathion dose-dependent BBB instability [202]. Employing the same in vitro BBB models, it was found that the cell viability of all the cell lines tested was reduced by paraoxon, malathion, and malaoxon exposition. Paraoxon directly affected the BBB in vitro, both at toxic and non-toxic concentrations, by attenuating tight junctional protein expression as well as increased ROS, leading to oxidative stress, which interferes with barrier integrity permeability across the BBB. Malathion (10 µM) and malaoxon at 1 µM decreased the expression of RBE4 tight junctions (occludins, claudin 5, Zonula occludens (ZO) 1, and ZO2) [203]. In addition, malathion (10 µM) and malaoxon (1 µM) affected the BBB in vitro by altering transendothelial electrical resistance since metabolic activities, quantities of neurotoxicants transferred (neuroblastoma cells SH-SY5Y), and sensitivity to test compounds differed between BMEC and RBE4 cells [204].
There are reports in the literature showing that malathion also induced concentration-dependent [Ca2+]i rises as well as cytotoxicity (5–25 μM) through the production of reactive oxidative species (ROS) in Gibco® Human Astrocytes (GHA cells) [205]. In a different study, malathion treatment (10–25 μM) for 24 h induced cytotoxicity and cell cycle arrest in a concentration-dependent manner in GHA cells. Regarding oxidative stress, malathion elevated the intracellular ROS levels and reduced glutathione and antioxidant enzyme levels [206]. In primary human fetal astrocytes treated with organophosphate insecticides, chlorpyrifos or cyfluthrin (100 µM) resulted in a complete inhibition of astrocyte growth and survival [207]. Cypermethrin, a class II pyrethroid insecticide administered at 10 and 25 mg/kg in rat astrocytes, provoked apoptosis by disrupting the autocrine/paracrine mode of Heparin-Binding Epidermal Growth Factor-Like Growth Factor (HB-EGF-EGFR) signaling at two levels, irreversible loss of basal EGFR, and downregulation of HB-EGF [208].
6.5.2. Microglia
Microglia are ubiquitously distributed in the brain and are the principal innate immune cells and the first responders to pathological insults [209]. Chlorpyrifos (0.3–300 µM) triggered oxidative stress and pro-inflammatory states. It promoted BV-2 microglial cell activation, proliferation, increased DNA damage, generation of oxidative markers, and overexpression of pro-inflammatory markers [210]. Pyrethroids, such as deltamethrin and permethrin at 25 µM or higher doses, significantly decreased microglial cell viability in a concentration- and time-dependent manner [211]. Meanwhile, cypermethrin (0.125 μM) activated rat primary microglial cells and released TNF-α and IL-1β as well as upregulated the expression of PKC-δ, iNOS, phosphorylated p38, and p42/44 MAPKs, MMP-3 and MMP-9 proteins, leading to neuroinflammation [212].
Male C57BL/6 mice, 9 weeks old, were injected intraperitoneally with paraquat twice a week for six weeks. To elucidate the role of microglia in paraquat-induced BBB disruption, microglial activation was pharmacologically inhibited by minocycline in the brains of exposed mice (intraperitoneal injection, once every two days). Microglial activation, BBB permeability, expression of tight junction (TJs) proteins, and matrix metalloproteinase (MMP), as well as the loss of dopaminergic neurons and neurological deficits assessment, were evaluated. The results demonstrated that exposure to this pesticide activated microglia and damaged the BBB; however, pharmacological inhibition of microglial activation with minocycline attenuated BBB disruption, loss of dopaminergic neurons, and alterations of neurological functions caused by paraquat. This chemical increased MMP-2 and MMP-9 expression in mice, a parameter that was significantly abrogated by minocycline treatment [213].
Neurotoxicity of pesticides in chronic exposure has been known as one of the most important human health problems, as most of these chemicals act by interacting with some elements of the CNS. Pesticide-induced neurotoxicity can be defined in different categories of neurological disorders, including neurodegenerative, neurodevelopmental, neurobehavioral, and neuropsychiatric disorders, some of which are among the most debilitating human health problems [103]. With the discovery of the role of neuroinflammation in developing many CNS disorders, the current search for potential therapeutics to combat these disorders has been extended to include substances with anti-inflammatory potential [214]. However, recent drug development projects were based on the emergence of new potential targets in different genomic and proteomic studies. Despite all the drug development efforts undertaken, the number of successful drugs and novel targets has been lower than expected during the past few decades [215,216]. Current treatments can address individual symptoms for some disorders, but no known cure for any neuroinflammatory disorder exists. While the cause, pathology, and symptoms of these disorders are extremely diverse, they all share a core inflammatory component [216].
6.6. Natural Products with Anti-Neuroinflammatory Effects
Natural herbs and unrefined medicinal substances possess therapeutic effects due to the presence of various potentially active compounds, which are closely connected to research and use in human healthcare. They are typically abundant in nature, easily accessible, and generally have lower toxicity. Their anti-inflammatory actions occur through the regulation of cells and signaling pathways associated with neuroinflammation [217].
Phytochemicals possess anti-inflammatory, antioxidant, and anti-amyloid properties and are capable of interacting with mediators involved in neuroinflammation. The traditional use of these natural products and the extensive data from animal studies demonstrate their ability to penetrate the BBB, which depends on the lipophilicity, polarity, and molecular weight of each compound [218], which may explain the natural products’ ability to reach areas of the brain implicated in neurodegenerative diseases.
Saponins are a diverse group of glycosides composed of a sugar unit—such as glucose, arabinose, galactose, glucuronic acid, xylose, rhamnose, or methyl pentose—attached to a nonpolar aglycone (sapogenin). They are generally classified into two main types: steroidal (C27) and triterpenoid (C30) [219]. Saponins are widely recognized for their neuroprotective properties. For instance, diosgenin, the aglycone form of a saponin derived from Dioscorea nipponica, has demonstrated anti-inflammatory effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages [220]. Diosgenin has been found to influence key inflammatory pathways, including the regulation of nuclear factor kappa B (NF-κB) [221], lipoxygenase, and cyclooxygenase-2 (COX-2) [222]. Another saponin, cantalasaponin-1 from Agave americana, was shown to lower levels of LPS-induced pro-inflammatory cytokines IL-6 and TNF-α in the brain while increasing levels of the anti-inflammatory cytokine IL-10 [223].
Alkaloids are naturally occurring nitrogen-containing compounds known for their basic (alkaline) properties and complex ring structures, and they exhibit a wide range of biological activities. Caffeine, a well-known alkaloid predominantly found in coffee, has demonstrated both anti-inflammatory and anti-apoptotic effects in models of LPS-induced neuroinflammation, particularly in RAW264.7 cells. It reduces the production of inflammatory mediators like nitric oxide (NO) and downregulates pro-inflammatory genes such as COX-2, iNOS, and interleukins (IL-3, IL-6, and IL-12). Additionally, studies have shown that caffeine consumption enhances cognitive performance in APPsw mice during the Morris water maze test [224], decreases β-amyloid accumulation in the hippocampus [225], and is capable of crossing the BBB [226].
Berberine, an isoquinoline alkaloid and a key active compound in Coptis chinensis extracts, has been shown to reduce pro-inflammatory cytokines such as COX-2, TNF-α, and IL-1β in models of scopolamine-induced memory impairment. It also helped to restore levels of cAMP response element-binding protein (CREB) and brain-derived neurotrophic factor (BDNF). In mice treated with amyloid-β (Aβ), pretreatment with berberine lowered IL-6 production and suppressed the expression of iNOS and COX-2 in both primary microglia and BV-2 cell lines [227].
Huperzine A (Hup A) is a purified alkaloid derived from the club moss Huperzia serrata. Research has highlighted its strong neuroprotective effects in Alzheimer’s disease, involving both cholinergic and non-cholinergic mechanisms [228]. Hup A can efficiently cross the blood–brain barrier and functions as a selective, reversible, and mixed-competitive inhibitor of acetylcholinesterase (AChE). It has also been shown to reduce amyloid-β (Aβ) buildup in the cortex and hippocampus in a dose-dependent fashion [229]. In a separate study, BV2 microglial cells pretreated with varying concentrations of β-sitosterol—a plant-based sterol—prior to LPS stimulation exhibited reduced expression of pro-inflammatory mediators such as IL-6, iNOS, TNF-α, and COX-2. β-sitosterol also blocked the activation of signaling pathways like p38, ERK, and NF-κB triggered by LPS [230].
Various flavonoids have been shown to have pharmacological effects on the central nervous system (CNS). For example, the flavanone Hesperidin demonstrates neuroprotective properties by significantly reducing LPS-induced increases in markers such as Toll-like receptor-4 (TLR4), Glial fibrillary acidic protein (GFAP), Ionized calcium-binding adapter molecule 1 (Iba-1), and phosphorylated nuclear factor-κB (p-NF-κB), as well as pro-inflammatory cytokines like TNF-α and IL-1β in the hippocampal and cortical regions [231]. Another flavonoid, Kaempferol, isolated from the rhizome of Kaempferia galanga (a ginger plant), inhibits neuroinflammation by suppressing NF-κB and p38 MAPK signaling pathways in LPS-stimulated BV2 microglial cells, thereby reducing the production of inflammatory mediators [232].
These findings suggest that medicinal plants could serve as valuable sources of neuroprotective compounds, which may be further explored to develop therapeutic strategies for treating neurodegenerative diseases linked to pesticide exposure. For instance, plants like Alternanthera sessilis, Stephania japonica, Eryngium foetidum, and Salix tetrasperma have been reported to protect against damage caused by Rotenone in SH-SY5Y cells [233,234].
7. Alternatives to Chemical Pesticides
While pesticides play a crucial role in agriculture by combating pests, they are also important for controlling disease vectors in public health. However, their use has led to soil, water, and air pollution, as well as health issues for farmers, consumers, and animals. As a result, there is a growing interest in developing strategies to reduce the use of chemical pesticides to help conserve ecosystems [235,236]. Some of these strategies include Agroecological Crop Protection (ACP), crop rotation, and biological pesticides, which are described below.
7.1. Agroecological Crop Protection (ACP)
ACP is a pest and disease management approach rooted in agroecology, which prioritizes sustainability, biological diversity, and the well-being of ecosystems. It integrates a variety of techniques to safeguard crops while minimizing the application of synthetic chemicals, focusing on maintaining ecological balance instead of relying on chemical solutions [237,238]. Additionally, agroecological practices play a crucial role in addressing climate change by enhancing carbon sequestration in soils and promoting agroforestry systems, and both help decrease greenhouse gas emissions [239].
The fundamental principles of ACP involve promoting plant diversity and creating diverse ecosystems that support natural predators, pollinators, and other beneficial organisms to naturally control pests [239,240]. Additionally, it emphasizes maintaining soil vitality through organic methods such as composting [241,242] and employing traditional farming methods such as crop rotation, intercropping, and polycultures to break pest and disease cycles, strengthen plant resilience, and reduce pest outbreaks [236,243]. Furthermore, physical barriers (e.g., nets, traps, or mulches) are used to prevent pests from accessing crops, while mechanical techniques like tilling or hand-picking help to reduce pest populations [244,245]. ACP also emphasizes the judicious use of environmentally friendly biological control methods, such as beneficial insects, microorganisms (bacteria and fungi), viruses, and entomopathogenic nematodes, thereby avoiding harmful chemicals and their negative impact on human health and the environment [236]. Additionally, this strategy provides a more sustainable and comprehensive alternative to traditional pest management approaches, focusing on long-term ecological balance and economic well-being [235].
7.2. Crop Rotation
Crop rotation is an ACP key principle essential for sustainable agriculture. It involves the systematic alternation of different crops in the same field across multiple growing seasons. This method enhances the diversity and structure of soil microbes by making use of different crop root systems, which help improve soil aeration, increase water uptake, and encourage deeper root growth, ultimately minimizing soil erosion [238]. Crop rotation also serves an important function in pest and disease control, as many pests have life cycles that are disrupted when their specific host plants are absent. It also helps in managing weeds, as some crops produce chemicals that inhibit the growth of weeds (allelopathy) [246]. Another benefit of crop rotation is its ability to lessen reliance on chemical fertilizers and pesticides since natural soil fertility is maintained, while pest control and disease control are achieved through ecological means. Furthermore, crop rotation strengthens the resilience of agricultural systems, making them more adaptable to climatic changes and variations in soil conditions [247] while also boosting long-term crop productivity [248].
7.3. Biological Pesticides (Biological Control)
Biological pesticides offer an environmentally sustainable solution for pest control as a viable alternative to chemical pesticides. These biopesticides arise from bacteria, algae, fungi, nematodes, protozoa, viruses, or plant extracts and are specifically formulated to attack and damage harmful pests [249]. They are highly selective since they act against target pests, generally posing little to no threat to human health or the environment [250]. Biopesticides serve an important role in crop protection; they are often integrated with other pest control methods as part of a biointensive integrated pest management (IPM) approach that also incorporates chemical pesticides [251]. The development of innovative biopesticides promotes the modernization of agricultural practices, and it is anticipated that they will replace many chemical pesticides in the near future [250]. Examples of biopesticides include Bacillus thuringiensis (Bt), which produces proteins known as Bt toxins. Upon ingestion by insects, these toxins become activated in the insect’s digestive system, causing cellular damage and resulting in the eventual mortality of the insect [252,253,254,255]. Another example is Beauveria bassiana, a fungus that targets insects through the production of toxins or by parasitizing them; this fungus can infect over 200 insect species across six orders and 15 families. The fungus invades the insect’s body, growing through it and causing its demise [256,257]. Some of the biopesticides are presented in Table 2.
To further enhance the environmental compatibility of biopesticides, specific application strategies can be implemented to minimize unintended effects on beneficial insects. These include selecting highly specific biopesticide formulations that exclusively target pest species, thereby reducing the risk to non-target organisms. Additionally, timing applications to coincide with periods of reduced activity among beneficial insects, such as early morning or late afternoon, can significantly lower the likelihood of exposure during peak foraging times [258,259,260].

Table 2.
Examples of biopesticides.
Table 2.
Examples of biopesticides.
| Organisms | Activity | Trade Name of the Product | Crops | Pest | Reference |
|---|---|---|---|---|---|
| Plants | |||||
| Jatropha curcas | Insecticide, nematicide, and molluscicide | Jatropha oil | Vegetables, fruit trees, and cereals | Moths, butterflies, aphids, bugs, beetles, flies, and cockroaches | [261] |
| Tagetes erecta | Nematicide | Nemagold | Tomato, potato, tobacco, cucumber, watermelon, melon, banana, etc. | Nematode root-knot | [262] |
| Eucalyptus globulus | Insecticide, fungicide | Eucalyptus oils | Tomatoes, cucumbers, carrots, lettuce, etc. | Flies, mosquito larvae, lice, etc. | [263] |
| Fungi | |||||
| Beauveria bassiana | Insecticide | BEA-SIN® | Pepper, bell pepper, tomato, peeled tomato, potato, and eggplant | Whiteflies | [257,264] |
| Metarhizium anisopliae | Insecticide | Met52® | Tomatoes, cucumbers, lettuce, carrots, apples, pears, citrus, corn, wheat, rice, oatmeal, etc. | Whiteflies, mosquitoes, thrips, aphids, mites, ticks, etc. | [265] |
| Metarhizium flavoviride | Insecticide | Green Muscle® | Tomatoes, cucumbers, lettuce, carrots, melons, grapes, apples, pears, citrus, corn, wheat, rice, etc. | Grasshoppers | [266] |
| Verticillium chlamydosporium | Nematicide | Ecocill® | Tomatoes, carrots, cucumbers, corn, wheat, oats, | Wireworms, rootworms, soil cockroaches, soil beetles, etc. | [267] |
| Lecanicillium longisporum | Insecticide | Eday®, Vertalec® | Tomatoes, cucumbers, lettuce, peppers, cabbage, spinach, etc. | Whitefly, blind hen, aphids, thrips, etc. | [268] |
| Bacteria | |||||
| Bacillus thuringiensis | Insecticide | Dipel® | Soybean, grapevines, and fruit trees (apple, peach, pear, and plum) | Lepidopteran larvae (Caterpillars) | [269] |
| Bacillus sphaericus | Insecticide | VectoLex® | - | Mosquito larvae | [270] |
| Pseudomonas aeruginosa | Fungicide | Gluticid® | Tomatoes | Fungicide | [271] |
| Pseudomonas fluorescens | Fertilizer and Fungicide | Bio Tak P® | Plant growth promoter | Fungicide | [272] |
| Nematodes | |||||
| Steinernema carpocapsae | Insecticide | ScanMask® | Tomatoes, apples, pears, citrus, corn, wheat, etc. | Weevils, root maggots, mosquitoes, cutworms, flea larvae, fire ants, etc. | [273] |
| Steinernema glaseri | Insecticide | Entonem® | Tomatoes, apples, pears, citrus, corn, wheat, rice, etc. | Fly larvae, beetle larvae, caterpillars, etc. | [274] |
| Steinernema feltiae | Insecticide, nematicide | Nemasys® | Tomatoes, apples, pears, citrus, corn, wheat, rice, oatmeal, etc. | Thrips, beetles, caterpillars, bedbugs, flies, etc. | [275] |
| Heterorhabditis bacteriophora | Insecticide | Grub-Guard® | Tomatoes, cucumbers, lettuce, apples, pears, citrus, corn, wheat, oats, etc. | Insect larvae, Japanese beetle larvae, etc. | [276] |
| Protozoan | |||||
| Nosema locustae | Insecticide | Nolo Bait® | Corn, wheat, oats, barley, tomatoes, carrots, lettuce, peppers, cucumbers, etc. | Grasshoppers and crickets | [277] |
8. Conclusions and Perspectives
This review provides evidence that pesticide exposure can lead to a broad range of neurological effects in humans and animals. Even low-level, chronic exposure has been linked to cognitive impairments, neurodegenerative diseases such as Parkinson’s and Alzheimer’s, as well as various behavioral disorders. The risk of neurotoxicity associated with pesticides is becoming an increasing concern. New research suggests possible connections between environmental exposure and neurological disorders that may not become apparent immediately but can have long-lasting impacts on health. Furthermore, studies indicate that pesticides may affect the nervous system through mechanisms that trigger oxidative stress, inflammation, and altered neurotransmission, leading to neurodegeneration. Populations at highest risk include agricultural workers and communities living near areas treated with pesticides, who face higher levels of exposure.
To reduce these risks, it is essential to develop and promote a holistic strategy projecting (a) pest control alternatives like Agricultural Crop Protection (ACP), organic farming practices, and the use of biopesticides, which can minimize dependence on harmful pesticides and reduce associated risks; (b) respond to the urgent demand for tougher regulations on pesticide application, along with more rigorous oversight of pesticide residue levels in both food and water supplies; (c) introduce thorough long-term health surveillance programs to detect early signs of neurotoxic effects; (d) sensitize the population by diffusing awareness of the neurological risks related to exposure to pesticides, as well as the ethnopharmacological resources provided by the medicinal plants available in the region, in order to protect against the damage that such exposure exerts on the CNS, especially among vulnerable groups; and finally, (e) emphasize the importance of providing farm workers with education on safety measures and proper protective equipment. To achieve these strategies, it is important to promote multidisciplinary research focused on the study of pest control, such as biopesticides, and the potential use of medicinal plants as pharmacological alternatives, with scientific support, to address environmental problems and their impact on public health.
Author Contributions
Conceptualization, M.L.C.-G. and N.M.-B.; writing—original draft preparation, A.R., M.L.C.-G. and N.M.-B.; writing—review and editing, A.R., M.L.C.-G. and N.M.-B.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI), through the project “Sistema de tratamiento para la eliminación de glifosato, paraquat y atrazina, herbicidas altamente contaminantes, basado en biomasa residual agrícola y de especies invasoras como soporte de microorganismos degradadores” grant number CBF2023-2024-2134.
Data Availability Statement
All relevant data are within the manuscript..
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chandran, C.S.; Thomas, S.; Unni, M.R. Pesticides: Classification, detection, and degradation. In Organic Farming; Chandran, C.S., Thomas, S., Unni, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 71–87. [Google Scholar] [CrossRef]
- Gomes, H.D.O.; Menezes, J.M.C.; da Costa, J.G.M.; Coutinho, H.D.M.; Teixeira, R.N.P.; do Nascimento, R.F. A socio-environmental perspective on pesticide use and food production. Ecotoxicol. Environ. Saf. 2020, 197, 110627. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, H.; da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, E.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef] [PubMed]
- Maksymiv, I. Pesticides: Benefits and hazards. J. Vasyl Stefanyk Precarpath. Nat. Univ. 2015, 2, 70–76. [Google Scholar] [CrossRef]
- Shattuck, A.; Werner, M.; Mempel, F.; Dunivin, Z.; Galt, R. Global pesticide use and trade database (GloPUT): New estimates show pesticide use trends in low-income countries substantially underestimated. Glob. Environ. Change 2023, 81, 102693. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Pesticides Use. Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 1 December 2024).
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Tang, F.H.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Narayanan, M.; Kandasamy, S.; He, Z.; Kumarasamy, S. Ecological impacts of pesticides on soil and water ecosystems and its natural degradation process. In Pesticides in the Natural Environment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–49. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, R.; Thakur, K.; Mahajan, D.; Brar, B.; Sharma, D.; Kumar, S.; Sharma, A.K. Impact of pesticides application on aquatic ecosystem and biodiversity: A review. Biol. Bull. 2023, 50, 1362–1375. [Google Scholar] [CrossRef]
- Weiss, F.T.; Ruepert, C.; Echeverría-Sáenz, S.; Eggen, R.I.; Stamm, C. Agricultural pesticides pose a continuous ecotoxicological risk to aquatic organisms in a tropical horticulture catchment. Environ. Adv. 2023, 11, 100339. [Google Scholar] [CrossRef]
- Beringue, A.; Queffelec, J.; Le Lann, C.; Sulmon, C. Sublethal pesticide exposure in non-target terrestrial ecosystems: From known effects on individuals to potential consequences on trophic interactions and network functioning. Environ. Res. 2024, 260, 119620. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Dhankhar, N.; Kumar, J. Impact of increasing pesticides and fertilizers on human health: A review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2024, 11, 100410. [Google Scholar] [CrossRef]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Coutinho, T.E.; Silva, T.L.; Andreani, T.; Silva, A.M. Neurotoxicity assessment of four different pesticides using in vitro enzymatic inhibition assays. Toxics 2022, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Honatel, K.F.; Arbo, B.D.; Leal, M.B.; da Silva Júnior, F.M.R.; Garcia, S.C.; Arbo, M.D. An update of the impact of pesticide exposure on memory and learning. Discov. Toxicol. 2024, 1, 11. [Google Scholar] [CrossRef]
- Mwaka, O.; Mwamahonje, A.; Nene, W.; Rweyemamu, E.; Maseta, Z. Pesticides use and its effects on grape production: A review. Sustain. Environ. 2024, 10, 2366555. [Google Scholar] [CrossRef]
- Saroop, S.; Tamchos, S. Impact of pesticide application: Positive and negative side. Pestic. Environ. 2024, 155–178. [Google Scholar] [CrossRef]
- Garud, A.; Pawar, S.; Patil, M.S.; Kale, S.R.; Patil, S. A Scientific Review of Pesticides: Classification, Toxicity, Health Effects, Sustainability, and Environmental Impact. Cureus 2024, 16, e67945. [Google Scholar] [CrossRef]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, history, and classification. In Natural Remedies for Pest, Disease and Weed Control; Academic Press: Cambridge, MA, USA, 2020; pp. 29–42. [Google Scholar] [CrossRef]
- Secretaría de Agricultura y Desarrollo Rural (SADER); Servicio Nacional de Sanidad Inocuidad y Calidad Agroalimentaria (SENASICA). Manual para el Buen uso y Manejo de Plaguicidas en Campo. 1a Edición. 2019; pp. 1–80. Available online: https://www.gob.mx/cms/uploads/attachment/file/452645/MANUAL_PARA_EL_BUEN_USO_Y_MANEJO_DE_PLAGUICIDAS_EN_CAMPO.pdf (accessed on 5 December 2024).
- Yadav, I.C.; Devi, N.L. Pesticides classification and its impact on human and environment. Environ. Sci. Eng. 2017, 6, 140–158. [Google Scholar]
- Libs, E.; Salim, E. Formulation of essential oil pesticides technology and their application. Agric. Res. Technol. 2017, 9, 555759. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019, 2019th ed.; World Health Organization: Geneva, Switzerland, 2020; Available online: https://iris.who.int/bitstream/handle/10665/332193/9789240005662-eng.pdf (accessed on 5 December 2024).
- Jayaprakas, C.A.; Tom, J.; Sreejith, S. Impact of Insecticides on Man and Environment. In Biomedical Applications and Toxicity of Nanomaterials; Springer Nature Singapore: Singapore, 2023; pp. 751–768. [Google Scholar] [CrossRef]
- EPA. Chemically-Related Groups of Active Ingredients. 2018. Available online: https://www.epa.gov/ingredients-used-pesticide-products/chemically-related-groups-active-ingredients (accessed on 5 December 2024).
- Rodríguez, A.; Castrejón-Godínez, M.L.; Sánchez-Salinas, E.; Mussali-Galante, P.; Tovar-Sánchez, E.; Ortiz-Hernández, M.L. Pesticide bioremediation: OMICs technologies for understanding the processes. In Pesticides Bioremediation; Springer International Publishing: Cham, Switzerland, 2022; pp. 197–242. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Fu, X.; Nageotte, J.R.; Silverman, J.; Bretsnyder, E.C.; Chen, D.; Rydel, T.J.; Bean, G.J.; Li, K.S.; et al. Bacillus thuringiensis Cry1Da_7 and Cry1B. 868 protein interactions with novel receptors allow control of resistant fall armyworms, Spodoptera frugiperda (JE Smith). Appl. Environ. Microbiol. 2019, 85, e00579-19. [Google Scholar] [CrossRef] [PubMed]
- Baratzhanova, G.; Fournier, A.; Delannoy, M.; Baubekova, A.; Altynova, N.; Djansugurova, L.; Cakir-Kiefer, C. The mode of action of different organochlorine pesticides families in mammalians. Environ. Toxicol. Pharmacol. 2024, 110, 104514. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and oxidative stress: A review. Med. Sci. Monit. 2004, 10, 141–147. [Google Scholar]
- Li, S.; Crooks, P.A.; Wei, X.; Leon, J. Toxicity of dipyridyl compounds and related compounds. Crit. Rev. Toxicol. 2004, 34, 447–460. [Google Scholar] [CrossRef]
- Agrawal, A.N.J.U.; Sharma, B. Pesticides induced oxidative stress in mammalian systems. Int. J. Biol. Med. Res. 2010, 1, 90–104. [Google Scholar]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Gupta, P.K. Toxicity of herbicides. In Veterinary Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 553–567. [Google Scholar] [CrossRef]
- Quinn, L.; de Vos, J.; Fernandes-Whaley, M.; Roos, C.; Bouwman, H.; Kylin, H.; Pieters, R.; van den Berg, J. Pesticide use in South Africa: One of the largest importers of pesticides in Africa. In Pesticides in the Modern World—Pesticides Use and Management; IntechOpen: London, UK, 2011; pp. 49–96. [Google Scholar]
- Rosic, N.; Bradbury, J.; Lee, M.; Baltrotsky, K.; Grace, S. The impact of pesticides on local waterways: A scoping review and method for identifying pesticides in local usage. Environ. Sci. Policy 2020, 106, 12–21. [Google Scholar] [CrossRef]
- Umar, A.M.; Aisami, A. Acetylcholinesterase enzyme (AChE) as a biosensor and biomarker for pesticides: A mini review. Bull. Environ. Sci. Sustain. Manag. 2020, 4, 7–12. [Google Scholar] [CrossRef]
- Moreira, S.; Silva, R.; Carrageta, D.F.; Alves, M.G.; Seco-Rovira, V.; Oliveira, P.F.; de Lourdes Pereira, M. Carbamate pesticides: Shedding light on their impact on the male reproductive system. Int. J. Mol. Sci. 2022, 23, 8206. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.; Sousa, S.F. The buzz on insecticides: A review of uses, molecular structures, targets, adverse effects, and alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef]
- Costa, L.G. Neurotoxicity of amitraz, a formamidine pesticide. In Advances in Neurotoxicology; Academic Press: Cambridge, MA, USA, 2020; Volume 4, pp. 255–276. [Google Scholar]
- Bhandarwar, A.; Burute, R.B.; Soni, N.P. A study of Clinical Profile and Management of Amitraz Poisoning: A Case Series of Not So (UN) Common Poisoning. Int. J. Med. Pharm. Res. 2023, 4, 87–90. [Google Scholar]
- Giorgini, M.; Taroncher, M.; Ruiz, M.J.; Rodríguez-Carrasco, Y.; Tolosa, J. In vitro and predictive computational toxicology methods for the neurotoxic pesticide amitraz and its metabolites. Brain Sci. 2023, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Iken, I.; Abdessadek, M.; El Attari, A.; Achour, S. Poisoning by Amitraz, uncommon pesticide revealed by high performance liquid chromatography: About two cases. Toxicol. Anal. Clin. 2020, 32, 200–204. [Google Scholar] [CrossRef]
- Reynoso, E.C.; Torres, E.; Bettazzi, F.; Palchetti, I. Trends and perspectives in immunosensors for determination of currently-used pesticides: The case of glyphosate, organophosphates, and neonicotinoids. Biosensors 2019, 9, 20. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.R.; Martínez, M.A. Neurotoxicity of neonicotinoids. In Advances in Neurotoxicology; Academic Press: Cambridge, MA, USA, 2020; Volume 4, pp. 167–207. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.F.; Liu, L.Y.; Zhu, F.J.; Ma, W.L. National-scale monitoring of historic used organochlorine pesticides (OCPs) and current used pesticides (CUPs) in Chinese surface soil: Old topic and new story. J. Hazard. Mater. 2023, 443, 130285. [Google Scholar] [CrossRef]
- Ali, U.; Syed, J.H.; Malik, R.N.; Katsoyiannis, A.; Li, J.; Zhang, G.; Jones, K.C. Organochlorine pesticides (OCPs) in South Asian region: A review. Sci. Total Environ. 2014, 476, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, H.A.; Yahaya, A.; Abdullahi, S.S.A.; Jagaba, A.H.; Birniwa, A.H. Mitigating water contamination by controlling anthropogenic activities of organochlorine pesticides (OCPs) for surface water quality assurance. Case Stud. Chem. Environ. Eng. 2023, 8, 100474. [Google Scholar] [CrossRef]
- Costa, L.G. The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 2015, 131, 135–148. [Google Scholar] [CrossRef]
- Uğurlu, P.; Satar, E.İ.; Ünlü, E. Toxic effects of commercial grade indoxacarb and endosulfan on Gammarus kischineffensis (Schellenberg, 1937) (Crustacea: Amphipoda). Chemosphere 2024, 360, 142387. [Google Scholar] [CrossRef]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; de Araujo Furtado, M.; Pidoplichko, V.I.; Braga, M.F. Mechanisms of organophosphate toxicity and the role of acetylcholinesterase inhibition. Toxics 2023, 11, 866. [Google Scholar] [CrossRef]
- Hodoșan, C.; Gîrd, C.E.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Nistor, L.; Bărbuică, I.S.; Marin, S.C.; Mihalache, A.; Popa, L. Pyrethrins and pyrethroids: A comprehensive review of natural occurring compounds and their synthetic derivatives. Plants 2023, 12, 4022. [Google Scholar] [CrossRef]
- Ranatunga, M.; Kellar, C.; Pettigrove, V. Toxicological impacts of synthetic pyrethroids on non-target aquatic organisms: A review. Environ. Adv. 2023, 12, 100388. [Google Scholar] [CrossRef]
- Arsuffi-Marcon, R.; Souza, L.G.; Santos-Miranda, A.; Joviano-Santos, J.V. Neurotoxicity of Pyrethroids in exacerbating neurodegenerative diseases: From animals’ models to humans’ studies. Chem. Biol. Interact. 2024, 391, 110911. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Skouras, P.; Varvarelis, O.P.; Aloizou, A.M.; Hernández, A.F.; Liampas, I.; Rikos, D.; Dasramani, M.; Golokhvast, K.S.; Bogdanos, D.P.; et al. Pesticides and tremor: An overview of association, mechanisms and confounders. Environ. Res. 2023, 229, 115442. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.S.; Lein, P.J. Neurotoxicity. In Encyclopedia of Toxicology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 6, pp. 727–740. [Google Scholar] [CrossRef]
- Klementova, S.; Keltnerova, L. Triazine herbicides in the environment. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; IntechOpen: Croatia, Balkan, 2015; pp. 71–96. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Ru, S.; Wang, J.; Xiong, J.; Yang, L.; Hao, L.; Zhang, J.; Zhang, X. Effects of co-exposure of the triazine herbicides atrazine, prometryn and terbutryn on Phaeodactylum tricornutum photosynthesis and nutritional value. Sci. Total Environ. 2022, 807, 150609. [Google Scholar] [CrossRef]
- LeBaron, H.M. The Triazine Herbicides. 50 Years Revolutionizing Agriculture; LeBaron, H.M., McFarland, J.E., Burnside, O.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; 600p. [Google Scholar]
- Chaudhary, N.; Choudhary, K.K.; Agrawal, S.B.; Agrawal, M. Pesticides usage, uptake and mode of action in plants with special emphasis on photosynthetic characteristics. In Pesticides in Crop Production: Physiological and Biochemical Action; Srivastava, P.K., Singh, V.P., Singh, A., Tripathi, D.K., Singh, S., Prasad, S.M., Chauhan, D.K., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 159–180. [Google Scholar] [CrossRef]
- Rao, I.S.; Madhulety, T.Y. Role of herbicides in improving crop yields. In Developments in Physiology, Biochemistry and Molecular Biology of Plants; Bose, B., Hemantaranjan, A., Eds.; New India Publishing Agency: New Delhi, India, 2005; Volume 1, pp. 203–287. [Google Scholar]
- Panigrahi, K.K.; Mohanty, A.; Padhan, S.R.; Guru, R.K.S. Genotoxicity and DNA damage induced by herbicides and toxins in plants. In Induced Genotoxicity and Oxidative Stress in Plants; Khan, Z., Ansari, M.Y.H., Shahwar, D., Eds.; Springer: Singapore, 2021; pp. 29–63. [Google Scholar] [CrossRef]
- Himani, P.U.; Mahawer, S.K.; Kumar, R.; Prakash, O. Plant protection through agrochemicals and its consequences. In Plant Protection: From Chemicals to Biologicals; Soni, R., Suyal, D.C., Goel, R., Eds.; De Gruyter: Beilin, Germany, 2022; pp. 25–44. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in drinking water-a review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Roy, D.; Ghosh, D.; Chander, S.; Saha, B.; Moulick, D. Herbicide Contamination in Ground Water and Their Probable Remediation. In Environmental Contaminants; Apple Academic Press: Boca Raton, FL, USA, 2024; pp. 111–140. [Google Scholar]
- Ahmad, S.; Chandrasekaran, M.; Ahmad, H.W. Investigation of the persistence, toxicological effects, and ecological issues of S-triazine herbicides and their biodegradation using emerging technologies: A Review. Microorganisms 2023, 11, 2558. [Google Scholar] [CrossRef]
- Yao, T.; Sun, P.; Zhao, W. Triazine herbicides risk management strategies on environmental and human health aspects using in-silico methods. Int. J. Mol. Sci. 2023, 24, 5691. [Google Scholar] [CrossRef]
- Arabi, S.; Heidari-Beni, M.; Poursafa, P.; Roshanaei, M.; Kelishadi, R. A review of the potential adverse health impacts of atrazine in humans. Rev. Environ. Health 2024. [Google Scholar] [CrossRef]
- Raj, A.; Dubey, A.; Malla, M.A.; Kumar, A. Pesticide Pestilence: Global Scenario and Recent Advances in Detection and Degradation Methods. J. Environ. Manag. 2023, 338, 117680. [Google Scholar] [CrossRef]
- Phat, C.; Ty, B.; Kuok, F.; Andrews, E.M.; Kurniawan, W.; Hinode, H. Residual Pesticides. In Water and Life in Tonle Sap Lake; Yoshimura, C., Khanal, R., Sovannara, U., Eds.; Springer: Singapore, 2022; pp. 387–397. [Google Scholar] [CrossRef]
- Bagheri, A.; Emami, N.; Damalas, C.A. Farmers’ behavior towards safe pesticide handling: An analysis with the theory of planned behavior. Sci. Total Environ. 2021, 751, 141709. [Google Scholar] [CrossRef]
- Sarker, S.; Akbor, M.A.; Nahar, A.; Hasan, M.; Islam, A.R.M.T.; Siddique, M.A.B. Level of pesticides contamination in the major river systems: A review on South Asian countries perspective. Heliyon 2021, 7, e07270. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Liu, J.; Xu, Y.; Liu, H.; Yang, G.; He, Y.; Xu, J.; Lu, Z. Suspecting screening “known unknown” pesticides and transformation products in soil at pesticide manufacturing sites. Sci. Total Environ. 2022, 808, 152074. [Google Scholar] [CrossRef] [PubMed]
- Khatib, I.; Rychter, P.; Falfushynska, H. Pesticide Pollution: Detrimental Outcomes and Possible Mechanisms of Fish Exposure to Common Organophosphates and Triazines. J. Xenobiot. 2022, 12, 236–265. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Sharma, P.; Pasrija, R.; Kaur, K.; Umesh, M.; Thazeem, B. Toxicity Analysis of Endocrine Disrupting Pesticides on Non-Target Organisms: A Critical Analysis on Toxicity Mechanisms. Toxicol. Appl. Pharmacol. 2023, 474, 116623. [Google Scholar] [CrossRef]
- Wan, N.F.; Fu, L.; Dainese, M.; Kiær, L.P.; Hu, Y.Q.; Xin, F.; Goulson, D.; Woodcock, B.A.; Vanbergen, A.J.; Spurgeon, D.J.; et al. Pesticides have negative effects on non-target organisms. Nat. Commun. 2025, 16, 1360. [Google Scholar] [CrossRef]
- Shahid, M.; Khan, M.S. Ecotoxicological Implications of Residual Pesticides to Beneficial Soil Bacteria: A Review. Pest. Biochem. Physiol. 2022, 188, 105272. [Google Scholar] [CrossRef]
- Beaumelle, L.; Tison, L.; Eisenhauer, N.; Hines, J.; Malladi, S.; Pelosi, C.; Thouvenot, L.; Phillips, H.R.P. Pesticide effects on soil fauna communities—A meta-analysis. J. Appl. Ecol. 2023, 60, 1239–1253. [Google Scholar] [CrossRef]
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-Effects of Pesticides on Non-Target Insects in Agriculture: A Mini-Review. Sci. Nat. 2022, 109, 17. [Google Scholar] [CrossRef]
- Goritschnig, L.; Burtscher-Schaden, H.; Durstberger, T.; Zaller, J.G. Ecotoxicity of Pesticides Approved for Use in European Conventional or Organic Agriculture for Honeybees, Birds, and Earthworms. Environments 2024, 11, 137. [Google Scholar] [CrossRef]
- Baweja, P.; Kumar, S.; Kumar, G. Fertilizers and Pesticides: Their Impact on Soil Health and Environment. In Soil Health, Soil Biology; Giri, B., Varma, A., Eds.; Springer: Cham, Switzerland, 2020; Volume 59. [Google Scholar] [CrossRef]
- Grung, M.; Lin, Y.; Zhang, H.; Steen, A.O.; Huang, J.; Zhang, G.; Larssen, T. Pesticide levels and environmental risk in aquatic environments in China: A review. Environ. Int. 2015, 81, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Farah, I.F.; dos Santos, C.R.; Pinto, M.C.F.; Araújo, C.R.; Amaral, M.C.S. Pesticides in aquatic environment: Occurrence, ecological implications and legal framework. J. Environ. Chem. Eng. 2024, 12, 114072. [Google Scholar] [CrossRef]
- Kadiru, S.; Patil, S.; D’Souza, R. Effect of pesticide toxicity in aquatic environments: A recent review. Int. J. Fish. Aquat. Stud. 2022, 10, 113–118. [Google Scholar] [CrossRef]
- Shahid, N.; Liess, M.; Knillmann, S. Environmental stress increases synergistic effects of pesticide mixtures on Daphnia magna. Environ. Sci. Technol. 2019, 53, 12586–12593. [Google Scholar] [CrossRef] [PubMed]
- Barathinivas, A.; Ramya, S.; Neethirajan, K.; Jayakumararaj, R.; Pothiraj, C.; Balaji, P.; Faggio, C. Ecotoxicological Effects of Pesticides on Hematological Parameters and Oxidative Enzymes in Freshwater Catfish, Mystus keletius. Sustainability 2022, 14, 9529. [Google Scholar] [CrossRef]
- Katagi, T. Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organisms. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2010; pp. 1–132. [Google Scholar] [CrossRef]
- Tongo, I.; Onokpasa, A.; Emerure, F.; Balogun, P.T.; Enuneku, A.A.; Erhunmwunse, N.; Asemota, O.; Ogbomida, E.; Ogbeide, O.; Ezemonye, L. Levels, bioaccumulation and biomagnification of pesticide residues in a tropical freshwater food web. Int. J. Environ. Sci. Technol. 2022, 19, 1467–1482. [Google Scholar] [CrossRef]
- Farcas, A.; Matei, A.V.; Florian, C.; Badea, M.; Coman, G. Health effects associated with acute and chronic exposure to pesticides. In Environmental Security Assessment and Management of Obsolete Pesticides in Southeast Europe; Springer: Berlin/Heidelberg, Germany, 2013; pp. 103–110. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, S.M.N.; Rahman, M.A.; Juraimi, A.S.; Uddin, M.K.; Brown, C.L.; Arshad, A. Chronic Effects of Organic Pesticides on the Aquatic Environment and Human Health: A Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100740. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, M.K. Soil pollution and human health. In Plant Responses to Soil Pollution; Singh, P., Singh, S.K., Prasad, S.M., Eds.; Springer: Singapore, 2020; pp. 205–220. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Iqubal, A.; Ahmed, M.; Ahmad, S.; Sahoo, C.R.; Iqubal, M.K.; Haque, S.E. Environmental neurotoxic pollutants. Environ. Sci. Pollut. Res. 2020, 27, 41175–41198. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Wakode, S.; Sharma, A.; Nair, N.; Dhobi, M.; Wani, M.A.; Pottoo, F.H. Effect of environmental toxicants on neuronal functions. Environ. Sci. Pollut. Res. 2020, 27, 44906–44921. [Google Scholar] [CrossRef]
- Costa, L.G. Interactions of neurotoxicants with neurotransmitter systems. Toxicology 1988, 49, 359–366. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Z.; Zhang, W.; Ren, Y. Investigating the neurotoxicity of environmental pollutants using zebrafish as a model organism: A review and recommendations for future work. Neurotoxicology 2023, 94, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, J.; Wang, H. Advancements in Targeting Ion Channels for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2024, 17, 1462. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Mostafalou, S. Neurotoxicity of pesticides in the context of CNS chronic diseases. Int. J. Environ. Health Res. 2022, 32, 2718–2755. [Google Scholar] [CrossRef]
- Vellingiri, B.; Chandrasekhar, M.; Sabari, S.S.; Gopalakrishnan, A.V.; Narayanasamy, A.; Venkatesan, D.; Iyer, M.; Kesari, K.; Dey, A. Neurotoxicity of pesticides–A link to neurodegeneration. Ecotoxicol. Environ. Saf. 2022, 243, 113972. [Google Scholar] [CrossRef]
- Akinaga, J.; García-Sáinz, J.A.; Pupo, A.S. Updates in the function and regulation of α1-adrenoceptors. Br. J. Pharmacol. 2019, 176, 2343–2357. [Google Scholar] [CrossRef]
- Costa, L.G.; Wu, D.S.; Olibet, G.; Murphy, S.D. Formamidine pesticides and alpha 2-adrenoceptors: Studies with amitraz and chlordimeform in rats and development of a radioreceptor binding assay. Neurotoxicol. Teratol. 1989, 11, 405–411. [Google Scholar] [CrossRef]
- Del Pino, J.; Moyano, P.; Ruiz, M.; Anadón, M.; Díaz, M.; García, J.; Labajo González, E.; Frejo, M. Amitraz changes NE, DA and 5-HT biosynthesis and metabolism mediated by alterations in estradiol content in CNS of male rats. Chemosphere 2017, 181, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Jacome, L.F.; Gautreaux, C.; Inagaki, T.; Mohan, G.; Alves, S.; Lubbers, L.S.; Luine, V. Estradiol and ERb agonists enhance recognition memory, and DPN, an ERb agonist, alters brain monoamines. Neurobiol. Learn Mem. 2010, 94, 488–498. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Levin, E.D. Developmental neurotoxicity of succeeding generations of insecticides. Environ. Inter. 2017, 99, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, B.; Siefert, P. Acetylcholine and its receptors in honeybees: Involvement in development and impairments by neonicotinoids. Insects 2019, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Sheets, L.P.; Li, A.A.; Minnema, D.J.; Collier, R.H.; Creek, M.R.; Peffer, R.C. A critical review of neonicotinoid insecticides for developmental neurotoxicity. Crit. Rev. Toxicol. 2016, 46, 153–190. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Yang, Y.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, X.W.; Anadón, A.; Martinez, M.A. Neonicotinoids: Mechanisms of systemic toxicity based on oxidative stress-mitochondrial damage. Arch. Toxicol. 2022, 96, 1493–1520. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; Goldstein, L.B.; Bullman, S.; Tu, T.; Khan, W.A.; Dechkovskaia, A.M.; Abdel-Rahman, A.A. Imidacloprid induces neurobehavioral deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure. J. Toxicol. Environ. Health Part A 2008, 71, 119–130. [Google Scholar] [CrossRef]
- Bal, R.; Erdogan, S.; Theophilidis, G.; Baydas, G.; Naziroglu, M. Assessing the effects of the neonicotinoid insecticide imidacloprid in the cholinergic synapses of the stellate cells of the mouse cochlear nucleus using whole-cell patch-clamp recording. Neurotoxicology 2010, 31, 113–120. [Google Scholar] [CrossRef]
- Tanaka, T. Effects of maternal clothianidin exposure on behavioral development in F1 generation mice. Toxico. Ind. Health 2012, 28, 697–707. [Google Scholar] [CrossRef]
- Tanaka, T. Reproductive and neurobehavioral effects of clothianidin administered to mice in the diet. Birth Defects Res. B Dev. Reprod. Toxicol. 2012, 95, 151–159. [Google Scholar] [CrossRef]
- Özdemir, H.H.; Kara, M.; Yumrutas, O.; Uckardes, F.; Eraslan, E.; Demir, C.F.; Bal, R. Determination of the effects on learning and memory performance and related gene expressions of clothianidin in rat models. Cogn. Neurodyn. 2014, 8, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ou, S.W.; Wang, Y.J. Distribution and function of voltage-gated sodium channels in the nervous system. Channels 2017, 11, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.G.E.; Field, L.M.; Usherwood, P.N.R.; Williamson, M.S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, Y.; Jiang, D.; Behnke, C.; Nomura, Y.; Zhorov, B.S.; Dong, K. The receptor site and mechanism of action of sodium channel blocker insecticides. J. Biol. Chem. 2016, 291, 20113–20124. [Google Scholar] [CrossRef]
- Zhorov, B.S.; Dong, K. Elucidation of pyrethroid and DDT receptor sites in the voltage-gated sodium channel. Neurotoxicology 2017, 60, 171–177. [Google Scholar] [CrossRef]
- Narahashi, T. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol. 1996, 78, 1–14. [Google Scholar] [CrossRef]
- Costas-Ferreira, C.; Faro, L.R. Systematic review of calcium channels and intracellular calcium signaling: Relevance to pesticide neurotoxicity. Int. J. Mol. Sci. 2021, 22, 13376. [Google Scholar] [CrossRef]
- Bloomquist, J.R.; Soderlund, D.M. Pyrethroid insecticides and DDT modify alkaloid-dependent sodium channel activation and its enhancement by sea anemone toxin. Mol. Pharmacol. 1988, 33, 543–550. [Google Scholar] [CrossRef]
- Silver, K.S.; Du, Y.; Nomura, Y.; Oliveira, E.E.; Salgado, V.L.; Zhorov, B.S.; Dong, K. Voltage-Gated Sodium Channels as Insecticide Targets. Adv. Insect Phys. 2014, 46, 389–433. [Google Scholar] [CrossRef]
- Penticoff, H.B.; Fortin, J.S. Toxic/metabolic diseases of the nervous system. In Neurobiology of Brain Disorders, 2nd ed.; Zigmond, M.J., Wiley, C.A., Chesselet, M.F., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 379–401. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Morris, R.H.; Counsell, S.J.; McGonnell, I.M.; Thornton, C. Early life exposure to air pollution impacts neuronal and glial cell function leading to impaired neurodevelopment. BioEssays 2021, 43, 2000288. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, A.M.; Camoni, L.; Calamandrei, G.; Chiarotti, F.; Venerosi, A. The contribution of environmental pollutants to the risk of autism and other neurodevelopmental disorders: A systematic review of case-control studies. Neurosci. Biobehav. Rev. 2024, 164, 105815. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Dong, X.; Zhang, X.; Ye, F.; Cheng, J.; Dan, G.; Zhao, Y.; Zou, Z.; Cao, J.; Sai, Y. Pesticides exposure and dopaminergic neurodegeneration. Expo. Health 2021, 13, 295–306. [Google Scholar] [CrossRef]
- Franjesevic, A.J.; Sillart, S.B.; Beck, J.M.; Vyas, S.; Callam, C.S.; Hadad, C.M. Resurrection and Reactivation of Acetylcholinesterase and Butyrylcholinesterase. Chemistry 2019, 25, 5337–5371. [Google Scholar] [CrossRef]
- Čadež, T.; Kolić, D.; Šinko, G.; Kovarik, Z. Assessment of four organophosphorus pesticides as inhibitors of human acetylcholinesterase and butyrylcholinesterase. Sci Rep. 2021, 11, 21486. [Google Scholar] [CrossRef]
- Yadav, B.; Kaur, S.; Yadav, A.; Verma, H.; Kar, S.; Sahu, B.K.; Pati, K.R.; Sarkar, B.; Dhiman, M.; Mantha, A.K. Implications of organophosphate pesticides on brain cells and their contribution toward progression of Alzheimer’s disease. J. Biochem. Mol. Toxicol. 2024, 38, e23660. [Google Scholar] [CrossRef]
- Go, R.C.P.; Corley, M.J.; Ross, G.W.; Petrovitch, H.; Masaki, K.H.; Maunakea, A.K.; He, Q.; Tiirikainen, M.I. Genome-wide epigenetic analyses in Japanese immigrant plantation workers with Parkinson’s disease and exposure to organochlorines reveal possible involvement of glial genes and pathways involved in neurotoxicity. BMC Neurosci. 2020, 21, 1–18. [Google Scholar] [CrossRef]
- Kalia, V.; Niedzwiecki, M.M.; Bradner, J.M.; Lau, F.K.; Anderson, F.L.; Bucher, M.L.; Manz, K.E.; Schlotter, A.P.; Fuentes, Z.C.; Pennell, K.D.; et al. Cross-species metabolomic analysis of tau- and DDT-related toxicity. PNAS Nexus 2022, 1, pgac050. [Google Scholar] [CrossRef]
- Mir, R.H.; Sawhney, G.; Pottoo, F.H.; Mohi-Ud-Din, R.; Madishetti, S.; Jachak, S.M.; Ahmed, Z.; Masoodi, M.H. Role of environmental pollutants in Alzheimer’s disease: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 44724–44742. [Google Scholar] [CrossRef]
- Torres-Sánchez, E.D.; Ortiz, G.G.; Reyes-Uribe, E.; Torres-Jasso, J.H.; Salazar-Flores, J. Effect of pesticides on phosphorylation of tau protein, and its influence on Alzheimer’s disease. World J. Clin. Cases. 2023, 11, 5628–5642. [Google Scholar] [CrossRef]
- Mohammadzadeh, L.; Abnous, K.; Razavi, B.M.; Hosseinzadeh, H. Crocin-protected malathion-induced spatial memory deficits by inhibiting TAU protein hyperphosphorylation and antiapoptotic effects. Nutr. Neurosci. 2020, 23, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.; Credle, J.; Oaks, A.W.; Duka, V.; Lee, J.H.; Jones, J.; Sidhu, A. Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS ONE 2012, 7, e30745. [Google Scholar] [CrossRef]
- Barbeau, A.; Dallaire, L.; Buu, N.T.; Poirier, J.; Rucinska, E. Comparative behavioral, biochemical and pigmentary effects of MPTP, MPP+ and paraquat in Rana pipiens. Life Sci. 1985, 37, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Liou, H.H.; Chen, R.C.; Tsai, Y.F.; Chen, W.P.; Chang, Y.C.; Tsai, M.C. Effects of paraquat on the substantia nigra of the Wistar rats: Neurochemical, histological, and behavioral studies. Toxicol. Appl. Pharmacol. 1996, 137, 34–41. [Google Scholar] [CrossRef]
- Brooks, A.I.; Chadwick, C.A.; Gelbard, H.A.; Cory-Slechta, D.A.; Federoff, H.J. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999, 823, 1–10. [Google Scholar] [CrossRef]
- Prasad, K.; Winnik, B.; Thiruchelvam, M.J.; Buckley, B.; Mirochnitchenko, O.; Richfield, E.K. Prolonged toxicokinetics and toxicodynamics of paraquat in mouse brain. Environ. Health Perspect. 2007, 115, 1448–1453. [Google Scholar] [CrossRef]
- Vaccari, C.; El Dib, R.; Gomaa, H.; Lopes, L.C.; de Camargo, J.L. Paraquat and Parkinson’s disease: A systematic review and meta-analysis of observational studies. J. Toxicol. Environ. Health B Crit. Rev. 2019, 22, 172–202. [Google Scholar] [CrossRef]
- Tong, T.; Duan, W.; Xu, Y.; Hong, H.; Xu, J.; Fu, G.; Wang, X.; Yang, L.; Deng, P.; Zhang, J.; et al. Paraquat exposure induces Parkinsonism by altering lipid profile and evoking neuroinflammation in the midbrain. Environ. Int. 2022, 169, 107512. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Tinkov, A.A.; Skalny, A.V.; Siokas, V.; Dardiotis, E.; Tsatsakis, A.; Bowman, A.B.; da Rocha, J.B.T.; Aschner, M. Brain diseases in changing climate. Environ. Res. 2019, 177, 108637. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Adeyinka, A.; Muco, E.; Regina, A.C.; Pierre, L. Organophosphates. In StatPearls [Internet]; StatPearls: Petersburg, FL, USA, 2023. [Google Scholar]
- Loewi, O. Über humorale übertragbarkeit der Herznervenwirkung. Pflüg. Arch. Eur. J. Phy. 1921, 189, 239–242. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Parsons, S.M.; Prior, C.; Marshall, I.G. Acetylcholine transport, storage, and release. Int Rev. Neurobiol. 1993, 35, 279–390. [Google Scholar] [CrossRef] [PubMed]
- Woolf, N.J.; Butcher, L.L. Cholinergic systems mediate action from movement to higher consciousness. Behav. Brain Res. 2011, 221, 488–498. [Google Scholar] [CrossRef]
- Boccia, M.M.; Blake, M.G.; Baratti, C.M.; McGaugh, J.L. Involvement of the basolateral amygdala in muscarinic cholinergic modulation of extinction memory consolidation. Neurobiol. Learn. Mem. 2009, 91, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, P.J.; Price, D.L.; Struble, R.G.; Clark, A.W.; Coyle, J.T.; Delon, M.R. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef]
- Millar, N.S.; Gotti, C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 2009, 56, 237–246. [Google Scholar] [CrossRef]
- Dineley, K.T.; Pandya, A.A.; Yakel, J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015, 36, 96–108. [Google Scholar] [CrossRef]
- Nathanson, N.M. A multiplicity of muscarinic mechanisms: Enough signaling pathways to take your breath away. Proc. Natl. Acad. Sci. USA 2000, 97, 6245–6247. [Google Scholar] [CrossRef]
- Kudo, Y.; Ogura, A.; Iijima, T. Stimulation of muscarinic receptor in hippocampal neuron induces characteristic increase in cytosolic free Ca2+ concentration. Neurosci. Lett. 1988, 85, 345–350. [Google Scholar] [CrossRef]
- Porter, A.C.; Bymaster, F.P.; DeLapp, N.W.; Yamada, M.; Wess, J.; Hamilton, S.E.; Nathanson, N.M.; Felder, C.C. M1 muscarinic receptor signaling in mouse hippocampus and cortex. Brain Res. 2002, 944, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Segal, M. Rat hippocampal neurons in culture: Responses to electrical and chemical stimuli. J. Neurophysiol. 1983, 50, 1249–1264. [Google Scholar] [CrossRef]
- Tobin, A.B. A golden age of muscarinic acetylcholine receptor modulation in neurological diseases. Nat. Rev. Drug Discov. 2024, 23, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Giordano, G.; Guizzetti, M.; Vitalone, A. Neurotoxicity of pesticides: A brief review. Front Biosci. 2008, 13, 1240–1249. [Google Scholar] [CrossRef]
- Kaur, S.; Chowdhary, S.; Kumar, D.; Bhattacharyya, R.; Banerjee, D. Organophosphorus and carbamate pesticides: Molecular toxicology and laboratory testing. Clin. Chim. Acta 2023, 551, 117584. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mercado, J.P.; Sierra-Santoyo, A.; Verdín-Betancourt, F.A.; Rojas-García, A.E.; Quintanilla-Vega, B. Temephos, an organophosphate larvicide for residential use: A review of its toxicity. Crit. Rev. Toxicol. 2022, 52, 113–124. [Google Scholar] [CrossRef]
- Musilek, K.; Dolezal, M.; Gunn-Moore, F.; Kuca, K. Design, evaluation and structure-Activity relationship studies of the AChE reactivators against organophosphorus pesticides. Med. Res. Rev. 2009, 31, 548–575. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Anticholinesterase insecticide retrospective. Chem. Biol. Interact. 2013, 203, 221–225. [Google Scholar] [CrossRef]
- Franco, R.; Li, S.; Rodriguez-Rocha, H.; Burns, M.; Panayiotidis, M.I. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem. Biol. Interact. 2010, 188, 289–300. [Google Scholar] [CrossRef]
- Nagami, H.; Nishigaki, Y.; Matsushima, S.; Matsushita, T.; Asanuma, S.; Yajima, N.; Usuda, M.; Hirosawa, M. Hospital-based survey of pesticide poisoning in Japan, 1998–2002. Int. J. Occup. Environ. Health 2005, 11, 180–184. [Google Scholar] [CrossRef]
- Mukherjee, S.; Gupta, R.D. Organophosphorus nerve agents: Types, toxicity, and treatments. J. Toxicol. 2020, 2020, 3007984. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Anwar, H.; Rasul, A.; Imran, A.; Qasim, M.; Zafar, S.; Imran, M.; Kamran, S.K.S.; Aziz, N.; Razzaq, A.; et al. Lipids as biomarkers of brain disorders. Crit. Rev. Food Sci. Nutr. 2020, 60, 351–374. [Google Scholar] [CrossRef]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The physiological roles of tau and Ab: Implications for Alzheimer’s disease pathology and therapeutics. Acta Neurophathol. 2020, 140, 417–447. [Google Scholar] [CrossRef]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Kimura-Kuroda, J.; Komuta, Y.; Kuroda, Y.; Hayashi, M.; Kawano, H. Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS ONE 2012, 7, e32432. [Google Scholar] [CrossRef]
- Estrada-Atehortúa, A.F.; Berrouet-Mejía, M.C.; Giraldo, J.A. Toxicidad por neonicotinoides: Revisión de tema y reporte de dos casos. Med. UPB 2016, 35, 41–46. [Google Scholar] [CrossRef]
- Venkatesan, D.; Iyer, M.; Narayanasamy, A.; Siva, K.; Vellingiri, B. Kynurenine pathway in Parkinson’s disease-an update. eNeurologicalSci 2020, 21, 100270. [Google Scholar] [CrossRef]
- Venkatesan, D.; Iyer, M.; Wilson, R.; Lakshmipathy, G.; Vellingiri, B. The association between multiple risk factors, clinical correlations and molecular insights in Parkinson’s disease patients from Tamil Nadu population, India. Neurosci. Lett. 2021, 755, 135903. [Google Scholar] [CrossRef]
- Gorell, J.M.; Peterson, E.L.; Rybicki, B.A.; Johnson, C.C. Multiple risk factors for Parkinson’s disease. J. Neurol. Sci. 2004, 217, 169–174. [Google Scholar] [CrossRef]
- Dardiotis, E.; Aloizou, A.M.; Siokas, V.; Tsouris, Z.; Rikos, D.; Marogianni, C.; Aschner, M.; Kovatsi, L.; Bogdanos, D.P.; Tsatsakis, A. Paraoxonase-1 genetic polymorphisms in organophosphate metabolism. Toxicology 2019, 411, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Iteire, K.A.; Sowole, A.T.; Ogunlade, B. Exposure to pyrethroids induces behavioral impairments, neurofibrillary tangles and tau pathology in Alzheimer’s type neurodegeneration in adult Wistar rats. Drug Chem. Toxicol. 2022, 45, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Niu, C.Y.; Lei, C.L.; Cui, J.J.; Desneux, N. Use of an innovative T-tube maze assay and the proboscis extension response assay to assess sublethal effects of GM products and pesticides on learning capacity of the honey bee Apis mellifera L. Ecotoxicology 2010, 19, 1612–1619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flaskos, J. The developmental neurotoxicity of organophosphorus insecticides: A direct role for the oxon metabolites. Toxicol. Lett. 2012, 209, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, F.; Ozturk, M.; Akdogan, M. The effect of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro). Arch. Toxicol. 2000, 74, 533–538. [Google Scholar] [CrossRef]
- Zhang, X.; Wallace, A.D.; Du, P.; Kibbe, W.A.; Jafari, N.; Xie, H.; Lin, S.; Baccarelli, A.; Soares, M.B.; Hou, L. DNA methylation alterations in response to pesticide exposure in vitro. Environ. Mol. Mutagen. 2012, 53, 542–549. [Google Scholar] [CrossRef]
- Zhang, X.; Wallace, A.D.; Du, P.; Lin, S.; Baccarelli, A.A.; Jiang, H.; Jafari, N.; Zheng, Y.; Xie, H.; Soares, M.B.; et al. Genome-wide study of DNA methylation alterations in response to diazinon exposure in vitro. Environ. Toxicol. Pharmacol. 2012, 34, 959–968. [Google Scholar] [CrossRef]
- Cortese, S.; Coghill, D. Twenty years of research on attention-deficit/hyperactivity disorder (ADHD): Looking back, looking forward. BMJ Ment. Health 2018, 21, 173–176. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- Tripp, G.; Wickens, J.R. Neurobiology of ADHD. Neuropharmacology 2009, 57, 579–589. [Google Scholar] [CrossRef]
- Asghar, U.; Malik, M.F.; Javed, A. Pesticide exposure and human health: A review. J. Ecosys. Ecograph S. 2016, 5. [Google Scholar] [CrossRef]
- Shah, R. Pesticides and human health. In Emerging Contaminants; Nuro, A., Ed.; InTech: Nappanee, IN, USA, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Nabi, M.; Tabassum, N. Role of environmental toxicants on neurodegenerative disorders. Front. Toxicol. 2022, 4, 837579. [Google Scholar] [CrossRef]
- Suarez-Lopez, J.R.; Himes, J.H.; Jacobs, D.R., Jr.; Alexander, B.H.; Gunnar, M.R. Acetylcholinesterase activity and neurodevelopment in boys and girls. Pediatrics 2013, 132, e1649–e1658. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.J.; Du, J.C.; Chiou, H.C.; Chung, M.Y.; Yang, W.; Chen, Y.S.; Fuh, M.R.; Chien, L.C.; Hwang, B.; Chen, M.L. Increased risk of attention-deficit/hyperactivity disorder associated with exposure to organophosphate pesticide in Taiwanese children. Andrology 2016, 4, 695–705. [Google Scholar] [CrossRef]
- Marks, A.R.; Harley, K.; Bradman, A.; Kogut, K.; Barr, D.B.; Johnson, C.; Calderon, N.; Eskenazi, B. Organophosphate pesticide exposure and attention in young Mexican American children: The CHAMACOS study. Environ. Health Perspect. 2010, 118, 1768–1774. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Jiménez-Ferrer, E.; González-Cortazar, M.; Zamilpa, A.; Cardoso-Taketa, A.; Arenas-Ocampo, M.L.; Jiménez-Aparicio, A.R.; Monterrosas-Brisson, N. Potential Use of Agave Genus in Neuroinflammation Management. Plants 2022, 11, 2208. [Google Scholar] [CrossRef]
- Cresto, N.; Forner-Piquer, I.; Baig, A.; Chatterjee, M.; Perroy, J.; Goracci, J.; Marchi, N. Pesticides at brain borders: Impact on the blood-brain barrier, neuroinflammation, and neurological risk trajectories. Chemosphere 2023, 324, 138251. [Google Scholar] [CrossRef] [PubMed]
- Sreekanthreddy, P.; Gromnicova, R.; Davies, H.; Phillips, J.; Romero, I.A.; Male, D. A three-dimensional model of the human blood-brain barrier to analyse the transport of nanoparticles and astrocyte/endothelial interactions. F1000Research 2015, 4, 1279. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol. Rev. 2018, 70, 278–314. [Google Scholar] [CrossRef]
- Archie, S.R.; Al Shoyaib, A.; Cucullo, L. Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics 2021, 13, 1779. [Google Scholar] [CrossRef] [PubMed]
- Brandl, S.; Reindl, M. Blood-Brain Barrier Breakdown in Neuroinflammation: Current In Vitro Models. Int. J. Mol. Sci. 2023, 24, 12699. [Google Scholar] [CrossRef]
- Ravid, O.; Elhaik Goldman, S.; Macheto, D.; Bresler, Y.; De Oliveira, R.I.; Liraz-Zaltsman, S.; Gosselet, F.; Dehouck, L.; Beeri, M.S.; Cooper, I. Blood-Brain Barrier Cellular Responses Toward Organophosphates: Natural Compensatory Processes and Exogenous Interventions to Rescue Barrier Properties. Front. Cell Neurosci. 2018, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, P.; Li, W.; Ehrich, M. Assessments of tight junction proteins occludin, claudin 5 and scaffold proteins ZO1 and ZO2 in endothelial cells of the rat blood-brain barrier: Cellular responses to neurotoxicants malathion and lead acetate. Neurotoxicology 2011, 32, 58–67. [Google Scholar] [CrossRef]
- Balbuena, P.; Li, W.; Magnin-Bissel, G.; Meldrum, J.B.; Ehrich, M. Comparison of two blood-brain barrier in vitro systems: Cytotoxicity and transfer assessments of malathion/oxon and lead acetate. Toxicol. Sci. 2010, 114, 260–271. [Google Scholar] [CrossRef]
- Hsu, S.S.; Jan, C.R.; Liang, W.Z. Uncovering malathion (an organophosphate insecticide) action on Ca2+ signal transduction and investigating the effects of BAPTA-AM (a cell-permeant Ca2+ chelator) on protective responses in glial cells. Pestic. Biochem. Physiol. 2019, 157, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Shieh, P.; Jan, C.R.; Liang, W.Z. The protective effects of the antioxidant N-acetylcysteine (NAC) against oxidative stress-associated apoptosis evoked by the organophosphorus insecticide malathion in normal human astrocytes. Toxicology 2019, 417, 1–14. [Google Scholar] [CrossRef]
- Mense, S.M.; Sengupta, A.; Lan, C.; Zhou, M.; Bentsman, G.; Volsky, D.J.; Whyatt, R.M.; Perera, F.P.; Zhang, L. The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol. Sci. 2006, 93, 125–135. [Google Scholar] [CrossRef]
- Maurya, S.K.; Rai, A.; Rai, N.K.; Deshpande, S.; Jain, R.; Mudiam, M.K.; Prabhakar, Y.S.; Bandyopadhyay, S. Cypermethrin induces astrocyte apoptosis by the disruption of the autocrine/paracrine mode of epidermal growth factor receptor signaling. Toxicol. Sci. 2012, 125, 473–487. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Weis, G.C.C.; Assmann, C.E.; Mostardeiro, V.B.; Alves, A.O.; da Rosa, J.R.; Pillat, M.M.; de Andrade, C.M.; Schetinger, M.R.C.; Morsch, V.M.M.; da Cruz, I.B.M.; et al. Chlorpyrifos pesticide promotes oxidative stress and increases inflammatory states in BV-2 microglial cells: A role in neuroinflammation. Chemosphere 2021, 278, 130417. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Liu, J.; Richardson, J.R. Pyrethroid Insecticides Directly Activate Microglia Through Interaction With Voltage-Gated Sodium Channels. Toxicol. Sci. 2017, 155, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Rajput, C.; Singh, M.P. Cypermethrin Induces the Activation of Rat Primary Microglia and Expression of Inflammatory Proteins. J. Mol. Neurosci. 2021, 71, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T.; Xu, Y.N.; Ren, X.Y.; Zhang, X.F. Inactivation of microglia dampens blood-brain barrier permeability and loss of dopaminergic neurons in paraquat-lesioned mice. Food Chem. Toxicol. 2023, 174, 113692. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Podolak, I. Anti-Neuroinflammatory Effects of Adaptogens: A Mini-Review. Molecules 2024, 29, 866. [Google Scholar] [CrossRef]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- Granger, K.T.; Barnett, J.H. Postoperative cognitive dysfunction: An acute approach for the development of novel treatments for neuroinflammation. Drug Discov. 2021, 26, 1111–1114. [Google Scholar] [CrossRef]
- Deng, M.; Yan, W.; Gu, Z.; Li, Y.; Chen, L.; He, B. Anti-Neuroinflammatory Potential of Natural Products in the Treatment of Alzheimer’s Disease. Molecules 2023, 28, 1486. [Google Scholar] [CrossRef]
- Rajadhyaksha, M.; Boyden, T.; Liras, J.; El-Kattan, A.; Brodfuehrer, J. Current advances in delivery of biotherapeutics across the blood-brain barrier. Curr. Drug Discov. Technol. 2011, 8, 87–101. [Google Scholar] [CrossRef]
- Juang, Y.P.; Liang, P.H. Biological and Pharmacological Effects of Synthetic Saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef]
- Chiu, F.L.; Lin, J.K. Tomatidine inhibits iNOS and COX-2 through suppression of NF-κB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008, 582, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Mao, Z.; Yang, S.; Wu, G.; Chen, Y.; Yin, L.; Qi, Y.; Han, L.; Xu, L. Dioscin alleviates Alzheimer’s disease through regulating RAGE/NOX4 mediated oxidative stress and inflammation. Biomed. Pharmacother. 2022, 152, 113248. [Google Scholar] [CrossRef] [PubMed]
- Lepage, C.; Liagre, B.; Cook-Moreau, J.; Pinon, A.; Beneytout, J.L. Cyclooxygenase-2 and 5-lipoxygenase pathways in diosgenin-induced apoptosis in HT-29 and HCT-116 colon cancer cells. Int. J. Oncol. 2010, 36, 1183–1191. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Jiménez-Ferrer, E.; Tortoriello, J.; Zamilpa, A.; Alegría-Herrera, E.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Martínez-Duncker, I.; Monterrosas-Brisson, N. Anti-neuroinflammatory effect of agaves and cantalasaponin-1 in a model of LPS-induced damage. Nat. Prod. Res. 2021, 35, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, L. The Neuroprotective Effects of Moderate and Regular Caffeine Consumption in Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 5568011. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7–28. [Google Scholar] [CrossRef]
- Jaturapatporn, D.; Isaac, M.G.; McCleery, J.; Tabet, N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst. Rev. 2012, 2, CD006378. [Google Scholar] [CrossRef]
- Shukla, M.; Wongchitrat, P.; Govitrapong, P. A Synopsis of Multitarget Potential Therapeutic Effects of Huperzine A in Diverse Pathologies-Emphasis on Alzheimer’s Disease Pathogenesis. Neurochem. Res. 2022, 47, 1166–1182. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zheng, W.; Wang, T.; Xie, J.W.; Wang, S.L.; Zhao, B.L.; Teng, W.P.; Wang, Z.Y. Huperzine A activates Wnt/β-catenin signaling and enhances the nonamyloidogenic pathway in an Alzheimer transgenic mouse model. Neuropsychopharmacology 2011, 36, 1073–1089. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. B-Sitosterol Alleviates Inflammatory Response via Inhibiting the Activation of ERK/p38 and NF-κB Pathways in LPS-Exposed BV2 Cells. BioMed. Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-κB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Sapkota, K.; Kim, S.; Kim, H.; Kim, S.J. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br. J. Pharmacol. 2011, 164, 1008–1025. [Google Scholar] [CrossRef]
- Hijam, A.C.; Tongbram, Y.C.; Nongthombam, P.D.; Meitei, H.N.; Koijam, A.S.; Rajashekar, Y.; Haobam, R. Neuroprotective Potential of Traditionally Used Medicinal Plants of Manipur against Rotenone-Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. J. Ethnopharmacol. 2024, 330, 118197. [Google Scholar] [CrossRef]
- Hijam, A.C.; Tongbram, Y.C.; Nongthombam, P.D.; Meitei, H.N.; Koijam, A.S.; Rajashekar, Y.; Haobam, R. Traditionally used edible medicinal plants protect against rotenone induced toxicity in SH-SY5Y cells-a prospect for the development of herbal nutraceuticals. Neurochem. Int. 2024, 180, 105855. [Google Scholar] [CrossRef] [PubMed]
- Rhioui, W.; Al Figuigui, J.; Lahlali, R.; Laasli, S.E.; Boutagayout, A.; El Jarroudi, M.; Belmalha, S. Towards sustainable vegetable farming: Exploring agroecological alternatives to chemical products in the Fez-Meknes region of Morocco. Sustainability 2023, 15, 7412. [Google Scholar] [CrossRef]
- Singh, M. Pesticide predicament: Exploring environmental and health impacts, and possible eco-friendly solutions. Int. J. Environ. Health Sci. 2024, 6, 38–47. [Google Scholar]
- Deguine, J.P.; Aubertot, J.N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]
- Deguine, J.P.; Aubertot, J.N.; Bellon, S.; Côte, F.; Lauri, P.E.; Lescourret, F.; Ratnadass, A.; Scopel, E.; Andrieu, N.; Bàrberi, P.; et al. Agroecological crop protection for sustainable agriculture. Adv. Agron. 2023, 178, 1–59. [Google Scholar] [CrossRef]
- Vikas; Ranjan, R. Agroecological approaches to sustainable development. Front. Sustain. Food Syst. 2024, 8, 1405409. [Google Scholar] [CrossRef]
- Jeanneret, P.; Aviron, S.; Alignier, A.; Lavigne, C.; Helfenstein, J.; Herzog, F.; Kay, S.; Petit, S. Agroecology landscapes. Landsc. Ecol. 2021, 36, 2235–2257. [Google Scholar] [CrossRef]
- Rastogi, M.; Verma, S.; Kumar, S.; Bharti, S.; Kumar, G.; Azam, K.; Singh, V. Soil health and sustainability in the age of organic amendments: A review. Int. J. Environ. Clim. Change 2023, 13, 2088–2102. [Google Scholar] [CrossRef]
- Somashekar, K.S.; Abhishek, G.J.; Kumar, V. Agroecology Principles, Practices and their Impact on Sustainable Food Systems. Eur. J. Nutr. Food Saf. 2024, 16, 249–260. [Google Scholar] [CrossRef]
- Hatt, S.; Döring, T.F. Designing pest suppressive agroecosystems: Principles for an integrative diversification science. J. Clean. Prod. 2023, 432, 139701. [Google Scholar] [CrossRef]
- Ganesh, B.M.; Ahmad, M.A.; Karthik, S.; Raju, B.J. Organic insect pest management: A sustainable approach for the Indian Farming Community. In Agriculture Entomology; Singh, K., Ed.; Rohini: Delhi, India, 2021; Volume 2, pp. 127–150. [Google Scholar]
- Sharma, S. Cultivating sustainable solutions: Integrated Pest Management (IPM) for safer and greener agronomy. Corp. Sustain. Manag. J. 2023, 1, 103–108. [Google Scholar] [CrossRef]
- Farooq, N.; Abbas, T.; Tanveer, A.; Jabran, K. Allelopathy for weed management. Co-Evol. Second. Metab. 2020, 505–519. [Google Scholar] [CrossRef]
- Yu, T.; Mahe, L.; Li, Y.; Wei, X.; Deng, X.; Zhang, D. Benefits of crop rotation on climate resilience and its prospects in China. Agronomy 2022, 12, 436. [Google Scholar] [CrossRef]
- Shah, K.K.; Modi, B.; Pandey, H.P.; Subedi, A.; Aryal, G.; Pandey, M.; Shrestha, J. Diversified crop rotation: An approach for sustainable agriculture production. Adv. Agric. 2021, 2021, 8924087. [Google Scholar] [CrossRef]
- Meena, R.K.; Mishra, P. Bio-pesticides for agriculture and environment sustainability. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer: Singapore, 2020; pp. 85–107. [Google Scholar] [CrossRef]
- Ximhai, R.; Nava-Pérez, E.; García-Gutiérrez, C.; Camacho-Báez, J.R.; Vázquez-Montoya, E.L. Bioplaguicidas: Una opción para el control biológico de plagas. Ra Ximhai 2012, 8, 17–29. [Google Scholar]
- Verma, D.K.; Guzmán, K.N.R.; Mohapatra, B.; Talukdar, D.; Chávez-González, M.L.; Kumar, V.; Srivastava, S.; Singh, V.; Yulianto, R.; Malar, S.E.; et al. Recent trends in plant-and microbe-based biopesticide for sustainable crop production and environmental security. In Recent Developments in Microbial Technologies; Prasad, R., Kumar, V., Singh, J., Upadhyaya, C.P., Eds.; Springer: Singapore, 2021; pp. 1–37. [Google Scholar] [CrossRef]
- Bel, Y.; Ferré, J.; Hernández-Martínez, P. Bacillus thuringiensis toxins: Functional characterization and mechanism of action. Toxins 2020, 12, 785. [Google Scholar] [CrossRef]
- Heckel, D.G. How do toxins from Bacillus thuringiensis kill insects? An evolutionary perspective. Arch. Insect Biochem. Physiol. 2020, 104, e21673. [Google Scholar] [CrossRef]
- Pinos, D.; Andrés-Garrido, A.; Ferré, J.; Hernández-Martínez, P. Response mechanisms of invertebrates to Bacillus thuringiensis and its pesticidal proteins. Microbiol. Mol. Biol. Rev. 2021, 85, 10–1128. [Google Scholar] [CrossRef]
- Azizoglu, U.; Salehi Jouzani, G.; Sansinenea, E.; Sanchis-Borja, V. Biotechnological advances in Bacillus thuringiensis and its toxins: Recent updates. Rev. Environ. Sci. Biotechnol. 2023, 22, 319–348. [Google Scholar] [CrossRef]
- Mwamburi, L.A. Beauveria. Benef. Microbes Agro-Ecol. 2020, 727–748. [Google Scholar] [CrossRef]
- Wang, H.; Peng, H.; Li, W.; Cheng, P.; Gong, M. The toxins of Beauveria bassiana and the strategies to improve their virulence to insects. Front. Microbiol. 2021, 12, 705343. [Google Scholar] [CrossRef]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as promising alternatives to chemical pesticides: A review of their current and future status. Online J. Biol. Sci. 2020, 20, 66–76. [Google Scholar] [CrossRef]
- Cappa, F.; Baracchi, D.; Cervo, R. Biopesticides and insect pollinators: Detrimental effects, outdated guidelines, and future directions. Sci. Total Environ. 2022, 837, 155714. [Google Scholar] [CrossRef] [PubMed]
- Daraban, G.M.; Hlihor, R.M.; Suteu, D. Pesticides vs. biopesticides: From pest management to toxicity and impacts on the environment and human health. Toxics 2023, 11, 983. [Google Scholar] [CrossRef]
- Eisa, M.A.S.; Hamid, H.A.; Ishag, A.E.S. Jatropha (Jatropha curcas) and Argel (Solenostemma argel) extracts as an oviposition inhibitor to Spiny Bollworm [Earias insulana (Lepidoptera: Noctuidae)]. Int. J. Life Sci. Res. 2020, 8, 5–11. [Google Scholar]
- Bratu, E.; Şovărel, G.; Cenuşă, A.E.; Velea, M. Nemagold”–bio-product of perspective in the control of root-knot nematode (Meloidogyne spp.) At tomato crops under plastic tunnel. Int. Multidiscip. Sci. GeoConference SGEM 2017, 17, 837–844. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Swathy, K.; Parmar, M.K.; Vivekanandhan, P. Biocontrol efficacy of entomopathogenic fungi Beauveria bassiana conidia against agricultural insect pests. Environ. Qual. Manag. 2024, 34, e22174. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Huang, S.T.; Chen, J.T.; Li, N.; Wei, Y.; Nawaz, A.; Deng, S.Q. An update of a green pesticide: Metarhizium anisopliae. All Life 2022, 15, 1141–1159. [Google Scholar] [CrossRef]
- Yapa, A.T.; Thambugala, K.M.; Samarakoon, M.C.; de Silva, N. Metarhizium species as bioinsecticides: Potential, progress, applications & future perspectives. N. Z. J. Bot. 2024, 63, 439–461. [Google Scholar] [CrossRef]
- Naz, I.; Khan, R.A.A.; Masood, T.; Baig, A.; Siddique, I.; Haq, S. Biological control of root knot nematode, Meloidogyne incognita, in vitro, greenhouse and field in cucumber. Biol. Control. 2021, 152, 104429. [Google Scholar] [CrossRef]
- Kim, J.J.; Goettel, M.S.; Gillespie, D.R. Evaluation of Lecanicillium longisporum, Vertalec® for simultaneous suppression of cotton aphid, Aphis gossypii, and cucumber powdery mildew, Sphaerotheca fuliginea, on potted cucumbers. Biol. Control. 2008, 45, 404–409. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Heckel, D.G.; Ferré, J. Mechanisms of resistance to insecticidal proteins from Bacillus thuringiensis. Annu. Rev. Entomol. 2021, 66, 121–140. [Google Scholar] [CrossRef]
- Jamal, Q.M.S.; Ahmad, V. Lysinibacilli: A biological factories intended for bio-insecticidal, bio-control, and bioremediation activities. J. Fungi. 2022, 8, 1288. [Google Scholar] [CrossRef]
- Terry Alfonso, E.; Ruiz Padrón, J.; Tejeda Peraza, T. Efecto de un bioproducto a base de Pseudomona aeruginosa en el cultivo del tomate (Solanum licopersicum Mill). Rev. Colomb. Biotecnol. 2010, 12, 32–38. [Google Scholar]
- Sarkar, B.; Kumar, C.; Pasari, S.; Goswami, B. Review on Pseudomonas fluorescens: A plant growth promoting rhizobacteria. J. Posit. Sch. Psychol. 2022, 6, 2701–2709. [Google Scholar]
- Nalinci, E.; Karagoz, M.; Gulcu, B.; Ulug, D.; Gulsen, S.H.; Cimen, H.; Touray, M.; Shapiro-Ilan, D.; Hazir, S. The effect of chemical insecticides on the scavenging performance of Steinernema carpocapsae: Direct effects and exposure to insects killed by chemical insecticides. J. Invertebr. Pathol. 2021, 184, 107641. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martínez, C.I.; Rodríguez-Hernández, A.I.; López-Cuellar, M.D.R.; Chavarría-Hernández, N.; De los Santos-Romero, R. Culture of Steinernema glaseri on three solid media and their virulence against Galleria mellonella larvae. Plant Prot. Sci. 2023, 59, 278–283. [Google Scholar] [CrossRef]
- Ropek, D.R.; Gospodarek, J. Entomopathogenic Nematode Steinernema feltiae as an Indicator of Soil Pollution with Oil Derivatives in Bioremediation Process. Agriculture 2022, 12, 2033. [Google Scholar] [CrossRef]
- Kary, N.E.; Sanatipour, Z.; Mohammadi, D.; Dillon, A.B. Combination effects of entomopathogenic nematodes, Heterorhabditis bacteriophora and Steinernema feltiae, with Abamectin on developmental stages of Phthorimaea operculella (Lepidoptera, Gelechiidae). Crop. Prot. 2021, 143, 105543. [Google Scholar] [CrossRef]
- Zhang, L.; Lecoq, M. Nosema locustae (Protozoa, Microsporidia), a biological agent for locust and grasshopper control. Agronomy 2021, 11, 711. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).