The Impact of Flooding on Soil Microbial Communities and Their Functions: A Review
Abstract
1. Introduction
2. Flooding Impacts on the Ecosystem
3. Mechanisms of Flooding-Induced Changes in Soil Microbial Communities
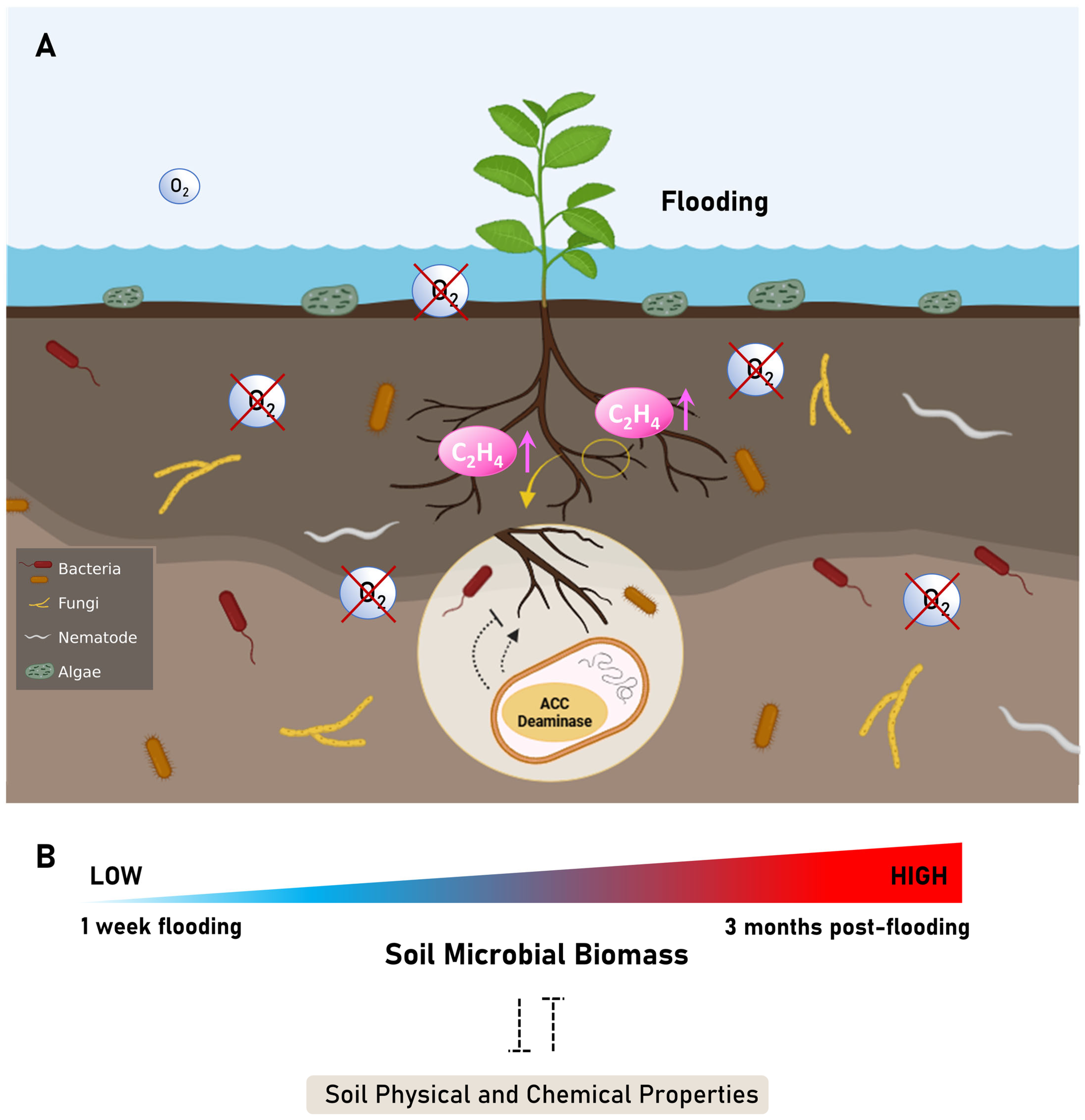
3.1. Flooding Leading to Soil Oxygen Reduction, Creating Anaerobic Environments
3.2. Adaptation of Microorganisms in Freshwater
3.3. Adaptation of Microorganisms to Saline Water
3.4. Impacts on Soil Microbial Structure and Biomass Under Flooded Conditions
3.5. Interconnected Influences Between Soil Microorganisms and Nutrients in Wetlands
4. Impact of Flooding on Long-Term Soil Health and Crop Productivity
5. Plant-Growth-Promoting Microorganism Roles in Plants Under Flooding Conditions
| Plants | Microbes | Groups | Flooding Types | Duration | Stress Condition | Impact on Plant Physiology | Impact on Ethylene Response/Gene | Impact on Soil Microorganisms | References |
|---|---|---|---|---|---|---|---|---|---|
| Lycopersicon esculentum | Enterobacter cloacae UW4, E. cloacae CAL2, Pseudomonas putida ATCC17399/pRKACC or P. putida ATCC17399/pRK415 | Bacteria | Waterlogging | 9 days | Pot | ACC deaminase-producing PGPR improved root growth, reduced shoot damage, and increased chlorophyll content | Reduced ethylene mitigated leaf epinasty and flooding-induced damage | Facilitated the proliferation of PGPR | [176] |
| Ocimum sanctum | Fd2, Achromobacter xylosoxidans (GenBank Accession No. JQ975414), Bac5, Serratia ureilytica (GenBank Accession No. JQ975415), Oci9, Herbaspirillum seropedicae (GenBank Accession No. JQ975416) and Oci13, Ochrobactrum rhizosphaerae (GenBank Accession No. JQ522946). | Bacteria | Waterlogging | 15 days | Pot | Reduced ROS production, increased root weight, shoot height, chlorophyll, and nutrient uptake | ACC deaminase-containing rhizobacteria reduced ethylene levels by degrading ACC, facilitating improved growth and yield | Promoted beneficial rhizobacterial activity, especially of strains like Achromobacter xylosoxidans and Serratia ureilytica | [177] |
| Cicer arietinum | Mesorhizobium ciceri LMS-1 | Bacteria | Waterlogging | 7 days | Pot | Enhanced growth under normal conditions. No significant improvements of LMS-1 (pRKACC) inoculated chickpea under waterlogging stress | LMS-1 (pRKACC) expressed an exogenous ACC deaminase gene (acdS) and reduced ethylene levels | Enhanced colonization of Mesorhizobium ciceri LMS-1 (pRKACC), increasing nodulation efficiency and nitrogen fixation | [178] |
| Rumex palustris | P. putida UW4 | Bacteria | Submergence | 72 h and 17 days | Pot | Decreased leaf elongation, reduced shoot weight, and root weight | Decreased ethylene production and altered flood escape strategies | Enhanced bacterial populations around plant roots | [179] |
| Vigna radiata | Streptomyces sp. GMKU 336 | Bacteria | Waterlogging | 21 days | Pot | Increased shoot and root elongation, biomass, leaf area, chlorophyll content, adventitious root formation, and survival rate | Reduced ethylene biosynthesis | Enhanced colonization by endophytic Streptomyces sp. in plant root | [180] |
| Sesamum indicum | Pseudomonas veronii KJ | Bacteria | Waterlogging | 10 days | Pot | Improved shoot and root length, biomass, chlorophyll content, and photosynthetic efficiency | Reduced ethylene level | Enhanced colonization by Pseudomonas veronii KJ in rhizosphere | [181] |
| Triticum aestivum | Trichoderma asperellum MAP1 | Fungi | Waterlogging | 5 days | Pot | Increased growth, photosynthetic efficiency, chlorophyll content, reduced electrolyte leakage, MDA, H2O2, and enhanced antioxidant enzyme | Reduced ethylene biosynthesis and ethylene signaling genes | Promoted colonization of Trichoderma asperellum MAP1 and influenced rhizosphere dynamics | [182] |
| Oryza sativa | Bacillus sp. (AR-ACC1), Microbacterium sp. (AR-ACC2), Methylophaga sp. (AR-ACC3), Paenibacillus sp. (ANR-ACC3) | Bacteria | Submergence | 7 days | Pot | Enhanced germination rates, seedling vigor index, improved root and shoot length, and total chlorophyll content | Reduced ethylene level | Promoted colonization of ACC deaminase-producing PGPR in the rhizosphere | [183] |
| Oryza sativa ssp. japonica | Phomopsis liquidambaris B3 | Fungi | Waterlogging | 9 days | Hydroponic | Improved root aerenchyma formation, chlorophyll content, soluble sugar accumulation, root respiration, and energy metabolism while increasing radial oxygen loss | Induced ethylene-mediated root aerenchyma formation | Interaction with rhizosphere microbial communities influenced nutrient dynamics and energy metabolism | [184] |
| Zea mays | Spergillus nomiae (MA1), Aspergillus fumigatus (MA4) | Fungi | Waterlogging | 7 days | Pot | Improved root and shoot length, biomass, chlorophyll content, antioxidant enzyme activity, soluble sugar content, reduced ROS production, and enhanced stomatal activity | Reduced ethylene production | Enhanced colonization of fungal endophytes | [185] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Soil microorganisms: Their role in enhancing crop nutrition and health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Raffa, C.M.; Chiampo, F. Bioremediation of agricultural soils polluted with pesticides: A review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.; Sharma, P.; Dwivedi, S.; Singh, A.; Gaur, V.K.; Varjani, S.; Srivastava, J.K.; Pandey, A.; Chang, J.-S.; Ngo, H.H. A review on control and abatement of soil pollution by heavy metals: Emphasis on artificial intelligence in recovery of contaminated soil. Environ. Res. 2023, 225, 115592. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef]
- Bai, Z.G.; Dent, D.L.; Olsson, L.; Schaepman, M.E. Proxy global assessment of land degradation. Soil Use Manag. 2008, 24, 223–234. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Compant, S.; Cassan, F.; Kostić, T.; Johnson, L.; Brader, G.; Trognitz, F.; Sessitsch, A. Harnessing the plant microbiome for sustainable crop production. Nat. Rev. Microbiol. 2024, 23, 9–23. [Google Scholar] [CrossRef]
- Trivedi, P.; Mattupalli, C.; Eversole, K.; Leach, J.E. Enabling sustainable agriculture through understanding and enhancement of microbiomes. New Phytol. 2021, 230, 2129–2147. [Google Scholar] [CrossRef]
- Zgadzaj, R.; Garrido-Oter, R.; Jensen, D.B.; Koprivova, A.; Schulze-Lefert, P.; Radutoiu, S. Root nodule symbiosis in Lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities. Proc. Natl. Acad. Sci. USA 2016, 113, E7996–E8005. [Google Scholar] [CrossRef]
- Bernhard, A. The nitrogen cycle: Processes. Play. Hum. 2010, 3, 25. [Google Scholar]
- Altaf, M.M. Functional diversity of nitrogen-fixing plant growth-promoting Rhizobacteria: The story so far. In Soil Nitrogen Ecology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 327–348. [Google Scholar]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef]
- Ribeiro, I.D.A.; Volpiano, C.G.; Vargas, L.K.; Granada, C.E.; Lisboa, B.B.; Passaglia, L.M.P. Use of mineral weathering bacteria to enhance nutrient availability in crops: A review. Front. Plant Sci. 2020, 11, 590774. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Wu, X.; Shao, C.; Zhang, H. Microbial enhancement of plant nutrient acquisition. Stress Biol. 2022, 2, 3. [Google Scholar] [CrossRef]
- Ezawa, T.; Saito, K. How do arbuscular mycorrhizal fungi handle phosphate? New insight into fine-tuning of phosphate metabolism. New Phytol. 2018, 220, 1116–1121. [Google Scholar] [CrossRef]
- Pellegrino, E.; Bedini, S. Enhancing ecosystem services in sustainable agriculture: Biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2014, 68, 429–439. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Fortuna, A.-M. The Soil Biota. Nat. Educ. Knowl. 2012, 3, 1. [Google Scholar]
- Kundzewicz, Z.W.; Kanae, S.; Seneviratne, S.I.; Handmer, J.; Nicholls, N.; Peduzzi, P.; Mechler, R.; Bouwer, L.M.; Arnell, N.; Mach, K. Flood risk and climate change: Global and regional perspectives. Hydrol. Sci. J. 2014, 59, 1–28. [Google Scholar] [CrossRef]
- Cressman, D.; Fortin, M.; Hensel, M.; Brubacher, P.; McBride, R. Estimation of cropland damages caused by overland flooding, two case studies. Can. Water Resour. J. 1988, 13, 15–25. [Google Scholar] [CrossRef]
- Nandy, A.; Kumar, A. Application of Geographic Information System and Remote Sensing on Flood Hazard Assessment and its Impact on Agriculture: A Review. In Flood Risk Management: Assessment and Strategy; Springer: Berlin/Heidelberg, Germany, 2024; pp. 287–308. [Google Scholar] [CrossRef]
- Raza, S.; Pandey, B.K.; Hawkesford, M.J.; Griffiths, S.; Bennett, M.J.; Mooney, S.J. Future crop breeding needs to consider future soils. Nat. Plants 2025. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Leal Filho, W.; Nagy, G.J.; Setti, A.F.F.; Sharifi, A.; Donkor, F.K.; Batista, K.; Djekic, I. Handling the impacts of climate change on soil biodiversity. Sci. Total Environ. 2023, 869, 161671. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Geng, W.; Sayer, E.J.; Zhou, G.; Zhou, P.; Liu, C. Soil microbial biomass and community responses to experimental precipitation change: A meta-analysis. Soil Ecol. Lett. 2020, 2, 93–103. [Google Scholar] [CrossRef]
- Panagos, P.; Borrelli, P.; Matthews, F.; Liakos, L.; Bezak, N.; Diodato, N.; Ballabio, C. Global rainfall erosivity projections for 2050 and 2070. J. Hydrol. 2022, 610, 127865. [Google Scholar] [CrossRef]
- FAO. Integrated Flood Management for Resilient Agrifood Systems and Rural Development; FAO: Rome, Italy, 2023. [Google Scholar]
- Winsemius, H.C.; Aerts, J.C.; Van Beek, L.P.; Bierkens, M.F.; Bouwman, A.; Jongman, B.; Kwadijk, J.C.; Ligtvoet, W.; Lucas, P.L.; Van Vuuren, D.P. Global drivers of future river flood risk. Nat. Clim. Change 2016, 6, 381–385. [Google Scholar] [CrossRef]
- Rentschler, J.; Salhab, M.; Jafino, B.A. Flood exposure and poverty in 188 countries. Nat. Commun. 2022, 13, 3527. [Google Scholar] [CrossRef]
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security: 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- Wallemacq, P.; Below, R.; McClean, D. Economic Losses, Poverty & Disasters: 1998–2017; United Nations Office for Disaster Risk Reduction: Geneva, Switzerland, 2018. [Google Scholar]
- Wax, E. For Flood-Prone Bangladesh, a Floating Future. Available online: https://www.nbcnews.com/id/wbna20982307 (accessed on 17 March 2025).
- Shalant, J. Bangladesh: A Country Underwater, a Culture on the Move. Available online: https://www.nrdc.org/stories/bangladesh-country-underwater-culture-move (accessed on 17 March 2025).
- Kamruzzaman, M.; Shariot-Ullah, M.; Islam, R.; Amin, M.G.M.; Islam, H.M.T.; Ahmed, S.; Yildiz, S.; Muktadir, A.; Shahid, S. Projections of future bioclimatic indicators using bias-corrected CMIP6 models: A case study in a tropical monsoon region. Environ. Sci. Pollut. Res. 2024, 31, 64596–64627. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kamruzzaman, M.; Deb, L.; Islam, H.M.T. Flood mapping, damage assessment, and susceptibility zonation in northeastern Bangladesh in 2022 using geospatial datasets. Prog. Disaster Sci. 2025, 25, 100402. [Google Scholar] [CrossRef]
- Khadka, N.S. The ‘Flying Rivers’ Causing Devastating Floods in India. Available online: https://www.bbc.com/news/articles/cv2g9x47441o (accessed on 17 March 2025).
- AlJazeera. Monsoon Floods Kill Dozens in India, Thousands in Relief Camps. Available online: https://www.aljazeera.com/news/2024/9/2/monsoon-floods-kill-dozens-in-india-thousands-in-relief-camps (accessed on 17 March 2025).
- Syldon, P.; Shrestha, B.B.; Miyamoto, M.; Tamakawa, K.; Nakamura, S. Assessing the impact of climate change on flood inundation and agriculture in the Himalayan Mountainous Region of Bhutan. J. Hydrol. Reg. Stud. 2024, 52, 101687. [Google Scholar] [CrossRef]
- Badamosi, A.P.; Olutumise, A.I.; Olukoya, O.P.; Adegoroye, A.; Aturamu, O.A. Socioeconomic impacts of flooding and its coping strategies in Nigeria: Evidence from Dagiri community, Gwagwalada area council of Abuja. Nat. Hazards Res. 2024, 4, 374–386. [Google Scholar] [CrossRef]
- FFC. Annual Flood Report 2012. Ministry of Water and Power, Government of Pakistan, Federal Flood Commission; FFC: Kowloon, Hong Kong, 2021. [Google Scholar]
- Baloch, S.M. ‘We Have No Dry Land Left’: Impact of Pakistan Floods to Be Felt for Years. The Guardian. 2022. Available online: https://www.theguardian.com/world/2022/oct/12/pakistan-floods-impact-years-crops-farms (accessed on 17 March 2025).
- Carrera, L.; Standardi, G.; Bosello, F.; Mysiak, J. Assessing direct and indirect economic impacts of a flood event through the integration of spatial and computable general equilibrium modelling. Environ. Model. Softw. 2015, 63, 109–122. [Google Scholar] [CrossRef]
- Djanibekov, U.; Polyakov, M.; Craig, H.; Paulik, R. Flood Impacts on Agriculture under Climate Change: The case of the Awanui Catchment, New Zealand. Econ. Disasters Clim. Change 2024, 8, 283–316. [Google Scholar] [CrossRef]
- Banerjee, L. Effects of Flood on Agricultural Productivity in Bangladesh. Oxf. Dev. Stud. 2010, 38, 339–356. [Google Scholar] [CrossRef]
- Chowdhury, R.B.; Moore, G.A. Floating agriculture: A potential cleaner production technique for climate change adaptation and sustainable community development in Bangladesh. J. Clean. Prod. 2017, 150, 371–389. [Google Scholar] [CrossRef]
- Van Steenbergen, F. Six Ways to Increase Productivity of Flood-Based Farming Systems. Risk and Variability, Research Program on Water, Land and Ecosystem. CGIAR. Available online: https://archive.iwmi.org/wle/solutions/six-ways-increase-productivity-flood-based-farming-systems/ (accessed on 3 January 2025).
- Drew, M. Sensing soil oxygen. Plant Cell Environ. 1990, 13, 681–693. [Google Scholar] [CrossRef]
- Erdmann, B.; Wiedenroth, E.M. Changes in the Root System of Wheat Seedlings Following Root Anaerobiosis: III. Oxygen Concentration in the Roots. Ann. Bot. 1988, 62, 277–286. [Google Scholar] [CrossRef]
- Sasidharan, R.; Bailey-Serres, J.; Ashikari, M.; Atwell, B.J.; Colmer, T.D.; Fagerstedt, K.; Fukao, T.; Geigenberger, P.; Hebelstrup, K.H.; Hill, R.D. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 2017, 214, 1403–1407. [Google Scholar] [CrossRef]
- Du, Y.; Guo, S.; Wang, R.; Song, X.; Ju, X. Soil pore structure mediates the effects of soil oxygen on the dynamics of greenhouse gases during wetting–drying phases. Sci. Total Environ. 2023, 895, 165192. [Google Scholar] [CrossRef]
- Chakraborty, P.; Guber, A.; Kravchenko, A. Influence of soil pore structure on the rate of microbial oxygen consumption. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 24–28 April 2023; p. EGU-9129. [Google Scholar]
- Burgin, A.J.; Groffman, P.M. Soil O2 controls denitrification rates and N2O yield in a riparian wetland. J. Geophys. Res. 2012, 117, G01010. [Google Scholar] [CrossRef]
- Oh, S.; Cho, K.; Park, S.; Kwon, M.J.; Chung, J.; Lee, S. Denitrification dynamics in unsaturated soils with different porous structures and water saturation degrees: A focus on the shift in microbial community structures. J. Hazard. Mater. 2023, 445, 130413. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liu, W.; Liu, R.; He, J.; Liu, D.; Feng, Z.; Feng, Z.; Li, R.; Li, C. Evolution of oxygen vacancies in cerium dioxide at atomic scale under CO2 reduction. Chem Catal. 2023, 3, 100759. [Google Scholar] [CrossRef]
- Borken, W.; Matzner, E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob. Change Biol. 2009, 15, 808–824. [Google Scholar] [CrossRef]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
- Zhu, K.; Bruun, S.; Larsen, M.; Glud, R.N.; Jensen, L.S. Heterogeneity of O2 dynamics in soil amended with animal manure and implications for greenhouse gas emissions. Soil Biol. Biochem. 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Graff, A.; Conrad, R. Impact of flooding on soil bacterial communities associated with poplar (Populus sp.) trees. FEMS Microbiol. Ecol. 2005, 53, 401–415. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Caruso, T. Soil microbial community responses to climate extremes: Resistance, resilience and transitions to alternative states. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190112. [Google Scholar] [CrossRef]
- Unger, I.M.; Motavalli, P.P.; Muzika, R.-M. Changes in soil chemical properties with flooding: A field laboratory approach. Agric. Ecosyst. Environ. 2009, 131, 105–110. [Google Scholar] [CrossRef]
- Dassonville, F.; Renault, P. Interactions between microbial processes and geochemical transformations under anaerobic conditions: A review. Agronomie 2002, 22, 51–68. [Google Scholar] [CrossRef]
- Pett-Ridge, J.; Firestone, M.K. Redox Fluctuation Structures Microbial Communities in a Wet Tropical Soil. Appl. Environ. Microbiol. 2005, 71, 6998–7007. [Google Scholar] [CrossRef]
- Wilson, J.S.; Baldwin, D.S.; Rees, G.N.; Wilson, B.P. The effects of short-term inundation on carbon dynamics, microbial community structure and microbial activity in floodplain soil. River Res. Appl. 2011, 27, 213–225. [Google Scholar] [CrossRef]
- Moche, M.; Gutknecht, J.; Schulz, E.; Langer, U.; Rinklebe, J. Monthly dynamics of microbial community structure and their controlling factors in three floodplain soils. Soil Biol. Biochem. 2015, 90, 169–178. [Google Scholar] [CrossRef]
- Ranatunga, T.; Hiramatsu, K.; Onishi, T.; Ishiguro, Y. Process of denitrification in flooded rice soils. Rev. Agric. Sci. 2018, 6, 21–33. [Google Scholar] [CrossRef]
- Francioli, D.; Cid, G.; Kanukollu, S.; Ulrich, A.; Hajirezaei, M.-R.; Kolb, S. Flooding Causes Dramatic Compositional Shifts and Depletion of Putative Beneficial Bacteria on the Spring Wheat Microbiota. Front. Microbiol. 2021, 12, 773116. [Google Scholar] [CrossRef]
- Randle-Boggis, R.J.; Ashton, P.D.; Helgason, T. Increasing flooding frequency alters soil microbial communities and functions under laboratory conditions. MicrobiologyOpen 2018, 7, e00548. [Google Scholar] [CrossRef]
- Coates, J.D.; Phillips, E.J.; Lonergan, D.J.; Jenter, H.; Lovley, D.R. Isolation of Geobacter species from diverse sedimentary environments. Appl. Environ. Microbiol. 1996, 62, 1531–1536. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, J.; Liu, X.; Zhong, H.; Wang, B.; Kong, Z.; Wu, L. Tracking the changes of wetland soil bacterial community and metabolic potentials under drought and flooding conditions in experimental microcosms. J. Soils Sediments 2021, 21, 2404–2417. [Google Scholar] [CrossRef]
- Herrmann, M.; Wegner, C.-E.; Taubert, M.; Geesink, P.; Lehmann, K.; Yan, L.; Lehmann, R.; Totsche, K.U.; Küsel, K. Predominance of Cand. Patescibacteria in Groundwater Is Caused by Their Preferential Mobilization From Soils and Flourishing Under Oligotrophic Conditions. Front. Microbiol. 2019, 10, 1407. [Google Scholar] [CrossRef]
- Dong, H.; Sun, H.; Fan, S.; Jiang, L.; Ma, D. Rhizobacterial communities, enzyme activity, and soil properties affect rice seedling’s nitrogen use. Agron. J. 2021, 113, 633–644. [Google Scholar] [CrossRef]
- Chimwamurombe, P.M.; Grönemeyer, J.L.; Reinhold-Hurek, B. Isolation and characterization of culturable seed-associated bacterial endophytes from gnotobiotically grown Marama bean seedlings. FEMS Microbiol. Ecol. 2016, 92, fiw083. [Google Scholar] [CrossRef]
- Soltani, A.-A.; Khavazi, K.; Asadi-Rahmani, H.; Omidvari, M.; Dahaji, P.A.; Mirhoseyni, H. Plant growth promoting characteristics in some Flavobacterium spp. isolated from soils of Iran. J. Agric. Sci. 2010, 2, 106. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Panjiar, N.; Kumar, S.; Saxena, A.K.; Suman, A. Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann. Microbiol. 2015, 65, 1885–1899. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Kachare, S.; Tiwari, S. Molecular diversity of 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing PGPR from wheat (Triticum aestivum L.) rhizosphere. Plant Soil 2017, 414, 213–227. [Google Scholar] [CrossRef]
- Bovio-Winkler, P.; Guerrero, L.D.; Erijman, L.; Oyarzúa, P.; Suárez-Ojeda, M.E.; Cabezas, A.; Etchebehere, C. Genome-centric metagenomic insights into the role of Chloroflexi in anammox, activated sludge and methanogenic reactors. BMC Microbiol. 2023, 23, 45. [Google Scholar] [CrossRef]
- Speirs, L.B.M.; Rice, D.T.F.; Petrovski, S.; Seviour, R.J. The Phylogeny, Biodiversity, and Ecology of the Chloroflexi in Activated Sludge. Front. Microbiol. 2019, 10, 2015. [Google Scholar] [CrossRef]
- da Silva, W.L.; Yang, K.-T.; Pettis, G.S.; Soares, N.R.; Giorno, R.; Clark, C.A. Flooding-Associated Soft Rot of Sweetpotato Storage Roots Caused by Distinct Clostridium Isolates. Plant Dis. 2019, 103, 3050–3056. [Google Scholar] [CrossRef]
- Torres-Martínez, L.; Sánchez-Julia, M.; Kimbrough, E.; Hendrix, T.C.; Hendrix, M.; Day, R.H.; Krauss, K.W.; Van Bael, S.A. Influence of soil microbiota on Taxodium distichum seedling performance during extreme flooding events. Plant Ecol. 2020, 221, 773–793. [Google Scholar] [CrossRef]
- Safavi-Rizi, V.; Friedlein, H.; Safavi-Rizi, S.; Krajinski-Barth, F. The impact of arbuscular mycorrhizal colonization on flooding response of Medicago truncatula. Front. Plant Sci. 2025, 15, 1512350. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Tebbe, C.C.; Zhao, Q.; Jia, J.; Wang, W.; Wang, X.; Yu, L. Salinity controls soil microbial community structure and function in coastal estuarine wetlands. Environ. Microbiol. 2021, 23, 1020–1037. [Google Scholar] [CrossRef]
- Verhoeven, J.T.A.; Laanbroek, H.J.; Rains, M.C.; Whigham, D.F. Effects of increased summer flooding on nitrogen dynamics in impounded mangroves. J. Environ. Manag. 2014, 139, 217–226. [Google Scholar] [CrossRef]
- Mukherji, S.; Haldar, S.; Ghosh, A. Investigation of the Structural and Functional Microbial Diversity in Indian Mangroves. In Microorganisms in Saline Environments: Strategies and Functions; Giri, B., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 93–130. [Google Scholar]
- Bai, S.; Li, J.; He, Z.; Van Nostrand, J.D.; Tian, Y.; Lin, G.; Zhou, J.; Zheng, T. GeoChip-based analysis of the functional gene diversity and metabolic potential of soil microbial communities of mangroves. Appl. Microbiol. Biotechnol. 2013, 97, 7035–7048. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Roy, S.; Mitra, A.; Roy, M. Isolation of multiple drug resistant and heavy metal resistant Stenotrophomonas maltophila strain BN1, a plant growth promoting Rhizobacteria, from Mangrove associate Ipomoea pes-caprae of Indian Sundarbans. J. Pure Appl. Microbiol. 2016, 10, 3131–3139. [Google Scholar] [CrossRef]
- Gaonkar, T.; Bhosle, S. Effect of metals on a siderophore producing bacterial isolate and its implications on microbial assisted bioremediation of metal contaminated soils. Chemosphere 2013, 93, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Pant, P.; Shanbhag, Y.P.; Sawant, S.V.; Ghadi, S.C. Genome Sequence of Microbulbifer mangrovi DD-13T Reveals Its Versatility to Degrade Multiple Polysaccharides. Mar. Biotechnol. 2017, 19, 116–124. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Saxena, R.; Mittal, P.; Gupta, A.; Sharma, V.K. Draft Genome Sequence of Pseudomonas hussainii Strain MB3, a Denitrifying Aerobic Bacterium Isolated from the Rhizospheric Region of Mangrove Trees in the Andaman Islands, India. Genome Announc. 2017, 5, 01527-16. [Google Scholar] [CrossRef]
- Ravikumar, S.; Gnanadesigan, M.; Ignatiammal, S.T.M.; Sumaya, S. Population dynamics of free living, nitrogen fixing bacteria Azospirillum in Manakkudi mangrove ecosystem, India. J. Environ. Biol. 2012, 33, 597–602. [Google Scholar]
- Das, S.; Kumar, J.T.; De, T.K. Vertical Profile of Phosphatase Activity in the Sundarban Mangrove Forest, North East Coast of Bay of Bengal, India. Geomicrobiol. J. 2014, 31, 716–725. [Google Scholar] [CrossRef]
- Sultana, M.; Nusrin, S.; Hasan, N.A.; Sadique, A.; Ahmed, K.U.; Islam, A.; Hossain, A.; Longini, I.; Nizam, A.; Huq, A.; et al. Biofilms Comprise a Component of the Annual Cycle of Vibrio cholerae in the Bay of Bengal Estuary. mBio 2018, 9, 00483-18. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bhattacharjee, S.; Bal, B.; Pal, R.; Niyogi, S.K.; Sarkar, K. Is Vibrio fluvialis emerging as a pathogen with epidemic potential in coastal region of eastern India following cyclone Aila? J. Health Popul. Nutr. 2010, 28, 311. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Plemenitaš, A.; Oren, A. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol. Rev. 2018, 42, 353–375. [Google Scholar] [CrossRef]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil Enzyme Activities and Biodiversity Measurements as Integrative Microbiological Indicators. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; SSSA Special Publications; WILEY: Washington, DC, USA, 1997; pp. 247–271. [Google Scholar]
- Sanaullah, M.; Blagodatskaya, E.; Chabbi, A.; Rumpel, C.; Kuzyakov, Y. Drought effects on microbial biomass and enzyme activities in the rhizosphere of grasses depend on plant community composition. Appl. Soil Ecol. 2011, 48, 38–44. [Google Scholar] [CrossRef]
- Burns, A.; Ryder, D. Response of bacterial extracellular enzymes to inundation of floodplain sediments. Freshw. Biol. 2001, 46, 1299–1307. [Google Scholar] [CrossRef]
- González Macé, O.; Steinauer, K.; Jousset, A.; Eisenhauer, N.; Scheu, S. Flood-Induced Changes in Soil Microbial Functions as Modified by Plant Diversity. PLoS ONE 2016, 11, e0166349. [Google Scholar] [CrossRef]
- Wagner, D.; Eisenhauer, N.; Cesarz, S. Plant species richness does not attenuate responses of soil microbial and nematode communities to a flood event. Soil Biol. Biochem. 2015, 89, 135–149. [Google Scholar] [CrossRef]
- Söderberg, K.H.; Probanza, A.; Jumpponen, A.; Bååth, E. The microbial community in the rhizosphere determined by community-level physiological profiles (CLPP) and direct soil– and cfu–PLFA techniques. Appl. Soil Ecol. 2004, 25, 135–145. [Google Scholar] [CrossRef]
- Wright, A.J.; Ebeling, A.; de Kroon, H.; Roscher, C.; Weigelt, A.; Buchmann, N.; Buchmann, T.; Fischer, C.; Hacker, N.; Hildebrandt, A.; et al. Flooding disturbances increase resource availability and productivity but reduce stability in diverse plant communities. Nat. Commun. 2015, 6, 6092. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Szili-Kovács, T.; Takács, T. Microbial Biomass and Rhizosphere Soil Properties in Response to Heavy Metal-Contaminated Flooding. Agriculture 2024, 14, 756. [Google Scholar] [CrossRef]
- Tang, J.; Liu, E.; Li, Y.; Tang, Y.; Tian, Y.; Du, S.; Li, H.; Wan, L.; Zhang, Q. Afforestation Promotes Soil Organic Carbon and Soil Microbial Residual Carbon Accrual in a Seasonally Flooded Marshland. Forests 2024, 15, 1542. [Google Scholar] [CrossRef]
- Petersen, S.O.; Klug, M.J. Effects of Sieving, Storage, and Incubation Temperature on the Phospholipid Fatty Acid Profile of a Soil Microbial Community. Appl. Environ. Microbiol. 1994, 60, 2421–2430. [Google Scholar] [CrossRef]
- Hackl, E.; Pfeffer, M.; Donat, C.; Bachmann, G.; Zechmeister-Boltenstern, S. Composition of the microbial communities in the mineral soil under different types of natural forest. Soil Biol. Biochem. 2005, 37, 661–671. [Google Scholar] [CrossRef]
- Bossio, D.A.; Fleck, J.A.; Scow, K.M.; Fujii, R. Alteration of soil microbial communities and water quality in restored wetlands. Soil Biol. Biochem. 2006, 38, 1223–1233. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Kennedy, A.C. Phospholipid fatty acid profiles and carbon utilization patterns for analysis of microbial community structure under field and greenhouse conditions. FEMS Microbiol. Ecol. 1998, 26, 151–163. [Google Scholar] [CrossRef]
- Ou, Y.; Rousseau, A.N.; Wang, L.; Yan, B.; Gumiere, T.; Zhu, H. Identification of the alteration of riparian wetland on soil properties, enzyme activities and microbial communities following extreme flooding. Geoderma 2019, 337, 825–833. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, H.; Huang, C. Assessing the effect of air-drying and storage on microbial biomass and community structure in paddy soils. Plant Soil 2009, 317, 213–221. [Google Scholar] [CrossRef]
- Wang, J.; Chapman, S.J.; Yao, H. The effect of storage on microbial activity and bacterial community structure of drained and flooded paddy soil. J. Soils Sediments 2015, 15, 880–889. [Google Scholar] [CrossRef]
- Shah, A.; Shah, S.; Shah, V. Impact of flooding on the soil microbiota. Environ. Chall. 2021, 4, 100134. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Beßler, H.; Engels, C.; Gleixner, G.; Habekost, M.; Milcu, A.; Partsch, S.; Sabais, A.C.W.; Scherber, C.; Steinbeiss, S.; et al. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 2010, 91, 485–496. [Google Scholar] [CrossRef]
- Strecker, T.; Macé, O.G.; Scheu, S.; Eisenhauer, N. Functional composition of plant communities determines the spatial and temporal stability of soil microbial properties in a long-term plant diversity experiment. Oikos 2016, 125, 1743–1754. [Google Scholar] [CrossRef]
- Milcu, A.; Thebault, E.; Scheu, S.; Eisenhauer, N. Plant diversity enhances the reliability of belowground processes. Soil Biol. Biochem. 2010, 42, 2102–2110. [Google Scholar] [CrossRef]
- Shen, R.; Lan, Z.; Rinklebe, J.; Nie, M.; Hu, Q.; Yan, Z.; Fang, C.; Jin, B.; Chen, J. Flooding variations affect soil bacterial communities at the spatial and inter-annual scales. Sci. Total Environ. 2021, 759, 143471. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Osanai, Y.; Lai, K.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Responses of the soil microbial community to nitrogen fertilizer regimes and historical exposure to extreme weather events: Flooding or prolonged-drought. Soil Biol. Biochem. 2018, 118, 227–236. [Google Scholar] [CrossRef]
- Koski-Vähälä, J.; Hartikainen, H. Assessment of the Risk of Phosphorus Loading Due to Resuspended Sediment. J. Environ. Qual. 2001, 30, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Qiqige, B.; Liu, J.; Li, M.; Hu, X.; Guo, W.; Wang, P.; Ding, Y.; Zhi, Q.; Wu, Y.; Guan, X.; et al. Different Flooding Conditions Affected Microbial Diversity in Riparian Zone of Huihe Wetland. Microorganisms 2025, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, Y.; Guo, J.; Li, X.; Rensing, C.; Wang, G. Dynamics of the rice rhizosphere microbial community under continuous and intermittent flooding treatment. J. Environ. Manag. 2019, 249, 109326. [Google Scholar] [CrossRef]
- Somenahally, A.C.; Hollister, E.B.; Yan, W.; Gentry, T.J.; Loeppert, R.H. Water Management Impacts on Arsenic Speciation and Iron-Reducing Bacteria in Contrasting Rice-Rhizosphere Compartments. Environ. Sci. Technol. 2011, 45, 8328–8335. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W.; Cao, Z.; Fiedler, S.; Frenzel, P.; Jahn, R.; Kalbitz, K.; Kölbl, A.; Schloter, M. Biogeochemistry of paddy soils. Geoderma 2010, 157, 1–14. [Google Scholar] [CrossRef]
- Chen, X.-P.; Kong, W.-D.; He, J.-Z.; Liu, W.-J.; Smith, S.E.; Smith, F.A.; Zhu, Y.-G. Do water regimes affect iron-plaque formation and microbial communities in the rhizosphere of paddy rice? J. Plant Nutr. Soil Sci. 2008, 171, 193–199. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. In Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.O., Scholes, R.J., Agard, J., Archer, E., Arneth, A., Bai, X., Barnes, D., Burrows, M., Chan, L., Cheung, W.L., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Reed, C.; Anderson, W.; Kruczkiewicz, A.; Nakamura, J.; Gallo, D.; Seager, R.; McDermid, S.S. The impact of flooding on food security across Africa. Proc. Natl. Acad. Sci. USA 2022, 119, e2119399119. [Google Scholar] [CrossRef]
- Ngumbi, E.N. Could flooding undermine progress in building climate-resilient crops? Trends Plant Sci. 2025, 30, 85–94. [Google Scholar] [CrossRef]
- Khan, I.; Fahad, S.; Wu, L.; Zhou, W.; Xu, P.; Sun, Z.; Salam, A.; Imran, M.; Jiang, M.; Kuzyakov, Y.; et al. Labile organic matter intensifies phosphorous mobilization in paddy soils by microbial iron (III) reduction. Geoderma 2019, 352, 185–196. [Google Scholar] [CrossRef]
- Rupngam, T.; Messiga, A.J. Unraveling the Interactions between Flooding Dynamics and Agricultural Productivity in a Changing Climate. Sustainability 2024, 16, 6141. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Croke, J.; Fryirs, K.; Thompson, C. Channel–floodplain connectivity during an extreme flood event: Implications for sediment erosion, deposition, and delivery. Earth Surf. Process. Landf. 2013, 38, 1444–1456. [Google Scholar] [CrossRef]
- Talbot, C.J.; Bennett, E.M.; Cassell, K.; Hanes, D.M.; Minor, E.C.; Paerl, H.; Raymond, P.A.; Vargas, R.; Vidon, P.G.; Wollheim, W.; et al. The impact of flooding on aquatic ecosystem services. Biogeochemistry 2018, 141, 439–461. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Merritt, D.M.; Olden, J.D.; Reynolds, L.V.; Lytle, D.A. Flow regime alteration degrades ecological networks in riparian ecosystems. Nat. Ecol. Evol. 2018, 2, 86–93. [Google Scholar] [CrossRef]
- Szejgis, J.; Nielsen, U.N.; Dijkstra, F.A.; Carrillo, Y. Prolonged drought moderates flood effects on soil nutrient pools across a rainfall gradient. Soil Biol. Biochem. 2024, 193, 109404. [Google Scholar] [CrossRef]
- Brammer, H. Floods in the agroecology of Bangladesh; Relief and Development Institute: London, UK; Mimeo: New York, NY, USA, 1988. [Google Scholar]
- Hofer, T.; Messerli, B. Floods in Bangladesh: History, dynamics and rethinking the role of the Himalayas. Ecology 2006, 29, 254–283. [Google Scholar] [CrossRef]
- Dar, M.H.; Chakravorty, R.; Waza, S.A.; Sharma, M.; Zaidi, N.W.; Singh, A.N.; Singh, U.S.; Ismail, A.M. Transforming rice cultivation in flood prone coastal Odisha to ensure food and economic security. Food Secur. 2017, 9, 711–722. [Google Scholar] [CrossRef]
- Rahaman, S. Flood and flood management in Bangladesh. In Proceedings of the Seminar Work: Course: DSMHT 403 Climate Modelling and Adaptation, Dhaka, Bangladesh, 17 January 2017. [Google Scholar]
- Tasleem, Z.; Tasleem, Z.; Hatim, M.; Muhammad, S. A case study of floods and its impact on agriculture, livestock and infrastructure in balochistan, Pakistan. J. Educ. Soc. Stud. 2023, 4, 859–873. [Google Scholar] [CrossRef]
- Wilbanks, T.; Fernandez, S.; Backus, G.; Garcia, P.; Jonietz, K.; Kirshen, P.; Savonis, M.; Solecki, W.; Toole, L. Climate change and infrastructure, urban systems and Vulnerabilities: Technical Report for the US Department of Energy in Support of the National Climate Assessment; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ploschuk, R.A.; Miralles, D.J.; Striker, G.G. A quantitative review of soybean responses to waterlogging: Agronomical, morpho-physiological and anatomical traits of tolerance. Plant Soil 2022, 475, 237–252. [Google Scholar] [CrossRef]
- Wu, C.; Mozzoni, L.A.; Moseley, D.; Hummer, W.; Ye, H.; Chen, P.; Shannon, G.; Nguyen, H. Genome-wide association mapping of flooding tolerance in soybean. Mol. Breed. 2019, 40, 4. [Google Scholar] [CrossRef]
- Hossain, M.A.; Uddin, S.N. Mechanisms of waterlogging tolerance in wheat: Morphological and metabolic adaptations under hypoxia or anoxia. Australian Journal of Crop Science 2011, 5, 1094–1101. [Google Scholar]
- Gao, J.; Yang, C.; Zhuang, S.; Gui, R. Effects of mulching and flooding on soil nutrients and bacterial community structure under Phyllostachys praecox. Front. For. Glob. Change 2024, 7, 1411297. [Google Scholar] [CrossRef]
- WMO. Associated Programme on Flood Management Technical Document No. 3, Flood Management Policy Series – Environmental Aspects of Integrated Flood Management; World Meteorological Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding Stress: Acclimations and Genetic Diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Principles of Biochemistry, 5th ed.; W. H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Zabalza, A.; van Dongen, J.T.; Froehlich, A.; Oliver, S.N.; Faix, B.; Gupta, K.J.; Schmälzlin, E.; Igal, M.; Orcaray, L.; Royuela, M.; et al. Regulation of Respiration and Fermentation to Control the Plant Internal Oxygen Concentration. Plant Physiol. 2008, 149, 1087–1098. [Google Scholar] [CrossRef]
- Kennedy, R.A.; Rumpho, M.E.; Fox, T.C. Anaerobic Metabolism in Plants 1. Plant Physiol. 1992, 100, 1–6. [Google Scholar] [CrossRef]
- Szal, B.; Drozd, M.; Rychter, A.M. Factors affecting determination of superoxide anion generated by mitochondria from barley roots after anaerobiosis. J. Plant Physiol. 2004, 161, 1339–1346. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Pedersen, O.; Colmer, T.D.; Sand-Jensen, K. Underwater Photosynthesis of Submerged Plants – Recent Advances and Methods. Front. Plant Sci. 2013, 4, 140. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, R.; Hartman, S.; Liu, Z.; Martopawiro, S.; Sajeev, N.; van Veen, H.; Yeung, E.; Voesenek, L.A.C.J. Signal Dynamics and Interactions during Flooding Stress. Plant Physiol. 2017, 176, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Tournaire-Roux, C.; Sutka, M.; Javot, H.; Gout, E.; Gerbeau, P.; Luu, D.-T.; Bligny, R.; Maurel, C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 2003, 425, 393–397. [Google Scholar] [CrossRef]
- Drew, M.C.; He, C.-J.; Morgan, P.W. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000, 5, 123–127. [Google Scholar] [CrossRef]
- Drew, M.C.; He, C.-J.; Morgan, P.W. Decreased Ethylene Biosynthesis, and Induction of Aerenchyma, by Nitrogen- or Phosphate-Starvation in Adventitious Roots of Zea mays L. Plant Physiol. 1989, 91, 266–271. [Google Scholar] [CrossRef]
- Hartman, S.; Liu, Z.; Van Veen, H.; Vicente, J.; Reinen, E.; Martopawiro, S.; Zhang, H.; Van Dongen, N.; Bosman, F.; Bassel, G.W. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019, 10, 4020. [Google Scholar] [CrossRef]
- Hartman, S.; Sasidharan, R.; Voesenek, L.A.C.J. The role of ethylene in metabolic acclimations to low oxygen. New Phytol. 2021, 229, 64–70. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 01473. [Google Scholar] [CrossRef]
- Maheshwari, S.K.; Ramyashree, D.G.S.; Meena, A.; Mawar, R.; Yadav, D.L. Role of PGPM in Managing Soil-Borne Plant Pathogens in Horticulture Crops. In Plant Growth Promoting Microorganisms of Arid Region; Mawar, R., Sayyed, R.Z., Sharma, S.K., Sattiraju, K.S., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 185–194. [Google Scholar]
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and pathogenic plant-microbe interactions during flooding stress. Plant, Cell Environ. 2022, 45, 2875–2897. [Google Scholar] [CrossRef]
- Ali, S.; Kim, W.-C. Plant Growth Promotion Under Water: Decrease of Waterlogging-Induced ACC and Ethylene Levels by ACC Deaminase-Producing Bacteria. Front. Microbiol. 2018, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gupta, A.; Vandana, P.; Tiwari, L.D.; Patel, M.K.; Siddique, K.H.M. Chapter 17 - Recent advances of plant growth-promoting rhizobacteria (PGPR)-mediated drought and waterlogging stress tolerance in plants for sustainable agriculture. In Microbial Biostimulants for Plant Growth and Abiotic Stress Amelioration; Chauhan, P.S., Bisht, N., Agarwal, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 315–344. [Google Scholar]
- Senko, H.; Kajić, S.; Huđ, A.; Palijan, G.; Petek, M.; Rajnović, I.; Šamec, D.; Udiković-Kolić, N.; Mešić, A.; Brkljačić, L.; et al. Will the beneficial properties of plant-growth promoting bacteria be affected by waterlogging predicted in the wake of climate change: A model study. Appl. Soil Ecol. 2024, 198, 105379. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Geng, S.; Zhang, X. A review of soil waterlogging impacts, mechanisms, and adaptive strategies. Front. Plant Sci. 2025, 16, 1545912. [Google Scholar] [CrossRef]
- Verma, K.K.; Joshi, A.; Song, X.-P.; Singh, S.; Kumari, A.; Arora, J.; Singh, S.K.; Solanki, M.K.; Seth, C.S.; Li, Y.-R. Synergistic interactions of nanoparticles and plant growth promoting rhizobacteria enhancing soil-plant systems: A multigenerational perspective. Front. Plant Sci. 2024, 15, 1376214. [Google Scholar] [CrossRef]
- Tyagi, A.; Ali, S.; Mir, R.A.; Sharma, S.; Arpita, K.; Almalki, M.A.; Mir, Z.A. Uncovering the effect of waterlogging stress on plant microbiome and disease development: Current knowledge and future perspectives. Front. Plant Sci. 2024, 15, 1407789. [Google Scholar] [CrossRef]
- Tuheteru, F.D.; Wu, Q.-S. Arbuscular Mycorrhizal Fungi and Tolerance of Waterlogging Stress in Plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.-S., Ed.; Springer: Singapore, 2017; pp. 43–66. [Google Scholar]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D. Arbuscular Mycorrhizal Fungi and Plant Stress Tolerance. In Plant Microbiome: Stress Response; Egamberdieva, D., Ahmad, P., Eds.; Springer: Singapore, 2018; pp. 81–103. [Google Scholar]
- Wu, Q.-S.; Zou, Y.-N.; Huang, Y.-M. The arbuscular mycorrhizal fungus Diversispora spurca ameliorates effects of waterlogging on growth, root system architecture and antioxidant enzyme activities of citrus seedlings. Fungal Ecol. 2013, 6, 37–43. [Google Scholar] [CrossRef]
- Zou, Y.; Srivastava, A.; Wu, Q.; Huang, Y. Increased tolerance of trifoliate orange (Poncirus trifoliata) seedlings to waterlogging after inoculation with arbuscular mycorrhizal fungi. J. Animal Plant Sci. 2014, 24, 1415–1420. [Google Scholar]
- Tuo, X.-Q.; Li, S.; Wu, Q.-S.; Zou, Y.-N. Alleviation of waterlogged stress in peach seedlings inoculated with Funneliformis mosseae: Changes in chlorophyll and proline metabolism. Sci. Hortic. 2015, 197, 130–134. [Google Scholar] [CrossRef]
- Grichko, V.P.; Glick, B.R. Amelioration of flooding stress by ACC deaminase-containingplant growth-promoting bacteria. Plant Physiol. Biochem. 2001, 39, 11–17. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol. Biochem. 2012, 58, 227–235. [Google Scholar] [CrossRef]
- Nascimento, F.; Brígido, C.; Alho, L.; Glick, B.R.; Oliveira, S. Enhanced chickpea growth-promotion ability of a Mesorhizobium strain expressing an exogenous ACC deaminase gene. Plant Soil 2012, 353, 221–230. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.C.J.; Kowalchuk, G.A.; Jousset, A. ACC deaminase-producing rhizosphere bacteria modulate plant responses to flooding. J. Ecol. 2017, 105, 979–986. [Google Scholar] [CrossRef]
- Jaemsaeng, R.; Jantasuriyarat, C.; Thamchaipenet, A. Positive role of 1-aminocyclopropane-1-carboxylate deaminase-producing endophytic Streptomyces sp. GMKU 336 on flooding resistance of mung bean. Agric. Nat. Resour. 2018, 52, 330–334. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.A.; Kim, W.-C. Pseudomonas veronii KJ mitigates flood stress-associated damage in Sesamum indicum L. Appl. Biol. Chem. 2018, 61, 575–585. [Google Scholar] [CrossRef]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular Mechanisms of the 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase Producing Trichoderma asperellum MAP1 in Enhancing Wheat Tolerance to Waterlogging Stress. Front. Plant Sci. 2021, 11, 614971. [Google Scholar] [CrossRef]
- Bal, H.B.; Adhya, T.K. Alleviation of Submergence Stress in Rice Seedlings by Plant Growth-Promoting Rhizobacteria With ACC Deaminase Activity. Front. Sustain. Food Syst. 2021, 5, 606158. [Google Scholar] [CrossRef]
- Hu, L.-Y.; Yang, Y.; Wu, H.; Tang, M.-J.; Xie, X.-G.; Dai, C.-C. Phomopsis liquidambaris Increases Rice Mineral Uptake Under Waterlogging Condition via the Formation of Well-Developed Root Aerenchyma. J. Plant Growth Regul. 2022, 41, 1758–1772. [Google Scholar] [CrossRef]
- Rahman, K.U.; Ali, K.; Rauf, M.; Arif, M. Aspergillus nomiae and fumigatus Ameliorating the Hypoxic Stress Induced by Waterlogging through Ethylene Metabolism in Zea mays L. Microorganisms 2023, 11, 2025. [Google Scholar] [CrossRef]
- Stevens, K.; Spender, S.; Peterson, R. Phosphorus, arbuscular mycorrhizal fungi and performance of the wetland plant Lythrum salicaria L. under inundated conditions. Mycorrhiza 2002, 12, 277–283. [Google Scholar]
- Oyarte Galvez, L.; Bisot, C.; Bourrianne, P.; Cargill, R.; Klein, M.; van Son, M.; van Krugten, J.; Caldas, V.; Clerc, T.; Lin, K.-K.; et al. A travelling-wave strategy for plant–fungal trade. Nature 2025, 639, 172–180. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Bacterial Modulation of Plant Ethylene Levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Pilet, P.-E.; Saugy, M. Effect on Root Growth of Endogenous and Applied IAA and ABA: A Critical Reexamination. Plant Physiol. 1987, 83, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y. The Function of Root Exudates in the Root Colonization by Beneficial Soil Rhizobacteria. Biology 2024, 13, 95. [Google Scholar] [CrossRef]
- Ansari, M.; Devi, B.M.; Sarkar, A.; Chattopadhyay, A.; Satnami, L.; Balu, P.; Choudhary, M.; Shahid, M.A.; Jailani, A.A.K. Microbial Exudates as Biostimulants: Role in Plant Growth Promotion and Stress Mitigation. J. Xenobiotics 2023, 13, 572–603. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.K.; Lee, D.-S.; Woo, Y.-J.; Sultana, S.; Mahmud, A.; Yun, B.-W. The Impact of Flooding on Soil Microbial Communities and Their Functions: A Review. Stresses 2025, 5, 30. https://doi.org/10.3390/stresses5020030
Das AK, Lee D-S, Woo Y-J, Sultana S, Mahmud A, Yun B-W. The Impact of Flooding on Soil Microbial Communities and Their Functions: A Review. Stresses. 2025; 5(2):30. https://doi.org/10.3390/stresses5020030
Chicago/Turabian StyleDas, Ashim Kumar, Da-Sol Lee, Youn-Ji Woo, Sharmin Sultana, Apple Mahmud, and Byung-Wook Yun. 2025. "The Impact of Flooding on Soil Microbial Communities and Their Functions: A Review" Stresses 5, no. 2: 30. https://doi.org/10.3390/stresses5020030
APA StyleDas, A. K., Lee, D.-S., Woo, Y.-J., Sultana, S., Mahmud, A., & Yun, B.-W. (2025). The Impact of Flooding on Soil Microbial Communities and Their Functions: A Review. Stresses, 5(2), 30. https://doi.org/10.3390/stresses5020030






