Abstract
The aim of the present study was to assess heavy metal tolerance and its accumulation potential in coastal nitrophilic species Rumex maritimus, and to study the possible effects of nitrogen fertilizer and salinity on the characteristics of metal-treated plants. Two experiments were performed in partially controlled greenhouse conditions: (1) gradual treatment with increasing concentrations of Cd, Pb, Cu, Mn, and Zn; and (2) acute treatment with Cd, Pb, and Cu on the background of different nitrogen fertilizer amendment rates (0.15 and 0.30 g L−1 N) and salinity (50 and 100 mM). R. maritimus plants were extremely tolerant to treatment with all metals, with no negative effect on total leaf biomass both in the case of gradual or acute treatment. However, the number and biomass of dry leaves increased under high doses of heavy metals, and the effect was more pronounced in the case of acute treatment. All studied metals were excluded from the roots and young leaves, predominantly accumulating in the dry leaves, reaching 250 mg kg−1 for Cd, 2000 mg kg−1 for Pb, and 500 mg kg−1 for Cu. In the second experiment, the presence of increased nitrogen in the substrate positively affected the growth of R. maritimus plants and their morphological response to heavy metals, but salinity affected metal accumulation. Photosynthesis-related parameters, leaf chlorophyll concentration, and the chlorophyll a fluorescence parameter Performance Index Total confirmed that heavy metals had no negative effect on the physiological state of photosynthetically active leaves. It is concluded that R. maritimus plants have exceptional potential for practical phytoremediation needs due to the high tolerance and accumulation potential for heavy metals.
1. Introduction
Heavy metals appear in the environment as a result of natural causes as well as due to anthropogenic pressure. These metals tend to accumulate in sediments, where they can persist for longer periods of time due to their low degradability. Their accumulation is a threat not only to plants but to entire ecosystems exposed to them [1,2,3]. Different wetlands, including the ones affected by seawater, are among the ecosystems with a high intensity of geochemical cycling. Active circulation and accumulation of heavy metals (Cd, Pb, Fe, Zn, As, Hg, etc.) is a characteristic feature of coastal habitats; therefore, many coastal wetland species are known for their metal tolerance [4,5,6,7]. In addition, the ability of a large number of coastal halophyte species to extract heavy metals from contaminated saltwater or saline soil has been shown [8,9,10].
Phytoremediation of degraded lands has gained large scientific and practical interest during the last decades [11]. Among different types of phytoremediation, phytoextraction is the most effective technique for removing metals from contaminated soil. This approach is considered to be especially environmentally friendly and requires the selection of plant species suitable for local conditions with certain physiological characteristics: high rate of biomass accumulation, resistance to various metals, and the ability to accumulate metals in the above-ground parts of plants [12,13,14].
It is logical to assume that changes in substrate salinity could affect heavy metal tolerance and/or accumulation potential in species native to saline environments. In respect to salinity, several halophyte species have been reported as being able to better tolerate high soil heavy metal concentrations in saline conditions, such as Juncus acutus [15] and Lepidium latifolium [16] in the case of Zn, as well as Kosteletzkya pentacarpos under polymetallic (As, Cd, Zn, Pb) treatment [17].
Plant mineral nutrition status is an important determinant of tolerance against different abiotic factors, including heavy metals [18,19]. In many species, additional nitrogen fertilization increases growth rate and heavy metal accumulation potential, as in Zn hyperaccumulator Noccaea caerulescens [20], Cd hyperaccumulator Sedum plumbizincicola [21], Cd hyperaccumulator Rorippa globosa [22], Ni hyperaccumulator Alyssum murale [23], or Zn/Cd hyperaccumulating Sedum alfredii [24]. In addition, several salt-tolerant species from coastal habitats can be considered as nitrophiles, because the additional supply of nitrogenous compounds promotes their growth [25,26]. However, the effects of nitrogen fertilization on heavy metal tolerance and accumulation in coastal halophytes have been assessed less often [27].
Rumex maritimus L. is a potentially salt-tolerant species frequently found in coastal habitats [28] that has been most extensively studied with respect to flooding tolerance [29,30,31]. Salinity tolerance in dependence on anion type has been also assessed in R. maritimus and other wetland Rumex species [26]. Another typical wetland species, Rumex hydrolapathum, frequently found in salt-affected coastal wetlands [32], has emerged as a metal-tolerant species with a high phytoremediation potential [33,34]. However, there is no information available on heavy metal tolerance and accumulation potential in plants from coastal R. maritimus populations. R. maritimus can be regarded as a nitrophilic species, as the species in natural conditions is “confined to the naturally N-rich soils” (with nitrogen availability indicator value 8 out of 9) [35], and plant growth is stimulated by additional nitrate doses in controlled conditions [34]. Therefore, it seems logical to expect that substrate amendment with nitrogen will lead to increased metal tolerance of R. maritimus plants due to increased nutrient uptake or enhanced metabolic processes.
The aim of the present study was to assess heavy metal tolerance and their accumulation potential in coastal nitrophilic species R. maritimus and to study the possible effects of nitrogen fertilizer amendment and salinity on plant characteristics. The hypothesis tested was that additional nitrogen will improve plant growth of control as well as metal-treated plants and that nitrogen and/or salinity treatment will affect metal accumulation capacity in plant tissues.
2. Results
2.1. Experiment 1: Gradual Treatment with Cd, Pb, Cu, Mn, and Zn
In the first experiment, R. maritimum plants were gradually treated with increasing concentrations of Cd, Pb, Cu, Mn, and Zn. In this case, the growth of plants changed relatively little, and no visual signs of toxicity were observed (Table 1, Supplementary Figures S1–S5). There was no significant effect of metal treatment on the total number of leaves and total dry biomass of leaves. However, total dry biomass of roots significantly decreased in plants treated with 500 mg L−1 Cu and 1000 mg L−1 Zn. In addition, the number of dry leaves per plant significantly increased at 100 mg L−1 Cd as well as 500 and 1000 mg L−1 Zn. Surprisingly, the biomass of the dry leaves did not significantly increase by any of the heavy metal treatments. Instead, there was a significant decrease of this parameter in several treatments (Cd 20 mg L−1, Pb 500 mg L−1, Cu 100 and 500 mg L−1, Mn 200 mg L−1, Zn 200 mg L−1).

Table 1.
Effect of gradual treatment with various metals on morphological parameters of Rumex maritimus plants.
Metal concentration was separately analyzed in the plant roots, roots outside the container, dry leaves, old leaves, middle leaves, and young leaves. A statistically significant gradient of metal accumulation in different plant parts was observed. All heavy metals predominantly accumulated in the dry leaves of R. maritimus plants, with the lowest accumulation potential in the roots (Figure 1). Also, the metal accumulation capacity in the old leaves was higher than that in the young leaves. For Cd and Cu, relatively high accumulation was evident also for the roots located outside the plant containers (Figure 1A,C).
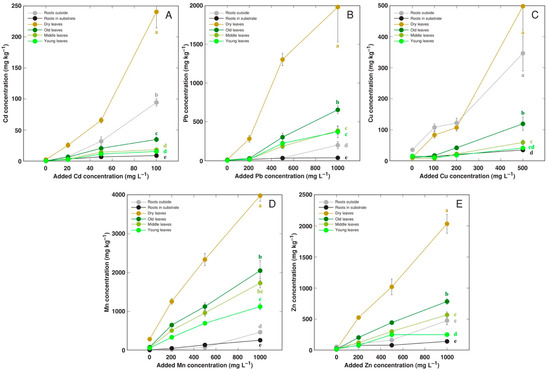
Figure 1.
Effect of increasing substrate concentration of various metals on metal accumulation in various parts of Rumex maritimus plants. (A): Cd; (B): Pb; (C): Cu; (D): Mn; (E): Zn. Data are means from 3 replicates ± SE. Means followed by the same letter are not significantly different according to the Tukey HSD test (p < 0.05).
The chlorophyll a fluorescence parameter, Performance Index Total, did not show pronounced changes in the middle leaves of R. maritimus plants under the influence of heavy metal treatment (Table 2). Only in the Zn-treated plants was an increasing trend of the parameter observed with increasing metal concentration, but this effect was statistically significant only at the highest Zn dose.

Table 2.
Effect of gradual treatment with various metals on Performance Index Total in middle leaves of Rumex maritimus plants.
2.2. Experiment 2: Acute Treatment with Cd, Pb, and Cu on the Background of Different N Content and Salinity
In the second experiment, acute treatment with the highest doses of three heavy metals (100 mg L−1 Cd, 1000 mg L−1 Pb, 500 mg L−1 Cu) on the background of different soil nitrogen amendment (0, 0.15, and 0.30 g of added N L−1; control, N1 and N2, respectively) and salinity level (0, 50, and 100 mmol of added NaCl L−1; control, S1 and S2, respectively) were used (Supplementary Figures S6–S9).
For plants in non-amended soil, treatment with Cu resulted in a significantly increased total number of leaves, but the dry biomass of the leaves was not significantly affected (Figure 2A). However, the number of dry leaves (Figure 2B) and their biomass (Figure 2D) significantly increased under the effect of Cd and Pb. Both the number of leaves and the total leaf biomass increased in the N-amended plants without a metal treatment, but the addition of NaCl had no effect on these parameters (Figure 2A,C). Surprisingly, also both the number of dry leaves and their biomass increased under the N amendment in the non-treated plants, with no significant effect of salinity (Figure 2B,D). Comparing only plants treated with respective metals, N-amended plants had higher leaf number and biomass than the non-amended plants (Figure 2A,C). However, for the N2-amended plants, the treatment with Pb and Cu resulted in significantly decreased leaf biomass in comparison to that of the plants without metal treatment. Under low salinity (50 mmol L−1 NaCl; S1), the treatment with Cd and Cu plants decreased leaf biomass, but this effect was not significant under moderate salinity (50 mmol L−1 NaCl; S2). Nitrogen amendment decreased both the number and biomass of dry leaves in the Cu-treated plants with significant effect only at the highest amendment rate, but not in the Cd-treated ones (Figure 2B,D). However, salinity treatment had no significant effect on the number and biomass of the dry leaves of metal-treated plants.
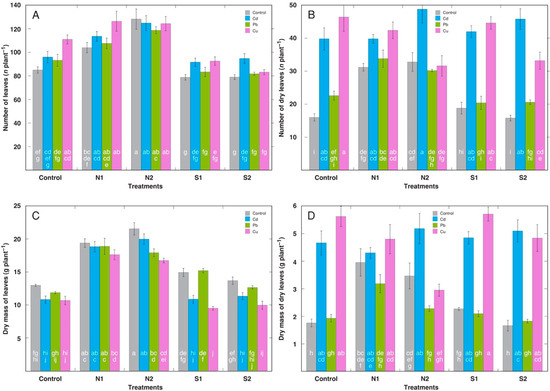
Figure 2.
Effect of various metals on number of leaves (A), number of dry leaves (B), dry mass of leaves (C), dry mass of dry leaves (D) of Rumex maritimus plants on the background of additional nitrogen (N1, N2) or salinity (S1, S2) treatment. N1, 0.15 g N L−1; N2, 0.30 g N L−1; S1, 50 mmol NaCl L−1; S2, 100 mmol NaCl L−1. Data are means from 5 replicates ± SE. Means followed by the same letter are not significantly different according to the Tukey HSD test (p < 0.05).
Analysis of a relative distribution of biomass among leaves of different ages indicated that the proportion of young leaves increased both in the N-amended as well as NaCl-treated plants without metal treatment (Figure 3A). N amendment decreased the proportion of dry leaves in the Cd- (Figure 3B) and Cu-treated (Figure 3D) plants, together with the increase in the proportion of both young and middle leaves.
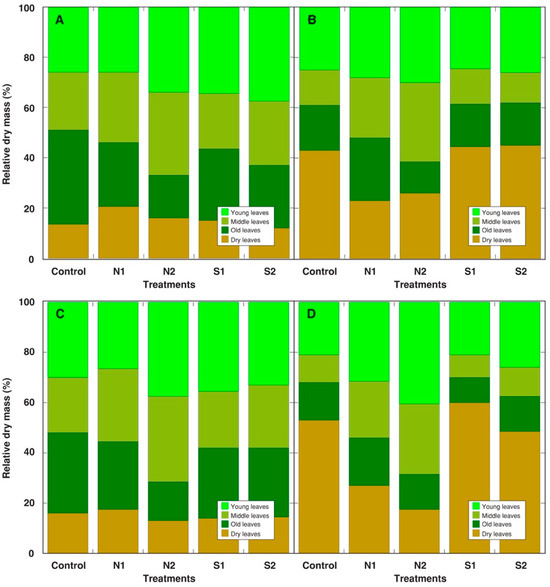
Figure 3.
Effect of additional nitrogen (N1, N2) or salinity (S1, S2) treatment on relative distribution of dry mass in leaves of different age groups of Rumex maritimus plants treated with different heavy metals. (A): no heavy metal; (B): Cd; (C): Pb; (D): Cu. N1, 0.15 g N L−1; N2, 0.30 g N L−1; S1, 50 mmol NaCl L−1; S2, 100 mmol NaCl L−1.
Metal concentration was separately analyzed in the dry leaves, old leaves, and middle leaves of R. maritimus plants (Figure 4). Accumulation of Cd was not significantly affected by N amendment, but it increased in the dry leaves under salinity (Figure 4A). Pb accumulation was decreased by salinity in the old leaves, with no significant effect by either soil amendment in the dry leaves (Figure 4B). Substrate amendment with N significantly decreased Cu accumulation in the dry leaves, but low salinity increased it (Figure 4C).
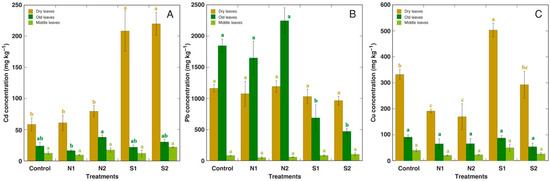
Figure 4.
Effect of various metals on metal accumulation in various parts of Rumex maritimus plants on the background of additional nitrogen (N1, N2) or salinity (S1, S2) treatment. (A): Cd; (B): Pb; (C): Cu. N1, 0.15 g N L−1; N2, 0.30 g N L−1; S1, 50 mmol NaCl L−1; S2, 100 mmol NaCl L−1. Data are means from 3 replicates ± SE. Means followed by the same letter are not significantly different according to the Tukey HSD test (p < 0.05). For each leaf age group, statistical significance is analyzed separately.
Chlorophyll concentration in the middle leaves of R. maritimus plants was relatively little affected by heavy metals, soil nitrogen, and salinity (Figure 5). There was a tendency for chlorophyll to increase in the plants receiving additional nitrogen, but the effect was significant only for the Pb- and Cu-treated plants at the higher N dose. This can also be interpreted as a nitrogen-dependent increase in Pb and Cu tolerance. Surprisingly, there was a statistically significant decrease of leaf chlorophyll concentration in the leaves of control plants at the lower salinity treatment, but such an effect was not evident for the plants treated with heavy metals or at the higher salinity.
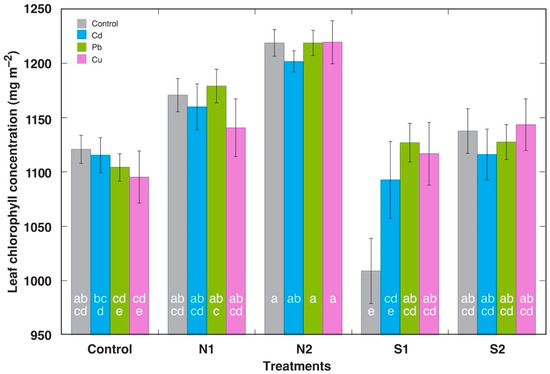
Figure 5.
Effect of increasing substrate concentration of various metals on chlorophyll concentration in middle leaves of Rumex maritimus plants on the background of additional nitrogen (N1, N2) or salinity (S1, S2) treatment. N1, 0.15 g N L−1; N2, 0.30 g N L−1; S1, 50 mmol NaCl L−1; S2, 100 mmol NaCl L−1. Data are means from 10 independent measurements on 5 plants ± SE. Means followed by the same letter are not significantly different according to the Tukey HSD test (p < 0.05).
The chlorophyll a fluorescence parameter, Performance Index Total, positively responded to substrate amendment with nitrogen fertilizer, but this effect decreased with time (Table 3). One and three weeks after the full treatment, plants receiving 0.30 g L−1 N showed a significant additional increase in the parameter due to the effect of Cd and Pb. In addition, plants under 100 mM salinity treated with Pb and Cu had significantly increased Performance Index Total values in comparison to those in the control plants.

Table 3.
Effect of various metals on the background of additional nitrogen (N1, N2) or salinity (S1, S2) treatment on Performance Index Total in middle leaves of Rumex maritimus plants.
3. Discussion
3.1. Metal Tolerance
R. maritimus plants showed extremely high tolerance against all heavy metals tested. Biomass of leaves was not negatively affected even at the highest metal doses used (100 mg L−1 Cd, 1000 mg L−1 Pb, 500 mg L−1 Cu, 1000 mg L−1 Mn, 1000 mg L−1 Zn), but root growth was significantly inhibited only at 500 mg L−1 Cu and 1000 mg L−1 Zn (Table 1). Moreover, no visual signs of toxicity were evident, as found, for example, in a related species Rumex hydrolapathum from a coastal salt marsh, showing dry yellow lesions already at 500 mg L−1 Zn and reddish-brown lesions at 500 mg L−1 Mn treatments [33].
Due to the absence of significant growth changes by gradual heavy metal treatment in the first experiment, single-dose acute treatment with Cd, Cu, and Pb was used in the second experiment. It has been demonstrated that acute treatment with heavy metals has more severe consequences on plant growth and physiology in comparison to the gradual one [36]. In the present study, acute treatment with Cd and Cu resulted in a higher increase in the number of dry leaves (Figure 2B) and their biomass (Figure 2D) in comparison to gradual treatment (Table 1). No other negative effects were evident, including leaf chlorophyll concentration (Figure 5) and the chlorophyll a fluorescence parameter Performance Index Total (Table 2 and Table 3), confirming the existence of excellent metal tolerance in R. maritimus plants, which could be attributed to the developmental characteristics of the species. Thus, gradual treatment with heavy metals within several weeks can improve plant metal tolerance in comparison to a single-dose acute treatment. It has been shown that each single time-separated treatment episode acts as a factor inducing increased tolerance at the biochemical level by antioxidative systems, as shown by associated changes in leaf peroxidase activity [36].
At the vegetative stage, Rumex plants form a rosette of leaves, with the relatively clear morphological distinction between the groups of leaves of different ages. The characteristic developmental feature of the plants is the programmed senescence of the older leaves, which is especially manifested in unfavorable conditions, accompanied by the stimulation of the formation of new leaves [26]. This phenomenon was evident also in the present study, where an increase in N fertilizer resulted in a significant increase in the number of dry leaves of control plants (Figure 2). Both salt ions [26] and heavy metals [33,34] accumulate in senescing leaves of Rumex plants, which can therefore be considered as an avoidance mechanism against chemical stress, since the accumulation capacity is markedly lower in actively photosynthesizing and, especially, growing younger leaves.
If low or moderate salinity is required for optimum growth of a halophytic plant (so-called “true halophytes” or “euhalophytes”), then it is logical to expect that these plants will have better heavy metal tolerance at optimal salinity. Thus, shoot growth was reduced at 20 mg kg−1 Cd in soil-grown true halophyte species Carpobrotus rosii, but additional treatment with 30 mM NaCl fully reversed Cd-dependent growth suppression of plants [37]. Similarly, NaCl increased Cd tolerance in a halophyte Salicornia fruticosa [38]. In the present study, increased salinity (50 and 100 mM NaCl) had no effect on the growth and development of R. maritimus plants not treated with heavy metals (Figure 2). Similarly, increased salinity did not affect plant tolerance to heavy metals, as Cd- and Cu-dependent increases in the number and biomass of dry leaves were the same as for plants growing in non-saline soil.
As R. maritimus can be regarded as nitrophilic species [26,35], it was assumed that substrate amendment with nitrogen fertilizer would lead to increased metal tolerance of R. maritimus plants. The presence of increased nitrogen in the substrate indeed affected the growth of R. maritimus plants and their morphological response to heavy metals. Both control and heavy metal-treated plants showed an increased total number of leaves and dry biomass of leaves, but at the highest N amendment rate, a significantly negative relative effect of Pb and Cu treatment on leaf biomass was evident (Figure 2C). However, this effect cannot be evaluated unambiguously, because the increase of nitrogen in the substrate contributed to the increase of dry leaf quantity and biomass in control plants, but in the presence of high N, the promoting effect of Cu on dry leaf biomass increase decreased. Thus, it appears that additional N reduced Cu-dependent leaf senescence.
Given the very high tolerance of R. maritimus plants to heavy metals as well as their high tolerance to salinity, the species can be characterized as a “facultative extremophile”. There was no evidence for euhalophytic (growth stimulated by salinity) or obligatory metallophytic characteristics of the species. Similarly, Armeria maritima can be designated as both a facultative metallophyte and a facultative halophyte [39]. Indeed, Armeria maritima accessions from a dry coastal meadow showed prominent salinity tolerance, with no negative effect on growth up to 100–200 mmol L−1 [40]. In addition, there was no adverse effect of several heavy metals on plant growth [41]. An interesting example of the extremophilic genus is represented by Cochlearia, which is composed only of halophytes or metallophytes [39]. These species possess multiple pre-adaptations to both high metal content and high salinity. Physiological and biochemical mechanisms of such cross-tolerance have been analyzed in detail elsewhere [8,10,42].
Photosynthesis-related parameters have been often used as indicators of plant physiological status in heavy metal studies (e.g., [43]) due to special vulnerability of the photosynthesis process to heavy metals [44]. Usually, the increase in the complex fluorescence-related parameters, such as the Performance Index, is regarded as an indicator of the improved metabolic and physiological state of plants, while their decrease has been associated with negative physiological changes and, in particular, heavy metal toxicity [45]. In contrast to leaf chlorophyll concentration that reflects late negative changes of physiological status associated with deleterious processes [46], the Performance Index has relatively early changes after exposure to suboptimal conditions and, therefore can be useful as a predictive indicator of negative physiological effects [47]. In the present study, both leaf chlorophyll concentration as well as the Performance Index Total of R. maritimus plants significantly increased with an increase in nitrogen amendment in the substrate (Figure 5, Table 2 and Table 3). Thus, the stable physiological state shows that the plants were in the stage of long-term metal tolerance.
3.2. Metal Accumulation
In the present study, the maximum metal accumulation capacity in dry leaves of gradually-treated R. maritimus plants reached 250 mg kg−1 Cd, 2000 mg kg−1 Pb, 500 mg kg−1 Cu, 4000 mg kg−1 Mn, and 2000 mg kg−1 Zn (Figure 1). For comparison, related species from a coastal wetland, Rumex hydrolapathum, accumulated up to 60 mg kg−1 Cd, 800 mg kg−1 Pb in dry leaves [34], 6400 mg kg−1 Mn and 1840 mg kg−1 Zn in old leaves [33]. Consequently, R. maritimus had higher accumulation potential for Cd and Pb in comparison to that of R. hydrolapathum, and hyperaccumulation threshold concentration for these metals (100 mg kg−1 for Cd and 1000 mg kg−1 for Pb), as well as that for Cu (300 mg kg−1) was exceeded [48]. However, by no means R. maritimus can be designated as a metal hyperaccumulating species as no evidence has been presented showing similar metal accumulation ability in natural conditions.
The predominant accumulation of metals in leaves of R. maritimus plants over roots seems to be a rather unique feature among halophyte species. Maximum Cu concentration in the roots and leaves of Kosteletzkya virginica was 1500 and 60 mg kg−1, respectively [49]. The maximum concentration of Pb in roots and shoots of Atriplex halimus was 30–70 and 10–20 mg kg−1, respectively, and that for Cd 2–5 and 1–3 mg kg−1, respectively [50]. Salicornia europaea plants accumulated 7000 and 3500 mg kg−1 Zn, 800 and 400 mg kg−1 Pb, and 80 and 35 mg kg−1 Cd in roots and shoots, respectively [51]. Mesembryanthemum crystallinum plants accumulated 300 and 65 mg kg−1 Cd in roots and shoots, respectively [52]. Maximum accumulation potential in roots and shoots of Sesuvium portulacastrum was 3815 and 251 mg kg−1 Cd and 2090 and 106 mg kg−1 Cu, respectively [53]. Also, several coastal halophytic species of the genus Cochlearia predominantly accumulated both Cd and Zn in roots [39]. However, in a taxonomically and functionally related coastal wetland species R. hydrolapathum, several heavy metals—Cd and Ni [34] as well as Zn and Mn [33]—predominantly accumulated in the older leaves.
An increase in a plant-available nitrogen concentration in the substrate has been shown to enhance Cd accumulation potential in several species, but the effect was species-specific and depended on the form of N fertilizer [54]. Thus, nitrogen (NH4NO3) application increased the shoot accumulation of Cd in halophyte Atriplex lentiformis [55]. However, no effect of increased N on Cd accumulation was evident in R. maritimus plants (Figure 4). It is possible that the differences are related to the form of N compound used, as it has been shown that only ammonium can have a positive effect on Cd accumulation, as in the species Carpobrotus rossii [56]. In species not requiring additional nitrogen for their optimal growth, such as Noccaea caerulescens, the addition of nitrate had no positive effect on the accumulation of Cd and Zn, but nitrogen in the form of ammonia even decreased it [57].
Another soil factor that was manipulated in the present study, NaCl-related salinity, increased Cd accumulation in the dry leaves of R. maritimus (Figure 4). From the point of chemical interactions in soil, it has been shown that salinity increases the bioavailability of several heavy metals [58], but this might be relevant only for metal indicator species and not for metal-excluding or hyperaccumulator species. However, the information mentioned in the literature about the effect of salinity on Cd accumulation is rather contradictory. For an obligate halophyte species Atriplex halimus, Cd accumulation in shoots was reduced by NaCl salinity both in leaves and roots [59]. In contrast, salinity increased Cd accumulation in shoots of halophytic species Tamarix smyrnensis [60], and both in shoots and roots of halophyte Salicornia fruiticosa [38]. From a mechanistic point of view, increased expression of several genes involved in a metal uptake by salinity can be involved in salinity-dependent elevation of Cd accumulation potential for halophyte Mesembryanthemum crystallinum [61].
Although it is logical to assume that the salinity tolerance of halophytic species is also related to their metal accumulation capacity, information on the effect of salinity on heavy metal accumulation in halophyte species is rather contradictory. Using perlite hydroponics, it was shown that moderate NaCl level (9 dS m−1) increased accumulation of heavy metals in relatively salt-tolerant coastal halophyte species Plantago coronopus and Inula crithmoides, but metal accumulation decreased at high NaCl (18 dS m−1) [62]. In hydroponic conditions, 50 mM NaCl decreased Cd and Zn accumulation in a salt marsh halophyte Kosteletzkya pentacarpos [63]. However, salinity increased Cd accumulation in a soil-grown obligate halophyte species, Carpobrotus rosii [37].
Another prominent effect found in the present study was increased accumulation of Cu in the dry leaves of R. maritimus plants at low (50 mmol L−1) salinity (Figure 4C). This effect is inconsistent with the results obtained in experiments with wetland halophyte species Kosteletzkya virginica, where NaCl treatment decreased Cu accumulation both in the stems and roots without any positive effect on plant growth [49]. In addition, a decrease in Cu concentration in the dry leaves of R. maritimus was the only observed effect of increased substrate nitrogen concentration on metal accumulation in the present study (Figure 4C). This effect could be attributed to the so-called “dilution” mechanism, which can be observed when the rate of biomass accumulation increases significantly, but the rate of uptake of the element remains unchanged [64,65].
In contrast to Cd and Cu, which accumulation was promoted by salinity in the dry leaves of R. maritimus, Pb accumulation was not affected in the dry leaves by salinity, but it decreased in the old living leaves (Figure 4B). However, Pb accumulation capacity was increased in halophyte Suaeda salsa plants by NaCl through increased translocation from roots to shoots [66], but not affected in the halophyte Atriplex halimus [50].
As for phytoextraction potential, it is reasonable to suggest that any conditions leading to increased shoot biomass even without a decrease in tissue metal concentration will increase the total amount of the extracted metal. Therefore, the total amount of shoot-accumulated metals per individual R. maritimus plant will increase in the case of Cd and Pb, because their concentration in tissues did not decrease in the situation where additional N stimulated biomass formation. In general, the plants had a relatively high rate of biomass formation, which is an essential prerequisite for ensuring efficient phytoextraction. In addition, its long-lived perennial status and ability to regrow even after multiple mowing during the season further confirm the practical phytoextraction potential of R. maritimus.
3.3. Possible Limitations and Perspectives
To understand the limitations of the experimental system used, at least two characteristics related to the growing substrate and their possible impact on the obtained results should be discussed. First, the level of mineral nutrient supply and availability is a critical factor in plant tolerance to adverse environmental factors [19]. Studies on the plant tolerance to heavy metals also need to take into account the correspondence of the actual mineral content to the optimal one, and this factor is most often overlooked in scientific studies. The soil substrate used in this study contained appropriate levels of minerals (Supplementary Table S1) and a regular supply of plants with universal mineral fertilizer was ensured. However, such differences may explain the contradictory results obtained in various studies on the effect of salinity on the tolerance and accumulation of heavy metals. At the same time, it is also clear that nitrophilic species such as R. maritimus require relatively higher amounts of nitrogen compounds for optimal growth, as shown by the results obtained. Therefore, it would be correct to conduct additional experiments to determine the optimal nitrogen level for this model species. Further experiments to evaluate the resistance and accumulation of heavy metals should be carried out against the background of optimal nitrogen levels.
Second, soil properties significantly affect the bioavailability of heavy metals and, consequently, plant tolerance and metal accumulation in plant tissues [67,68]. In particular, the organic matter content plays a decisive role in influencing the bioavailability of metals. In soils with high content of organic matter, low mobility of heavy metals results in relatively low bioavailability [69]. It could be suggested that the substrate used in this experiment with a very high organic matter content could have reduced the availability of metals to plants, thus positively affecting their tolerance to high doses of added metals. On the other hand, the metal accumulation capacity in the aboveground parts of plants was relatively high, especially for the non-biogenic metals Cd and Pb, and the biogenic metal Cu, and it increased with increasing amounts of added metal in the soil (Figure 1). This suggests that the observed relatively high metal tolerance of R. maritimus plants is not related to a metal exclusion strategy and soil properties therefore have a relatively small impact on plant tolerance in this experimental system. In future studies with this halophyte species, it is necessary to optimize the growth conditions, which are of great importance in ensuring the phytoextraction potential [10,14]. In order to move to field trials and assess the potential of R. maritimus for practical application, the conditions affecting the bioavailability of metals should be evaluated [13], including the presence of growth-promoting bacteria [70].
4. Materials and Methods
4.1. Plant Material
Seeds of R. maritimus were collected from plants naturally growing on coastal shingle habitat in Oheasaare, island of Saaremaa, Estonia (58°00′06.9″ N 22°01′26.0″ E) and stored at 4 °C. For experiments, the seeds were sown in plastic containers (1 L) containing autoclaved (1 atm, 20 min) commercial garden soil (Biolan, Eura, Finland) and kept in a growth cabinet at 20 °C, photoperiod 16 h, the photon flux density of photosynthetically active radiation 100 μmol m−2 s−1. Two weeks later, established seedlings were individually transplanted into 250 mL plastic containers filled with a mixture of quartz sand (Saulkalne S, Saulkalne, Latvia) and garden soil (Biolan, Eura, Finland) 1:3 (v/v). The containers were placed inside closed plastic boxes (48 L) and acclimatized to greenhouse conditions by gradually increased ventilation. Experimental automated greenhouse (HortiMaX, Maasdijk, The Netherlands) with natural and supplemented light provided by Master SON-TPIA Green Power CG T 400 W (Philips, Amsterdam, The Netherlands) and Powerstar HQI-BT 400 W/D PRO (Osram, Munich, Germany) lamps (photon flux density of photosynthetically active radiation 380 mol m−2 s−1 at the plant level, 16 h photoperiod, day/night temperature 24/16 °C, relative air humidity 60 to 70%) was used. After two weeks, a second transplantation was performed on 1.2 L plastic containers filled with a mixture of quartz sand (Saulkalne S, Saulkalne, Latvia) and garden soil (Biolan, Eura, Finland) 1:4 (v/v). The containers with plants were randomly positioned on a greenhouse table using a randomization algorithm and repositioned once a week. Watering was performed with deionized water so that there was always water on the bottom plates of the containers. One week after the final transplanting, the plants were randomly selected for experiments. From a larger number of plants, morphologically uniform, medium-sized plants were selected and divided into experimental groups using a randomization algorithm. Two separate experiments were performed.
4.2. Experiment 1
In Experiment 1, the treatment of R. maritimus plants with heavy metals was performed gradually within two weeks, with five plants per treatment (Table 4). The range of concentration for particular heavy metals was based on results from earlier studies and corresponded to the range in which physiological effects and subsequent negative consequences may potentially occur for most coastal plant species [25,33,34,41,71,72]. The second treatment was performed one week after the first treatment. The necessary amount of the respective metal salts was dissolved in 150 mL of deionized water and applied to the substrate. The plants that were not to be treated received 150 mL of deionized water. As a result, 16 different treatment combinations were obtained. After the full treatment, the plants were fertilized once a week with Yara Tera Kristalon Red and Yara Tera Calcinit fertilizers (Yara International, Norway). A stock solution (100 g L−1) was prepared for each fertilizer, and a working solution contained 25 mL of each per 10 L deionized water, applied at a rate of 100 mL per container. Three weeks after the full treatment, an analysis of chlorophyll a fluorescence was performed as described below. Four weeks after the full treatment the experiment was terminated.

Table 4.
Treatments used in Experiment 1 with Rumex maritimus plants (gradual heavy metal treatment) in the present study.
4.3. Experiment 2
In Experiment 2, all R. maritimus plants except the control were pretreated either with nitrogen fertilizer (0.15 g N L−1 soil) or NaCl (50 mmol L−1) (Table 5). One week later, acute treatment with a single dose of heavy metals Cd, Pb, and Cu was performed at a maximum concentration as used in Experiment 1. After another week, a part of the plants was treated with an additional dose of nitrogen fertilizer (0.15 g N L−1) or NaCl (50 mmol L−1). Such nitrogen doses correspond to possible nitrogen variations in coastal soils, which are also characteristic of nitrophilic species. The two salinity levels (50 and 100 mmol L−1) reflected low and moderate salinity characteristics for coastal soils, respectively [25,26,34,40,73]. The necessary amount of the respective salts was dissolved in 150 mL of deionized water and applied to the substrate. The plants that were not to be treated received 150 mL of deionized water. As a result, 16 different treatment combinations were obtained. After the full treatment, the plants were fertilized once a week with Yara Tera Kristalon Red and Yara Tera Calcinit fertilizers (Yara International, Norway). A stock solution (100 g L−1) was prepared for each fertilizer, and a working solution contained 25 mL of each per 10 L deionized water, applied at a rate of 100 mL per container. Three weeks after the full treatment, an analysis of leaf chlorophyll concentration and chlorophyll a fluorescence was performed as described below. Four weeks after the full treatment the experiment was terminated.

Table 5.
Treatments used in Experiment 2 with Rumex maritimus plants (acute heavy metal treatment).
4.4. Measurement of Photosynthesis-Related Parameters
At each time point, photosynthesis-related parameters were measured non-destructively on two randomly selected photosynthetically most active middle leaves for each individual plant, one measurement per leaf, with 10 independent measurements per treatment. Chlorophyll concentration was measured by a chlorophyll meter CCM-300 (Opti-Sciences, USA). For chlorophyll a fluorescence measurement, the leaves were dark adapted at least for 20 min and measurement was performed using a Handy PEA fluorimeter (Hansatch Instruments, UK). The photochemical activity of photosynthesis was characterized using a parameter Performance Index Total, which combines information on the status of both photosystems as well as properties of electron flow between the two systems on an absorption basis, and can be used as an indicator of plant vitality [74].
4.5. Termination of the Experiments
All leaves were detached from individual plants separately according to their age/developmental groups as the dry leaves, the old leaves, the middle leaves, and the young leaves. All leaves by these groups were counted and weighed separately. Roots were harvested only for Experiment 1, first collecting the roots grown outside the container (designated as the “roots outside”) followed by separation of the remaining roots (“roots in substrate”) from the soil and washing to remove any adhered substrate particles. Plant tissues were dried at 60 °C for 72 h or until the stable mass was reached and weighed to measure dry biomass. The tissue water content was estimated as a mass of water in grams per gram of tissue dry mass.
4.6. Metal Analysis
For Experiment 1, the content of Cd, Pb, Cu, Mn, and Zn was analyzed separately in the roots outside, roots in the substrate, dry leaves, old leaves, middle leaves, and young leaves, each in triplicate. For Experiment 2, the content of Cd, Cu, and Pb was analyzed only in dry leaves, old leaves, and middle leaves. About 2 g of plant material was ground and dry-ashed with HNO3 vapor and redissolved in a 3% HCl solution. Metal analysis was performed using a microwave plasma atomic emission spectrometer (4200 MP-AES, Agilent, Santa Clara, CA, USA). Metal concentration in plant tissues was expressed as mg kg−1 dry mass.
Analytical calibration was carried out using multi-element standard solutions. A 100 mg L−1 Multi-element standard solution for ICP (Fisher Chemical, Göteborg, Sweden) was used to prepare calibration standards at the following concentrations: 0.1, 0.5, 1.0, and 2.5 μg mL−1 of Cu, Mn, Zn, Cd, and Pb. Working standards and a blank were matrix-matched with the samples to be analyzed and prepared in the 3% HCl deionized water solution. Linear calibration curves were obtained for all five elements across the calibrated range with calibration coefficients greater than 0.999. To ensure the quality of the detection and validate the calibration, the highest calibration standard was reset every 20 samples. The recoveries for all elements were within 100 ± 5%.
The detection wavelength (nm) used for the sample analysis: Cu 324.754, Zn 213.857, Mn 257.610, Cd 228.802, Pb 405.781, as no spectral interferences were expected. The limit of quantification was 0.15 mg kg−1 of the element in the plant sample. The spectral intensity was the mean of 5 replicate readings per sample. Adequate rinse time (45 s) between solutions was performed.
Spike experiments were also carried out to check the method’s performance. To verify the accuracy of the MP-AES results, three additional plant samples were used for a spike recovery study. Two spikes were completed for each sample with 20 and 100 mg/kg of Cu, Mn, Zn, Cd, and Pb using a multi-element standard solution. The recovery results were within ± 10% of the expected values for all three samples, indicating the suitability of the method for application in the analysis of metals in plant material.
4.7. Data Analysis
Results were analyzed using KaleidaGraph (v. 5.0, Synergy Software, USA). One-way ANOVA and Tukey’s honestly significant difference post hoc test were used for evaluation of the statistical significance of differences (p < 0.05).
5. Conclusions
R. maritimus plants showed an exceptional tolerance to several heavy metals, associated with an ability to preferentially accumulate metals in older leaves, inducing the senescence sequence of these leaves and stimulating the formation of new leaves. Accumulation of metals in the older leaves and their exclusion from the roots and young leaves is an essential part of the physiological tolerance strategy. The relative accumulation potential of R. maritimus for Cd, Pb, and Cu can be characterized as extremely high, as actual concentration values exceeded these established for the hyperaccumulation threshold. Therefore, the species can be characterized as a hypertolerant metal accumulator. Nitrogen amendment increased the biomass of R. maritimus plants grown in heavy metal spiked soil, thus, increasing the overall amount of accumulated Cd and Pb. Salinity had no negative effect on plant growth but increased accumulation capacity for Cd and, to some extent, for Cu. Thus, combined treatment of R. maritimus plants with nitrogen fertilizer in different forms on the background of increased salinity seems to be a promising approach with respect to heavy metal extraction and practical phytoremediation application of the species that needs to be studied further.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/stresses5020029/s1, Table S1: Plant-available mineral nutrient concentrations and other characteristics of commercial garden soil used in the study; Figure S1: Typical gradually Cd-treated Rumex maritimus plants 20 days after the full treatment; Figure S2: Typical gradually Pb-treated Rumex maritimus plants 20 days after the full treatment; Figure S3: Typical gradually Cu-treated Rumex maritimus plants 20 days after the full treatment; Figure S4: Typical gradually Mn-treated Rumex maritimus plants 20 days after the full treatment; Figure S5: Typical gradually Zn-treated Rumex maritimus plants 20 days after the full treatment; Figure S6: Typical control Rumex maritimus plants in different soil treatments; Figure S7: Typical acutely Cd-treated (100 mg L−1) Rumex maritimus plants in different soil treatments; Figure S8: Typical acutely Pb-treated (1000 mg L−1) Rumex maritimus plants in different soil treatments; Figure S9: Typical acutely Cu-treated (500 mg L−1) Rumex maritimus plants in different soil treatments.
Author Contributions
Conceptualization, L.N. and G.I.; methodology, G.I. and A.O.; investigation, L.N., U.A.-O., L.B., A.J., A.K. and A.O.; writing—original draft preparation, G.I.; writing—review and editing, L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data generated during this study are included in this paper and its Supplementary Files.
Acknowledgments
L.B. conducted this research while supported by the Erasmus+ Scholarship for PhD Students (grant number: 503-4220-0861-23).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 56–66. [Google Scholar]
- Ahamad, M.I.; Yao, Z.; Ren, L.; Zhang, C.; Li, T.; Lu, H.; Mehmood, M.; Rehman, A.; Muhammad, A.; Lu, S.; et al. Impact of heavy metals on aquatic life and human health: A case study of River Ravi Pakistan. Front. Mar. Sci. 2024, 11, 1374835. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, M.; Tian, W.; Hui, H. The content and potential ecological risk assessment of heavy metals in coastal wetlands around the Bohai Sea. Plant Soil Environ. 2024, 70, 356–365. [Google Scholar] [CrossRef]
- Orson, R.A.; Simpson, R.J.; Good, R.E. A mechanism for the accumulation and retention of heavy metals in tidal freshwater marshes of the upper Delawere river. Estuar. Coast. Shelf Sci. 1992, 34, 171–186. [Google Scholar] [CrossRef]
- Williams, T.P.; Bubb, J.M.; Lester, J.N. The occurrence and distribution of trace metals in halophytes. Chemosphere 1994, 28, 1189–1199. [Google Scholar] [CrossRef]
- Doyle, M.O.; Otte, M.L. Organism-induced accumulation of iron, zinc and arsenic in wetland soils. Environ. Pollut. 1998, 96, 1–11. [Google Scholar] [CrossRef]
- Sruthi, P.; Shackira, A.M.; Puthur, J.T. Heavy metal detoxification mechanisms in halophytes: An overview. Wetl. Ecol. Manag. 2017, 25, 129–148. [Google Scholar] [CrossRef]
- Lutts, S.; Lefèvre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 115, 509–528. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Maggio, A. Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environ. Exp. Bot. 2015, 111, 135–146. [Google Scholar] [CrossRef]
- Caparrós, P.G.; Ozturk, M.; Gul, A.; Batool, T.S.; Pirasteh-Anosheh, H.; Unal, B.T.; Altay, V.; Toderich, K.N. Halophytes have potential as heavy metal phytoremediators: A comprehensive review. Environ. Exp. Bot. 2022, 193, 104666. [Google Scholar] [CrossRef]
- Tonelli, F.M.P.; Bhat, R.A.; Dar, G.H.; Hakeem, K.R. The history of phytoremediation. In Phytoremediation. Biotechnological Strategies for Promoting Invigorating Environs; Bhat, R.A., Tonelli, F.M.P., Dar, G.H., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–18. [Google Scholar]
- Buscaroli, A. An overview of indexes to evaluate terrestrial plants for phytoremediation purposes (Review). Ecol. Indic. 2017, 82, 367–380. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Liang, Z.; Neményi, A.; Kovács, G.P.; Gyuricza, C. Incorporating functional traits into heavy metals phytoremediation: The future of field-based phytoremediation. Ecol. Indic. 2024, 166, 112262. [Google Scholar] [CrossRef]
- Mateos-Naranjo, E.; Pérez-Romero, J.A.; Redondo-Gómez, S.; Mesa-Marín, J.; Castellanos, E.M.; Davy, A.J. Salinity alleviates zinc toxicity in the saltmarsh zinc-accumulator Juncus acuttus. Ecotoxicol. Environ. Saf. 2018, 163, 478–485. [Google Scholar] [CrossRef]
- Nezhadasad-Aghbash, B.; Radjabian, T.; Hajiboland, R. Tolerance to Zn toxicity in the halophyte Lepidium latifolium L. and the effect of salt on Zn tolerance and accumulation. Acta Agric. Slov. 2023, 191, 1–17. [Google Scholar] [CrossRef]
- Zhou, M.; Engelmann, T.; Lutts, S. Salinity modifies heavy metals and arsenic absorption by the halophyte plant species Kosteletzkya pentacarpos and pollutant leaching from a polycontaminated substrate. Ecotoxicol. Environ. Saf. 2019, 182, 109460. [Google Scholar] [CrossRef]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Nazir, F.; Maheshwari, C.; Chopra, P.; Chhillar, H.; Sreenivasulu, N. Mineral nutrients in plants under changing environments: A road to future food and nutrition security. Plant Genome 2023, 16, e20362. [Google Scholar] [CrossRef]
- Monsant, A.C.; Wang, Y.; Tang, C. Nitrate nutrition enhances zinc hyperaccumulation in Noccaea caerulescens (Prayon). Plant Soil 2010, 336, 391–404. [Google Scholar] [CrossRef]
- Hu, P.; Yin, Y.-G.; Ishikawa, S.; Suzui, N.; Kawachi, N.; Fujimaki, S.; Igura, M.; Yuan, C.; Huang, J.; Li, Z.; et al. Nitrate facilitates cadmium uptake, transport and accumulation in the hyperaccumulator Sedum plumbizincicola. Environ. Sci. Pollut. Res. 2013, 20, 6306–6316. [Google Scholar] [CrossRef]
- Wei, S.; Ji, D.; Twardowska, I.; Li, W.; Zhu, J. Effect of different nitrogenous nutrients on the cadmium hyperaccumulation efficiency of Rorippa globosa (Turcz.) Thell. Environ. Sci. Pollut. Res. 2015, 22, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Kanso, A.; Azoury, S.; Benizri, E.; Kobaissi, A.; Echevarria, G.; Sirguey, C. Improvement of Ni phytoextaction by Alyssum murale and its rhizosphere microbial activities by applying nitrogen fertilizer. Ecol. Res. 2018, 33, 811–821. [Google Scholar] [CrossRef]
- Lin, Z.; Dou, C.; Li, Y.; Wang, H.; Niazi, N.K.; Zhang, S.; Liu, D.; Zhao, K.; Fu, W.; Li, Y.; et al. Nitrogen fertilizer enhances zinc and cadmium uptake by hyperaccumulator Sedum alfredii Hance. J. Soils Sedim. 2020, 20, 320–329. [Google Scholar] [CrossRef]
- Ievinsh, G.; Landorfa-Svalbe, Z.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A. Salinity and heavy metal tolerance, and phytoextraction potential of Ranunculus sceleratus plants from a sandy coastal beach. Life 2022, 12, 1959. [Google Scholar] [CrossRef]
- Landorfa-Svalbe, Z.; Andersone-Ozola, U.; Ievinsh, G. Type of anion largely determines salinity tolerance in four Rumex species. Plants 2023, 12, 92. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, C.; Hu, P.; Luo, W.; Wu, L.; Sale, P.; Tang, C. Influence of nitrogen form on the phytoextraction of cadmium by a newly discovered hyperaccumulator Carpobrotus rossii. Environ. Sci. Pollut. Res. 2016, 23, 1246–1253. [Google Scholar] [CrossRef]
- Ievinsh, G.; Ieviņa, S.; Andersone-Ozola, U.; Samsone, I. Leaf sodium, potassium and electrolyte accumulation capacity of plant species from salt-affected coastal habitats of the Baltic Sea: Towards a definition of Na hyperaccumulation. Flora 2021, 274, 151748. [Google Scholar] [CrossRef]
- Van der Sman, A.J.M.; Blom, C.W.P.; Barendse, G.W.M. Flooding resistance and shoot elongation in relation to developmental stage and environmental conditions in Rumex maritimus L. and Rumex palustris Sm. New Phytol. 1993, 125, 73–84. [Google Scholar] [CrossRef]
- Van der Sman, A.J.M.; van Tongeren, O.F.R.; Blom, C.W.P.M. Growth and reproduction of Rumex maritimus and Chenopodium rubrum under different waterlogging regimes. Acta Bot. Neerl. 1988, 37, 439–460. [Google Scholar] [CrossRef]
- Van der Sman, A.J.M.; Voesenek, L.A.C.J.; Blom, C.W.P.M.; Harren, F.J.M.; Reuss, J. The role of ethylene in shoot elongation with respect to survival and seed output of flooded Rumex maritimus L. plants. Funct. Ecol. 1991, 5, 304–313. [Google Scholar] [CrossRef]
- Samsone, I.; Ievinsh, G. Different plant species accumulate various concentration of Na+ in a sea-affected coastal wetland during a vegetation season. Environ. Exp. Biol. 2018, 16, 117–127. [Google Scholar]
- Ievinsh, G.; Dišlere, E.; Karlsons, A.; Osvalde, A.; Vikmane, M. Physiological responses of wetland species Rumex hydrolapathum to increased concentration of biogenous heavy metals Zn and Mn in substrate. Proc. Latv. Acad. Sci. B 2020, 7, 35–47. [Google Scholar] [CrossRef]
- Ieviņa, S.; Karlsons, A.; Osvalde, A.; Andersone-Ozola, U.; Ievinsh, G. Coastal wetland species Rumex hydrolapathum: Tolerance against flooding, salinity and heavy metals for its potential use in phytoremediation and environmental restoration technologies. Life 2023, 13, 1604. [Google Scholar] [CrossRef] [PubMed]
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and trait values for Swedish vascular plants. Ecol. Indic. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Samsone, I.; Ievinsh, G. Comparison of the effects of gradual and acute treatment with Mn on physiological responses of Rumex hydrolapathum plants. Stresses 2024, 4, 225–237. [Google Scholar] [CrossRef]
- Zhang, C.; Sale, P.W.G.; Tang, C. Cadmium uptake by Carpobrotus rossii (Haw.) Schwantes under different saline conditions. Environ. Sci. Pollut. Res. 2016, 23, 13480–13488. [Google Scholar] [CrossRef]
- Salama, F.M.; al-Huqail, A.A.; Ali, M.; Abeed, A.H.A. Cd phytoextraction potential in halophyte Salicornia fruticosa: Salinity impact. Plants 2022, 11, 2556. [Google Scholar] [CrossRef]
- Nawaz, I.; Iqbal, M.; Bliek, M.; Schat, H. Salt and heavy metal tolerance and expression levels of candidate tolerance genes among extremophile Cochlearia species with contrasting habitat preferences. Sci. Total Environ. 2017, 584–585, 731–741. [Google Scholar] [CrossRef]
- Purmale, L.; Jēkabsone, A.; Andersone-Ozola, U.; Ievinsh, G. Salinity tolerance, ion accumulation potential and osmotic adjustment in vitro and in planta of different Armeria maritima accessions from a dry coastal meadow. Plants 2022, 11, 2570. [Google Scholar] [CrossRef]
- Purmale, L.; Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A.; Ievinsh, G. Comparison of in vitro and in planta heavy metal tolerance and accumulation potential of different Armeria maritima accessions from a dry coastal meadow. Plants 2022, 11, 2104. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Suprasanna, P. Coping with metal toxicity—Cues from halophytes. Front. Plant Sci. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of Sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, C.; Feng, Y.; Zhang, X.; Lu, Y.; Ying, R.; Yin, A.; Ji, W. Heavy metals can affect plant morphology and limit plant growth and photosynthesis processes. Agronomy 2023, 13, 2601. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Ayeni, O.O.; Ndakidemi, P.A.; Snyman, R.G.; Odendaal, J.P. Chemical, biological and physiological indicators of metal pollution in wetlands. Sci. Res. Essays 2010, 5, 1938–1949. [Google Scholar]
- Singh, H.; Kumar, D.; Soni, V. Performance of chlorophyll a fluorescence parameters in Lemna minor under heavy metal stress induced by various concentrations of copper. Sci. Rep. 2022, 12, 10620. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef]
- Han, R.-M.; Lefèvre, I.; Ruan, C.-J.; Beukelaers, N.; Qin, P.; Lutts, S. Effects of salinity on the response of the wetland halophyte Kosteletzkya virginica (L.) Presl. to copper toxicity. Water Air Soil Pollut. 2012, 223, 1137–1150. [Google Scholar] [CrossRef]
- Manousaki, E.; Kalogerakis, N. Phytoextraction of Pb and Cd by the Mediteranean saltbush (Atriplex halimus L.): Metal uptake in relation to salinity. Environ Sci. Pollut Res. 2009, 16, 844–854. [Google Scholar] [CrossRef]
- Khanlarian, M.; Roshanfar, M.; Rashchi, F.; Motesharezadeh, B. Phyto-extraction of zinc, lead, nickel, and cadmium from zinc leach residue by a halophyte: Salicornia europaea. Ecol. Eng. 2020, 148, 105797. [Google Scholar] [CrossRef]
- Śliva-Cebula, M.; Kaszycki, P.; Aczmarczyk, A.; Nosek, M.; Lis-Krzyścin, A.; Miszalski, Z. The common ice plant (Mesembryanthemum crystallinum L.)—phytoremediation potential for cadmium and chromate-contaminated soils. Plants 2020, 9, 1230. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lin, Y.; Yang, Y.; Shen, Q.; Huang, J.; Wang, S.; Zhu, X.; Li, Z. Tolerance and bioaccumulation of Cd and Cu in Sesuvium portulacastrum. Ecotoxicol. Environ. Saf. 2018, 147, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiong, J.; Tao, L.; Cao, Z.; Tang, W.; Zhang, J.; Yu, X.; Fu, G.; Zhang, X.; Lu, Y. Regulatory mechanisms of nitrogen (N) on cadmium (Cd) uptake and accumulation in plants: A review. Sci. Total Environ. 2020, 708, 135186. [Google Scholar] [CrossRef]
- Eissa, M.A.; Roshdy, N.M.K. Nitrogen fertilization: Effect on Cd-phytoextraction by the halophytic plant quail bush [Atriplex lentiformis (Torr.) S. Wats]. S. Afr. J. Bot. 2018, 115, 126–131. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, P.; Kopittke, P.M.; Wang, A.; Sale, P.W.G.; Tang, C. Cadmium accumulation is enhanced by ammonium compared to nitrate in two hyperaccumulators, without affecting speciation. J. Exp. Bot. 2016, 67, 5041–5050. [Google Scholar] [CrossRef]
- Jacobs, A.; Noret, N.; Van Baekel, A.; Liénard, A.; Colinet, G.; Drouet, T. Influence of edaphic conditions and nitrogen fertilizers on cadmium and zinc phytoextraction efficiency of Noccaea caerulescens. Sci. Total Environ. 2019, 665, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef]
- Lefèvre, I.; Marchal, G.; Meerts, P.; Corréal, E.; Lutts, S. Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L. Environ. Exp. Bot. 2009, 65, 142–152. [Google Scholar] [CrossRef]
- Manousaki, E.; Kokkali, F.; Kalogerakis, N. Influence of salinity on lead and cadmium accumulation by the salt cedar (Tamarix smyrnensis Bunge). J. Chem. Technol. Biotechnol. 2009, 84, 877–883. [Google Scholar] [CrossRef]
- Nosek, M.; Kaczmarczyk, A.; Jędrzejczyk, R.J.; Supel, P.; Kaszycki, P.; Miszalski, Z. Expression of genes involved in heavy metal trafficking in plants exposed to salinity stress and elevated Cd concentrations. Plants 2020, 9, 475. [Google Scholar] [CrossRef]
- Zurayk, R.A.; Khoury, N.F.; Talhouk, S.N.; Baalbaki, R.Z. Salinity-heavy metal interactions in four salt-tolerant plant species. J. Plant Nutr. 2001, 24, 1773–1786. [Google Scholar] [CrossRef]
- Zhou, M.-X.; Dailly, H.; Renard, M.-E.; Han, R.-M.; Lutts, S. NaCl impact on Kosteletzkya pentacarpos seedlings simultaneously exposed to cadmium and zinc toxicities. Environ. Sci. Pollut. Res. 2018, 25, 17444–17456. [Google Scholar] [CrossRef] [PubMed]
- Jarell, W.M.; Beverly, R.B. The dilution effect in plant nutrition studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: The role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 8, 1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Song, N. Salinity-induced alterations in plant growth, antioxidant enzyme activities, and lead transportation and accumulation in Suaeda salsa: Implications for phytoremediation. Ecotoxicology 2019, 28, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, J.; Baran, A.; Bubak, A. Mobility, bioaccumulation in plants, and risk assessment of metals in soils. Sci. Total Environ. 2023, 882, 163574. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H. Heavy metals in agricultural soils: Sources, influencing factors, and remediation strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of heavy metals in soil: Impact of microbial biodegradation of organic compounds and possible improvement strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef]
- Alves, A.R.A.; Yin, Q.; Oliveira, R.S.; Silva, E.F.; Novo, L.A.B. Plant growth-promoting bacteria in phytoremediation of metal-polluted soils: Current knowledge and future directions. Sci. Total Environ. 2022, 838, 156435. [Google Scholar] [CrossRef]
- Ievinsh, G.; Osvalde, A.; Karlsons, A.; Andersone-Ozola, U. Hylotelephium maximum from coastal drift lines is a promising Mn and Zn accumulator with a high tolerance to biogenous heavy metals. Stresses 2022, 2, 450–465. [Google Scholar] [CrossRef]
- Andersone-Ozola, U.; Jēkabsone, A.; Karlsons, A.; Osvalde, A.; Banaszczyk, L.; Samsone, I.; Ievinsh, G. Heavy metal tolerance and accumulation potential of a rare coastal species, Anthyllis vulneraria subsp. maritima. Stresses 2025, 5, 6. [Google Scholar] [CrossRef]
- Jēkabsone, A.; Kuļika, J.; Romanovs, M.; Andersone-Ozola, U.; Ievinsh, G. Salt tolerance and ion accumulation in several halophytic plant species depending on the type of anion. Int. J. Plant Biol. 2023, 14, 1131–1154. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M. Revisting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).