Elevated Soil Temperatures Impact Nematode Reproduction Biology
Abstract
1. Introduction
2. Results
2.1. Impact of Soil Temperature on Reproduction of Nematodes
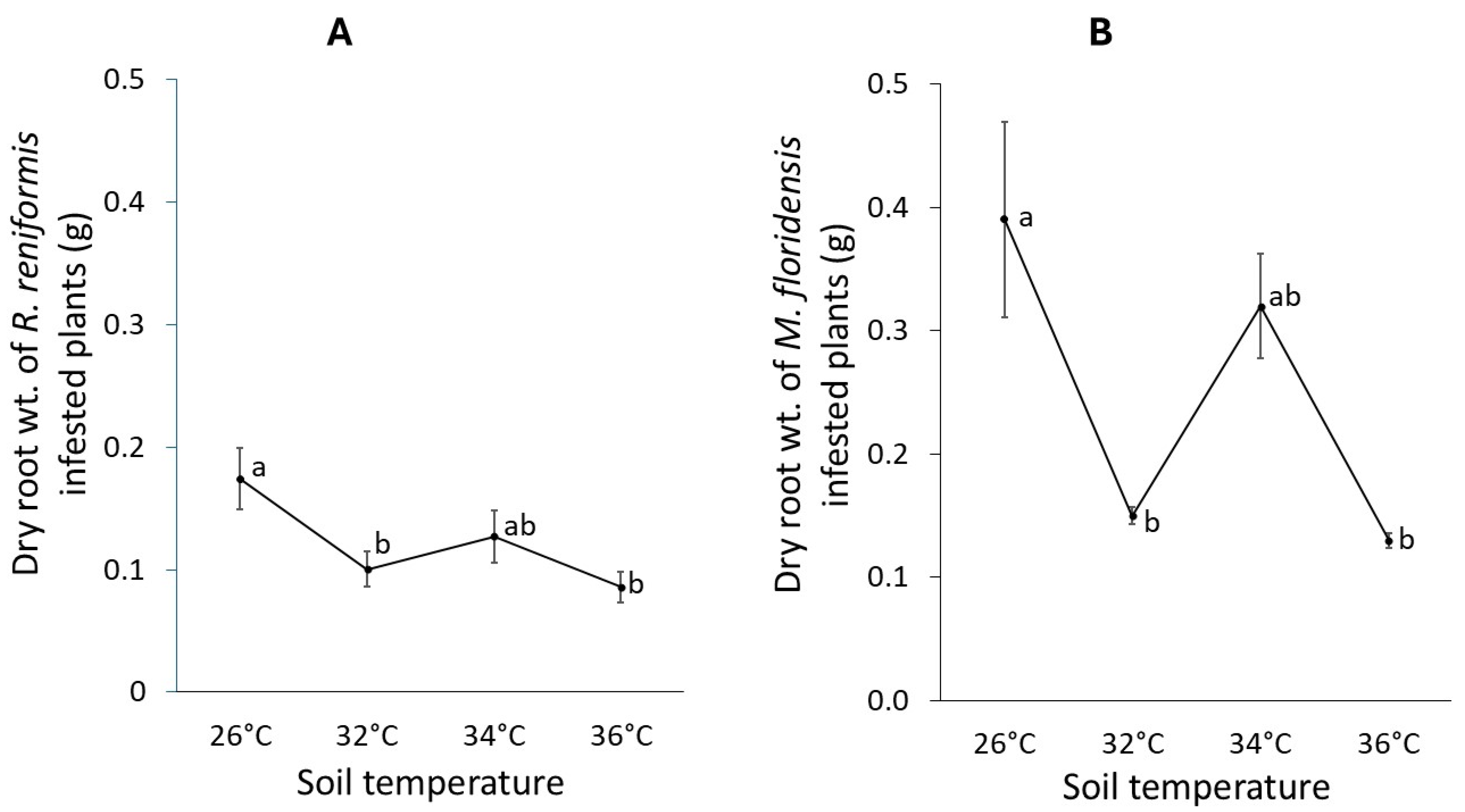
2.2. Impact of Soil Temperature and Nematode Species on Root Biomass
2.3. Impact of Soil Temperature on Nematode Virulence
3. Discussion
4. Materials and Methods
4.1. Preparation of Nematode Inoculum
4.2. Establishment of Experiment
4.3. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Dutta, T.K.; Phani, V. The pervasive impact of global climate change on plant-nematode interaction continuum. Front. Plant Sci. 2023, 14, 1143889. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–pathogen warfare under changing climate conditions. Curr. Biol. 2018, 28, 619–634. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar]
- Backlund, P.; Schimel, D.; Janetos, A.; Hatfield, J.; Ryan, M.G.; Archer, S.R.; Lettenmaier, D. Introduction. In The Effects of Climate Change on Agriculture, Land Resources, Water Resources, and Biodiversity in the United States. A Report by the U.S. Climate Change Science Program and the Subcommittee on Global Change Research; U.S. Environmental Protection Agency: Washington, DC, USA, 2008; Volume 362. Available online: https://www.usda.gov/sites/default/files/documents/CCSPFinalReport.pdf (accessed on 11 November 2024).
- Rosenzweig, C.; Liverman, D. Predicted effects of climate change on agriculture: A comparison of temperate and tropical regions. In Global Climate Change: Implications, Challenges, and Mitigation Measures; Majumdar, S.K., Ed.; Pennsylvania Academy of Science: East Stroudsburg, PA, USA, 1992; pp. 342–361. [Google Scholar]
- Khanal, C.; Land, J. Study on two nematode species suggests climate change will inflict greater crop damage. Sci. Rep. 2023, 13, 14185. [Google Scholar] [CrossRef]
- Lahlali, R.; Mohammed, T.; Laasli, S.E.; Gachara, G.; Ezzouggari, R.; Belabess, Z.; Aberkani, K.; Assouguem, A.; Meddich, A.; El Jarroudi, M.; et al. Effects of climate change on plant pathogens and host-pathogen interactions. Crop Environ. 2024, 3, 159–170. [Google Scholar] [CrossRef]
- Baron, C.; Domke, N.; Beinhofer, M.; Hapfelmeier, S. Elevated temperature differentially affects virulence, VirB protein accumulation, and T-pilus formation in different Agrobacterium tumefaciens and Agrobacterium vitis strains. J. Bacteriol. 2001, 183, 6852–6861. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Castroverde, C.D.M.; Velásquez, A.C.; Hubbard, E.; Pulman, J.A.; Yao, J.; Childs, K.L.; Tsuda, K.; Montgomery, B.L.; He, S.Y. Dual impact of elevated temperature on plant defence and bacterial virulence in Arabidopsis. Nat. Commun. 2017, 8, 1808. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbio. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, M.M.M.; Askary, T.H. Impact of phytonematodes on agriculture economy. In Biocontrol Agents of Phytonematodes; Askary, T.H., Martinelli, P.R.P., Eds.; CAB International: Wallingford, UK, 2015; pp. 3–49. [Google Scholar]
- Robinson, A.F.; Inserra, R.N.; Caswell-Chen, E.P.; Vovlas, N.; Troccoli, A. Rotylenchulus species: Identification, distribution, host ranges, and crop plant resistance. Nematropica 1997, 27, 127–180. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.R.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-Lopez, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.; Faske, T. Estimates of Crop Yield Losses Due to Diseases and Invertebrate Pests. Crop Protection Network. 2024. Available online: https://loss.cropprotectionnetwork.org/ (accessed on 14 November 2024).
- Handoo, Z.A.; Nyczepir, A.P.; Esmenjaud, D.; Van Der Beek, J.G.; Castagnone-Sereno, P.; Carta, L.K.; Skantar, A.M.; Higgins, J.A. Morphological, molecular, and differential-host characterization of Meloidogyne floridensis n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitizing peach in Florida. J. Nematol. 2004, 36, 20. [Google Scholar] [PubMed]
- Marquez, J.; Hajihassani, A. Identification and virulence of five isolates of root-knot nematode Meloidogyne floridensis on vegetables. Plant Dis. 2023, 107, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.D.; Brito, J.A.; Kokalis-Burelle, N.; Frank, J.H.; Dickson, D.W. Biological evaluation and comparison of four Florida isolates of Meloidogyne floridensis. Nematropica 2009, 39, 255–271. [Google Scholar]
- Sapir, A. Why are nematodes so successful extremophiles? Commun. Integr. Biol. 2021, 14, 24–26. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Webster, J.M. The genetics of plant-nematode parasitic systems. Bot. Rev. 1981, 47, 387–419. [Google Scholar] [CrossRef]
- Guo, X.; Endler, A.; Poll, C.; Marhan, S.; Ruess, L. Independent effects of warming and altered precipitation pattern on nematode community structure in an arable field. Agric. Ecosyst. Environ. 2021, 316, 107467. [Google Scholar] [CrossRef]
- Klusmann, C.; Cesarz, S.; Ciobanu, M.; Ferlian, O.; Jochum, M.; Schädler, M.; Scheu, S.; Sunnemann, M.; Wall, D.H.; Eisenhauer, N. Climate-change effects on the sex ratio of free-living soil nematodes—Perspective and prospect. Soil Org. 2022, 94, 15–28. [Google Scholar]
- Boag, B. Effect of temperature on the times to hatching of eggs of the plant parasitic nematode Longidorus elongatus. Nematol. Mediterr. 1985, 13, 61–66. [Google Scholar]
- Colagiero, M.; Ciancio, A. Climate changes and nematodes: Expected effects and perspectives for plant protection. Redia 2012, 94, 113–118. [Google Scholar]
- Khanal, C.; Galbieri, R.; Timper, P. Rotations with Crotalaria spp. do not suppress populations of Meloidogyne incognita in cotton. Nematology 2021, 23, 929–937. [Google Scholar] [CrossRef]
- Khanal, C.; Desaeger, J.A. On-farm evaluations of non-fumigant nematicides on cucurbits. Crop Prot. 2020, 133, 105152. [Google Scholar] [CrossRef]
- Omwega, C.O.; Roberts, P.A. Inheritance of resistance to Meloidogyne spp. In common bean and the genetic basis of its sensitivity to temperature. Theor. Appl. Genet. 1992, 83, 720–726. [Google Scholar] [CrossRef]
- Jatala, P.; Russell, C.C. Nature of sweet potato resistance to Meloidogyne incognita and the effects of temperature on parasitism. J. Nematol. 1972, 4, 1–7. [Google Scholar] [PubMed]
- Ferris, H.; Zheng, L.; Walker, M.A. Soil temperature effects on the interaction of grape rootstocks and plant-parasitic nematodes. J. Nematol. 2013, 45, 49–57. [Google Scholar]
- Baum, T.J.; Fortnum, B.A.; Lewis, S.A. Effects of Meloidogyne arenaria infection on M. incognita resistance in tobacco. Fundament. Appl. Nematol. 1995, 18, 583–588. [Google Scholar]
- Pollok, J.R.; Johnson, C.S.; Eisenback, J.D.; David, R.T.; Adamo, N. Effect of soil temperature on reproduction of root-knot nematodes in flue-cured tobacco with homozygous Rk1 and/or Rk2 resistance genes. J. Nematol. 2023, 55, 20230032. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, W.; Fan, J.; Li, Z.; Yang, R.; Zhao, F.; Wang, J.L.; Wang, S. Protective enzymes and genes related to the JA pathway are involved in the response to root-knot nematodes at high soil temperatures in tomatoes carrying Mi-1. Hort. Environ. Biotech. 2015, 56, 546–554. [Google Scholar] [CrossRef]
- Bell, A.A.; Robinson, A.F.; Quintana, J.; Dighe, N.D.; Menz, M.A.; Stelly, D.M.; Zheng, X.; Jones, J.E.; Overstreet, C.; Burris, E.; et al. Registration of LONREN-1 and LONREN-2 germplasm lines of upland cotton resistant to reniform nematode. J. Plant Regist. 2014, 8, 187–190. [Google Scholar] [CrossRef]
- Sikkens, R.B.; Weaver, D.B.; Lawrence, K.S.; Moore, S.R.; van Santen, E. LONREN upland cotton germplasm response to Rotylenchulus reniformis inoculum level. Nematropica 2011, 41, 68–74. [Google Scholar]
- Linford, M.B.; Oliveira, J.M. Rotylenchulus reniformis, nov. gen. n. sp., a nematode parasite of roots. Proc. Helminthol. Soc. Wash. 1940, 7, 35–42. [Google Scholar]
- Tietjen, J.H.; Lee, J.J. Life cycles of marine nematodes. Oecologia 1972, 10, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.S.; Osborne, W.W.; Webber, A.J.J. Effect of temperature on development and reproduction of Globodera solanacearum (Osborne’s cyst nematode, tobacco pest, Virginia). Nematropica 1982, 12, 305–331. [Google Scholar]
- Okada, H.; Ferris, H. Effect of temperature on growth and nitrogen mineralization of fungi and fungal-feeding nematodes. Plant Soil 2001, 234, 253–262. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. A comparison of methods for collecting inocula for Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Khanal, C.; McGawley, E.C.; Overstreet, C.; Stetina, S.R.; Myers, G.O.; Kularathna, M.T.; McInnes, B.; Godoy, F.M.C. Reproduction and pathogenicity of endemic populations of Rotylenchulus reniformis on cotton. Nematropica 2018, 48, 68–81. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020. [Google Scholar]
- JMP® PRO; Version 17; SAS Institute Inc.: Cary, NC, USA, 2023.


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
GC, S.; Banakar, P.; Harshman, D.; Khanal, C. Elevated Soil Temperatures Impact Nematode Reproduction Biology. Stresses 2025, 5, 2. https://doi.org/10.3390/stresses5010002
GC S, Banakar P, Harshman D, Khanal C. Elevated Soil Temperatures Impact Nematode Reproduction Biology. Stresses. 2025; 5(1):2. https://doi.org/10.3390/stresses5010002
Chicago/Turabian StyleGC, Sagar, Prakash Banakar, David Harshman, and Churamani Khanal. 2025. "Elevated Soil Temperatures Impact Nematode Reproduction Biology" Stresses 5, no. 1: 2. https://doi.org/10.3390/stresses5010002
APA StyleGC, S., Banakar, P., Harshman, D., & Khanal, C. (2025). Elevated Soil Temperatures Impact Nematode Reproduction Biology. Stresses, 5(1), 2. https://doi.org/10.3390/stresses5010002





