Childhood Adversity and White Matter Microstructure: White Matter Differences Associated with Trauma Exposure
Abstract
1. Introduction
2. Results
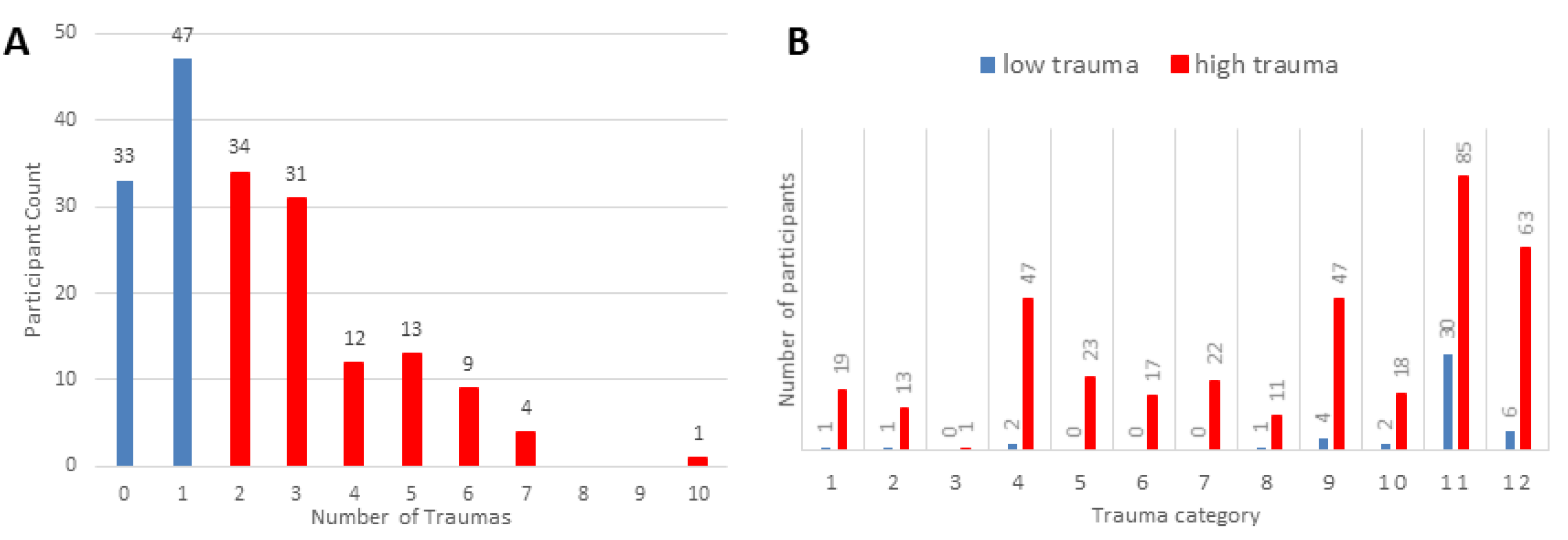
2.1. Participants Description
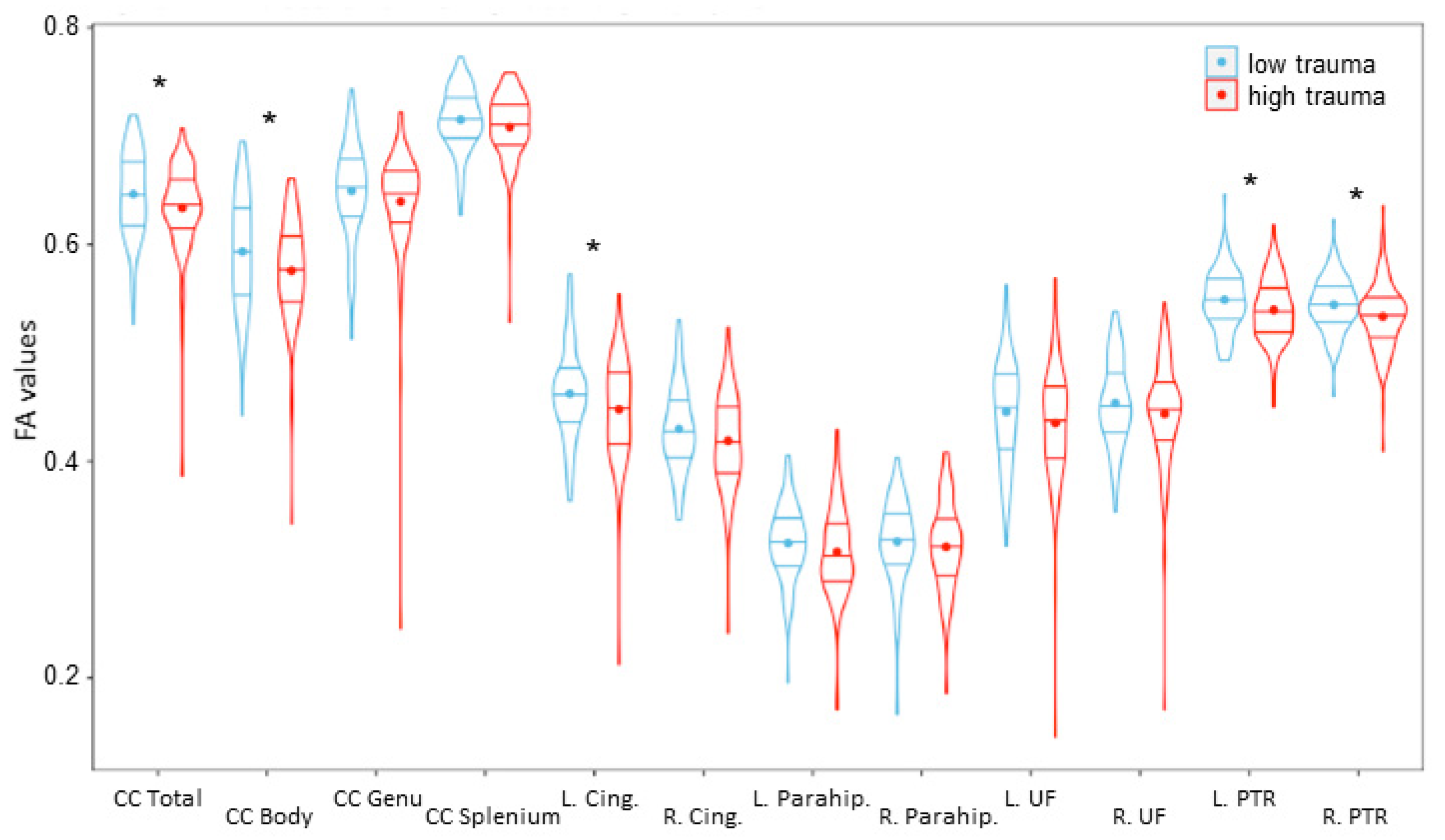
Brain Region Analysis: Trauma-and-FA Association
2.2. Corpus Callosum
2.3. Posterior Thalamic Radiation (Including Optic Radiation)
2.4. Cingulate Gyrus
2.5. Parahippocampal Cingulum
2.6. Uncinate Fasciculus (UF)
2.7. Brain Region Analysis: PTS and FA Association
3. Discussion
3.1. Posttraumatic Stress Symptoms
3.2. Limitations
3.3. Conclusions
4. Materials and Methods
4.1. Participants
4.2. Assessments
4.3. Diffusion Imaging Acquisition Parameters and Processing
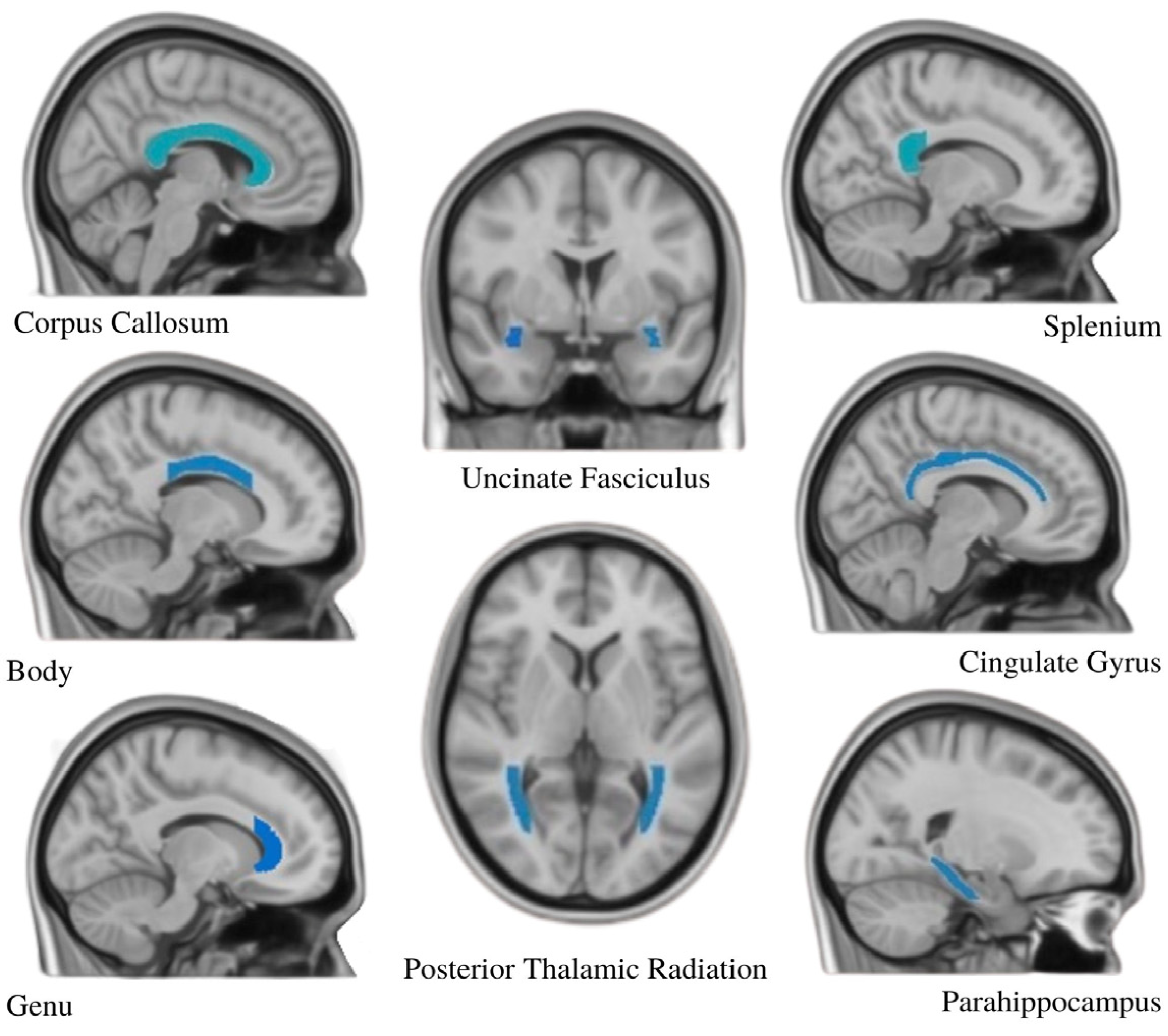
4.4. ROI Selection and FA Value Extraction
4.5. Statistics and Grouping
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.apa.org/pi/families/resources/update.pdf (accessed on 10 December 2024).
- Hetzel-Riggin, M.; Roby, R. Trauma Type and Gender Effects on PTSD, General Distress, and Peritraumatic Dissociation. J. Loss Trauma 2012, 18, 41–53. [Google Scholar] [CrossRef]
- Kira, I.; Lewandowski, L.; Somers, C.L.; Yoon, J.S.; Chiodo, L. The Effects of Trauma Types, Cumulative Trauma, and PTSD on IQ in Two Highly Traumatized Adolescent Groups. Psychol. Trauma Theory Res. Pract. Policy 2012, 4, 128–139. [Google Scholar] [CrossRef]
- De Bellis, M.D.; Keshavan, M.S.; Shifflett, H.; Iyengar, S.; Beers, S.R.; Hall, J.; Moritz, G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: A sociodemographically matched study. Biol. Psychiatry 2002, 52, 1066–1078. [Google Scholar] [CrossRef]
- Tendolkar, I.; Martensson, J.; Kuhn, S.; Klumpers, F.; Fernandez, G. Physical neglect during childhood alters white matter connectivity in healthy young males. Hum. Brain Mapp. 2018, 39, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jeong, B.; Polcari, A.; Rohan, M.L.; Teicher, M.H. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage 2012, 59, 1071–1079. [Google Scholar] [CrossRef]
- Jackowski, A.P.; Douglas-Palumberi, H.; Jackowski, M.; Win, L.; Schultz, R.T.; Staib, L.W.; Krystal, J.H.; Kaufman, J. Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Res. 2008, 162, 256–261. [Google Scholar] [CrossRef]
- Choi, J.; Jeong, B.; Rohan, M.L.; Polcari, A.M.; Teicher, M.H. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol. Psychiatry 2009, 65, 227–234. [Google Scholar] [CrossRef]
- Blakemore, S.J. Imaging brain development: The adolescent brain. Neuroimage 2012, 61, 397–406. [Google Scholar] [CrossRef]
- Sowell, E.R.; Thompson, P.M.; Holmes, C.J.; Batth, R.; Jernigan, T.L.; Toga, A.W. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage 1999, 9, 587–597. [Google Scholar] [CrossRef]
- Edwards, V.J.; Holden, G.W.; Felitti, V.J.; Anda, R.F. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. Am. J. Psychiatry 2003, 160, 1453–1460. [Google Scholar] [CrossRef]
- Heim, C.; Binder, E.B. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 2012, 233, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Cisler, J.M.; Begle, A.M.; Amstadter, A.B.; Resnick, H.S.; Danielson, C.K.; Saunders, B.E.; Kilpatrick, D.G. Exposure to interpersonal violence and risk for PTSD, depression, delinquency, and binge drinking among adolescents: Data from the NSA-R. J. Trauma. Stress 2012, 25, 33–40. [Google Scholar] [CrossRef]
- Duncan, N.W.; Hayes, D.J.; Wiebking, C.; Tiret, B.; Pietruska, K.; Chen, D.Q.; Rainville, P.; Marjanska, M.; Ayad, O.; Doyon, J.; et al. Negative childhood experiences alter a prefrontal-insular-motor cortical network in healthy adults: A preliminary multimodal rsfMRI-fMRI-MRS-dMRI study. Hum. Brain Mapp. 2015, 36, 4622–4637. [Google Scholar] [CrossRef]
- Lu, S.; Pan, F.; Gao, W.; Wei, Z.; Wang, D.; Hu, S.; Huang, M.; Xu, Y.; Li, L. Neural correlates of childhood trauma with executive function in young healthy adults. Oncotarget 2017, 8, 79843–79853. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wei, Z.; Gao, W.; Wu, W.; Liao, M.; Zhang, Y.; Li, W.; Li, Z.; Li, L. White matter integrity alterations in young healthy adults reporting childhood trauma: A diffusion tensor imaging study. Aust. N. Z. J. Psychiatry 2013, 47, 1183–1190. [Google Scholar] [CrossRef]
- Daniels, J.K.; Lamke, J.P.; Gaebler, M.; Walter, H.; Scheel, M. White matter integrity and its relationship to PTSD and childhood trauma--a systematic review and meta-analysis. Depress. Anxiety 2013, 30, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Rinne-Albers, M.A.; van der Werff, S.J.; van Hoof, M.J.; van Lang, N.D.; Lamers-Winkelman, F.; Rombouts, S.A.; Vermeiren, R.R.; van der Wee, N.J. Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: A DTI study. Eur. Child Adolesc. Psychiatry 2016, 25, 869–878. [Google Scholar] [CrossRef]
- Teicher, M.H.; Anderson, C.M.; Ohashi, K.; Polcari, A. Childhood maltreatment: Altered network centrality of cingulate, precuneus, temporal pole and insula. Biol. Psychiatry 2014, 76, 297–305. [Google Scholar] [CrossRef]
- Huang, H.; Gundapuneedi, T.; Rao, U. White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology 2012, 37, 2693–2701. [Google Scholar] [CrossRef]
- Ceponiene, R.; Rinne, T.; Naatanen, R. Maturation of cortical sound processing as indexed by event-related potentials. Clin. Neurophysiol. 2002, 113, 870–882. [Google Scholar] [CrossRef]
- Fani, N.; King, T.Z.; Jovanovic, T.; Glover, E.M.; Bradley, B.; Choi, K.; Ely, T.; Gutman, D.A.; Ressler, K.J. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology 2012, 37, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Eluvathingal, T.J.; Chugani, H.T.; Behen, M.E.; Juhasz, C.; Muzik, O.; Maqbool, M.; Chugani, D.C.; Makki, M. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics 2006, 117, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.T.; Yeatman, J.D.; Wandell, B.A.; Buonocore, M.H.; Amaral, D.G.; Nordahl, C.W. Diffusion properties of major white matter tracts in young, typically developing children. Neuroimage 2014, 88, 143–154. [Google Scholar] [CrossRef]
- Poletti, S.; Mazza, E.; Bollettini, I.; Locatelli, C.; Cavallaro, R.; Smeraldi, E.; Benedetti, F. Adverse childhood experiences influence white matter microstructure in patients with schizophrenia. Psychiatry Res. 2015, 234, 35–43. [Google Scholar] [CrossRef]
- Frieling, H.; Fischer, J.; Wilhelm, J.; Engelhorn, T.; Bleich, S.; Hillemacher, T.; Dorfler, A.; Kornhuber, J.; de Zwaan, M.; Peschel, T. Microstructural abnormalities of the posterior thalamic radiation and the mediodorsal thalamic nuclei in females with anorexia nervosa—A voxel based diffusion tensor imaging (DTI) study. J. Psychiatr. Res. 2012, 46, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Gratiot, M.; Owen, J.P.; Brandes-Aitken, A.; Desai, S.S.; Hill, S.S.; Arnett, A.B.; Harris, J.; Marco, E.J.; Mukherjee, P. White Matter Microstructure is Associated with Auditory and Tactile Processing in Children with and without Sensory Processing Disorder. Front. Neuroanat. 2015, 9, 169. [Google Scholar] [CrossRef]
- Payabvash, S.; Palacios, E.M.; Owen, J.P.; Wang, M.B.; Tavassoli, T.; Gerdes, M.; Brandes-Aitken, A.; Marco, E.J.; Mukherjee, P. Diffusion tensor tractography in children with sensory processing disorder: Potentials for devising machine learning classifiers. NeuroImage Clin. 2019, 23, 101831. [Google Scholar] [CrossRef]
- Zwicker, J.G.; Missiuna, C.; Harris, S.R.; Boyd, L.A. Developmental coordination disorder: A pilot diffusion tensor imaging study. Pediatr. Neurol. 2012, 46, 162–167. [Google Scholar] [CrossRef]
- Bremner, J.D. The relationship between cognitive and brain changes in posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 2006, 1071, 80–86. [Google Scholar] [CrossRef]
- De Bellis, M.D.; Keshavan, M.S.; Clark, D.B.; Casey, B.J.; Giedd, J.N.; Boring, A.M.; Frustaci, K.; Ryan, N.D.A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol. Psychiatry 1999, 45, 1271–1284. [Google Scholar] [CrossRef]
- Badura-Brack, A.S.; Mills, M.S.; Embury, C.M.; Khanna, M.M.; Klanecky Earl, A.; Stephen, J.M.; Wang, Y.P.; Calhoun, V.D.; Wilson, T.W. Hippocampal and parahippocampal volumes vary by sex and traumatic life events in children. J. Psychiatry Neurosci. 2020, 45, 190013. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.L.; Knodt, A.R.; Brigidi, B.D.; Hariri, A.R. Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev. Psychopathol. 2015, 27, 1611–1619. [Google Scholar] [CrossRef]
- Costanzo, M.E.; Jovanovic, T.; Pham, D.; Leaman, S.; Highland, K.B.; Norrholm, S.D.; Roy, M.J. White matter microstructure of the uncinate fasciculus is associated with subthreshold posttraumatic stress disorder symptoms and fear potentiated startle during early extinction in recently deployed Service Members. Neurosci. Lett. 2016, 618, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.B.J.; van Zuiden, M.; Nawijn, L.; Frijling, J.L.; Veltman, D.J.; Olff, M. Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: A diffusion tensor imaging study. J. Psychiatry Neurosci. 2017, 42, 331–342. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Pandya, D.N.; Wang, R.; Dai, G.; D’Arceuil, H.E.; de Crespigny, A.J.; Wedeen, V.J. Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain 2007, 130 Pt 3, 630–653. [Google Scholar] [CrossRef]
- Costanzo, M.E.; Chou, Y.Y.; Leaman, S.; Pham, D.L.; Keyser, D.; Nathan, D.E.; Coughlin, M.; Rapp, P.; Roy, M.J. Connecting combat-related mild traumatic brain injury with posttraumatic stress disorder symptoms through brain imaging. Neurosci. Lett. 2014, 577, 11–15. [Google Scholar] [CrossRef]
- Lacey, R.E.; Howe, L.D.; Kelly-Irving, M.; Bartley, M.; Kelly, Y. The Clustering of Adverse Childhood Experiences in the Avon Longitudinal Study of Parents and Children: Are Gender and Poverty Important? J. Interpers. Violence 2022, 37, 2218–2241. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, Y.; Cao, C.; Zhang, K.; Wang, L.; Zhang, L. The relationship between response inhibition and posttraumatic stress symptom clusters in adolescent earthquake survivors: An event-related potential study. Sci. Rep. 2015, 5, 8844. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, J.; Sun, X.; Zhang, K. Enhanced mismatch negativity in adolescents with posttraumatic stress disorder (PTSD). Int. J. Psychophysiol. 2011, 79, 231–235. [Google Scholar] [CrossRef]
- Stephen, J.M.; Solis, I.; Janowich, J.; Stern, M.; Frenzel, M.R.; Eastman, J.A.; Mills, M.S.; Embury, C.M.; Coolidge, N.M.; Heinrichs-Graham, E.; et al. The Developmental Chronnecto-Genomics (Dev-CoG) study: A multimodal study on the developing brain. Neuroimage 2020, 225, 117438. [Google Scholar] [CrossRef]
- Pynoos, R.; Steinberg, A. UCLA Trauma History Profile; National Child Traumatic Stress Network: Los Angeles, CA, USA, 2002. [Google Scholar]
- Briere, J. Trauma Symptom Checklist for Children: Professional Manual; Psychological Assessment Resources, Inc.: Odessa, FL, USA, 1996. [Google Scholar]
- Goodman, R.; Meltzer, H.; Bailey, V. The Strengths and Difficulties Questionnaire: A pilot study on the validity of the self-report version. Eur. Child Adolesc. Psychiatry 1998, 7, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R. The Strengths and Difficulties Questionnaire: A research note. J. Child Psychol. Psychiatry 1997, 38, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T. Manual for Child Behavior Checklist/4-18 and 1991 Profile; University of Vermont Department of Psychiatry: Burlington, VT, USA, 1991. [Google Scholar]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence, 2nd ed.; Pearson: Bloomington, MN, USA, 2011. [Google Scholar]
- Bathelt, J.; Scerif, G.; Nobre, A.C.; Astle, D.E. Whole-brain white matter organization, intelligence, and educational attainment. Trends Neurosci. Educ. 2019, 15, 38–47. [Google Scholar] [CrossRef]
- Andersson, J.L.; Skare, S.; Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 2003, 20, 870–888. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016, 125, 1063–1078. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Graham, M.S.; Zsoldos, E.; Sotiropoulos, S.N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 2016, 141, 556–572. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Graham, M.S.; Drobnjak, I.; Zhang, H.; Filippini, N.; Bastiani, M. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage 2017, 152, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A.; Saad, Z.S. FATCAT: (an efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain Connect. 2013, 3, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Oishi, K.; Jiang, H.; Jiang, L.; Li, X.; Akhter, K.; Hua, K.; Faria, A.V.; Mahmood, A.; Woods, R.; et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008, 40, 570–582. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
| Low-Trauma Mean (Range) (n = 80) | High-Trauma Mean (Range) (n = 104) | p-Value | |
|---|---|---|---|
| Age | 11.92 (9.3–14.83) | 11.82 (9.02–14.93) | 0.6458 |
| Female/Male | 38/42 | 53/51 | 0.6424 |
| Handedness(%RH) | 97% | 94% | 0.29 |
| MRN/UNMC | 39/41 | 52/52 | 0.8668 |
| Race (N) | 0.4504 | ||
| Caucasian | 85% | 80.77% | 0.4504 |
| African American | 0% | 5.77% | |
| Asian | 1.25% | 0.96% | |
| Native American/Alaska Native | 6.25% | 1.92% | |
| Native Hawaiian/Pacific Islander | 1.25% | 1.92% | |
| More than 1 race | 6.25% | 6.73% | |
| Unknown | 0% | 1.92% | |
| Number of Traumatic event categories | 0.5875 | 3.15192 | <0.001 |
| Low-Trauma Mean (Range) (n = 80) | High-Trauma Mean (Range) (n = 104) | p-Value | Cohen’s D | |
|---|---|---|---|---|
| CBCL-Parent | ||||
| Anxious/Depressed | 2.17 | 2.65 | NS | 0.2 |
| Withdrawn/Depressed | 1.48 | 1.14 | NS | −0.18 |
| Somatic Complaints | 0.91 | 1.44 | 0.02612 | 0.33 |
| Social Problems | 1.06 | 1.39 | NS | 0.23 |
| Thought Problems | 0.93 | 1.44 | 0.02324 | 0.33 |
| Attention Problems | 1.85 | 3.03 | 0.0028834 | 0.44 |
| Rule-Breaking Behavior | 0.96 | 1.09 | NS | 0.1 |
| Aggressive Behavior | 2.44 | 3.13 | NS | 0.23 |
| Other Problems | 2.06 | 2.69 | 0.02798 | 0.33 |
| Internalizing | 4.57 | 5.16 | NS | 0.13 |
| Externalizing | 3.41 | 4.235 | NS | 0.21 |
| Strengths and Difficulties—Child | ||||
| Emotional Problems | 1.93 | 3.17 | p < 0.001 | 0.6 |
| Conduct Problems | 1.18 | 2.13 | p < 0.001 | 0.67 |
| Hyperactivity | 3.09 | 4.07 | 0.003494 | 0.44 |
| Peer Problems | 1.63 | 2.03 | NS | 0.27 |
| Prosocial | 7.96 | 8.00 | NS | 0.03 |
| Internalizing | 3.57 | 5.21 | p < 0.001 | 0.57 |
| Externalizing | 4.27 | 6.21 | p < 0.001 | 0.61 |
| Total | 7.84 | 11.42 | p < 0.001 | 0.73 |
| TSCC | ||||
| Anxiety | 3.75 | 6.25 | p < 0.001 | 0.74 |
| Anger | 3.15 | 5.80 | p < 0.001 | 0.66 |
| Depression | 2.60 | 3.77 | p < 0.001 | 0.44 |
| Dissociation | 3.83 | 6.75 | p < 0.001 | 0.77 |
| Overt Dissociation | 2.35 | 4.20 | p < 0.001 | 0.69 |
| Fantasy Dissociation | 1.48 | 2.54 | p < 0.001 | 0.67 |
| Post-Traumatic Stress | 3.93 | 7.59 | p < 0.001 | 0.86 |
| Under-Reporting | 4.35 | 2.59 | p < 0.001 | −0.69 |
| Hyper-Reporting | 0.03 | 0.15 | p < 0.001 | 0.33 |
| F-Statistic | p-Value | R2 adj | |
|---|---|---|---|
| Corpus Callosum | |||
| Total | 4.67 | 0.032 | 0.094 |
| Body | 5.99 | 0.015 | 0.085 |
| Genu | 0.397 | 0.529 | 0.137 |
| Splenium | 2.34 | 0.127 | 0.135 |
| Cingulum | |||
| L Cingulate Gyrus | 4.5 | 0.035 | 0.208 |
| R Cingulate Gyrus | 3.36 | 0.068 | 0.267 |
| Parahippocampus | |||
| L Parahippocampus | 1.79 | 0.182 | 0.0274 |
| R Parahippocampus | 0.525 | 0.449 | 0.0268 |
| Uncinate Fasciculus | |||
| Left UF | 2.11 | 0.147 | −0.006 |
| Right UF | 1.83 | 0.177 | 0.005 |
| Posterior Thalamic Radiation | |||
| Left PTR | 5.64 | 0.02 | 0.112 |
| Right PTR | 7.009 | 0.009 | 0.160 |
| F-Statistic | p-Value | R2 adj | |
|---|---|---|---|
| Corpus Callosum | |||
| Total | 1.47 | 0.226 | 0.003 |
| Body | 2.25 | 0.135 | 0.006 |
| Genu | 0.853 | 0.357 | 0.000 |
| Splenium | 0.199 | 0.656 | −0.004 |
| Cingulum | |||
| L Cingulate Gyrus | 0.814 | 0.368 | −0.001 |
| R Cingulate Gyrus | 0.305 | 0.582 | −0.003 |
| L Parahippocampus | 0.55 | 0.459 | −0.002 |
| R Parahippocampus | 0.482 | 0.488 | −0.002 |
| Uncinate Fasciculus | |||
| Left UF | 0.613 | 0.435 | −0.002 |
| Right UF | 1.5 | 0.222 | 0.002 |
| Posterior Thalamic Radiation | |||
| Left PTR | 1.28 | 0.258 | 0.001 |
| Right PTR | 2.13 | 0.145 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, A.; Petropoulos, H.; Sanjuan, P.M.; Wang, Y.-P.; Wilson, T.W.; Calhoun, V.D.; Stephen, J.M. Childhood Adversity and White Matter Microstructure: White Matter Differences Associated with Trauma Exposure. Stresses 2025, 5, 19. https://doi.org/10.3390/stresses5010019
Rodriguez A, Petropoulos H, Sanjuan PM, Wang Y-P, Wilson TW, Calhoun VD, Stephen JM. Childhood Adversity and White Matter Microstructure: White Matter Differences Associated with Trauma Exposure. Stresses. 2025; 5(1):19. https://doi.org/10.3390/stresses5010019
Chicago/Turabian StyleRodriguez, Andrea, Helen Petropoulos, Pilar M. Sanjuan, Yu-Ping Wang, Tony W. Wilson, Vince D. Calhoun, and Julia M. Stephen. 2025. "Childhood Adversity and White Matter Microstructure: White Matter Differences Associated with Trauma Exposure" Stresses 5, no. 1: 19. https://doi.org/10.3390/stresses5010019
APA StyleRodriguez, A., Petropoulos, H., Sanjuan, P. M., Wang, Y.-P., Wilson, T. W., Calhoun, V. D., & Stephen, J. M. (2025). Childhood Adversity and White Matter Microstructure: White Matter Differences Associated with Trauma Exposure. Stresses, 5(1), 19. https://doi.org/10.3390/stresses5010019






