Abstract
Magnesium is a key element for plant growth and development. Plant responses to Mg deficiency were well investigated, especially in glycophytes. Such responses include a reduction in plant growth and biomass allocation between shoots and roots, photosynthates partitioning from source to sink organs, the accumulation of carbohydrates, and an induction of several Mg transporters. Some physiological and biochemical parameters are good markers of Mg deficiency stress even though they are not well investigated. In the present study, the halophyte Cakile maritima was subjected to Mg shortage, and several Mg stress indices were analyzed. Our data showed that Mg starvation affected shoot and plant length, leaf number, and plant organ growth. A significant decrease in chlorophyll synthesis and photosynthetic activity was also recorded. Mg deficiency triggered oxidative damage as electrolyte leakage and lipid peroxidation were increased by Mg deficiency while the membrane stability index decreased. For a deeper understanding of the effect of Mg starvation on C. maritima, several tolerance stress indices were evaluated, demonstrating a negative impact of Mg stress on almost all those parameters. This study provided important insights on several markers of Mg deficiency stress, which were informative by themselves as unique and early signals of Mg deficiency stress in this halophyte.
1. Introduction
Magnesium (Mg) is a key element in plant physiology and biochemistry since it is classified as the second most prevalent free divalent cation in plant cells [1]. Nevertheless, it is considered a forgotten element for plant production and development as compared to other essential elements [2,3,4]. The common function associated with Mg is its critical contribution to photosynthesis and the long-distance transport of photoassimilates [5]. The implication of Mg in the photosynthetic process is evident as the main steps of photosynthesis are Mg-dependent. Indeed, Mg serves as a key promoter of chlorophyll synthesis and is involved in CO2 fixation and assimilation. Mg is required for the growth and development of plants [6] as a promoter of cell elongation and division. According to Niu et al. [7], in the root cell, Mg is involved in several biochemical processes, including the activation of many enzymes, the generation of energy, and the metabolism of carbohydrates among others and thus, a disruption of Mg uptake by roots affects the metabolism responsible for energy generation leading to an impairment of the biomass allocation. Besides, Mg is an essential component of many enzymes involved in several primary and secondary metabolic pathways, including lipid, amino acid and energy metabolism, biosynthesis of secondary metabolites, and vitamin synthesis [8] as it is a cofactor of more than 300 enzymes including ATPases, phosphatases, carboxylases, and RNA polymerases [9,10].
Mg deficiency is a common problem affecting many agricultural soils worldwide [11], especially sandy soils, which are known for their low mineral nutrient availability [12]. Mg deficiency is often associated with a decrease in plant growth because Mg is involved directly in the photosynthetic pathway and, consequently, the production of photosynthetic metabolites [13]. Under these conditions, Mg, as a relatively mobile microelement, is transported from old to young tissues [14] to sustain their photosynthetic activity. Such quick response led to visual chlorosis symptoms in older leaves. Chlorosis could result not only from low Mg content but can be a direct consequence of leaf chlorophyll reduction upon long-term Mg shortage stress [15].
Roots are the primary organs responsible for Mg deficiency sensing and signaling in plants. Thus, low Mg availability in the soil solution promotes root exudation of organic acids aimed at enhancing the release of Mg from the soil and improving its uptake, a key adaptive trait to Mg stress [13]. Besides, an induction of root plasma membrane-localized high-affinity Mg2+ transporters (i.e., MGT6 and MGT7) was also demonstrated under Mg-limiting conditions [16,17]. Interestingly, Mg deficiency can affect the uptake of other nutrients, notably potassium (K) as OsHAK1, a gene encoding a high-affinity K transporter was up-regulated in rice plants exposed to Mg shortage. However, upon Mg starvation, the reduction in root growth and number as a consequence of their low sugar contents impairs the uptake of other mineral nutrients and negatively affects their use efficiency [18,19].
As Mg is an important component of the photosynthetic machinery, its deficiency underpins the photosynthetic performance of most crop species [20,21,22]. The impairment of the electron transport chain is a common effect of Mg shortage widely described in a wide range of plant species. Under such circumstances, the production of reactive oxygen species is unavoidable as the free electrons can interact with the oxygen molecule to generate superoxide anions, hydrogen peroxide, and hydrogen radicals in different plant organelles, including mitochondria, peroxisomes and chloroplasts with harmful impacts to lipids, proteins and nucleic acids [23].
Despite the well-known importance of Mg for plant growth and development, it is regarded as an overlooked element in many plant species [4]. Indeed, information on plant responses to its deficiency is still scarce; only limited data is available for some glycophyte species with emphasis on its effect on the biomass allocation between shoots and roots, photosynthates and carbohydrates, and the activation of several Mg transporters. Several physiological and biochemical biomarkers are good indicators of the intensity of Mg deficiency stress and could be informative by themselves; nonetheless, their investigation is limited. Besides, information regarding halophytes is scarcely described. Only a few studies conducted in our laboratory on the halophyte Sulla carnosa revealed an alteration of the activity of photosystems and chloroplast ultrastructure upon Mg shortage [20,24].
Cakile maritima is an annual diploid plant species and a moderate salt-requiring halophyte (needs 50 to 100 mM NaCl to express its maximal growth potential) widely distributed on sandy soils throughout the world [25,26,27]. In Tunisia, it is found along the Tunisian seashore, with climates ranging from humid to arid [28]. C. maritima is also regarded as an edible succulent herb commonly used for human consumption with several ethnomedicinal purposes as it exhibits a good source and high potential for bioactive secondary metabolite production [29]. Besides, this putative cash crop is a good genetic model of halophytes as its genome is small and its life cycle is short [26]. Recently, C. maritima has been deemed to be an excellent model for decorticating plant responses to multiple abiotic stresses, including salinity, drought, and nutrient deficiencies [30,31,32,33]. Its tolerance to salinity and/or potassium shortage was due to its powerful antioxidant system being modulated by plant organs and stress treatment duration [32,33,34]. Under salinity stress, maintaining the stability of the thylakoid protein complexes and the preservation of the photosynthetic efficiency are the two main adaptive processes enabling the plant to survive following 200 mM NaCl exposure [31]. It is worth noting that in its natural habitat, C. maritima is challenged not only with high salinity reaching 500 mM NaCl (equal to NaCl seawater concentration) but, also with nutrient deficiencies (i.e., macro and microelements) because of the poor soil fertility [25]. Thus, the current study aims to decipher the responses of C. maritima to Mg deficiency with particular emphasis on several biometric, physiological, and biochemical markers of long-term Mg deficiency, enabling us to provide new insights into their role in detecting the intensity of Mg stress at the early development stage. We hypothesize that Mg as a vital macroelement and specific nutrient signal [35] will induce a wide range of responses in C. maritima once omitted from the nutrient solution.
2. Results
2.1. Biometric and Physiological Markers of Mg Deficiency Stress in C. maritima
2.1.1. Shoot Length, Plant Length and Total Leaf Number
Given that Mg is a crucial macroelement for plant growth and development, several attributes related to biomass production, including leaf number, shoot, and whole plant length, were determined in C. maritima upon long-term Mg deficiency stress. Our data depicted that plants suffering Mg shortage were unable to achieve their optimal development in terms of shoot elongation growth as a significant decline in shoot length was noted, reaching 31.8% (Figure 1A). Whole plant length followed the same trend, being reduced by 20% as compared to control plants (Figure 1B). Interestingly, the negative effect of Mg deprivation on total leaf number was greater than on shoot and plant length (−46%, Figure 1C).
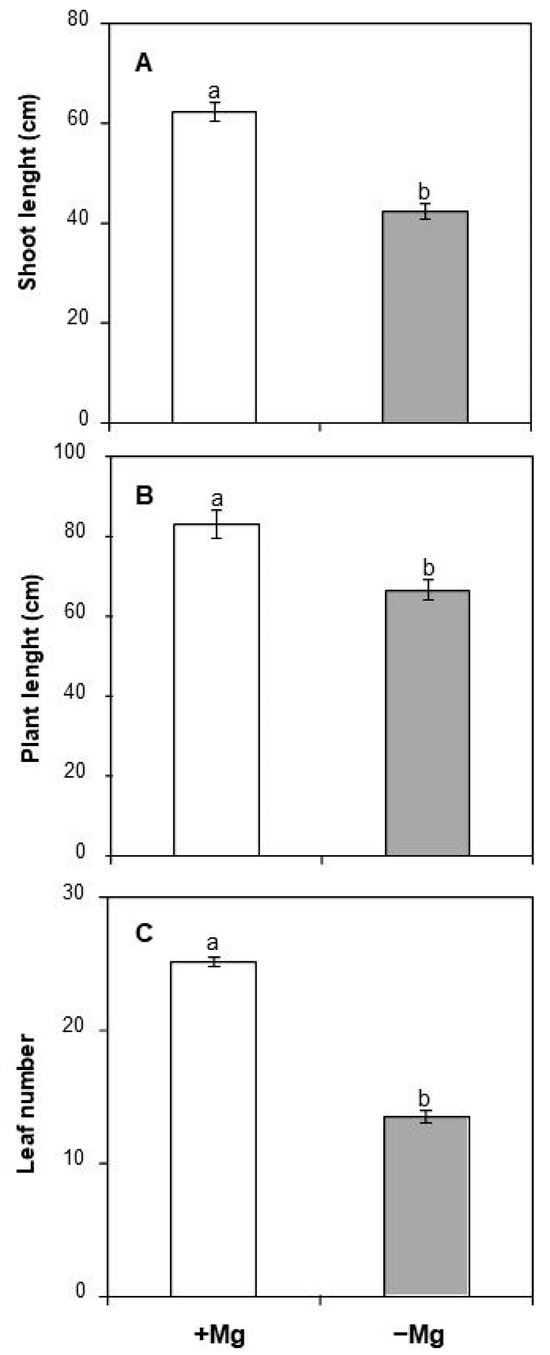
Figure 1.
Effect of Mg deficiency on (A) shoot length, (B) plant length, and (C) leaf number of C. maritma plants grown hydroponically in a complete or free–Mg medium. Values are means of 9 replicates. Means followed by the different letters are significantly different at p ≤ 0.05 according to Duncan’s test.
2.1.2. Leaf, Stem, and Root Biomass Accumulation
To further investigate the effect of Mg starvation on C. maritima growth, the biomass production of the main plant organ was determined (Figure 2). In plants sufficiently supplied with Mg, the whole plant’s fresh weight was about 11.82 g, being distributed between the main plant organs as follows: leaves (48%), stems (43%), and roots (8%). Upon Mg deficiency, the whole plant growth dropped from 11.82 g to 6.99 g. Similarly, the growth of the different plant organs was obviously reduced by Mg availability as shoot fresh weight was significantly impaired by Mg deprivation, with decreases reaching 38% and 41% in leaves and stems, respectively (Figure 2). Roots were more affected by low Mg supply (Figure 2). The decrease was about 52% as compared to plants sufficiently fed with Mg. Interestingly, the allocation of biomass between the different plant organs was not affected by Mg depletion (Figure 2).
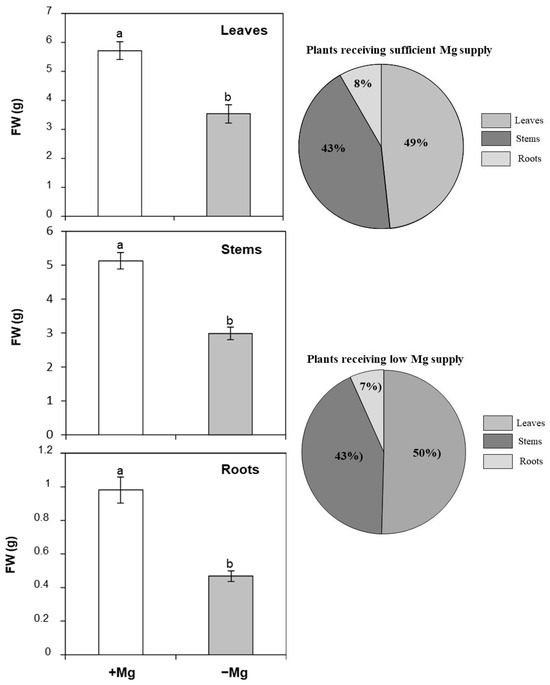
Figure 2.
Effect of Mg deficiency on leaf, stem, and root fresh weight (FW), and the allocation of the plant biomass between the different plant organs of C. maritma plants grown hydroponically in a complete or free–Mg medium. In the right, Mg-starved plants. Values are means of 9 replicates. Means followed by the different letters are significantly different at p ≤ 0.05 according to Duncan’s test.
2.1.3. Tolerance Stress Indices
For a deeper understanding of the effect of Mg shortage on C. maritima growth, several indices were calculated (Table 1). Our results demonstrated that all the studied parameters were significantly affected by Mg deficiency (Table 1). The decrease in shoot and whole plant elongation growth was concomitant with the decline in shoot (SHSI) and plant height (PHSI) stress tolerance index. The reduction in shoot biomass production was associated with a decrease in shoot fresh weight stress tolerance index (SFSI, −41.57%). In the same way, root fresh weight was in line with a significant decline in root fresh weight stress tolerance index (RFSI), reaching 50% of the control. On the basis of shoot (SDSI) and root dry weight stress tolerance index (SDSI), our data indicated a significant reduction (−32% and −44%, respectively). Data regarding the dry matter stress tolerance index (DMSI) revealed a decrease of about 33%.

Table 1.
Effect of Mg deficiency on SHSI, PHSI, SFSI, RFSI, SDSI, RDSI and DMSI. Values are means of 9 replicates. Means followed by the different letters are significantly different at p ≤ 0.05 according to Duncan’s test.
2.2. Effect of Mg Deficiency on Chlorophyll Content
Mg shortage severely decreased the concentrations of photosynthetic pigments. C. maritima seedlings grown in the total absence of Mg in the nutrient solution first showed Mg-deficient symptoms in the basal older leaves. Those later exhibited chlorosis, a typical symptom of magnesium deficiency in plants resulting from chlorophyll degradation and loss. The determination of chlorophyll concentration in the same chlorotic leaves revealed a significant impact of Mg starvation as Chla, Chlb, and total chlorophyll were reduced by 54%, 60%, and 56%, respectively (Table 2). The Chla/Chlb ratio was not affected by Mg shortage, suggesting that both pigments were similarly affected by Mg depletion (Table 2). Chlorophyll stability index (CSI) as an indicator of the stress tolerance capacity of plants was also determined. We found that this parameter was reduced by 55.5% in plants suffering Mg shortage compared to those sufficiently supplemented with Mg (Table 2). In contrast to chlorophyll, no difference in carotenoid concentrations was observed between control and Mg-stressed plants (Table 2).

Table 2.
Changes in chlorophyll a (Chla), b (Chlb), total chlorophyll (Chla+Chlb), carotenoids (Car), Chla/Chlb, and chlorophyll stability index (CSI) in C. maritima subjected to high (+Mg) or low Mg supply (−Mg). Values are means of 4 replicates. Means followed by the different letters are significantly different at p ≤ 0.05 according to Duncan’s test.
2.3. Effect of Mg Deficiency on Leaf Gas Exchange
To study the effect of Mg deficiency on photosynthesis, some gas exchange parameters including net CO2 assimilation (A), stomatal conductance (gs), and transpiration rate (E) were monitored in fully expanded leaves of C. maritima plants conducted under optimal or low Mg supply. Our data demonstrated that plants fed with adequate Mg supply displayed a net CO2 assimilation of 4.67 μmol CO2 m−2 s−1, a stomatal conductance of 0.16 mol H2O m−2 s−1 and a transpiration rate of 2.92 mmol H2O m−2 s−1 (Figure 3). Mg deficiency decreased those parameters by 85%, 216%, and 82%, respectively, justifying the importance of Mg for the photosynthetic process.
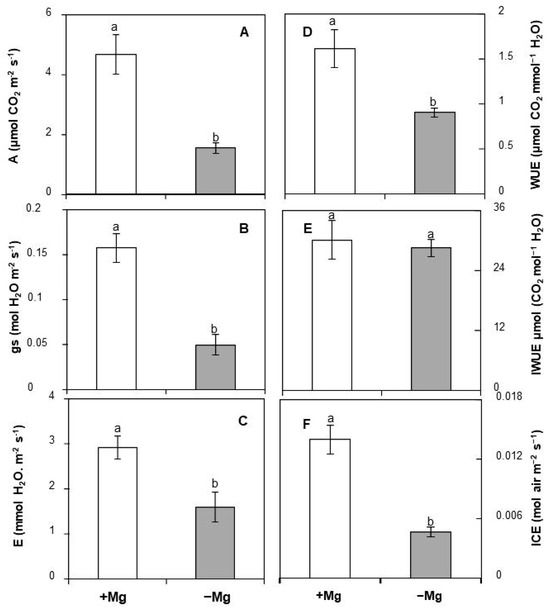
Figure 3.
Changes in (A) net CO2 assimilation, (B) stomatal conductance (gs), (C) transpiration rate (E), (D) water use efficiency (WUE), (E) intrinsic water use efficiency (IWUE), and (F) instantaneous carboxylation efficiency (ICE) in response of C. maritima to Mg supply. Values are means of 5 replicates. Means followed by the different letters are significantly different at p ≤ 0.05 according to Duncan’s test.
Several parameters were calculated based on A, E, and gs. They include water use efficiency (WUE), intrinsic water use efficiency (IWUE), and instantaneous carboxylation efficiency (ICE). Our findings indicated that Mg limitation reduced WUE and ICE by 44% and 66.5%, respectively (Figure 3). Data regarding IWUE showed that this parameter remained unaffected by the availability of Mg (Figure 3).
2.4. Effect of Mg Deficiency on Some Indicators of Oxidative Damage
Mg deficiency can trigger oxidative damage to several biological components, including lipids, proteins, and nucleic acids. In our study, electrolyte leakage (EL), malondialdehyde (MDA) content, and membrane stability index (MSI) were evaluated as three potential biochemical indicators of oxidative stress. As shown in Figure 4A, Mg deficiency resulted in a significant increase in EL (+34%). Likewise, the lowest values of MDA content were registered in control plants (1.98 nmol g−1 FW). Upon Mg shortage, this value displayed three-fold increases reaching 6.41 nmol g−1 FW (Figure 4B). A contrasting trend was observed regarding MSI, whose value was decreased by 32.8% in Mg-deficient leaves (Figure 4C).
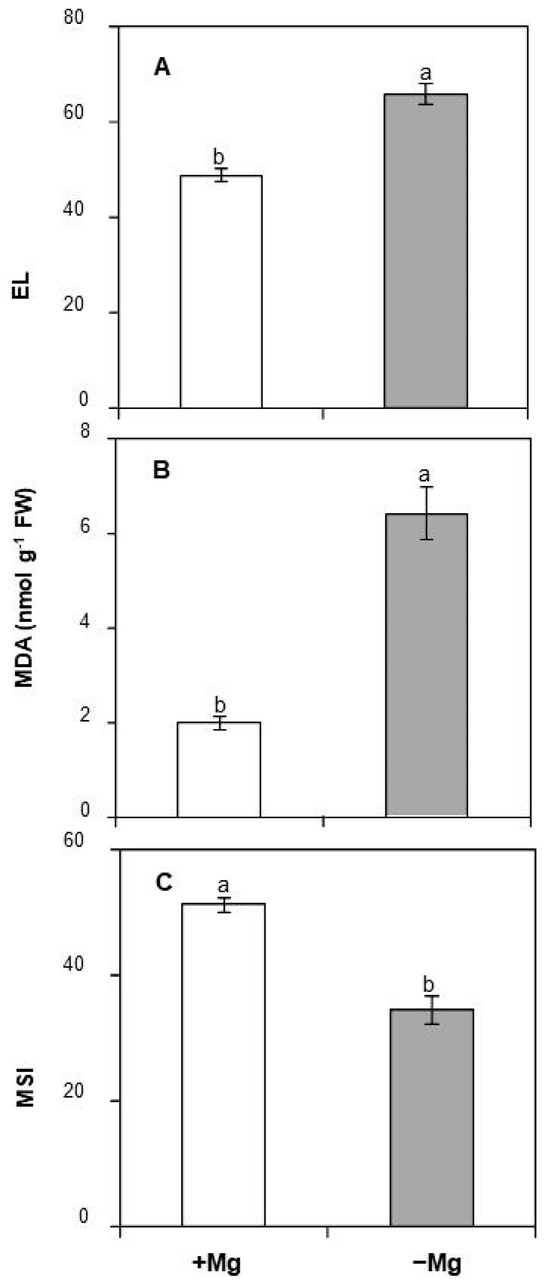
Figure 4.
Effect of Mg deficiency on (A) Electrolyte leakage, (B) MDA, and (C) MSI of C. maritma plants grown hydroponically in a complete or free–Mg medium. Values are means of 4 replicates. Means followed by the different letters are significantly different at p ≤ 0.05 according to Duncan’s test.
2.5. Correlation Analysis and Principal Component Analysis (PCA)
A complimentary PCA and correlation analysis were also used to investigate correlations among control and Mg-deficient plants in terms of the measured stress-related parameters (Figure 5, Table 3). The first two principal components, F1 and F2, explained 75.81% and 7.91% of the cumulative variability of all the measured parameters (Figure 4). Many positive and negative correlations between the different stress markers used in this study were obtained. When Mg was omitted from the nutrient solution, numerous negative and significant correlations were observed for several biometric and physiological parameters including shoot length, plant length, leaf number, leaf, stem and root fresh weights, SHSI, PHSI, SFSI, RFSI, SDSI, RDSI, DMSI, photosynthetic pigments (Chla, Chlb and Chla+Chlb), CSI, MSI, A, gs, E, WUE, and ICE (Table 3) with R2 equal to 0.9, 0.69, 0.98, 0.77, 0.87, 0.84, 0.89, 0.67, 0.95, 0.92, 0.96, 0.86, 0.97, 0.93, 0.98, 0.96, 0.98, 0.94, 0.85, 0.89, 0.75, 0.76 and 0.91 for each parameter respectively. Such data support the negative impact of Mg starvation on those physiological attributes that can be recognized as useful markers of Mg deficiency stress in C. maritima. By contrast, positive correlations were observed for some biochemical parameters, notably EL and MDA (Table 3), with R2 equal to 0.94 and 0.95, respectively, depicting that Mg starvation-induced oxidative damage in leaves of C. maritima.
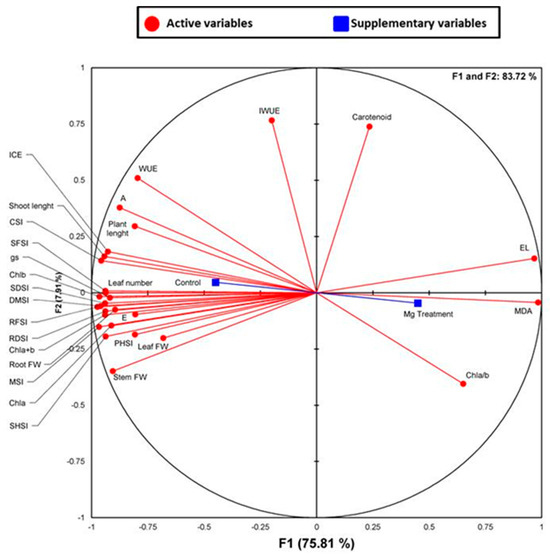
Figure 5.
Principal component analysis (PCA). Circles (•) represent different analysis parameters. Squares (■) represent different treatments (Control and (−) Mg). All studied parameters and the different treatments are projected onto the F1–F2 principal factorial plane that explains 83.72% of the variation. The analyzed parameters include shoot length, plant length, leaf number, leaf, stem and root fresh weights, SHSI, PHSI, SFSI, RFSI, SDSI, RDSI, DMSI, photosynthetic pigments (Chla, Chlb, Chl a+Chlb, Chla/b and Car, CSI, A, gs, E, WUE, IWUE, ICE, MSI, EL and MDA.

Table 3.
Pearson’s correlation matrix analyzes ‘Control (+Mg)’ and Mg deficiency (−) treatments and different studied parameters. The values mentioned in the table represent correlation coefficients (r), with negative correlations in red and positive correlations in blue. Correlations are statistically significant at the 0.05 (*), 0.01 (**), and 0.001 (***) levels. The bold entries mean that the difference is significant.
3. Discussion
Mg is an essential element for plant growth and crop production. Yet, Mg nutrition is often forgotten. In glycophytes, some effects of Mg deficiency were elucidated; meanwhile, in halophytes, little information is available. Plants display an array of physiological and biochemical responses to Mg shortage. Thus, scientists are generally turning to use such tools as markers to study the effect of Mg deficiency in plants. In the present investigation, several attributes were clarified in the halophyte C. maritima upon exposure to Mg deficiency conditions. Our data demonstrated a significant impact of Mg shortage on plant growth as leaf number, shoot and plant length, leaf; stem and root fresh weights were reduced significantly by the lack of Mg in the medium. In fact, such parameters are considered as important markers of stress effects on plant morphology [36]. Our results corroborated previous findings obtained in different plant species. In Eustoma grandiflorum, a native species of alkaline soils in Texas, Mg deficiency was found to reduce root dry weight without affecting shoot dry matter [21]. Besides, low Mg supply impeded the growth of both rice and cucumber cultivars [22]. As described previously in many plant species, Mg deficiency altered the plant organ growth, leading to a significant decrease in the root/shoot ratio [37,38,39,40]. In the halophyte S. carnosa, Mg deficiency lowered the accumulation of biomass in the main plant organs [20]. Indeed, among the earliest and noticeable effects of Mg deficiency in plants, a shrinkage and a reduction of the root system were recently described by Ishfaq et al. [10], which may partially explain the decrease in root-to-shoot dry weight ratio [40,41].
Photosynthesis is the main driver of biomass production in plants and is regarded as among the most important Mg-requiring physiological processes because many photosynthetic enzymes are Mg-dependent [21,42]. In the present study, subjecting C. maritima to long-term Mg deprivation decreased the net CO2 assimilation, the stomatal conductance, and the transpiration rate. Similar findings were obtained in barley, where a limitation of CO2 assimilation was noticed when plants encountered Mg deficiency [43]. Likewise, Farhat et al. studied the effect of Mg deficiency on the halophyte S. carnosa and found a significant decrease in the photosynthetic performance, especially when plants were subjected to severe Mg shortage [20,44]. Such findings could be explained by the fact that a high fraction of Mg is found in chloroplasts where it ensures an optimal functioning of photosynthesis [21] and that Mg is an important component of the photosynthetic machinery as a cofactor involved in the biosynthesis of various enzymes, including those involved in respiration and photosynthesis [45] and consequently its deficiency underpins the photosynthetic performance of most crop species [20,21,22]. As shown recently by Pang et al. and Jamali et al., Mg shortage adversely impacted the ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), the main enzyme involved in CO2 fixation resulting in a decrease in photosynthetic performance [46,47]. According to Meng et al., the reduction in the photosynthetic activity observed in rice and cucumber upon Mg deprivation could be due to a restriction in mesophyll conductance, maximum rate of electron transport as well as the rate of ribulose 1,5-bisphosphate carboxylation [22].
The biochemical limitation of photosynthesis under Mg deficiency stress was associated with low carboxylation efficiency suggesting a reduction in the maximum rate of ribulose-1,5- bisphosphate (RuBP) carboxylation and the low regeneration rate of RuBP driven by the electron transport rate as expected previously by Pena-Rojas [48]. Our data corroborated those of Santos et al., who found that ICE decreased by 50% upon Mg deficiency stress [49]. The decrease in ICE was also demonstrated under other nutrient deficiencies, such as potassium deprivation [50].
Water use efficiency (WUE) represents the ratio of carbon gained through photosynthesis per unit of water lost [51]. WUE was reduced by Mg deficiency, evidencing the important role of Mg in maintaining the homeostasis of the ratio of water loss to carbon gain (i.e., rate of CO2 uptake for a given rate of water loss). Indeed, according to Waraich et al., Mg can improve the WUE of crops through different mechanisms and thus should be included in agricultural practices for improving agricultural water use efficiency [52].
Mg is the most essential element constituent in chlorophyll molecules, and its metabolism regulates the photosynthetic process [45]. The reduction in chlorophyll concentration was generally followed by a significant decline in photosynthesis because chlorophyll metabolism is connected with photosynthesis [53]. In the current investigation, we found that Mg shortage lowered the chlorophyll concentration of C. maritima plants. It has been established that plants suffering Mg deprivation exhibited chlorosis phenomenon due to the relative mobility of Mg from old to young leaves and the degradation of the chlorophyll molecule by Mg-dechelatase which removes Mg2+ from chlorophylls or chlorophyllides to produce pheophytin or pheophorbide, respectively [54,55]. As reported previously by the research group of Hermans, Mg deficiency led to an over-accumulation of sucrose and starch, which reduced leaf chlorophyll content [56,57,58]. According to those authors, once accumulated in the leaves, sucrose inhibits CHLOROPHYLL A/B BINDING PROTEIN 2 (CAB2) gene encoding a class of light-harvesting proteins which are defined by Chen et al. as an antenna complexes of photosystem II (PSII) displaying important roles in the light-harvesting which are likely complexed with the chlorophyll molecule [59] and thus represents a crucial component of the light-harvesting complex II [59,60]. The inhibition of CAB2 by Mg deprivation results in chlorophyll degradation.
The decrease of chlorophyll content in Mg-deficient leaves could be used as a marker of oxidative damage because it results from the degradation of the chlorophyll molecule by the excessive accumulation of reactive oxygen species (ROS) or the induction of Mg dechelatase which initiates the first step of chlorophyll breakdown [53,61]. Such assumption was confirmed by the recent findings of Peng et al., who found that subjecting rice to Mg shortage induced the accumulation of H2O2, which regulated the expression of STAY-GREEN (OsSGR), a Mg de-chelatase gene involved in chlorophyll degradation [14]. Based on these observations, those authors concluded that ROS acts via feedback to promote the expression of genes encoding chlorophyll degradation [14]. Moreover, according to Kobayashi and Tanoi, the degradation of the chlorophyll molecule upon Mg shortage is attributed to ROS accumulation in chloroplasts rather than the lack of the element itself [62].
It has been reported that macronutrient deficiencies trigger oxidative stress [63] with significant damage to several cell biological components. Our data depicted an increase in MDA and El over a decrease in MSI upon Mg stress, indicating the induction of oxidative stress in the leaves of C. maritima. We can speculate that Mg starvation-induced oxidative stress via a decline of the effectiveness of the Calvin cycle, the utilization of reductive powers (i.e., NADPH), and impaired photosynthetic electron transport rate [63,64,65]. Indeed, such disruptions in the photosynthetic functioning are the main driver of ROS generation given that free electrons can interact with the oxygen molecule to produce dangerous reactive species such as superoxide anion and hydrogen peroxide [66] with harmful impacts on lipids, proteins and nucleic acids.
4. Materials and Methods
4.1. Plant Material and Growth Conditions
C. maritima is a halophyte species widely distributed in sandy coasts throughout Europe, the Canary Islands, North Africa, and West Asia. It is known as an edible plant for its high tolerance to several environmental cues [32,33,34,67]. Seeds of C. maritima were germinated in Petri dishes for 7 days. Seedlings were later transferred to hydroponic medium containing ½ Hoagland solution with the following composition: 2.5 mM Ca(NO3)2 · 4H2O, 2.5 mM KNO3, 0.5 mM KH2PO4, and 1 mM MgSO4 · 7H2O for the macronutrients and 23.2 μM H3BO3, 4.6 μM MnCl2 · 4H2O, 1.2 μM ZnSO4 · 7H2O, 0.185 μM CuSO4 · 5H2O, and 0.06 μM Na2MoO4 · 2H2O for the micronutrients. After a pretreatment phase (15 d), the seedlings were subjected to Mg deficiency by omitting the element from the nutrient solution. 1 mM Na2SO4 was used instead of 1 mM MgSO4 to maintain the concentrations of SO4. The culture was carried out in a greenhouse located at the Center of Biotechnology of Borj Cedria (North-East of Tunisia, 36°42′32.9″ N, 10°25′40.9″ E) under sunlight conditions (23/25 °C). When visual symptoms of Mg deficiency and differences in biomass production appeared (30 d), plants were harvested and separated into leaves, stems, and roots, weighted for fresh weight determination, and then dried at 60 °C. Figure 6 illustrates the experimental design used to investigate the responses of C. maritima to Mg deficiency.

Figure 6.
A summary of the experimental design used to investigate several biometric, physiological, and biochemical responses of C. maritima to Mg deficiency.
4.2. Physiological Stress Markers
4.2.1. Photosynthetic Pigment Analysis
Chlorophyll concentration was assayed according to the Arnon method [68]. 100 mg of fresh leaves were extracted in 5 mL of 80% (v/v) acetone. Absorbance was read at 665, 649, and 470 nm.
Chla was calculated as: (13.95 × A665) − (6.88 × A649).
Chlb was calculated as: (24.96 × A649) − (7.32 × A665).
Total chlorophyll was calculated as: Chla + Chlb
Carotenoids were calculated as: (1000 × A470) − (2.05 × Chla − 114.8 × Chlb/245).
4.2.2. Chlorophyll Stability Index (CSI)
CSI was assayed using the method of Sairam et al. [69] and calculated as follows:
CSI = (Total chlorophyll of stressed plants/Total chlorophyll of control plants) × 100
4.2.3. Gas Exchange Analysis
Gas exchange parameters were measured in fully expanded leaves of C. maritima. Net CO2 assimilation rate (A), stomatal conductance (gs), and transpiration rate (E) were assayed. All measurements were carried out in the growth chamber under sunlight conditions and using a portable photosynthesis system (Licor gas analyzer; LC pro+, ADC BioScientific Ltd. Hoddesdon, UK).
4.2.4. Water Use Efficiency (WUE), Intrinsic Water Use Efficiency (IWUE), and Instantaneous Carboxylation Efficiency (ICE)
WUE, IWUE and ICE Were Calculated according to Benslima et al. [50].
4.3. Biochemical Stress Markers
4.3.1. Relative Electrolyte Leakage (EL)
EL was determined using an electrical conductivity meter as described previously by [70]. Fresh leaf samples were placed in 10 mL of deionized water and incubated in a water bath at 25 °C for 2 h. The initial electrical conductivity C1 was read. Then, samples were kept in the same solution, and the temperature was raised to 100 °C. After 30 mn, samples were cooled at 25 °C, and the final electrical conductivity C2 was measured. EL was quantified using the following equation:
EL = (C1/C2) × 100
4.3.2. Membrane Stability Index (MSI)
The membrane stability index was calculated as described previously by Maghsoudi et al. [71] using the following equation:
MSI = (1 − C1/C2) × 100
4.3.3. Lipid Peroxidation
The quantification of malondialdehyde (MDA), the final product of lipid peroxidation, was determined to assess the oxidative stress-induced degradation of unstable lipid peroxides using the thiobarbituric acid method [72]. Briefly, leaf samples were homogenized in trichloroacetic acid (TCA, 0.1%) and then centrifuged at 15,000× g for 10 min. The supernatant was collected and mixed with thiobarbituric acid (TBA, 0.5%), and the obtained mixture was heated at 95 °C for 30 min. The samples were cooled in an ice bath and then centrifuged at 10.000× g for 10 min at 4 °C. Finally, the absorbance was read at 532 nm and 600 nm. MDA content was calculated using the molar extinction coefficient of MDA (155 mM−1 cm−1).
4.4. Physiological Stress Tolerance Indices
Several indexes of stress tolerance were evaluated as described by Kausar et al. [73] and Majeed et al. [74] using the following equations
PHSI = (Plant height of stressed plants/Plant height of control plants) × 100
RLSI = (Root length of stressed plants/Root length of control plants) × 100
SFSI = (Shoot fresh weights of stressed plants/Shoot fresh weights of control plants) × 100
RFSI = (Root fresh weights of stressed plants/Root fresh weights of control plants) × 100
SDSI = (Shoot dry weights of stressed plants/Shoot dry weights of control plants) × 100
RDSI = (Root dry weights of stressed plants/Root dry weights of control plants) × 100
DMSI = (Dry matter of stressed plants/Dry matter of control plants) × 100
4.5. Statistical Analysis
The different biometric, physiological, and biochemical parameters were analyzed statistically by one-way ANOVA (SPSS, Statgraphics Centurion 16.103), and the different treatments were compared using Duncan’s test. Different letters denoted significant differences at p < 0.05.
4.6. Principal Component Analysis
Correlations among the measured physiological and biochemical parameters and treatments were explored by a Principal Component Analysis (PCA) using the XLSTAT software (v. 2014).
5. Conclusions
Mg is a key macroelement for plant growth and development. Yet, few reports emphasizing the detection of its deficiency at the early development stage are available in the literature. Using the halophyte C. maritima, several physiological and biochemical markers were used for Mg shortage diagnostic. Overall, many biometric and physiological attributes such as plant height, leaf number, biomass accumulation in the different plant organs, photosynthetic parameters, and several stress tolerance indices, as well as biochemical markers including EL, MDA, and MSI are useful tools to detect the degree of Mg deficiency in C. maritima. Based on our findings, soil nutrient improvement should be included in the management programs of coastal zones, which represent vegetation-rich biotopes for several edible halophytes: a potential source of food with promising benefits, especially under the current climate fluctuations to satisfy the increasing needs of populations.
Author Contributions
Conceptualization, H.H.; methodology, H.H.; software, H.H.; validation, A.D. and N.F.; formal analysis, H.H. and R.H.; investigation, H.H.; resources, N.F.; data curation, R.H.; writing—original draft preparation, H.H.; writing—review and editing, A.D. and N.F.; visualization, N.F.; supervision, A.D.; project administration, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are included in the main text.
Acknowledgments
Authors would like to thank the Tunisian Ministry of Higher Education and Scientific Research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chaudhry, A.H.; Nayab, S.; Hussain, S.B.; Ali, M.; Pan, Z. Current understandings on magnesium deficiency and future outlooks for sustainable agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Guo, W. Magnesium homeostasis mechanisms and magnesium use efficiency in plants. In Plant Macronutrient Use Efficiency; Academic Press: Cambridge, MA, USA, 2017; pp. 197–213. [Google Scholar]
- Baloch, F.S.; Nadeem, M.A.; Sönmez, F.; Habyarimana, E.; Mustafa, Z.; Karaköy, T.; Cömertpay, G.; Alsaleh, A.; Çiftçi, V.; Sun, S.; et al. Magnesium-a forgotten element: Phenotypic variation and genome wide association study in Turkish Common Bean Germplasm. Front. Genet. 2022, 13, 848663. [Google Scholar] [CrossRef] [PubMed]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Niu, Y.; Jin, G.; Zhang, Y.S. Root development under control of magnesium availability. Plant Signal Behav. 2014, 9, e29720. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ackah, M.; Wang, L.; Amoako, F.K.; Shi, Y.; Essoh, L.G.; Li, J.; Zhang, Q.; Li, H.; Zhao, W. Magnesium Nutrient Application Induces Metabolomics and Physiological Responses in Mulberry (Morus alba). Int. J. Mol. Sci. 2023, 24, 9650. [Google Scholar] [CrossRef] [PubMed]
- Billard, V.; Maillard, A.; Coquet, L.; Jouenne, T.; Cruz, F.; Garcia-Mina, J.M.; Yvin, J.C.; Ourry, A.; Etienne, P. Mg deficiency affects leaf Mg remobilization and the proteome in Brassica napus. Plant Physiol. Biochem. 2016, 107, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef]
- Zhang, B.; Cakmak, I.; Feng, J.; Yu, C.; Chen, X.; Xie, D.; Wu, L.; Song, Z.; Cao, J.; He, Y. Magnesium deficiency reduced the yield and seed germination in wax gourd by affecting the carbohydrate translocation. Front. Plant Sci. 2020, 11, 797. [Google Scholar] [CrossRef]
- Omar, M.M.; Abdrabou, H.A.; Elghamry, A.M. Response of lettuce plant grown on sandy soil to organic and inorganic amendments. J. Soil Sci. Agric. Eng. 2022, 13, 33–38. [Google Scholar] [CrossRef]
- Qu, S.; Li, H.; Zhang, X.; Gao, J.; Ma, R.; Ma, L.; Ma, J. Effects of Magnesium Imbalance on Root Growth and Nutrient Absorption in Different Genotypes of Vegetable Crops. Plants 2023, 12, 3518. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Liao, L.L.; Liu, S.; Nie, M.M.; Li, J.; Zhang, L.D.; Ma, J.F.; Chen, Z.C. Magnesium deficiency triggers SGR–mediated chlorophyll degradation for magnesium remobilization. Plant Physiol. 2019, 181, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, X.F.; Deng, C.L.; Yang, L.T.; Lai, N.W.; Guo, J.X.; Chen, L.S. Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in Citrus sinensis seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Chen, J.; Tian, L.; Liu, Z.; Yang, L.; Tang, R.; Li, J.; Lu, C.; Yang, Y.; Shi, J.; et al. Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. Plant Cell 2014, 26, 2234–2248. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.W.; Mao, D.D.; Yang, L.; Qi, J.L.; Zhang, X.X.; Tang, Q.L.; Li, Y.P.; Tang, R.J.; Luan, S. Magnesium transporter MGT6 plays an essential role in maintaining magnesium homeostasis and regulating high magnesium tolerance in Arabidopsis. Front. Plant Sci. 2018, 9, 274. [Google Scholar] [CrossRef]
- Ogura, T.; Kobayashi, N.I.; Hermans, C.; Ichihashi, Y.; Shibata, A.; Shirasu, K.; Aoki, N.; Sugita, R.; Ogawa, T.; Suzuki, H.; et al. Short-term magnesium deficiency triggers nutrient retranslocation in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 563. [Google Scholar] [CrossRef]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 495191. [Google Scholar] [CrossRef]
- Farhat, N.; Ivanov, A.G.; Krol, M.; Rabhi, M.; Smaoui, A.; Abdelly, C.; Hüner, N.P. Preferential damaging effects of limited magnesium bioavailability on photosystem I in Sulla carnosa plants. Planta 2015, 241, 1189–1206. [Google Scholar] [CrossRef]
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef]
- Meng, X.; Bai, S.; Wang, S.; Pan, Y.; Chen, K.; Xie, K.; Wang, M.; Guo, S. The sensitivity of photosynthesis to magnesium deficiency differs between rice (Oryza sativa L.) and cucumber (Cucumis sativus L.). Front. Plant Sci. 2023, 14, 1164866. [Google Scholar] [CrossRef] [PubMed]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Farhat, N.; Rabhi, M.; Krol, M.; Barhoumi, Z.; Ivanov, A.G.; McCarthy, A.; Abdelly, C.; Smaoui, A.; Hüner, N.P. Starch and sugar accumulation in Sulla carnosa leaves upon Mg2+ starvation. Acta Physiol. Plant. 2014, 36, 2157–2165. [Google Scholar] [CrossRef]
- Debez, A.; Rejeb, K.B.; Ghars, M.A.; Gandour, M.; Megdiche, W.; Hamed, K.B.; Amor, N.B.; Brown, S.C.; Savouré, A.; Abdelly, C. Ecophysiological and genomic analysis of salt tolerance of Cakile maritima. Environ. Exp. Bot. 2013, 92, 64–72. [Google Scholar] [CrossRef]
- Arbelet-Bonnin, D.; Ben-Hamed-Louati, I.; Laurenti, P.; Abdelly, C.; Ben-Hamed, K.; Bouteau, F. Cakile maritima, a promising model for halophyte studies and a putative cash crop for saline agriculture. Adv. Agron. 2019, 155, 45–78. [Google Scholar]
- Clausing, G.; Vickers, K.; Kadereit, J.W. Historical biogeography in linear system: Genetic variation of Sea Rocket (Cakile maritima) and Sea Holly (Eryngium maritimum) along European coasts. Mol. Ecol. 2000, 9, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Amor, N.B.; Jiménez, A.; Megdiche, W.; Lundqvist, M.; Sevilla, F.; Abdelly, C. Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol. Plant. 2006, 126, 446–457. [Google Scholar] [CrossRef]
- Placines, C.; Castañeda-Loaiza, V.; João Rodrigues, M.; Pereira, C.G.; Stefanucci, A.; Mollica, A.; Zengin, G.; Llorent-Martínez, E.J.; Castilho, P.C.; Custódio, A.L. Phenolic profile, toxicity, enzyme inhibition, in silico studies, and antioxidant properties of Cakile maritima Scop. (Brassicaceae) from Southern Portugal. Plants 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Farhat, N.; Belghith, I.; Senkler, J.; Hichri, S.; Abdelly, C.; Braun, H.P.; Debez, A. Recovery aptitude of the halophyte Cakile maritima upon water deficit stress release is sustained by extensive modulation of the leaf proteome. Ecotoxicol. Environ. Saf. 2019, 179, 198–211. [Google Scholar] [CrossRef]
- Farhat, N.; Kouas, W.; Braun, H.P.; Debez, A. Stability of thylakoid protein complexes and preserving photosynthetic efficiency are crucial for the successful recovery of the halophyte Cakile maritima from high salinity. Plant Physiol. Biochem. 2021, 166, 177–190. [Google Scholar] [CrossRef]
- Houmani, H.; Debez, A.; Freitas-Silva, L.D.; Abdelly, C.; Palma, J.M.; Corpas, F.J. Potassium (K+) starvation-induced oxidative stress triggers a general boost of antioxidant and NADPH-generating systems in the halophyte Cakile maritima. Antioxidants 2022, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Houmani, H.; Palma, J.M.; Corpas, F.J. High Salinity Stimulates the Adaptive Response to Potassium Deficiency Through the Antioxidant and the NADPH-Generating Systems in the Roots and Leaves of the Halophyte Cakile maritima. J. Plant Growth Regul. 2022, 42, 6286–6306. [Google Scholar] [CrossRef]
- Houmani, H.; Rodríguez-Ruiz, M.; Palma, J.M.; Abdelly, C.; Corpas, F.J. Modulation of superoxide dismutase (SOD) isozymes by organ development and high long-term salinity in the halophyte Cakile maritima. Protoplasma 2016, 253, 885–894. [Google Scholar] [CrossRef]
- Houmani, H.; Corpas, F.J. Can nutrients act as signals under abiotic stress? Plant Physiol. Biochemistry 2023, 206, 108313. [Google Scholar] [CrossRef] [PubMed]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, physiological and molecular markers for salt-stressed plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef]
- Li, D.; Ma, W.; Wei, J.; Mao, Y.; Peng, Z.; Zhang, J.; Kong, X.; Han, Q.; Fan, W.; Yang, Y.; et al. Magnesium promotes root growth and increases aluminum tolerance via modulation of nitric oxide production in Arabidopsis. Plant Soil 2020, 457, 83–95. [Google Scholar] [CrossRef]
- Koch, M.; Busse, M.; Naumann, M.; Jákli, B.; Smit, I.; Cakmak, I.; Hermans, C.; Pawelzik, E. Differential effects of varied potassium and magnesium nutrition on production and partitioning of photoassimilates in potato plants. Physiol. Plant 2019, 166, 921–935. [Google Scholar] [CrossRef]
- Koch, M.; Winkelmann, M.K.; Hasler, M.; Pawelzik, E.; Naumann, M. Root growth in light of changing magnesium distribution and transport between source and sink tissues in potato (Solanum tuberosum L.). Sci. Rep. 2020, 10, 8796. [Google Scholar] [CrossRef]
- Ishfaq, M.; Zhong, Y.; Wang, Y.; Li, X. Magnesium limitation leads to transcriptional down-tuning of auxin synthesis, transport, and signaling in the tomato root. Front. Plant Sci. 2021, 12, 802399. [Google Scholar] [CrossRef]
- Jia, Y.; Xu, H.; Wang, Y.; Ye, X.; Lai, N.; Huang, Z.; Yang, L.; Li, Y.; Chen, L.S.; Guo, J. Differences in morphological and physiological features of citrus seedlings are related to Mg transport from the parent to branch organs. BMC Plant Biol. 2021, 21, 239. [Google Scholar] [CrossRef]
- Schneider, G.; Lindqvist, Y.; Branden, C.I. Rubisco—Structure and mechanism. Annu. Rev. Biophys. Biomol. Struct. 1992, 21, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Jaghdani, S.J.; Jahns, P.; Tränkner, M. Mg deficiency induces photo-oxidative stress primarily by limiting CO2 assimilation and not by limiting photosynthetic light utilization. Plant Sci. 2021, 302, 110751. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- El-Ezz, S.F.A.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Allam, H.M.; Abdein, M.A.; Abdelgawad, Z.A. A Comparison of the effects of several foliar forms of magnesium fertilization on ‘Superior Seedless’ (Vitis vinifera L.) in saline soils. Coatings 2022, 12, 201. [Google Scholar] [CrossRef]
- Pang, J.J.; Shin, J.S.; Li, S.Y. The Catalytic Role of RuBisCO for in situ CO2 recycling in Escherichia coli. Front. Bioeng. Biotechnol. 2020, 8, 543807. [Google Scholar] [CrossRef] [PubMed]
- Jamali Jaghdani, S.; Jahns, P.; Tränkner, M. The impact of magnesium deficiency on photosynthesis and photoprotection in Spinacia oleracea. Plant Stress 2021, 2, 100040. [Google Scholar] [CrossRef]
- Pena-Rojas, K.; Aranda, X.; Fleck, I. Stomatal limitation to CO2 assimilation and down-regulation of photosynthesis in Quercus ilex resprouts in response to slowly imposed drought. Tree Physiol. 2004, 24, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Mateus, N.S.; Rabêlo, F.H.S.; Macedo, F.G.; Lavres, J. Diagnosing early disorders in Jatropha curcas to calcium, magnesium and sulfur deficiency. J. Plant Nutr. 2020, 43, 1604–1616. [Google Scholar] [CrossRef]
- Benslima, W.; Zorrig, W.; Bagues, M.; Abdelly, C.; Hafsi, C. Silicon mitigates potassium deficiency in Hordeum vulgare by improving growth and photosynthetic activity but not through polyphenol accumulation and the related antioxidant potential. Plant Soil 2022, 477, 153–170. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y.; Saifullah; Ahmad, M. Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric. Scand Sect. B-Soil Plant Sci. 2011, 61, 291–304. [Google Scholar] [CrossRef]
- Wang, P.; Grimm, B. Connecting chlorophyll metabolism with accumulation of the photosynthetic apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Okazawa, A.; Fukusaki, E.; Kobayashi, A. Removal of magnesium by Mg-dechelatase is a major step in the chlorophyll-degrading pathway in Ginkgo biloba in the process of autumnal tints. Z. Naturforsch. C. 2000, 55, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Bourgis, F.; Faucher, M.; Strasser, R.J.; Delrot, S.; Verbruggen, N. Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Yu, Y.; Kou, X.; Periakaruppan, R.; Chen, X.; Li, X. STAY-GREEN and light-harvesting complex II chlorophyll a/b binding protein are involved in albinism of a novel albino tea germplasm ‘Huabai 1’. Sci. Hortic. 2022, 293, 110653. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, H.; Guo, Y.; Zou, Z. Light-harvesting chlorophyll a/b-binding protein-coding genes in jatropha and the comparison with castor, cassava and arabidopsis. PeerJ 2020, 8, e8465. [Google Scholar] [CrossRef]
- Shimoda, Y.; Ito, H.; Tanaka, A. Arabidopsis STAY-GREEN, Mendel’s green cotyledon gene, encodes magnesium-dechelatase. Plant Cell 2016, 28, 2147–2160. [Google Scholar] [CrossRef]
- Tanoi, K.; Kobayashi, N.I. Leaf senescence by magnesium deficiency. Plants 2015, 4, 756–772. [Google Scholar] [CrossRef]
- Tewari, R.K.; Yadav, N.; Gupta, R.; Kumar, P. Oxidative stress under macronutrient deficiency in plants. J. Soil Sci. Plant Nutr. 2021, 21, 832–859. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci. Hortic. 2006, 108, 7–14. [Google Scholar] [CrossRef]
- Tränkner, M.; Jaghdani, S.J. Minimum magnesium concentrations for photosynthetic efficiency in wheat and sunflower seedlings. Plant Physiol. Biochem. 2019, 144, 234–243. [Google Scholar] [CrossRef]
- Cai, Y.T.; Zhang, H.; Qi, Y.P.; Ye, X.; Huang, Z.R.; Guo, J.X.; Chen, L.S.; Yang, L.T. Responses of reactive oxygen species and methylglyoxal metabolisms to magnesium-deficiency differ greatly among the roots, upper and lower leaves of Citrus sinensis. BMC Plant Biol. 2019, 9, 76. [Google Scholar]
- Houmani, H.; Rodriguez-Ruiz, M.; Palma, J.M.; Corpas, F.J. Mechanical wounding promotes local and long distance response in the halophyte Cakile maritima through the involvement of the ROS and RNS metabolism. Nitric Oxide 2018, 74, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.T. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Deshmukh, P.S.; Shukla., D.S. Tolerance of Drought and Temperature Stress in Relation to Increased Antioxidant Enzyme Activity in Wheat. J. Agron. Crop Sci. 1997, 178, 171–178. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C. Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci. 2002, 162, 897–904. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. [43] Malondialdehyde determination as index of lipid Peroxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 421–431. [Google Scholar]
- Kausar, A.; Ashraf, M.Y.; Ali, I.; Niaz, M.; Abbass, Q. Evaluation of sorghum varieties/lines for salt tolerance using physiological indices as screening tool. Pak. J. Bot. 2012, 44, 47–52. [Google Scholar]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y. Effect of exogenous nitric oxide on sulfur and nitrate assimilation pathway enzymes in maize (Zea mays L.) under drought stress. Acta Physiol. Plant. 2018, 40, 206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).