Assessing Combining Abilities of Popcorn Inbred Lines for Agronomic and Root Traits under Contrasting Water Conditions: Towards Developing Drought-Tolerant Genotypes
Abstract
:1. Introduction
2. Results
2.1. Agronomic and Root Traits Response to Water Conditions: Significant Differences and Reductions in Phenotypic Responses
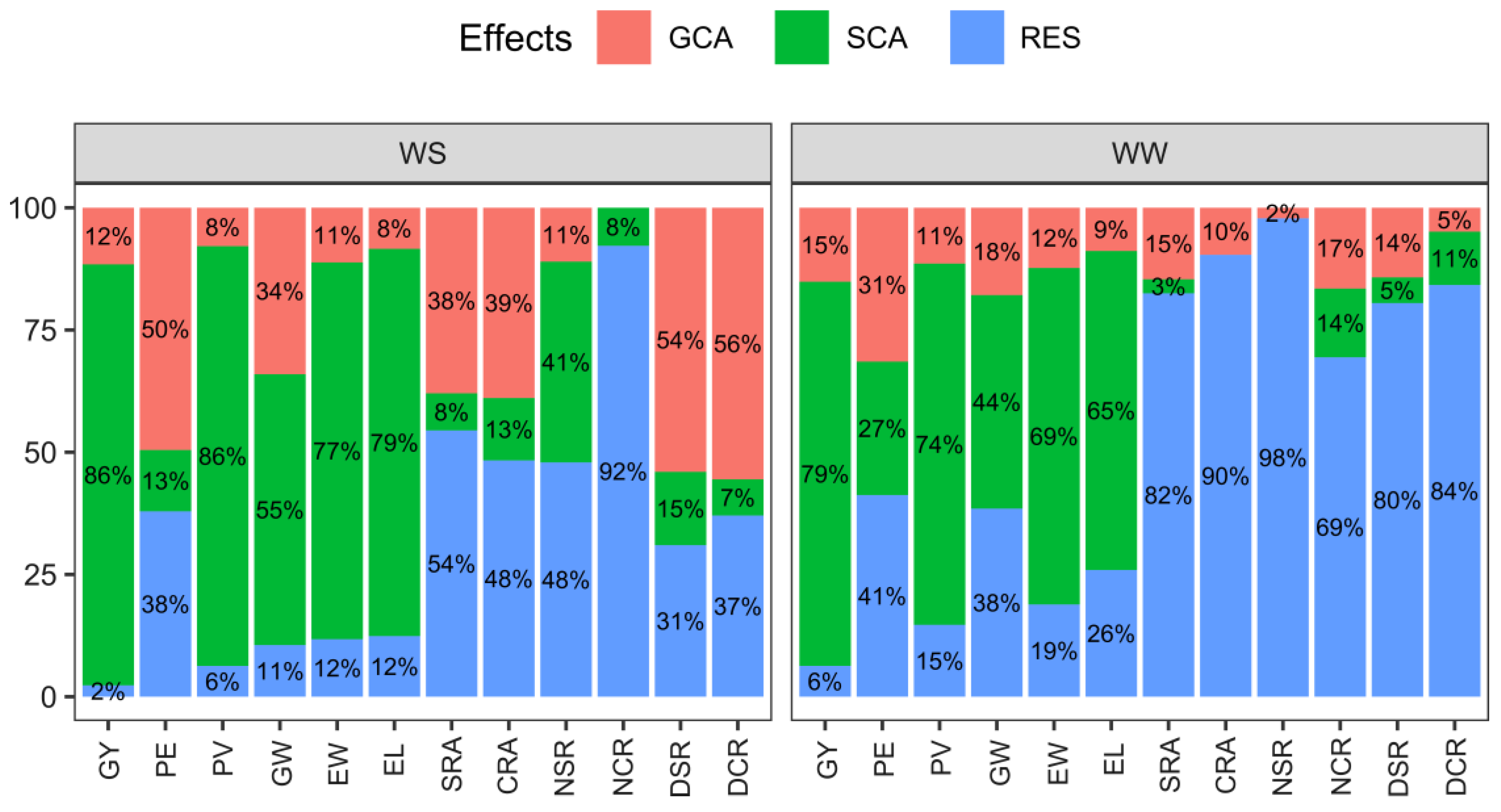
2.2. Estimates of General and Specific Combining Ability and Quadratic Components under Varying Water Conditions: Implications for Trait Variability
2.3. General Combining Ability Values in Popcorn Hybrids under Contrasting Water Conditions
2.4. Specific Combining Ability Value in Popcorn Hybrids under Contrasting Water Conditions
3. Discussion
3.1. Genetic Variability in Different WCs
3.2. Effect of Water Limitation
3.3. The Influence of Genetics Effects on Plant Response to Contrasting Water Conditions
3.4. Combining Ability: Exploring Trait Interactions in Contrasting Water Conditions
4. Materials and Methods
4.1. Plant Material
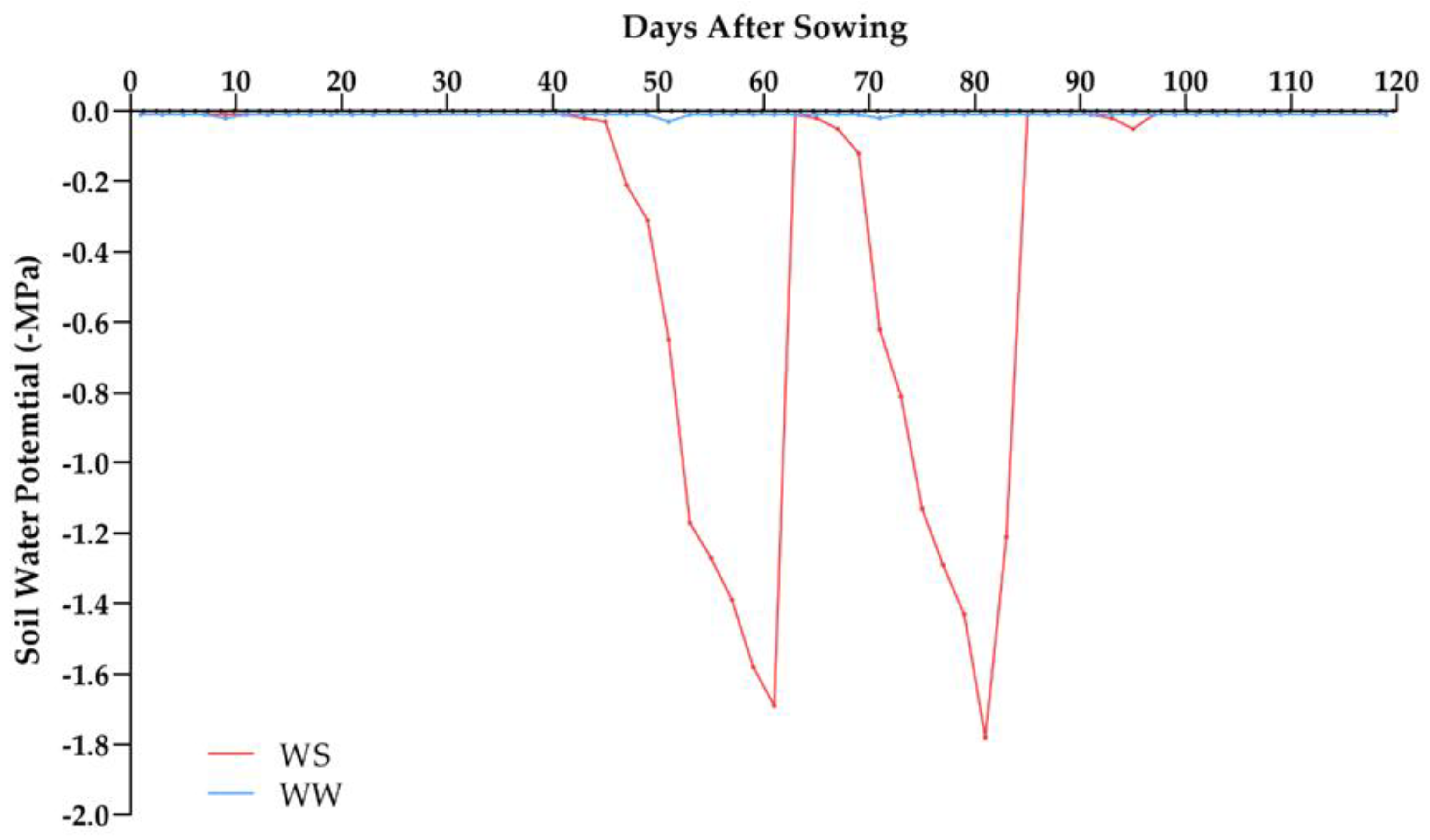
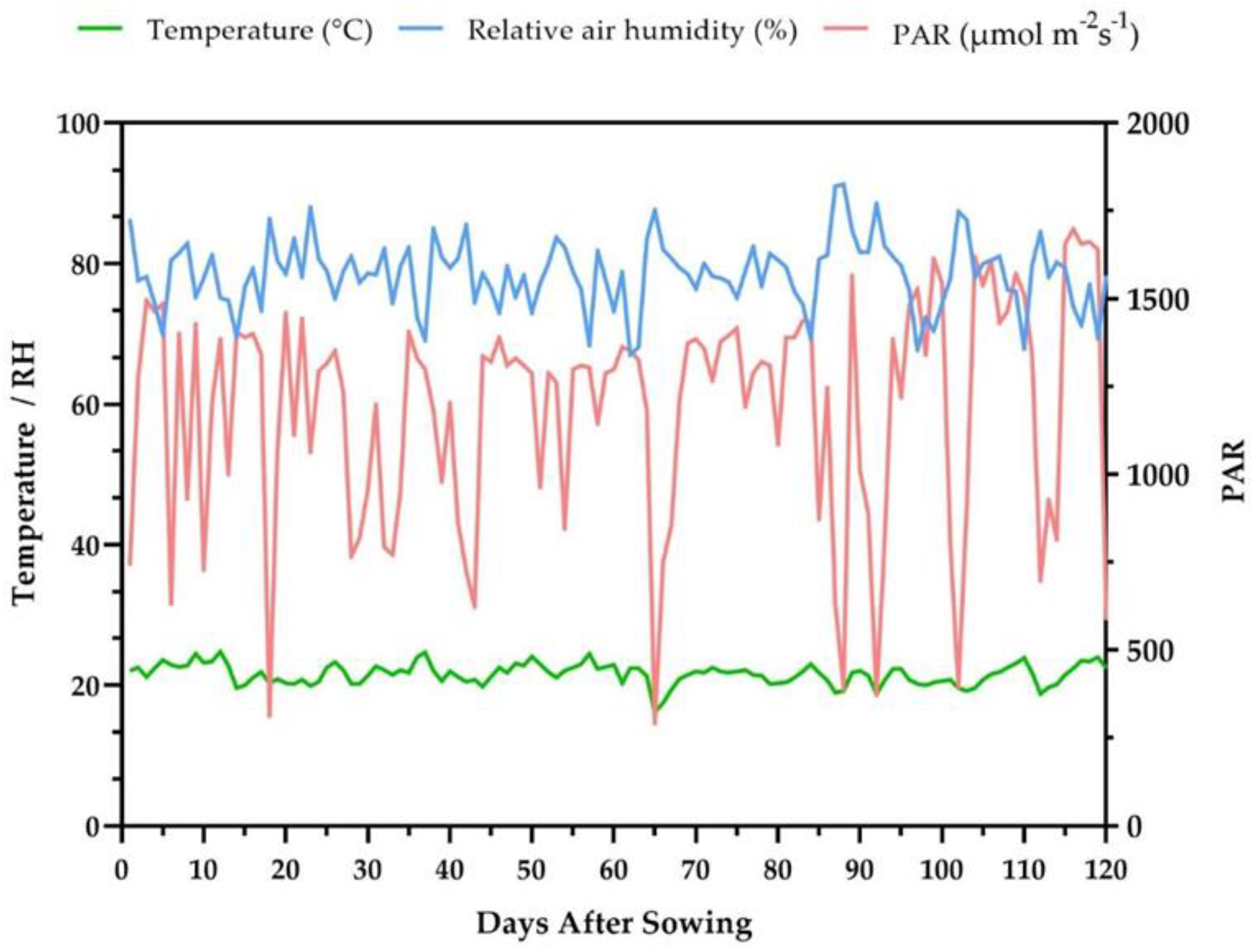
4.2. Environmental Conditions and Experimental Design
4.3. Evaluated Traits
4.3.1. Agronomic Traits
4.3.2. Root Traits
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wheeler, T.; Von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Durand, J.L. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Sabagh, A.; Hossain, A.; Barutçular, C.; Khaled, A.A.A.; Fahad, S.; Anjorin, F.B.; Islam, M.S.; Ratnasekera, D.; Kizilgeçi, F.; Yadav, G.S.; et al. Sustainable maize (Zea mays L.) production under drought stress by understanding its adverse effect, survival mechanism and drought tolerance indices. J. Exp. Biol. Agric. Sci. 2018, 6, 282–295. [Google Scholar] [CrossRef]
- Zilli, M.; Scarabello, M.; Soterroni, A.C.; Valin, H.; Mosnier, A.; Leclère, D.; Havlík, P.; Kraxner, F.; Lopes, M.A.; Ramos, F.M. The Impact of Climate Change on Brazil’s Agriculture. Sci. Total Environ. 2020, 740, 139384. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sahu, N.C.; Kumar, S.; Ansari, M.A. Impact of climate change on cereal production: Evidence from lower-middle-income countries. Environ. Sci. Pollut. Res. 2021, 28, 51597–51611. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Mishra, R.; Maurya, K.K.; Singh, R.B.; Wilson, D.W. Estimates for World Population and Global Food Availability for Global Health. In The Role of Functional Food Security in Global Health; Academic Press: Cambridge, MA, USA, 2019; pp. 3–24. [Google Scholar] [CrossRef]
- Avramova, V.; Nagel, K.A.; Abdelgawad, H.; Bustos, D.; Duplessis, M.; Fiorani, F.; Beemster, G.T. Screening for drought tolerance of maize hybrids by multi-scale analysis of root and shoot traits at the seedling stage. J. Exp. Bot. 2016, 67, 2453–2466. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Cheng, M.; Jiang, H.; Zhang, X.; Peng, C.; Jin, J. Effect of drought on agronomic traits of rice and wheat: A meta-analysis. Int. J. Environ. Res. Public Health 2018, 15, 839. [Google Scholar] [CrossRef] [Green Version]
- Lunduka, R.W.; Mateva, K.I.; Magorokosho, C.; Manjeru, P. Impact of adoption of drought-tolerant maize varieties on total maize production in south Eastern Zimbabwe. Clim. Dev. 2019, 11, 35–46. [Google Scholar] [CrossRef]
- Wang, C.; Linderholm, H.W.; Song, Y.; Wang, F.; Liu, Y.; Tian, J.; Ren, G. Impacts of drought on maize and soybean production in northeast China during the past five decades. Int. J. Environ. Res. Public Health 2020, 17, 2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamphorst, S.H.; Amaral, A.T., Jr.; Vergara-Diaz, O.; Gracia-Romero, A.; Fernandez-Gallego, J.A.; Chang-Espino, M.C.; Buchaillot, M.L.; Rezzouk, F.Z.; de Lima, V.J.; Serret, M.D.; et al. Heterosis and reciprocal effects for physiological and morphological traits of popcorn plants under different water conditions. Agric. Water Manag. 2022, 261, 107371. [Google Scholar] [CrossRef]
- Safian, N.; Naderi, M.R.; Torabi, M.; Soleymani, A.; Salemi, H.R. Corn (Zea mays L.) and sorghum (Sorghum bicolor (L.) Moench) yield and nutritional quality affected by drought stress. Biocatal. Agric. Biotechnol. 2022, 45, 102486. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; Amaral, A.T., Jr.; Lima, V.J.; Guimarães, L.J.M.; Schmitt, K.F.M.; Leite, J.T.; Cruz, C.D. Can Genetic Progress for Drought Tolerance in Popcorn Be Achieved by Indirect Selection? Agronomy 2019, 9, 792. [Google Scholar] [CrossRef] [Green Version]
- Santos, T.d.O.; Amaral, A.T., Jr.; Bispo, R.B.; de Lima, V.J.; Kamphorst, S.H.; Leite, J.T.; Santos, D.R., Jr.; Santos, P.H.A.D.; de Oliveira, U.A.; Schmitt, K.F.M.; et al. Phenotyping Latin American Open-Pollinated Varieties of Popcorn for Environments with Low Water Availability. Plants 2021, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant Breeding and Drought in C3 Cereals: What Should We Breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Snowdon, R.J.; Wittkop, B.; Chen, T.-W.; Stahl, A. Crop Adaptation to Climate Change as a Consequence of Long-Term Breeding. Theor. Appl. Genet. 2021, 134, 1613–1623. [Google Scholar] [CrossRef]
- de Lima, V.J.; Amaral, A.T., Jr.; Kamphorst, S.H.; Bispo, R.B.; Leite, J.T.; Santos, T.d.O.; Schmitt, K.F.M.; Chaves, M.M.; de Oliveira, U.A.; Santos, P.H.A.D.; et al. Combined Dominance and Additive Gene Effects in Trait Inheritance of Drought-Stressed and Full Irrigated Popcorn. Agronomy 2019, 9, 782. [Google Scholar] [CrossRef] [Green Version]
- Kamphorst, S.H.; Amaral, A.T., Jr.; de Lima, V.J.; Carena, M.J.; Azeredo, V.C.; Mafra, G.S.; Santos, P.H.A.D.; Leite, J.T.; Schmitt, K.F.M.; Santos, D.R., Jr.; et al. Driving Sustainable Popcorn Breeding for Drought Tolerance in Brazil. Front. Plant Sci. 2021, 12, 1942. [Google Scholar] [CrossRef]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 2011, 341, 75–87. [Google Scholar] [CrossRef]
- Gao, Y.; Lynch, J.P. Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J. Exp. Bot. 2016, 67, 4545–4557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, J.P. Rightsizing root phenotypes for drought resistance. J. Exp. Bot. 2018, 69, 3279–3292. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, A.; Schneider, H.; Lynch, J.P. Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol. 2015, 168, 1603–1615. [Google Scholar] [CrossRef] [Green Version]
- Alahmad, S.; El Hassouni, K.; Bassi, F.M.; Dinglasan, E.; Youssef, C.; Quarry, G.; Aksoy, A.; Mazzucotelli, E.; Juhasz, A.; Able, J.A.; et al. A Major Root Architecture QTL Responding to Water Limitation in Durum Wheat. Front. Plant Sci. 2019, 10, 436. [Google Scholar] [CrossRef] [Green Version]
- Kamphorst, S.H.; Lima, V.J.; Amaral, A.T., Jr.; Schmitt, K.F.M.; Leite, J.T.; Carvalho, C.M.; Silva, R.M.R. Popcorn breeding for water-stress tolerance or for agronomic water-use efficiency? Genet. Mol. Res. 2018, 17, 4. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; Lima, V.J.; Schmitt, K.F.M.; Leite, J.T.; Azeredo, V.C.; Pena, G.F.; Santos, P.H.D.; Santos, D.R., Jr.; Silva, S.B., Jr.; Bispo, R.B.; et al. Water stress adaptation of popcorn roots and association with agronomic traits. Genet. Mol. Res. 2018, 17, 3. [Google Scholar] [CrossRef]
- Leite, J.T.; Amaral, A.T., Jr.; Kamphorst, S.H.; de Lima, V.J.; Santos, D.R., Jr.; Schmitt, K.F.M.; Souza, Y.P.; Santos, T.O.; Bispo, R.B.; Mafra, G.S.; et al. Water use efficiency in Popcorn (Zea mays L. var. Everta): Which physiological traits would be useful for breeding? Plants 2021, 10, 1450. [Google Scholar] [CrossRef]
- Viana, F.N.; Chaves, M.M.; Kamphorst, S.H.; Amaral, A.T., Jr.; de Lima, V.J.; Leite, J.T.; Schmidt, K.F.M.; de Oliveira, U.A.; Lamego, D.L.; Pereira, J.L.; et al. Heritability of Morphophysiological Traits in Popcorn for Drought Tolerance and Their Use as Breeding Indicators of Superior Genotypes. Agronomy 2022, 12, 1517. [Google Scholar] [CrossRef]
- Griffing, B. Concept of general and specific combining ability in relation to diallel crossing systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef] [Green Version]
- Sprague, G.F.; Tatum, L.A. General vs. specific combining ability in single crosses of corn. Agron. J. 1942, 34, 923–932. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Carena, M.J.; Miranda Filho, J.B. Quantitative Genetics in Maize Breeding; Springer: New York, NY, USA, 2010; ISBN 978–1-4419–0765–3. [Google Scholar]
- Fonseca, A.E.; Westgate, M.E. Relationship between desiccation and viability of maize polle. Field Crops Res. 2005, 94, 114–125. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Huang, J. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Begcy, K.; Dresselhaus, T. Epigenetic responses to abiotic stresses during reproductive development in cereals. Plant Reprod. 2018, 31, 343–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Jiang, M.; Guo, C. Crop pollen development under drought: From the phenotype to the mechanism. Int. J. Mol. Sci. 2019, 20, 1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, F. Genetic dissection of maize drought tolerance for trait improvement. Mol. Breed. 2021, 41, 8. [Google Scholar] [CrossRef]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Relatórios Científicos 2020, 10, 2944. [Google Scholar] [CrossRef] [Green Version]
- Leite, J.T.; Amaral, A.T.d., Jr.; Kamphorst, S.H.; de Lima, V.J.; Santos, D.R.D., Jr.; Alves, U.O.; Azeredo, V.C.; Pereira, J.L.; Bispo, R.B.; Schmidt, K.F.M.; et al. All Are in a Drought, but Some Stand Out: Multivariate Analysis in the Selection of Agronomic Efficient Popcorn Genotypes. Plants 2022, 11, 2275. [Google Scholar] [CrossRef]
- da Silva, W.J.; Vidal, B.C.; Martins, M.E.Q.; Vargas, H.; Pereira, C.; Zerbetto, M.; Miranda, L.C.M. What makes popcorn pop. Nature 1993, 362, 417. [Google Scholar] [CrossRef]
- Ali, M.M.A. Estimation of some breeding parameters for improvement grain yield in yellow maize under water stress. J. Plant Prod. 2016, 7, 1509–1521. [Google Scholar] [CrossRef]
- Maazou, A.R.S.; Tu, J.; Qiu, J.; Liu, Z. Breeding for drought tolerance in maize (Zea mays L.). Am. J. Plant Sci. 2016, 7, 1858. [Google Scholar] [CrossRef] [Green Version]
- Ribaut, J.M.; Betran, J.; Monneveux, P.; Setter, T. Drought Tolerance in Maize. In Handbook of Maize: Its Biology; Bennetzen, J.L., Hake, S.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Messina, C.; McDonald, D.; Poffenbarger, H.; Clark, R.; Salinas, A.; Fang, Y.; Gho, C.; Tang, T.; Graham, G.; Hammer, G.L.; et al. Reproductive Resilience but Not Root Architecture Underpins Yield Improvement under Drought in Maize. J. Exp. Bot. 2021, 72, 5235–5245. [Google Scholar] [CrossRef] [PubMed]
- Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Large root cortical cell size improves drought tolerance in maize. Plant Physiol. 2014, 166, 2166–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiol. 2014, 166, 1943–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, H.M.; Lor, V.S.N.; Hanlon, M.T.; Perkins, A.; Kaeppler, S.M.; Borkar, A.N.; Lynch, J.P. Root angle in maize influences nitrogen capture and is regulated by calcineurin B-like protein (CBL)-interacting serine/threonine-protein kinase 15 (ZmCIPK15). Plant Cell Environ. 2022, 45, 837–853. [Google Scholar] [CrossRef]
- Pereira, M.G.; Amaral, A.T., Jr. Estimation of Genetic Components in Popcorn Based on the Nested Design. Crop. Breed. Appl. Biotechnol. 2001, 1, 3–10. [Google Scholar] [CrossRef]
- Vieira, R.A.; Scapim, C.A.; Tessmann, D.J.; Hata, F.T. Diallel analysis of yield, popping expansion, and southern rust resistance in popcorn lines. Rev. Ciência Agronômica 2011, 42, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Moterle, L.M.; Braccini, A.D.L.; Scapim, C.A.; Pinto, R.J.B.; Gonçalves, L.S.A.; Rodrigues, R.; Do Amaral, A.T., Jr. Combining ability of popcorn lines for seed quality and agronomic traits. Euphytica 2012, 185, 337–347. [Google Scholar] [CrossRef]
- Gerhardt, I.F.S.; Do Amaral, A.T., Jr.; Pena, G.F.; Guimaraes, L.J.M.; De Lima, V.J.; Vivas, M.; Kamphorst, S.H. Genetic effects on the efficiency and responsiveness to phosphorus use in popcorn as estimated by diallel analysis. PLoS ONE 2019, 14, 5. [Google Scholar] [CrossRef]
- Liu, W.; Tollenaar, M. Physiological mechanisms underlying heterosis for shade tolerance in maize. Crop Sci. 2009, 49, 1817–1826. [Google Scholar] [CrossRef]
- Munaro, E.M.; Eyhérabide, G.H.; D’Andrea, K.E.; Cirilo, A.G.; Otegui, M.E. Heterosis × environment interaction in maize: What drives heterosis for grain yield? Field Crop. Res. 2011, 124, 441–449. [Google Scholar] [CrossRef]
- Ertiro, B.T.; Beyene, Y.; Das, B.; Mugo, S.; Olsen, M.; Oikeh, S.; Prasanna, B.M. Combining ability and testcross performance of drought-tolerant maize inbred lines under stress and non-stress environments in Kenya. Plant Breed. 2017, 136, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Makore, F.; Magorokosho, C.; Dari, S.; Gasura, E.; Mazarura, U.; Kamutando, C.N. Genetic Potential of New Maize Inbred Lines in Single-Cross Hybrid Combinations under Low-Nitrogen Stress and Optimal Conditions. Agronomy 2022, 12, 2205. [Google Scholar] [CrossRef]
- Hao, Z.; Liu, X.; Li, X.; Xie, C.; Li, M.; Zhang, D.; Zhang, S.; Xu, Y. Identification of quantitative trait loci for drought tolerance at seedling stage by screening a large number of introgression lines in maize. Plant Breed. 2009, 128, 337–341. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.; Shah, T.; Xie, C.; Hao, Z.; Li, X.; Farkhari, M.; Ribaut, J.M.; Cao, M.; Rong, T.; et al. Joint linkage-linkage disequilibrium mapping is a powerful approach to detecting quantitative trait loci underlying drought tolerance in maize. Proc. Natl. Acad. Sci. USA 2010, 107, 19585–19590. [Google Scholar] [CrossRef]
- Rahman, H.; Pekic, S.; Lazic-Jancic, V.; Quarrie, S.A.; Shah, S.M.A.; Pervez, A.; Shah, M.M. Molecular mapping of quantitative trait loci for drought tolerance in maize plants. Genet. Mol. Res. 2011, 10, 889–901. [Google Scholar] [CrossRef]
- Schwantes, I.A.; Do Amaral, A.T., Jr.; Gerhardt, I.F.S.; Vivas, M.; Silva, F.H.L.; Hamphorst, S.H. Diallel analysis of resistance to Fusarium ear rot in Brazilian popcorn genotypes. Trop. Plant Pathol. 2017, 42, 70–75. [Google Scholar] [CrossRef]
- Mafra, G.S.; Do Amaral, A.T., Jr.; Vivas, M.; Santos, J.S.; Silva, F.H.L.; Guimarães, A.G.; Pena, G.F. The combining ability of popcorn S7 lines for Puccinia polysora resistance purposes. Bragantia 2018, 77, 519–526. [Google Scholar] [CrossRef]
- Coan, M.M.D.; Pinto, R.J.B.; Kuki, M.C.; Do Amaral, A.T., Jr.; Figueiredo, A.S.T.; Scapim, C.A.; Warburton, M.L. Inheritance study for popping expansion in popcorn vs. flint corn genotypes. Agron. J. 2019, 3, 2174–2183. [Google Scholar] [CrossRef]
- Schmitt, K.F.M.; Lima, V.J.; Do Amaral, A.T., Jr.; Santos, J.S.; Mafra, G.S.; Vivas, M.; Kamphorst, S.H.; Souza, Y.P.; Oliveira, F.T.; Ferreira, F.R.A.; et al. Combining ability of popcorn lines for resistance to the fungus Puccinia polysora (Pucciniaceae). Genet. Mol. Res. 2019, 18, gmr18330. [Google Scholar] [CrossRef]
- Possatto, O., Jr.; Pinto, R.J.B.; Rossi, E.S.; Kuki, M.C.; Bertagna, F.A.B.; Santos, P.H.A.D.; Scapim, C.A. Evidence of Additive Inheritance of Popping Expansion in Popcorn. Funct. Plant Breed. J. 2021, 3, 51–59. Available online: http://fpbjournal.com/fpbj/index.php/fpbj/article/view/121 (accessed on 9 July 2023). [CrossRef]
- Ribeiro, R.M.; Do Amaral, A.T., Jr.; Pena, G.F.; Vivas, M.; Kurosawa, R.N.; Gonçalves, L.S.A. Effect of recurrent selection on the variability of the UENF-14 popcorn population. Crop Breed. Appl. Biotechnol. 2016, 16, 123–131. [Google Scholar] [CrossRef]
- Wattoo, F.M.; Saleem, M.; Sajjad, M. Identification of potential F1 hybrids in maize responsive to water deficient condition. Am. J. Plant Sci. 2014, 5, 1945–1955. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; Amaral, A.T.d., Jr.; de Lima, V.J.; Santos, P.H.A.D.; Rodrigues, W.P.; Vivas, J.M.S.; Gonçalves, G.M.B.; Schmitt, K.F.M.; Leite, J.T.; Vivas, M.; et al. Comparison of selection traits for effective popcorn (Zea mays L. var. Everta) breeding under water limiting conditions. Front. Plant Sci. 2020, 11, 1289. [Google Scholar] [CrossRef] [PubMed]
- Vittorazzi, C.; Júnior, A.T.A.; Guimarães, A.G.; Silva, F.H.L.; Pena, G.F.; Daher, R.F.; Gerhardt, I.F.S.; Oliveira, G.H.F.; Santos, P.H.A.D.; Souza, Y.P.; et al. Evaluation of Genetic Variability to Form Heterotic Groups in Popcorn. Genet. Mol. Res. 2018, 17, 18083. [Google Scholar] [CrossRef]
- Kempthorne, O.; Curnow, R.N. The partial diallel cross. Biometrics 1961, 17, 229–250. [Google Scholar] [CrossRef]
- Cruz, C.D. GENES-A software package for analysis in experimental statistics and quantitative genetics. Acta Sci. Agron. 2013, 35, 271–276. [Google Scholar] [CrossRef]



| SV | WS | WW | JA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | RE | MS | AV | SD | CVe | G | RE | MS | AV | SD | CVe | G | WC | G × WC | ||
| (df = 24) | (df = 48) | (df = 24) | (df = 48) | |||||||||||||
| Agronomic traits | GY | 1606.11 | 5.07 | ** | 1.68 | ±2.25 | 13.4 | 3505.08 | 99.56 | ** | 2.77 | ±9.98 | 11.38 | ** | ** | ** |
| PE | 40.21 | 12.69 | ** | 22.71 | ±3.56 | 15.69 | 1469.25 | 10.78 | ** | 25.16 | ±3.28 | 13.05 | ** | ** | ns | |
| PV | 846 | 74.17 | ** | 37.75 | ±8.61 | 22.81 | 2027.86 | 145.7 | ** | 68.61 | ±12.07 | 17.59 | ** | ** | ** | |
| GW | 6.34 | 0.7 | ** | 14.03 | ±0.84 | 5.95 | 7.86 | 1.51 | ** | 15.29 | ±1.23 | 8.03 | ** | ** | * | |
| EW | 22.62 | 3.47 | ** | 28 | ±1.86 | 6.65 | 27.59 | 2.56 | ** | 31.2 | ±1.60 | 5.12 | ** | ** | ns | |
| EL | 6.61 | 1.1 | ** | 10.65 | ±1.05 | 9.85 | 8.3 | 1.14 | ** | 12.2 | ±1.07 | 8.76 | ** | ** | ns | |
| Root architecture | SRA | 53.25 | 24.97 | * | 59.73 | ±5.00 | 8.37 | 69.74 | 37.42 | * | 56.93 | ±6.12 | 10.74 | ** | ** | ns |
| CRA | 47.73 | 20.11 | ** | 69.23 | ±4.48 | 6.48 | 65.96 | 47.68 | ns | 66.54 | ±6.91 | 10.38 | ** | ** | ns | |
| NSR | 2.67 | 1.43 | * | 7.4 | ±1.20 | 16.19 | 1.47 | 1.81 | ns | 7.91 | ±1.35 | 16.99 | ns | ns | ns | |
| NCR | 11.08 | 3.87 | ** | 13.76 | ±1.97 | 14.3 | 10.3 | 4.2 | ** | 15.29 | ±2.05 | 13.4 | ** | ** | ns | |
| DSR | 2.42 | 0.62 | ** | 4.73 | ±0.79 | 16.63 | 2.05 | 1.07 | * | 5.39 | ±1.03 | 19.21 | ** | ** | ns | |
| DCR | 1.02 | 0.3 | ** | 3.48 | ±0.55 | 15.76 | 1.82 | 0.71 | ** | 4.05 | ±0.84 | 20.79 | ** | * | ns | |
| SV | WS | WW | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GCA | SCA | Residual | GCA | SCA | Residual | ||||||||||||
| MS | % | MS | % | MS | % | MS | % | MS | % | MS | % | ||||||
| Agronomic traits | GY | ** | 84,352.97 | 11.58 | ** | 627,108.88 | 86.10 | 50,701.06 | 2.32 | ** | 239,258.44 | 15.13 | ** | 1,242,056.56 | 78.57 | 99,563.94 | 6.30 |
| PE | ** | 5.53 | 49.55 | ns | 1.40 | 12.54 | 12.69 | 37.91 | ** | 8.23 | 31.47 | ** | 7.14 | 27.30 | 10.78 | 41.23 | |
| PV | ** | 30.89 | 7.86 | ** | 337.51 | 85.85 | 74.17 | 6.29 | ** | 113.18 | 11.42 | ** | 732.20 | 73.88 | 145.70 | 14.70 | |
| GW | ** | 0.75 | 34.05 | ** | 1.22 | 55.39 | 0.70 | 10.56 | ** | 0.70 | 17.87 | ** | 1.71 | 43.66 | 1.51 | 38.47 | |
| EW | ** | 1.10 | 11.19 | ** | 7.57 | 77.06 | 3.47 | 11.76 | ** | 1.67 | 12.32 | ** | 9.33 | 68.82 | 2.56 | 18.86 | |
| EL | ** | 0.24852 | 8.40 | ** | 2.34 | 79.19 | 1.10 | 12.40 | ** | 0.39 | 8.84 | ** | 2.88 | 65.25 | 1.14 | 25.91 | |
| Root architecture | SRA | ** | 5.80 | 37.95 | ns | 1.16 | 7.59 | 24.97 | 54.46 | ** | 6.64 | 14.64 | ns | 1.30 | 2.87 | 37.42 | 82.50 |
| CRA | ** | 5.40 | 38.92 | ns | 1.77 | 12.76 | 20.11 | 48.32 | * | 5.08 | 9.63 | ns | −2.45 | 0.00 | 47.68 | 90.37 | |
| NSR | ns | 0.11 | 11.02 | ns | 0.41 | 41.07 | 1.43 | 47.91 | ns | 0.04 | 2.17 | ns | −0.27 | 0.00 | 1.81 | 97.83 | |
| NCR | ** | −3.10 | 0.00 | ns | 2.17 | 7.78 | 77.14 | 92.22 | ** | 1.00 | 16.54 | ns | 0.85 | 14.06 | 4.20 | 69.41 | |
| DSR | ** | 0.36 | 54.01 | ns | 0.10 | 15.00 | 0.62 | 30.99 | ** | 0.19 | 14.25 | ns | 0.07 | 5.25 | 1.07 | 80.50 | |
| DCR | ** | 0.15 | 55.54 | ns | 0.02 | 7.41 | 0.30 | 37.05 | ** | 0.13 | 4.89 | * | 0.29 | 10.91 | 2.24 | 84.20 | |
| Inbred Lines | L75 | P2 | P6 | L76 | P3 | P7 |
|---|---|---|---|---|---|---|
| L61 | X | X | X | |||
| L63 | X | X | X | |||
| L65 | X | X | X | |||
| L71 | X | X | X | |||
| L75 | X | X | ||||
| P2 | X |
| Days after Sowing | Amount of Water (mm) | ||||
|---|---|---|---|---|---|
| Rainfall (mm) | WS | WW | |||
| Irrigation (mm) | Total | Irrigation (mm) | Total | ||
| 1 | 0.00 | 2.70 | 2.70 | 2.60 | 2.60 |
| 7 | 17.00 | 3.50 | 20.50 | 3.60 | 20.60 |
| 14 | 6.00 | 10.20 | 16.20 | 11.00 | 17.00 |
| 21 | 0.00 | 9.90 | 9.90 | 10.10 | 10.10 |
| 28 | 10.60 | 10.30 | 20.90 | 10.70 | 21.30 |
| 35 | 5.20 | 8.40 | 13.60 | 8.40 | 13.60 |
| 42 | 2.00 | 12.20 | 14.20 | 11.60 | 13.60 |
| 49 | 0.00 | 12.10 | 12.10 | 12.90 | 12.90 |
| 56 | 0.00 | - | 0.00 | 10.90 | 10.90 |
| 63 | 0.00 | - | 0.00 | 18.80 | 18.80 |
| 70 | 0.00 | - | 0.00 | 18.90 | 18.90 |
| 77 | 30.80 | - | 30.80 | 1.10 | 31.90 |
| 84 | 0.00 | - | 0.00 | 16.70 | 16.70 |
| 91 | 0.00 | - | 0.00 | 14.00 | 14.00 |
| 98 | 65.00 | - | 65.00 | 2.00 | 67.00 |
| 105 | 0.00 | - | 0.00 | 13.50 | 13.50 |
| 112 | 9.20 | - | 9.20 | 10.00 | 19.20 |
| 119 | 2.40 | - | 2.40 | 10.00 | 12.40 |
| Final Total | 148.20 mm | 69.30 mm | 217.50 mm | 186.0 mm | 335.10 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bispo, R.B.; Junior, A.T.d.A.; Kamphorst, S.H.; Lima, V.J.d.; Pena, G.F.; Santos, T.d.O.; Leite, J.T.; Viana, F.N.; Júnior, D.R.d.S.; Lamêgo, D.L.; et al. Assessing Combining Abilities of Popcorn Inbred Lines for Agronomic and Root Traits under Contrasting Water Conditions: Towards Developing Drought-Tolerant Genotypes. Stresses 2023, 3, 586-604. https://doi.org/10.3390/stresses3030041
Bispo RB, Junior ATdA, Kamphorst SH, Lima VJd, Pena GF, Santos TdO, Leite JT, Viana FN, Júnior DRdS, Lamêgo DL, et al. Assessing Combining Abilities of Popcorn Inbred Lines for Agronomic and Root Traits under Contrasting Water Conditions: Towards Developing Drought-Tolerant Genotypes. Stresses. 2023; 3(3):586-604. https://doi.org/10.3390/stresses3030041
Chicago/Turabian StyleBispo, Rosimeire Barboza, Antônio Teixeira do Amaral Junior, Samuel Henrique Kamphorst, Valter Jário de Lima, Guilherme Ferreira Pena, Talles de Oliveira Santos, Jhean Torres Leite, Flávia Nicácio Viana, Divino Rosa dos Santos Júnior, Danielle Leal Lamêgo, and et al. 2023. "Assessing Combining Abilities of Popcorn Inbred Lines for Agronomic and Root Traits under Contrasting Water Conditions: Towards Developing Drought-Tolerant Genotypes" Stresses 3, no. 3: 586-604. https://doi.org/10.3390/stresses3030041
APA StyleBispo, R. B., Junior, A. T. d. A., Kamphorst, S. H., Lima, V. J. d., Pena, G. F., Santos, T. d. O., Leite, J. T., Viana, F. N., Júnior, D. R. d. S., Lamêgo, D. L., Oliveira, U. A. d., Ribeiro, R. M., Pereira, T. N. S., & Khan, S. (2023). Assessing Combining Abilities of Popcorn Inbred Lines for Agronomic and Root Traits under Contrasting Water Conditions: Towards Developing Drought-Tolerant Genotypes. Stresses, 3(3), 586-604. https://doi.org/10.3390/stresses3030041













