Bioinput Inoculation in Common Beans to Mitigate Stresses Caused by a Period of Drought
Abstract
1. Introduction
2. Results
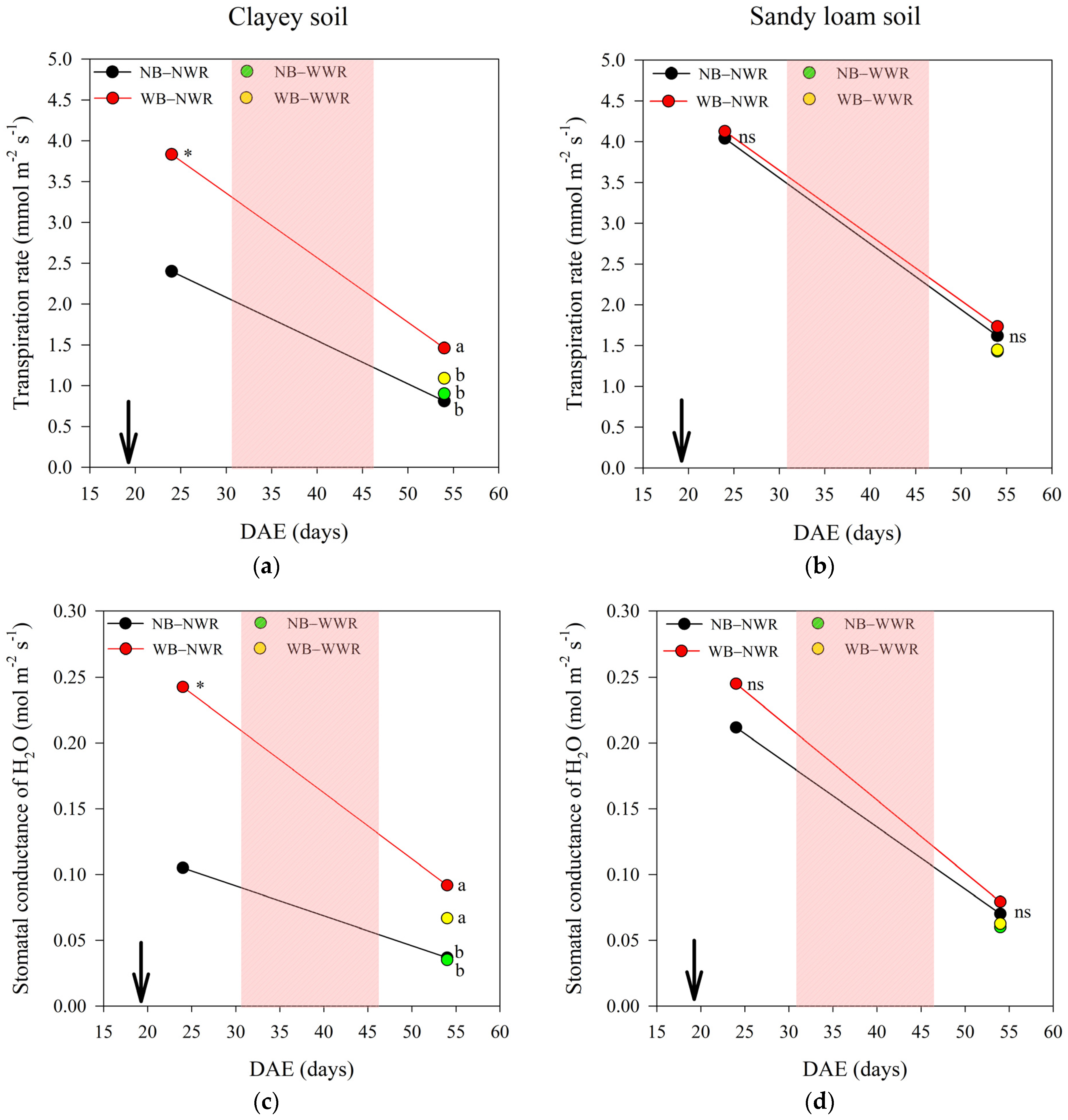
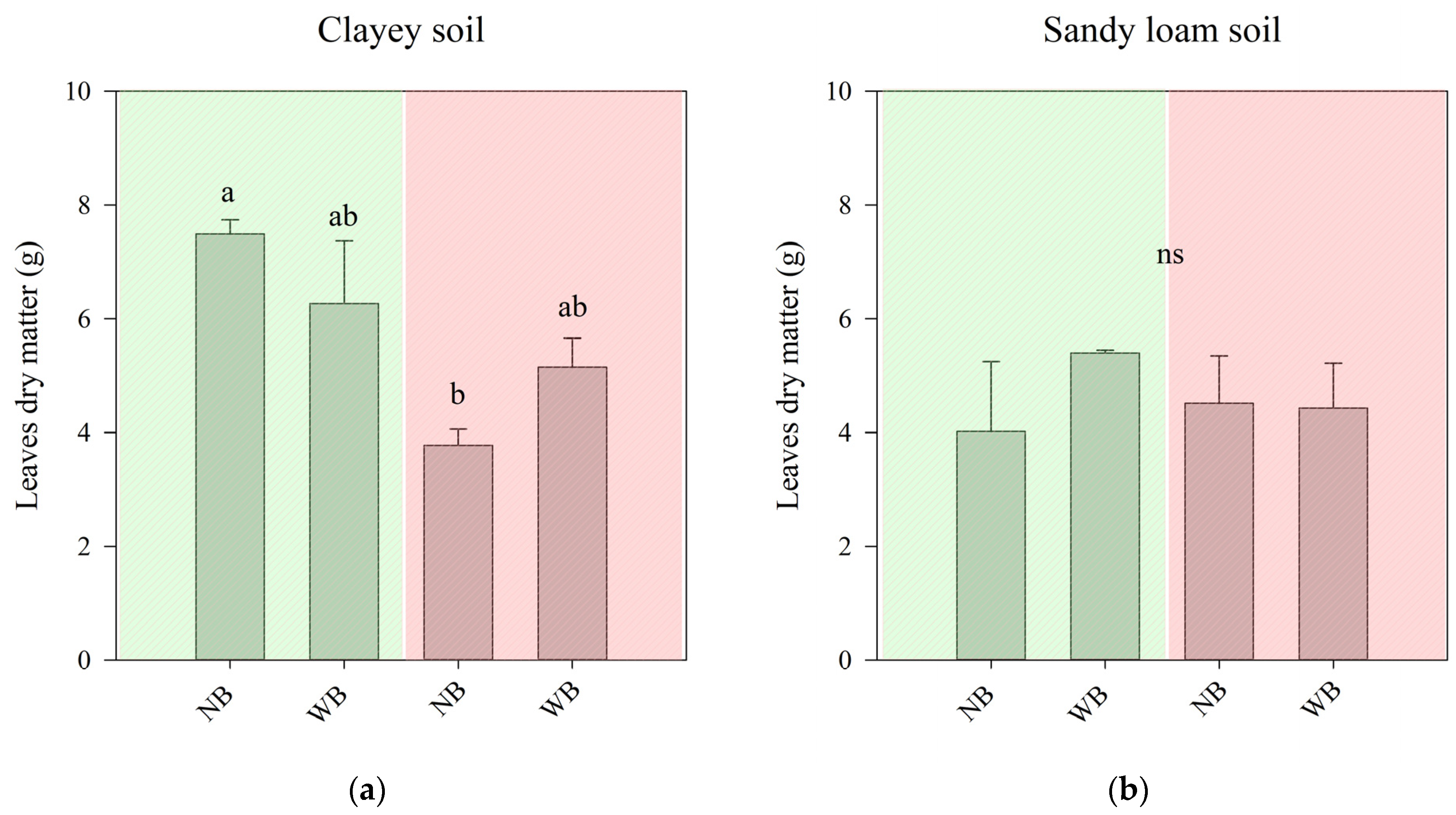
2.1. The Effect of Herbaspirillum frisingense AP21 in Common Beans Cultivated in Soils with Different Textures after a Drought Period
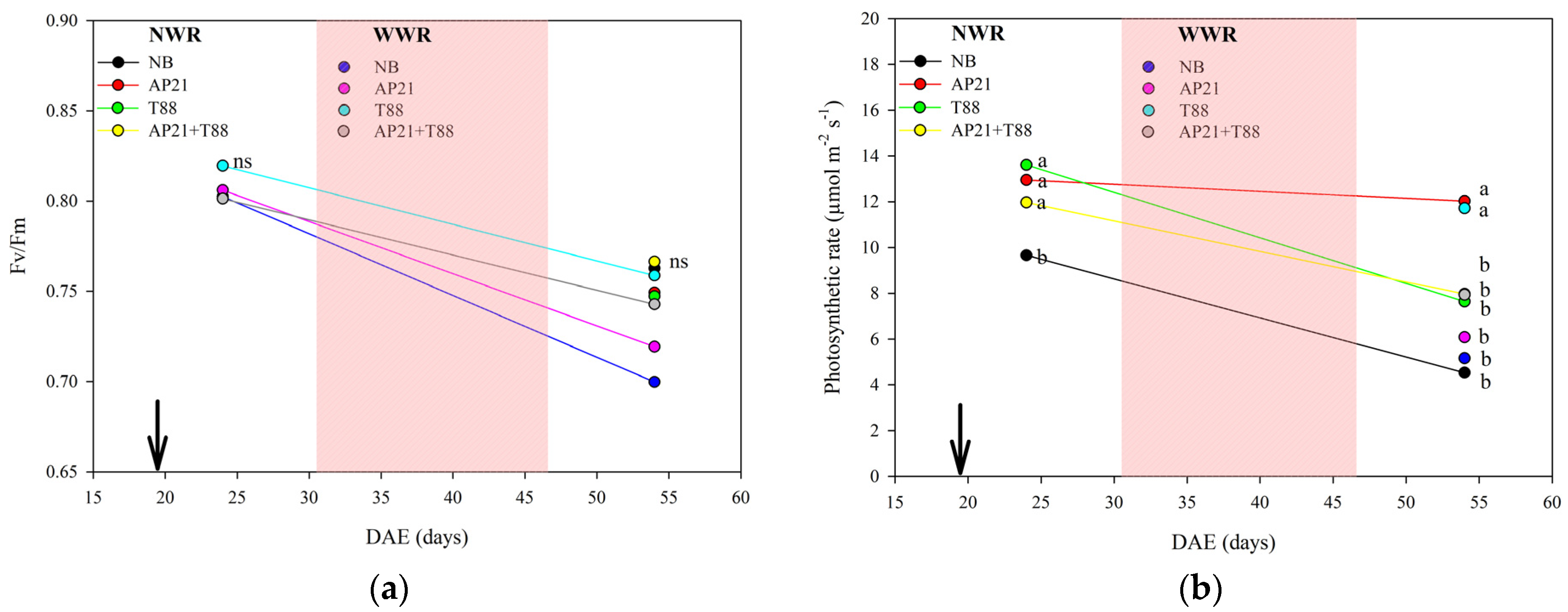
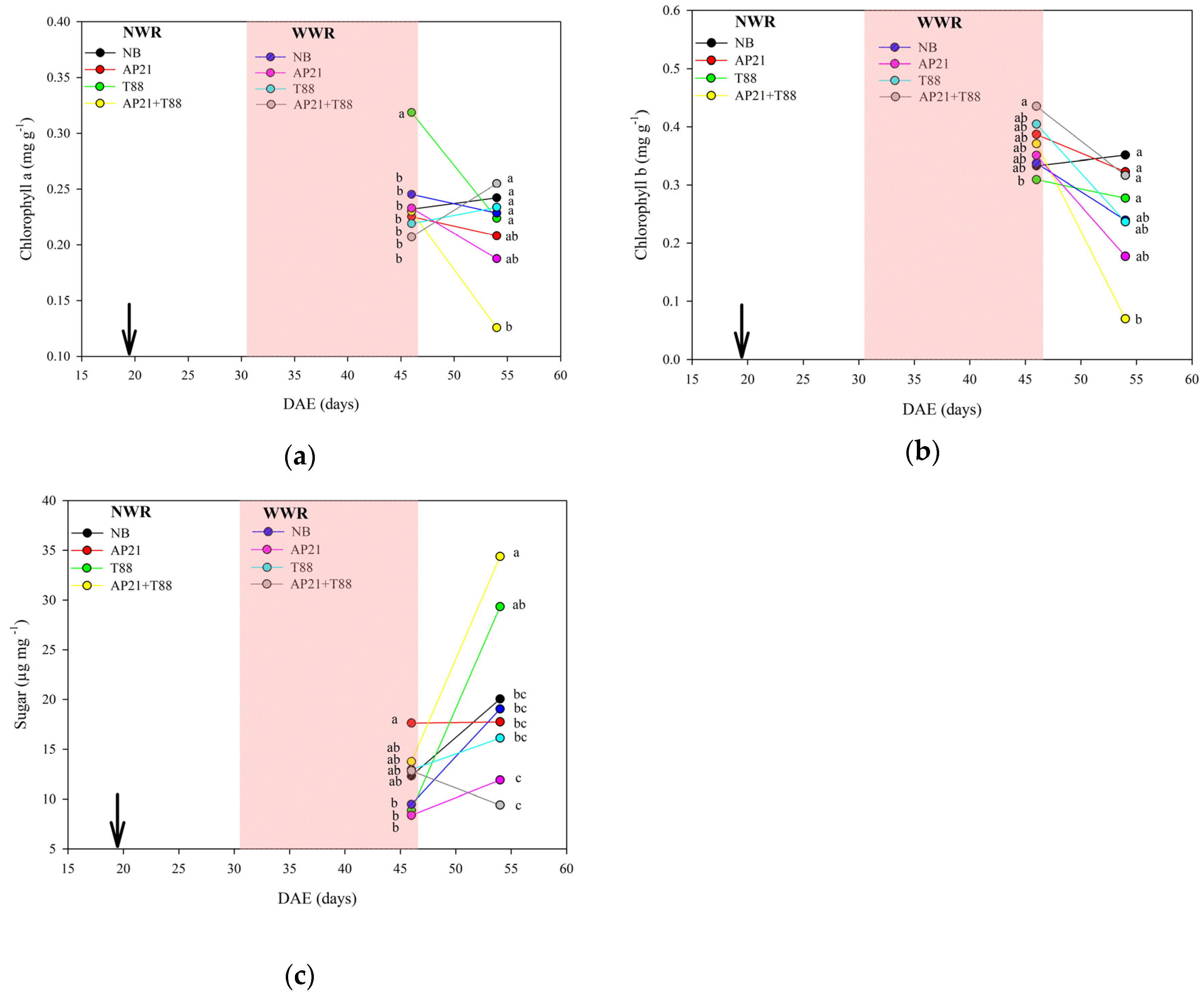
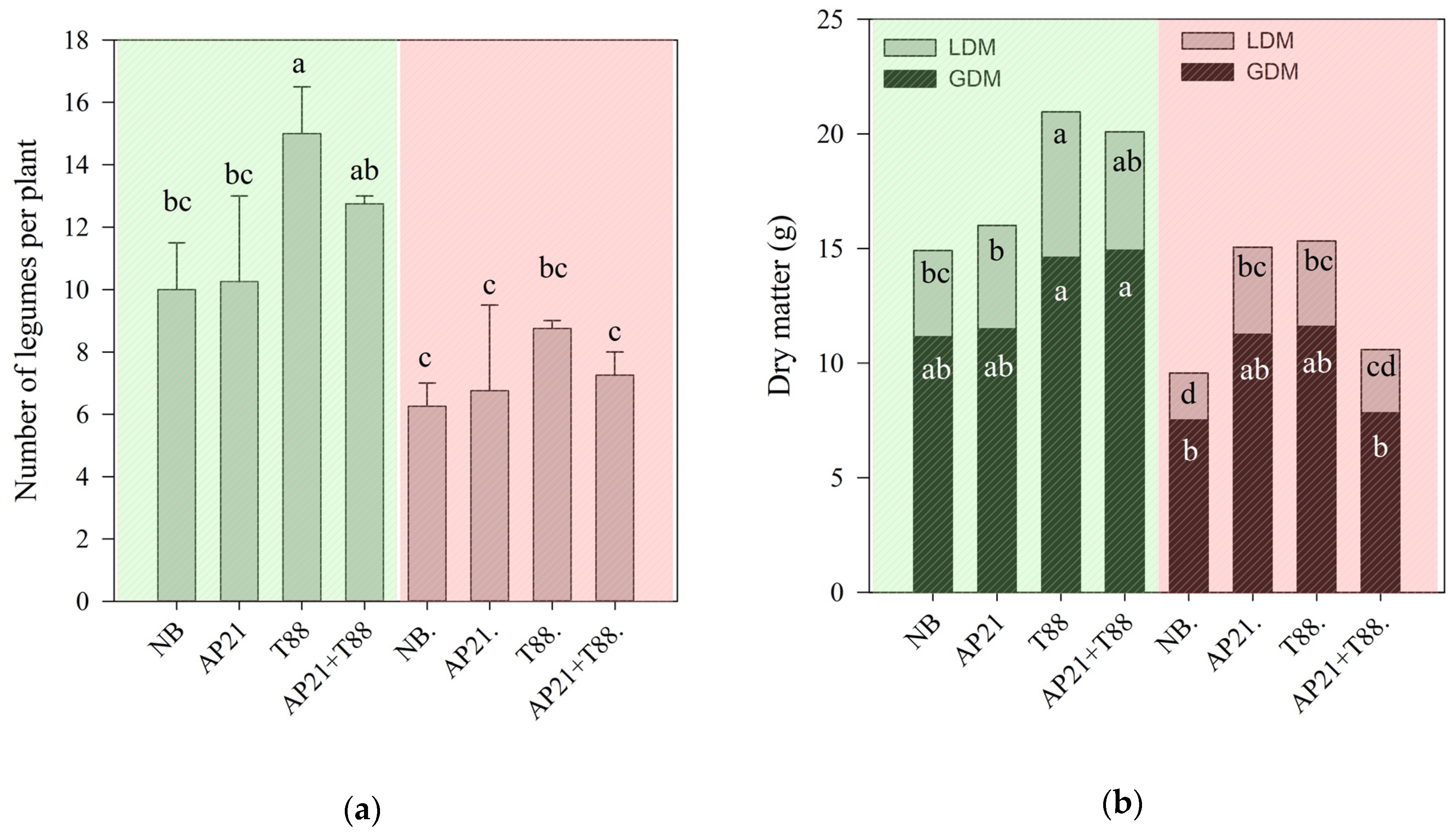
2.2. The Effect of Bioinput Inoculation, Alone or in Consortium, in Common Beans after a Drought Stress
3. Discussion
3.1. The Effect of Herbaspirillum frisingense AP21 in Common Beans Cultivated in Soils with Different Textures after a Drought Period
3.2. The Effect of Bioinput Inoculation, Alone or in Consortium, in Common Beans after a Drought Stress
4. Materials and Methods
4.1. The Effect of Herbaspirillum sp. AP21 in Common Beans Cultivated in Soils with Different Textures after a Drought Period
4.2. The Effect of Bioinput Inoculation, Alone or in Consortium, in Common Beans after a Drought Stress
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gitz, V.; Meybeck, A.; Lipper, L.; Young, C.D.; Braatz, S. Climate change and food security: Risks and responses. Food Agric. Organ. United Nations (FAO) Rep. 2016, 122. [Google Scholar]
- Brady, N.C.; Weil, R.R. Elements of the Nature and Properties of Soils; Pearson Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Fraire-Velázquez, S.; Balderas-Hernández, V.E. Abiotic stress in plants and metabolic responses. Abiotic Stress—Plant Responses Appl. Agric. 2013, 25–48. [Google Scholar]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Reichert, J.M.; Rodrigues, M.F.; Awe, G.O.; Riquelme, U.F.B.; Kaiser, D.R.; Reinert, D.J. Common bean in highly variable weather conditions, on sandy soils, and food security in a subtropical environment. Food Energy Secur. 2015, 4, 219–237. [Google Scholar] [CrossRef]
- Buitrago, R.B.; de Bashan, L.E.G.; Pedraza, R.O. Bacterias Promotoras de Crecimiento Vegetal; Corporacion Colombiana de Investigacion Agropecuaria (Corpoica): Mosquera, Colombia, 2021; 372p. [Google Scholar]
- Steiner, F.; da Silva Oliveira, C.-E.; Zoz, T.; Zuffo, A.-M.; de Freitas, R. Co-Inoculation of common bean with Rhizobium and Azospirillum enhance the drought tolerance. Russ. J. Plant Physiol. 2020, 67, 923–932. [Google Scholar] [CrossRef]
- Kirchhof, G.; Eckert, B.; Stoffels, M.; Baldani, J.I.; Reis, V.M.; Hartmann, A. Herbaspirillum frisingense sp. nov., a new nitrogen-fixing bacterial species that occurs in C4-fibre plants. Int. J. Syst. Evol. Microbiol. 2001, 51, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Curá, J.A.; Franz, D.R.; Filosofía, J.E.; Balestrasse, K.B.; Burgueño, L.E. Inoculation with Azospirillum sand Herbaspirillum sp. bacteria increases the tolerance of maize to drought stress. Microorganisms 2017, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Patino, S.; Vargas, C.; Álvarez-Flórez, F.; Bonilla, R.; Estrada-Bonilla, G. Potential of Herbaspirillum and Azospirillum consortium to promote growth of perennial ryegrass under water deficit. Microorganisms 2021, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Itaily, 2015; pp. 153–154. [Google Scholar]
- Sikuku, P.; Netondo, G.; Onyango, J.; Musyimi, D. Effects of Water Deficit on Physiology and Morphology of Three Varieties of NERICA Rainfed Rice (Oryza sativa L.); Maseno University: Kisumu, Kenya, 2010. [Google Scholar]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S. Turgor and the transport of CO2 and water across the cuticle (epidermis) of leaves. J. Exp. Bot. 2015, 66, 2625–2633. [Google Scholar] [CrossRef]
- Roche, D. Stomatal conductance is essential for higher yield potential of C3 crops. Crit. Rev. Plant Sci. 2015, 34, 429–453. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Perveen, S.; Mahmood, S. Dynamic proline metabolism: Importance and regulation in water-limited environments. In Plant Metabolites and Regulation under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 323–336. [Google Scholar]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Hare, P.; Cress, W. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- El-Saadony, F.M.; Mazrou, Y.S.; Khalaf, A.E.; El-Sherif, A.M.; Osman, H.S.; Hafez, E.M.; Eid, M.A. Utilization efficiency of growth regulators in wheat under drought stress and sandy soil conditions. Agronomy 2021, 11, 1760. [Google Scholar] [CrossRef]
- Da Conquista, V. Association of Growth-Promoting Microorganisms and Fertilizers Improves the Quality of Banana Seedlings; UESB: Vitóriada Conquista, Brazil, 2021; 73p. [Google Scholar]
- Pinheiro, C.; Chaves, M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Ramos, A.C.; Melo, J.; de Souza, S.B.; Bertolazi, A.A.; Silva, R.A.; Rodrigues, W.P.; Campostrini, E.; Olivares, F.L.; Eutrópio, F.J.; Cruz, C. Inoculation with the endophytic bacterium Herbaspirillum seropedicae promotes growth, nutrient uptake and photosynthetic efficiency in rice. Planta 2020, 252, 1–8. [Google Scholar] [CrossRef]
- Argaw, A.; Akuma, A. Rhizobium leguminosarum bv. viciae sp. inoculation improves the agronomic efficiency of N of common bean (Phaseolus vulgaris L.). Environ. Syst. Res. 2015, 4, 1–13. [Google Scholar]
- García de Salamone, I.E.; Esquivel-Cote, R.; Hernández-Melchor, D.J.; Alarcón, A. Manufacturing and quality control of inoculants from the paradigm of circular agriculture. In Microbial Interventions in Agriculture and Environment: Volume 2: Rhizosphere, Microbiome and Agro-Ecology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 37–74. [Google Scholar]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Brígido, C.; Duan, J.; Glick, B.R. Methods to study 1-aminocyclopropane-1-carboxylate (ACC) deaminase in plant growth-promoting bacteria. In Handbook for Azospirillum: Technical Issues and Protocols; Springer: Berlin/Heidelberg, Germany, 2015; pp. 287–305. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Wahyudi, A.T.; Priyanto, J.A.; Afrista, R.; Kurniati, D.; Astuti, R.I.; Akhdiya, A. Plant growth promoting activity of actinomycetes isolated from soybean rhizosphere. Online J. Biol. Sci. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Radwan, T.E.-S.E.-D.; Mohamed, Z.K.; Reis, V. Production of indole-3-acetic acid by different strains of Azospirillum and Herbaspirillum spp. Symbiosis 2002, 32, 39–54. [Google Scholar]
- Simonetti, E.; Roberts, I.N.; Montecchia, M.S.; Gutierrez-Boem, F.H.; Gomez, F.M.; Ruiz, J.A. A novel Burkholderia ambifaria strain able to degrade the mycotoxin fusaric acid and to inhibit Fusarium spp. growth. Microbiol. Res. 2018, 206, 50–59. [Google Scholar] [CrossRef]
- da Piedade Melo, A.; Olivares, F.L.; Médici, L.O.; Torres-Neto, A.; Dobbss, L.B.; Canellas, L.P. Mixed rhizobia and Herbaspirillum seropedicae inoculations with humic acid-like substances improve water-stress recovery in common beans. Chem. Biol. Technol. Agric. 2017, 4, 1–9. [Google Scholar] [CrossRef][Green Version]
- Köppen, W.; Geiger, R. Klimate der Erde. Wall-Map 150 cm × 200 cm; Verlag Justus Perthes: Gotha, Germany, 1928; pp. 91–102. [Google Scholar]
- Peech, M. Hydrogen-ion activity. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Wiley: Hoboken, NJ, USA, 1965; Volume 9, pp. 914–926. [Google Scholar]
- Walkley, A.; Black, I. Rapid titration method of organic carbon of soils. Soil Sci. 1934, 37, 29–33. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Sparks, D.; Bartels, J. Part 3: Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Mehlich, A. New extractant for soil test evaluation of phosphorus, potassium, magnesium, calcium, sodium, manganese and zinc. Commun. Soil Sci. Plant Anal. 1978, 9, 477–492. [Google Scholar] [CrossRef]
- Raij, B.v.; Andrade, J.d.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001; p. 285. [Google Scholar]
- Raij, B.v.; Cantarella, H.; Quaggio, J.A.; Furlani, A. Recomendações de adubação e calagem para o Estado de São Paulo; Instituto Agronômico/Fundação IAC Campinas: Campinas, Brazil, 1997. [Google Scholar]
- Fernández, F. Etapas de Desarrollo de la Planta de Fríjol Común: Guía de Estudio; CIAT: Singapore, 1982. [Google Scholar]
- Bates, L.S.; Waldren, R.a.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]

| Soil Classification 1 | Sampling Location | Sand | Silt | Clay | Textural Classification |
|---|---|---|---|---|---|
| % | |||||
| Andisol | Pasto–Nariño | 6 | 19 | 75 | Clayey |
| Inceptisol | Sibundoy–Putumayo | 66 | 16 | 18 | Sandy loam |
| Soil 1 | pHH2O | EC | OC | OM | P | S | CEC | B | Al + H | Al | Ca | Mg | K | Na | Fe | Cu | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | g/100 g | mg kg−1 | cmol(+)kg−1 | mg kg−1 | cmol(+) kg−1 | mg kg−1 | ||||||||||||
| Clayey | 6.98 | 1.05 | 3.24 | 5.59 | 92.56 | 27.51 | 20.37 | 0.54 | - 2 | - | 14.16 | 2.97 | 3.03 | 0.21 | 144.44 | 3.15 | 7.85 | 6.95 |
| Sandy loam | 5.18 | 1.04 | 4.58 | 7.90 | 27.28 | 15.88 | 12.84 | 0.21 | 0.30 | 0.11 | 10.44 | 1.33 | 0.69 | <0.14 | 552.26 | 2.38 | 28.51 | 9.35 |
| Soil | Bacteria 1 Inoculation 19 DAE | Water Differentiation 31 DAE | Replicates (n) 2 | ||
|---|---|---|---|---|---|
| 1st Analysis 24 DAE | Sampling 46 DAE | 2nd Analysis 54 DAE | |||
| Clayey | NB | NWR | 4 | 4 | 4 |
| Clayey | NB | WWR | - | 4 | 4 |
| Clayey | WB | NWR | 4 | 4 | 4 |
| Clayey | WB | WWR | - | 4 | 4 |
| Sandy loam | NB | NWR | 4 | 4 | 4 |
| Sandy loam | NB | WWR | - | 4 | 4 |
| Sandy loam | WB | NWR | 4 | 4 | 4 |
| Sandy loam | WB | WWR | - | 4 | 4 |
| Bacteria Inoculation 19 and 55 DAE | Water Differentiation 31 DAE | Replicates (n) 1 | |||
|---|---|---|---|---|---|
| 1st Analysis 24 DAE | Sampling 46 DAE | 2nd Analysis 54 DAE | Harvest 97 DAE | ||
| NB | NWR | 4 | 4 | 4 | 4 |
| AP21 | NWR | - | 4 | 4 | 4 |
| T88 | NWR | 4 | 4 | 4 | 4 |
| AP21 + T88 | NWR | - | 4 | 4 | 4 |
| NB | WWR | 4 | 4 | 4 | 4 |
| AP21 | WWR | - | 4 | 4 | 4 |
| T88 | WWR | 4 | 4 | 4 | 4 |
| AP21 + T88 | WWR | - | 4 | 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arruda, B.; Bejarano-Herrera, W.F.; Ortega-Cepeda, M.C.; Campo-Quesada, J.M.; Toro-Tobón, G.; Estrada-Bonilla, G.A.; Silva, A.M.M.; Ferrari Putti, F. Bioinput Inoculation in Common Beans to Mitigate Stresses Caused by a Period of Drought. Stresses 2023, 3, 842-857. https://doi.org/10.3390/stresses3040057
Arruda B, Bejarano-Herrera WF, Ortega-Cepeda MC, Campo-Quesada JM, Toro-Tobón G, Estrada-Bonilla GA, Silva AMM, Ferrari Putti F. Bioinput Inoculation in Common Beans to Mitigate Stresses Caused by a Period of Drought. Stresses. 2023; 3(4):842-857. https://doi.org/10.3390/stresses3040057
Chicago/Turabian StyleArruda, Bruna, Wilfrand Ferney Bejarano-Herrera, Maria Camila Ortega-Cepeda, Jose Manuel Campo-Quesada, Gabriela Toro-Tobón, German Andres Estrada-Bonilla, Antonio Marcos Miranda Silva, and Fernando Ferrari Putti. 2023. "Bioinput Inoculation in Common Beans to Mitigate Stresses Caused by a Period of Drought" Stresses 3, no. 4: 842-857. https://doi.org/10.3390/stresses3040057
APA StyleArruda, B., Bejarano-Herrera, W. F., Ortega-Cepeda, M. C., Campo-Quesada, J. M., Toro-Tobón, G., Estrada-Bonilla, G. A., Silva, A. M. M., & Ferrari Putti, F. (2023). Bioinput Inoculation in Common Beans to Mitigate Stresses Caused by a Period of Drought. Stresses, 3(4), 842-857. https://doi.org/10.3390/stresses3040057










