Dynamic Dysregulation of Ribosomal Protein Genes in Mouse Brain Stress Models
Abstract
1. Introduction
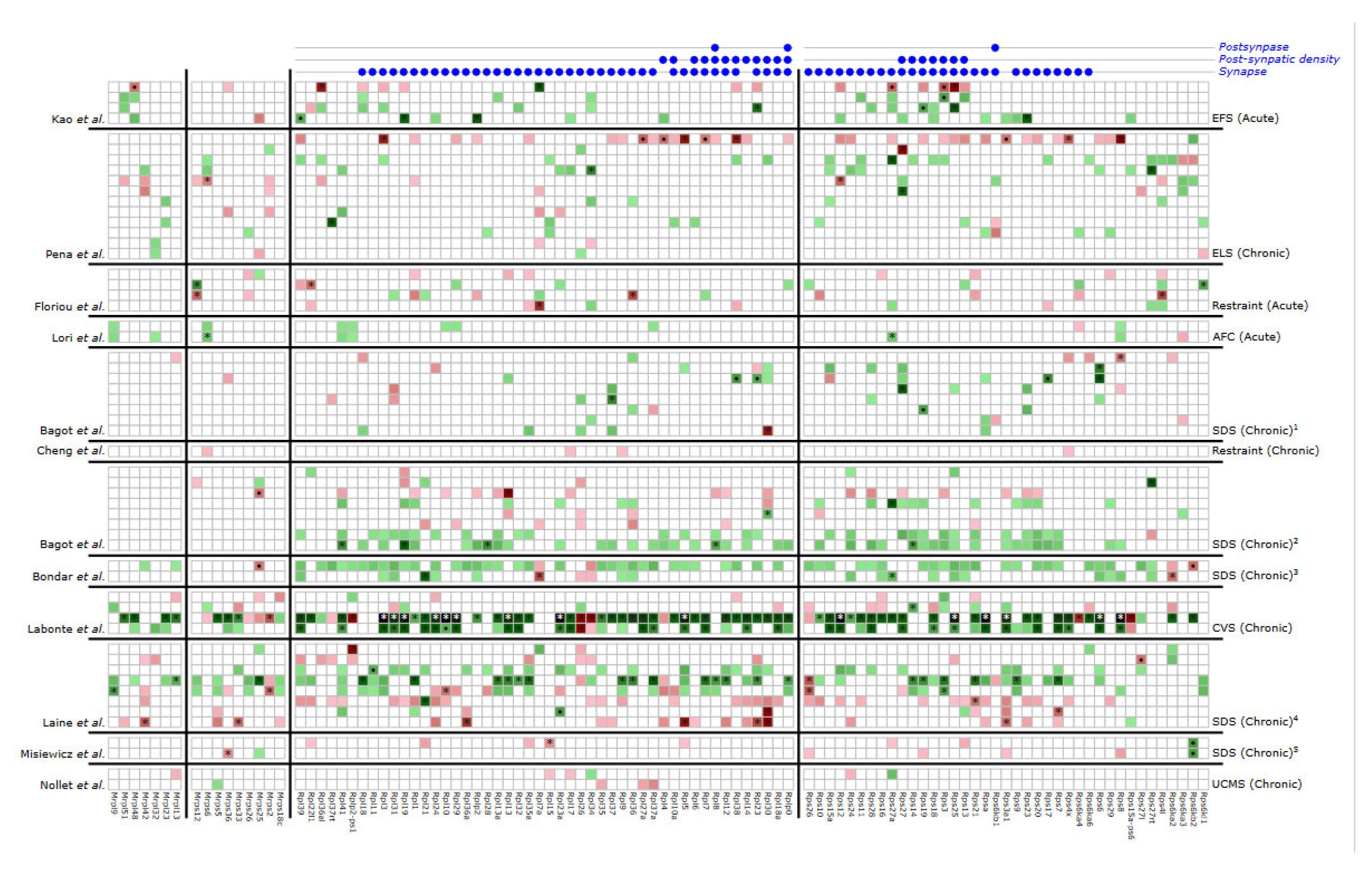
2. Results
3. Discussion
4. Material and Methods
4.1. Data Processing and Differentially Expressed Genes (DEGs) Selection Method
4.2. Calculation of Signed Enrichment Score of Differentially Expressed RPGs
4.3. SynGO Enrichment Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- An, H.; Ordureau, A.; Körner, M.; Paulo, J.A.; Harper, J.W. Systematic Quantitative Analysis of Ribosome Inventory during Nutrient Stress. Nature 2020, 583, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Swaminathan, K.; Shukla, R. The Ribosome Hypothesis: Decoding Mood Disorder Complexity. Int. J. Mol. Sci. 2024, 25, 2815. [Google Scholar] [CrossRef] [PubMed]
- Navarro, I.C.; Tuorto, F.; Jordan, D.; Legrand, C.; Price, J.; Braukmann, F.; Hendrick, A.G.; Akay, A.; Kotter, A.; Helm, M.; et al. Translational Adaptation to Heat Stress Is Mediated by RNA 5-methylcytosine in Caenorhabditis Elegans. EMBO J. 2021, 40, e105496. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Guang, H.; Gualerzi, C.O. The Ribosome as a Switchboard for Bacterial Stress Response. Front. Microbiol. 2020, 11, 619038. [Google Scholar] [CrossRef] [PubMed]
- Hang, R.; Wang, Z.; Deng, X.; Liu, C.; Yan, B.; Yang, C.; Song, X.; Mo, B.; Cao, X. Ribosomal RNA Biogenesis and Its Response to Chilling Stress in Oryza Sativa. Plant Physiol. 2018, 177, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Kim, J.; Jeong, H.; Kwon, D.; Moon, Y. Xenobiotic-Induced Ribosomal Stress Compromises Dysbiotic Gut Barrier Aging: A One Health Perspective. Redox Biol. 2023, 59, 102565. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Hein, M.Y.; Cox, J.; Mann, M. A “Proteomic Ruler” for Protein Copy Number and Concentration Estimation without Spike-in Standards. Mol. Cell. Proteom. 2014, 13, 3497–3506. [Google Scholar] [CrossRef]
- Wyant, G.A.; Abu-Remaileh, M.; Frenkel, E.M.; Laqtom, N.N.; Dharamdasani, V.; Lewis, C.A.; Chan, S.H.; Heinze, I.; Ori, A.; Sabatini, D.M. NUFIP1 Is a Ribosome Receptor for Starvation-Induced Ribophagy. Science 2018, 360, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Smriga, M.; Kameishi, M.; Uneyama, H.; Torii, K. Dietary L-Lysine Deficiency Increases Stress-Induced Anxiety and Fecal Excretion in Rats. J. Nutr. 2002, 132, 3744–3746. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Gao, X.; Li, L.; Ren, Z.; Lui, L.M.W.; McIntyre, R.S.; Teopiz, K.M.; Deng, P.; Cao, B. The Association Between Concentrations of Arginine, Ornithine, Citrulline and Major Depressive Disorder: A Meta-Analysis. Front. Psychiatry 2021, 12, 686973. [Google Scholar] [CrossRef] [PubMed]
- Ali-Sisto, T.; Tolmunen, T.; Viinamäki, H.; Mäntyselkä, P.; Valkonen-Korhonen, M.; Koivumaa-Honkanen, H.; Honkalampi, K.; Ruusunen, A.; Nandania, J.; Velagapudi, V.; et al. Global Arginine Bioavailability Ratio Is Decreased in Patients with Major Depressive Disorder. J. Affect. Disord. 2018, 229, 145–151. [Google Scholar] [CrossRef]
- Zhang, X.; Eladawi, M.A.; Ryan, W.G.; Fan, X.; Prevoznik, S.; Devale, T.; Ramnani, B.; Malathi, K.; Sibille, E.; Mccullumsmith, R.; et al. Ribosomal Dysregulation: A Conserved Pathophysiological Mechanism in Human Depression and Mouse Chronic Stress. PNAS Nexus 2023, 2, pgad299. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, F.; van Nierop, P.; Andres-Alonso, M.; Byrnes, A.; Cijsouw, T.; Coba, M.P.; Cornelisse, L.N.; Farrell, R.J.; Goldschmidt, H.L.; Howrigan, D.P.; et al. SynGO: An Evidence-Based, Expert-Curated Knowledge Base for the Synapse. Neuron 2019, 103, 217–234.e4. [Google Scholar] [CrossRef] [PubMed]
- Flati, T.; Gioiosa, S.; Chillemi, G.; Mele, A.; Oliverio, A.; Mannironi, C.; Rinaldi, A.; Castrignanò, T. A Gene Expression Atlas for Different Kinds of Stress in the Mouse Brain. Sci. Data 2020, 7, 1–18. [Google Scholar] [CrossRef]
- Kao, C.Y.; He, Z.; Zannas, A.S.; Hahn, O.; Kühne, C.; Reichel, J.M.; Binder, E.B.; Wotjak, C.T.; Khaitovich, P.; Turck, C.W. Fluoxetine Treatment Prevents the Inflammatory Response in a Mouse Model of Posttraumatic Stress Disorder. J. Psychiatr. Res. 2016, 76, 74–83. [Google Scholar] [CrossRef]
- Peña, C.J.; Smith, M.; Ramakrishnan, A.; Cates, H.M.; Bagot, R.C.; Kronman, H.G.; Patel, B.; Chang, A.B.; Purushothaman, I.; Dudley, J.; et al. Early Life Stress Alters Transcriptomic Patterning across Reward Circuitry in Male and Female Mice. Nat. Commun. 2019, 10, 5098. [Google Scholar] [CrossRef]
- Floriou-Servou, A.; von Ziegler, L.; Stalder, L.; Sturman, O.; Privitera, M.; Rassi, A.; Cremonesi, A.; Thöny, B.; Bohacek, J. Distinct Proteomic, Transcriptomic, and Epigenetic Stress Responses in Dorsal and Ventral Hippocampus. Biol. Psychiatry 2018, 84, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Lori, A.; Maddox, S.A.; Sharma, S.; Andero, R.; Ressler, K.J.; Smith, A.K. Dynamic Patterns of Threat-Associated Gene Expression in the Amygdala and Blood. Front. Psychiatry 2019, 10, 425511. [Google Scholar] [CrossRef] [PubMed]
- Bagot, R.C.C.; Cates, H.M.M.; Purushothaman, I.; Lorsch, Z.S.S.; Walker, D.M.M.; Wang, J.; Huang, X.; Schlüter, O.M.M.; Maze, I.; Peña, C.J.J.; et al. Circuit-Wide Transcriptional Profiling Reveals Brain Region-Specific Gene Networks Regulating Depression Susceptibility. Neuron 2016, 90, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, M.; Chen, L.; Li, Y.; Lin, L.; Yao, B.; Li, Z.; Wang, Z.; Chen, J.; Miao, Z.; et al. Ten-Eleven Translocation Proteins Modulate the Response to Environmental Stress in Mice. Cell Rep. 2018, 25, 3194–3203.e4. [Google Scholar] [CrossRef] [PubMed]
- Bagot, R.C.; Cates, H.M.; Purushothaman, I.; Vialou, V.; Heller, E.A.; Yieh, L.; LaBonté, B.; Peña, C.J.; Shen, L.; Wittenberg, G.M.; et al. Ketamine and Imipramine Reverse Transcriptional Signatures of Susceptibility and Induce Resilience-Specific Gene Expression Profiles. Biol. Psychiatry 2017, 81, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Bondar, N.; Bryzgalov, L.; Ershov, N.; Gusev, F.; Reshetnikov, V.; Avgustinovich, D.; Tenditnik, M.; Rogaev, E.; Merkulova, T. Molecular Adaptations to Social Defeat Stress and Induced Depression in Mice. Mol. Neurobiol. 2018, 55, 3394–3407. [Google Scholar] [CrossRef] [PubMed]
- Labonté, B.; Engmann, O.; Purushothaman, I.; Menard, C.; Wang, J.; Tan, C.; Scarpa, J.R.; Moy, G.; Loh, Y.H.E.; Cahill, M.; et al. Sex-Specific Transcriptional Signatures in Human Depression. Nat. Med. 2017, 23, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.A.; Trontti, K.; Misiewicz, Z.; Sokolowska, E.; Kulesskaya, N.; Heikkinen, A.; Saarnio, S.; Balcells, I.; Ameslon, P.; Greco, D.; et al. Genetic Control of Myelin Plasticity after Chronic Psychosocial Stress. eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz, Z.; Iurato, S.; Kulesskaya, N.; Salminen, L.; Rodrigues, L.; Maccarrone, G.; Martins, J.; Czamara, D.; Laine, M.A.; Sokolowska, E.; et al. Multi-Omics Analysis Identifies Mitochondrial Pathways Associated with Anxiety-Related Behavior. PLoS Genet. 2019, 15, e1008358. [Google Scholar] [CrossRef] [PubMed]
- Nollet, M.; Hicks, H.; McCarthy, A.P.; Wu, H.; Möller-Levet, C.S.; Laing, E.E.; Malki, K.; Lawless, N.; Wafford, K.A.; Dijk, D.J.; et al. REM Sleep’s Unique Associations with Corticosterone Regulation, Apoptotic Pathways, and Behavior in Chronic Stress in Mice. Proc. Natl. Acad. Sci. USA 2019, 116, 2733–2742. [Google Scholar] [CrossRef]
- Cajigas, I.J.; Tushev, G.; Will, T.J.; Tom Dieck, S.; Fuerst, N.; Schuman, E.M. The Local Transcriptome in the Synaptic Neuropil Revealed by Deep Sequencing and High-Resolution Imaging. Neuron 2012, 74, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Gumy, L.F.; Yeo, G.S.H.; Tung, Y.C.L.; Zivraj, K.H.; Willis, D.; Coppola, G.; Lam, B.Y.H.; Twiss, J.L.; Holt, C.E.; Fawcett, J.W. Transcriptome Analysis of Embryonic and Adult Sensory Axons Reveals Changes in MRNA Repertoire Localization. RNA 2011, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.M.; Choi, S.H.; Jamieson, C.A.M.; Geschwind, D.H.; Martin, K.C. Identification of Process-Localized MRNAs from Cultured Rodent Hippocampal Neurons. J. Neurosci. 2006, 26, 13390–13399. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, T.; Bloch, L.M. Dendritic MRNAs Encode Diversified Functionalities in Hippocampal Pyramidal Neurons. BMC Neurosci. 2006, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.D.; Dieck, S.T.; Alvarez-Castelao, B.; Tushev, G.; Chan, I.C.W.; Schuman, E.M. Subcellular Sequencing of Single Neurons Reveals the Dendritic Transcriptome of GABAergic Interneurons. Elife 2021, 10, e63092. [Google Scholar] [CrossRef] [PubMed]
- Zencir, S.; Dilg, D.; Rueda, M.P.; Shore, D.; Albert, B. Mechanisms Coordinating Ribosomal Protein Gene Transcription in Response to Stress. Nucleic Acids Res. 2020, 48, 11408. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Fujii, K.; Kovary, K.M.; Genuth, N.R.; Röst, H.L.; Teruel, M.N.; Barna, M. Heterogeneous Ribosomes Preferentially Translate Distinct Subpools of MRNAs Genome-Wide. Mol. Cell 2017, 67, 71–83.e7. [Google Scholar] [CrossRef]
- Genuth, N.R.; Barna, M. The Discovery of Ribosome Heterogeneity and Its Implications for Gene Regulation and Organismal Life. Mol. Cell 2018, 71, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Nold, A.; Fusco, C.M.; Rangaraju, V.; Tchumatchenko, T.; Heilemann, M.; Schuman, E.M. The Prevalence and Specificity of Local Protein Synthesis during Neuronal Synaptic Plasticity. Sci. Adv. 2021, 7, 790–807. [Google Scholar] [CrossRef] [PubMed]
- Turrigiano, G. Homeostatic Synaptic Plasticity: Local and Global Mechanisms for Stabilizing Neuronal Function. Cold Spring Harb. Perspect. Biol. 2012, 4, a005736. [Google Scholar] [CrossRef]
- Pamplona, F.; Henes, K.; Micale, V.; Mauch, C.; Takahashi, R.N.; Wotjak, C. Prolonged fear incubation leads to generalized avoidance behavior in mice. J. Psychiatr. Res. 2011, 45, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.J.; Kronman, H.G.; Walker, D.M.; Cates, H.M.; Bagot, R.C.; Purushothaman, I.; Issler, O.; Loh, Y.-H.E.; Leong, T.; Kiraly, D.D.; et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 2017, 356, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, K.R.; Reul, J.M. Acute Stress Enhances Heterodimerization and Binding of Corticosteroid Receptors at Glucocor-ticoid Target Genes in the Hippocampus. Proc. Natl. Acad. Sci. USA 2016, 113, 11336–11341. [Google Scholar] [CrossRef] [PubMed]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential Role of BDNF in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.-H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Borrow, A.P.; Heck, A.L.; Miller, A.M.; Sheng, J.A.; Stover, S.A.; Daniels, R.M.; Bales, N.J.; Fleury, T.K.; Handa, R.J. Chronic variable stress alters hypothalamic-pituitary-adrenal axis function in the female mouse. Physiol. Behav. 2019, 209, 112613. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, V.; Shukla, R. Dynamic Dysregulation of Ribosomal Protein Genes in Mouse Brain Stress Models. Stresses 2024, 4, 916-922. https://doi.org/10.3390/stresses4040061
Sharma V, Shukla R. Dynamic Dysregulation of Ribosomal Protein Genes in Mouse Brain Stress Models. Stresses. 2024; 4(4):916-922. https://doi.org/10.3390/stresses4040061
Chicago/Turabian StyleSharma, Vandana, and Rammohan Shukla. 2024. "Dynamic Dysregulation of Ribosomal Protein Genes in Mouse Brain Stress Models" Stresses 4, no. 4: 916-922. https://doi.org/10.3390/stresses4040061
APA StyleSharma, V., & Shukla, R. (2024). Dynamic Dysregulation of Ribosomal Protein Genes in Mouse Brain Stress Models. Stresses, 4(4), 916-922. https://doi.org/10.3390/stresses4040061






