Productivity and Nutrient Quality of Lemna minor as Affected by Microbiome, CO2 Level, and Nutrient Supply
Abstract
1. Introduction
2. Results and Discussion
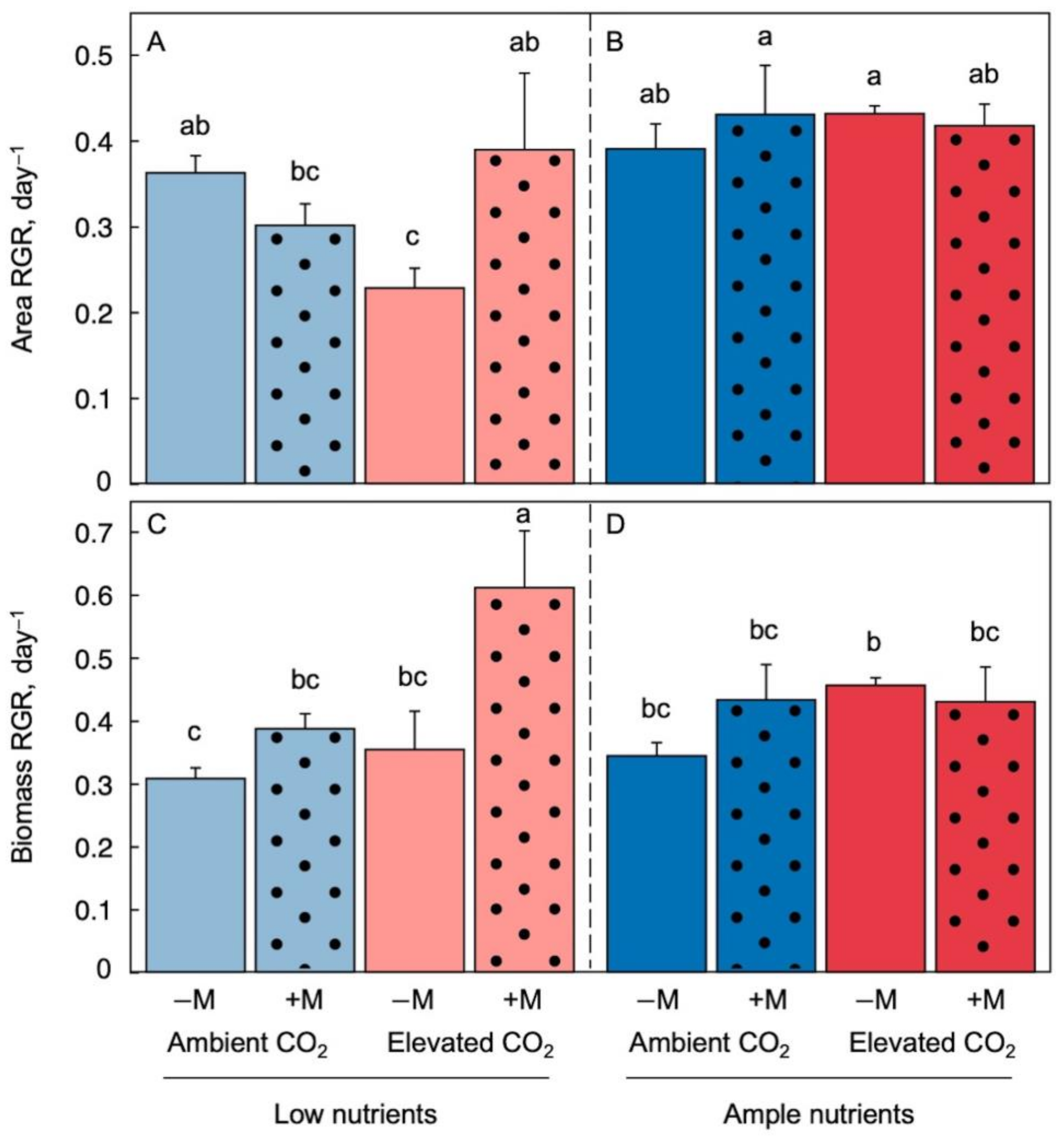
2.1. Effects of Growth Environment and Inoculation on Plant Growth Rate
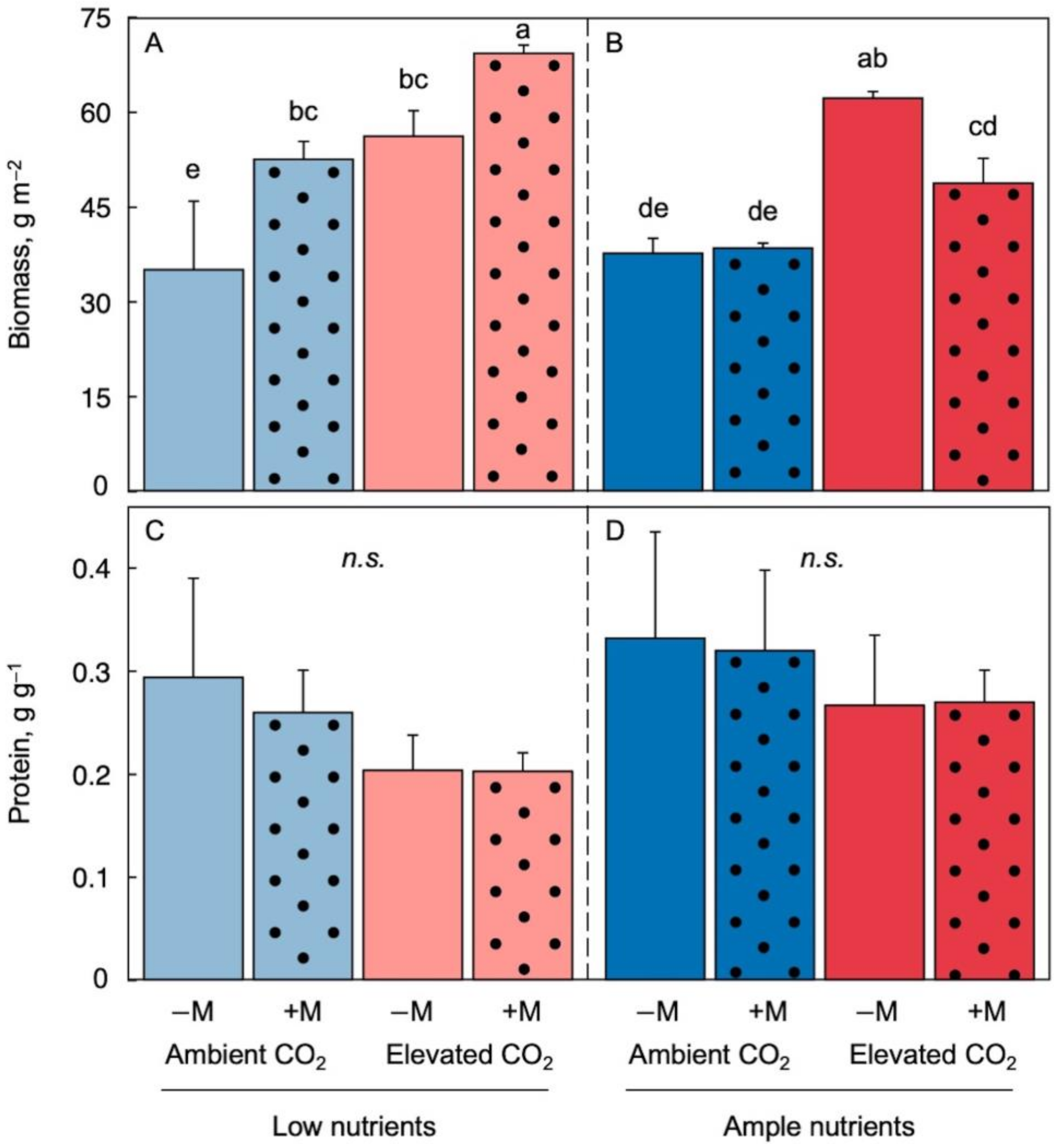
2.2. Effect of Growth Environment and Inoculation on Biomass per Area and Protein per Biomass
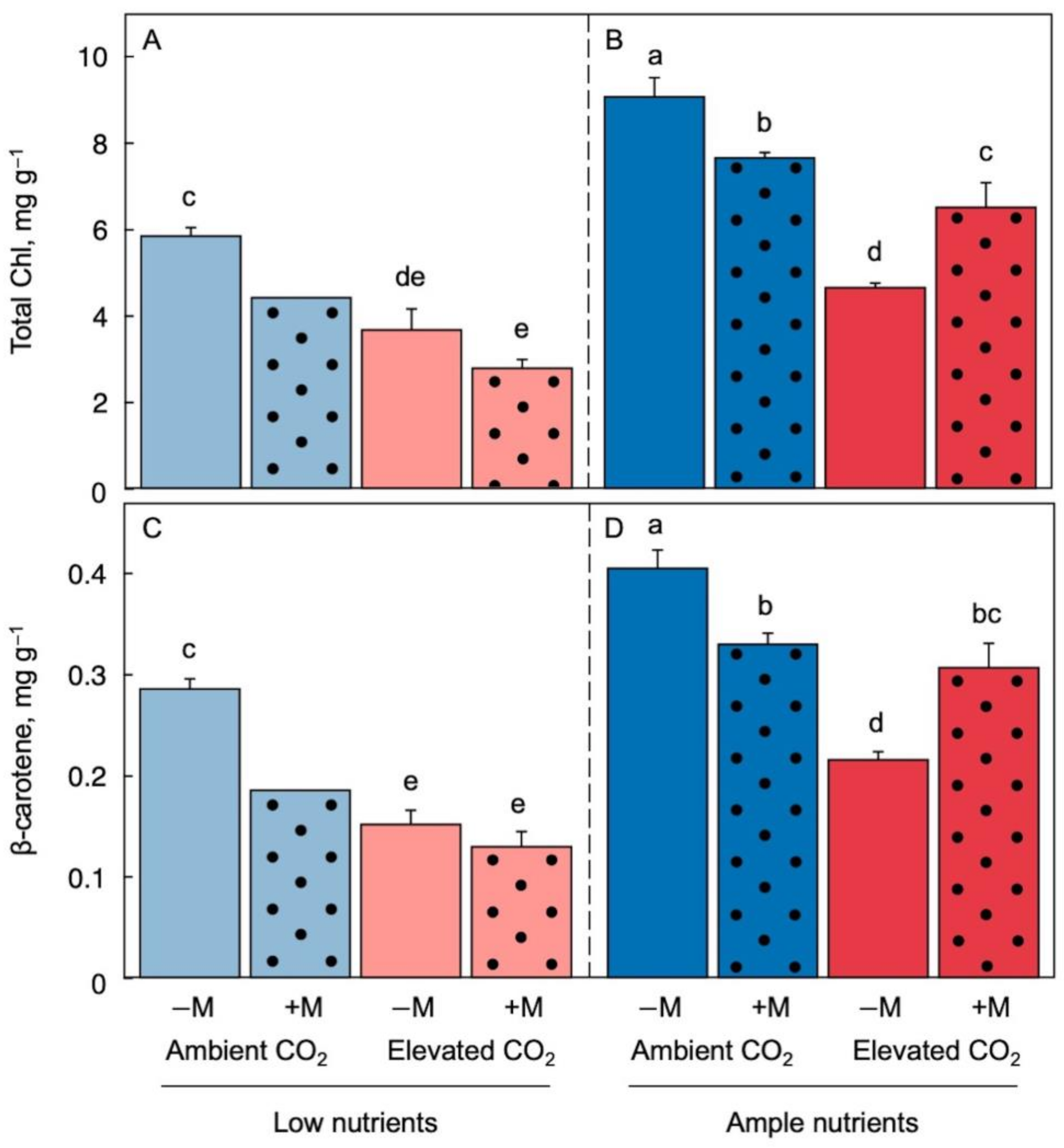
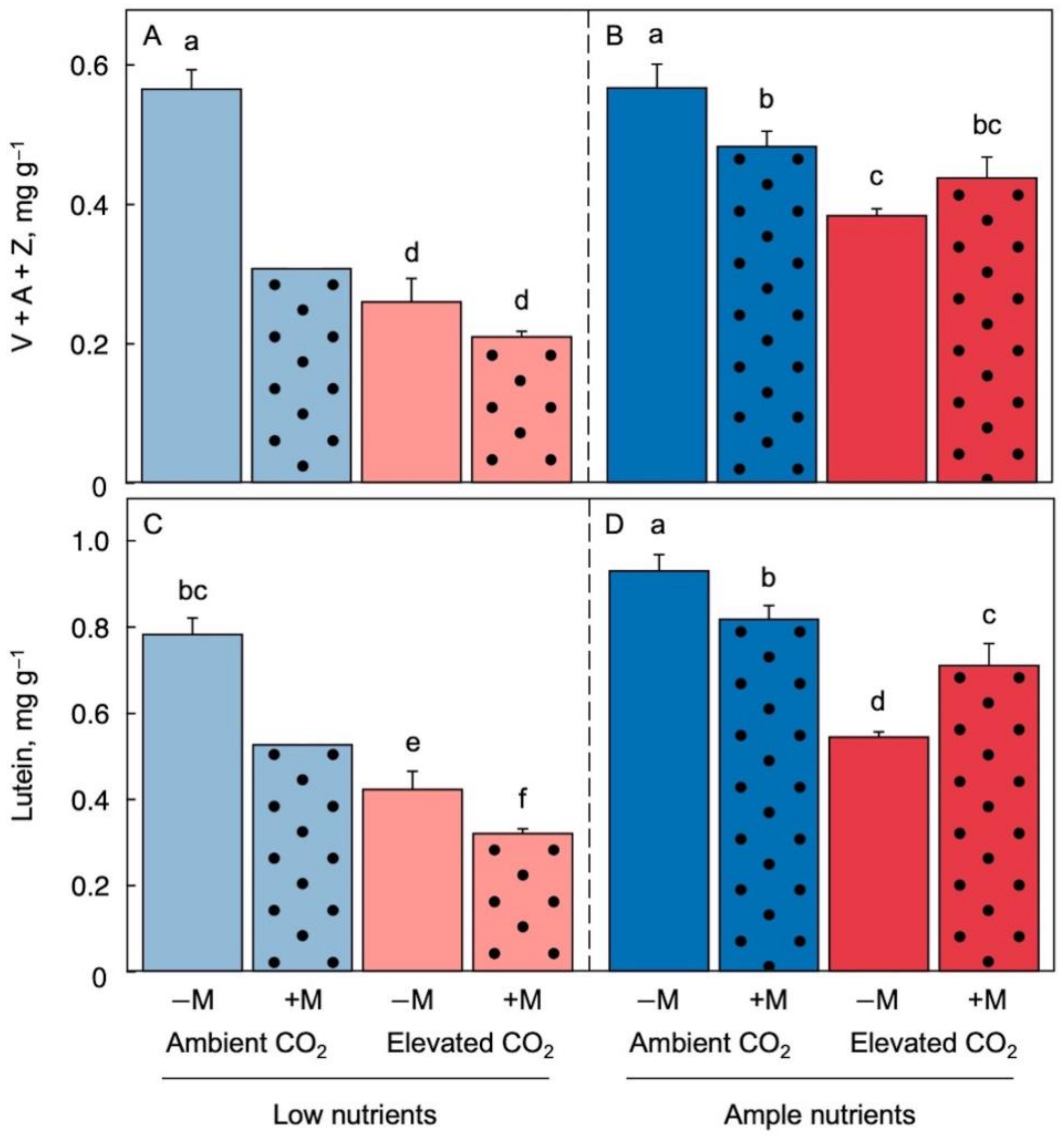
2.3. Effect of Growth Environment and Inoculation on Chlorophyll and Carotenoid Micronutrients
3. Materials and Methods
3.1. Plant Species and Growth Conditions
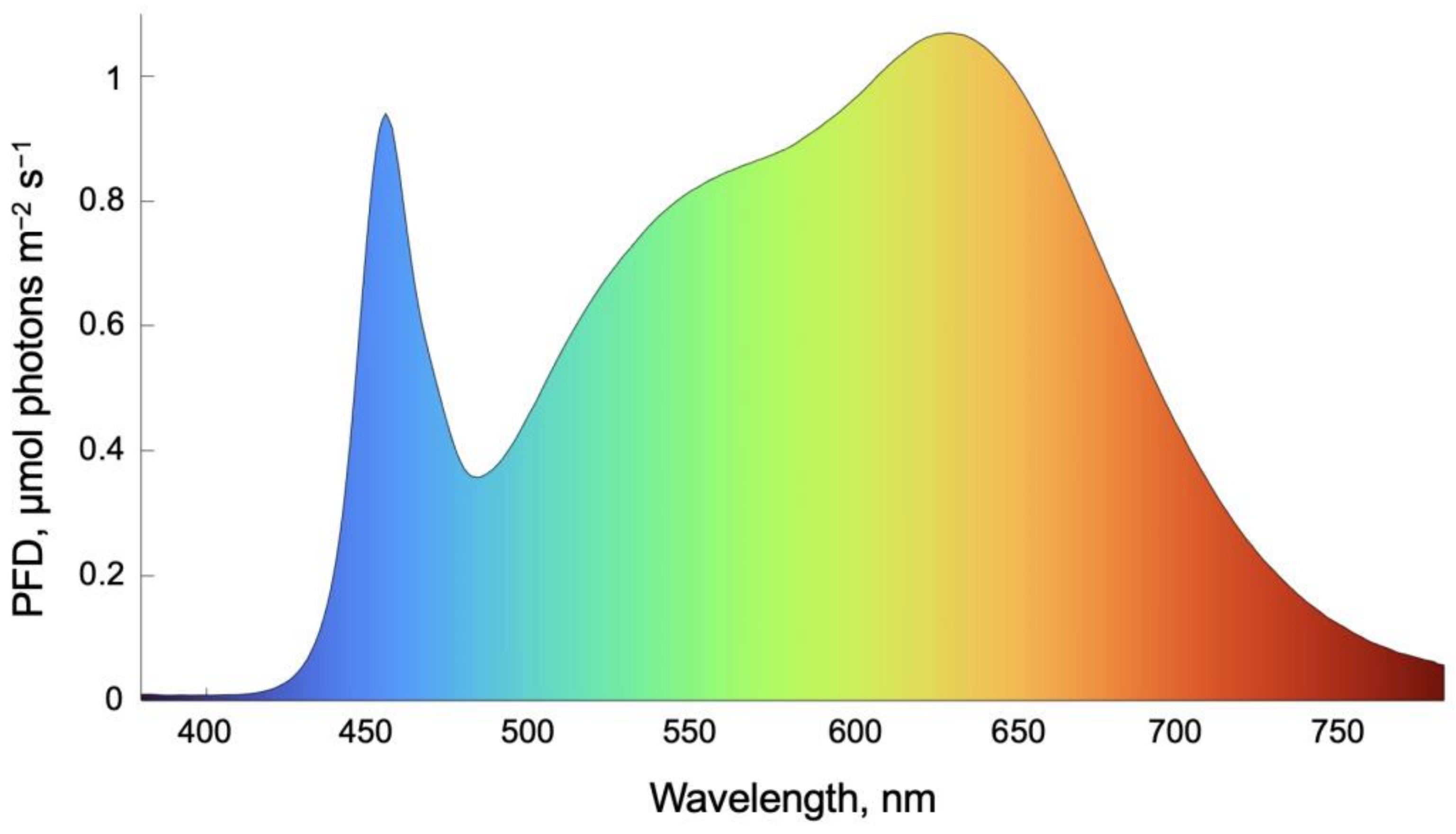
3.2. Light and CO2 Supply for Plant Growth
3.3. Inoculation Treatment
3.4. Protein Extraction and Analysis
3.5. Dry Frond Mass and Frond Area
3.6. Relative Growth Rate
3.7. Pigment Extraction and Analysis
3.8. Statistical Analysis
4. Conclusions and Recommendations
4.1. Costs and Benefits of Plant-Microbiome Interaction at a Glance
4.2. Future Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef] [PubMed]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Change Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.R.; Ro-Poulsen, H.; Mikkelsen, T.N.; Michelsen, A.; van der Linden, L.; Beier, C. Interactive effects of elevated CO2, warming, and drought on photosynthesis of Deschampsia flexuosa in a temperate heath ecosystem. J. Exp. Bot. 2011, 62, 4253–4266. [Google Scholar] [CrossRef] [PubMed]
- Agüera, E.; De la Haba, P. Leaf senescence in response to elevated atmospheric CO2 concentration and low nitrogen supply. Biol. Plant 2018, 62, 401–408. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of Elevated CO2 on Nutritional Quality of Vegetables: A Review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef]
- Dhankher, O.P.; Foyer, C.H. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef]
- Taub, D.R.; Miller, B.; Allen, H. Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Glob. Change Biol. 2008, 14, 565–575. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Fang, Y.; Huang, M.; Chen, X.; Zhang, Y.; Zhao, H. The effects of photoperiod and nutrition on duckweed (Landoltia punctata) growth and starch accumulation. Ind. Crops Prod. 2018, 115, 243–249. [Google Scholar] [CrossRef]
- Joy, K.W. Carbon and nitrogen sources for protein synthesis and growth of sugar-beet leaves. J. Exp. Bot. 1967, 18, 140–150. [Google Scholar] [CrossRef]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef]
- Cruz, J.L.; Mosquim, P.R.; Pelacani, C.R.; Araujo, W.L.; DaMatta, F.M. Carbon Partitioning and Assimilation as Affected by Nitrogen Deficiency in Cassava. Photosynthetica 2003, 41, 201–207. [Google Scholar] [CrossRef]
- Araya, T.; Noguchi, K.; Terashima, I. Effect of nitrogen nutrition on the carbohydrate repression of photosynthesis in leaves of Phaseolus vulgaris L. J. Plant Res. 2010, 123, 371–379. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Nelson, R.; Long, S.P. Testing the “source–sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric. For. Meteorol. 2004, 122, 85–94. [Google Scholar] [CrossRef]
- Padhan, B.K.; Sathee, L.; Meena, H.S.; Adavi, S.B.; Jha, S.K.; Chinnusamy, V. CO2 Elevation Accelerates Phenology and Alters Carbon/Nitrogen Metabolism Vis-à-Vis ROS Abundance in Bread Wheat. Front. Plant Sci. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Adavi, S.B.; Sathee, L. Elevated CO2 alters tissue balance of nitrogen metabolism and downregulates nitrogen assimilation and signalling gene expression in wheat seedlings receiving high nitrate supply. Protoplasma 2021, 258, 219–233. [Google Scholar] [CrossRef]
- Wingler, A.; Purdy, S.; MacLean, J.A.; Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J. Exp. Bot. 2006, 57, 391–399. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Polutchko, S.K.; Fourounjian, P.; Stewart, J.J.; Zenir, M.C.; Adams, W.W., III. Growth and Nutritional Quality of Lemnaceae Viewed Comparatively in an Ecological and Evolutionary Context. Plants 2022, 11, 145. [Google Scholar] [CrossRef]
- Krapp, A.; Stitt, M. An evaluation of direct and indirect mechanisms for the ”sink-regulation” of photosynthesis in spinach: Changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 1995, 195, 313–323. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Murata, N. Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol. Biotechnol. 2014, 98, 8777–8796. [Google Scholar] [CrossRef]
- Faria, T.; Wilkins, D.; Besford, R.T.; Vaz, M.; Pereira, J.S.; Chaves, M.M. Growth at elevated CO2 leads to down-regulation of photosynthesis and altered response to high temperature in Quercus suber L. seedlings. J. Exp. Bot. 1996, 47, 1755–1761. [Google Scholar] [CrossRef]
- Tausz-Posch, S.; Tausz, M.; Bourgault, M. Elevated [CO2] effects on crops: Advances in understanding acclimation, nitrogen dynamics and interactions with drought and other organisms. Plant Biol. 2020, 22, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.Y.; Long, S.P.; Garcia, R.L.; Kimball, B.A.; Lamorte, R.L.; Pinter, P.J.; Wall, G.W.; Webber, A.N. Effects of free-air CO2 enrichment on the development of the photosynthetic apparatus in wheat, as indicated by changes in leaf proteins. Plant Cell Environ. 1995, 18, 855–864. [Google Scholar] [CrossRef]
- Bahrami, H.; De Kok, L.J.; Armstrong, R.; Fitzgerald, G.J.; Bourgault, M.; Henty, S.; Tausz, M.; Tausz-Posch, S. The proportion of nitrate in leaf nitrogen, but not changes in root growth, are associated with decreased grain protein in wheat under elevated [CO2]. J. Plant Physiol. 2017, 216, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Karthikeyapandian, V.; Jain, V.; Srivastava, A.C.; Raj, A.; Sengupta, U.K. Biomass production and nutritional levels of berseem (Trifolium alexandrium) grown under elevated CO2. Agric. Ecosyst. Environ. 2004, 101, 31–38. [Google Scholar] [CrossRef]
- Paul, M.J.; Foyer, C.H. Sink regulation of photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef]
- Gojon, A.; Cassan, O.; Bach, L.; Lejay, L.; Martin, A. The decline of plant mineral nutrition under rising CO2: Physiological and molecular aspects of a bad deal. Trends Plant Sci. 2022. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Polutchko, S.K.; Adams, W.W., III. Structure-Function-Environment Relationship of the Isomers Zeaxanthin and Lutein. Photochem 2022, 2, 308–325. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and Lutein: Photoprotectors, Anti-Inflammatories, and Brain Food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Adams, W.W., III; Escobar, C.M.; Demmig-Adams, B. Conquering Space with Crops That Produce Ample Oxygen and Antioxidants. Oxygen 2022, 2, 211–226. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Glime, G.N.E.; Demmig-Adams, B. Synergistic Action of Membrane-Bound and Water-Soluble Antioxidants in Neuroprotection. Molecules 2021, 26, 5385. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Cazzonelli, C.I. Environmental impacts on carotenoid metabolism in leaves. Plant Growth Regul 2020, 92, 455–477. [Google Scholar] [CrossRef]
- Nie, M.; Bell, C.; Wallenstein, M.D.; Pendall, E. Increased plant productivity and decreased microbial respiratory C loss by plant growth-promoting rhizobacteria under elevated CO2. Sci. Rep. 2015, 5, 9212. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Polutchko, S.K.; Zenir, M.C.; Fourounjian, P.; Stewart, J.J.; López-Pozo, M.; Adams, W.W., III. Intersections: Photosynthesis, abiotic stress, and the plant microbiome. Photosynthetica 2022, 60, 59–69. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Goicoechea, N.; Antolín, M.C.; Pascual, I.; Sánchez-Díaz, M.; Aguirreolea, J.; Morales, F. Growth, photosynthetic acclimation and yield quality in legumes under climate change simulations: An updated survey. Plant Sci. 2014, 226, 22–29. [Google Scholar] [CrossRef]
- O’Brien, A.M.; Yu, Z.H.; Luo, D.; Laurich, J.; Passeport, E.; Frederickson, M.E. Resilience to multiple stressors in an aquatic plant and its microbiome. Am. J. Bot. 2020, 107, 273–285. [Google Scholar] [CrossRef]
- O’Brien, A.M.; Laurich, J.; Lash, E.; Frederickson, M.E. Mutualistic Outcomes Across Plant Populations, Microbes, and Environments in the Duckweed Lemna minor. Microb. Ecol. 2020, 80, 384–397. [Google Scholar] [CrossRef]
- Moreau, D.; Pivato, B.; Bru, D.; Busset, H.; Deau, F.; Faivre, C.; Matejicek, A.; Strbik, F.; Philippot, L.; Mougel, C. Plant traits related to nitrogen uptake influence plant-microbe competition. Ecology 2015, 96, 2300–2310. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Adams, W.W., III; Stewart, J.J.; Demmig-Adams, B. Photosynthetic Modulation in Response to Plant Activity and Environment. In The Leaf: A Platform for Performing Photosynthesis; Adams, W.W., III, Terashima, I., Eds.; Advances in Photosynthesis and Respiration; Springer International Publishing: Cham, Switzerland, 2018; pp. 493–563. ISBN 978-3-319-93594-2. [Google Scholar]
- Yamakawa, Y.; Jog, R.; Morikawa, M. Effects of co-inoculation of two different plant growth-promoting bacteria on duckweed. Plant Growth Regul. 2018, 86, 287–296. [Google Scholar] [CrossRef]
- Ishizawa, H.; Kuroda, M.; Morikawa, M.; Ike, M. Evaluation of environmental bacterial communities as a factor affecting the growth of duckweed Lemna minor. Biotechnol. Biofuels 2017, 10, 62. [Google Scholar] [CrossRef]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M.; Hritcu, L. Seed inoculation with plant growth promoting rhizobacteria enhances photosynthesis and yield of runner bean (Phaseolus coccineus L.). Sci. Hortic. 2013, 151, 22–29. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Asha, A.D.; Nivetha, N.; Krishna, G.K.; Thakur, J.K.; Rathi, M.S.; Manjunatha, B.S.; Chinnusamy, V.; Paul, S. Amelioration of short-term drought stress during different growth stages in Brassica juncea by rhizobacteria mediated maintenance of ROS homeostasis. Physiol. Plant. 2021, 172, 1880–1893. [Google Scholar] [CrossRef]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.K.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a model plant system in the genomics and postgenomics era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef]
- Cedergreen, N.; Madsen, T.V. Nitrogen uptake by the floating macrophyte Lemna minor. New Phytol. 2002, 155, 285–292. [Google Scholar] [CrossRef]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.-J. Relative in vitro growth rates of duckweeds (Lemnaceae)—the most rapidly growing higher plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef]
- Liu, C.; Dai, Z.; Sun, H. Potential of duckweed (Lemna minor) for removal of nitrogen and phosphorus from water under salt stress. J. Environ. Manag. 2017, 187, 497–503. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef]
- Rusoff, L.L.; Blakeney, E.W.; Culley, D.D. Duckweeds (Lemnaceae family): A potential source of protein and amino acids. J. Agric. Food Chem. 1980, 28, 848–850. [Google Scholar] [CrossRef]
- Mohedano, R.A.; Costa, R.H.R.; Tavares, F.A.; Belli Filho, P. High nutrient removal rate from swine wastes and protein biomass production by full-scale duckweed ponds. Bioresour. Technol. 2012, 112, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; Lopez-Pozo, M.; Demmig-Adams, B. Growth and Essential Carotenoid Micronutrients in Lemna gibba as a Function of Growth Light Intensity. Front. Plant Sci. 2020, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.J.; Stomp, A.-M. Growing Duckweed to Recover Nutrients from Wastewaters and for Production of Fuel Ethanol and Animal Feed. CLEAN Soil Air Water 2009, 37, 17–26. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, H.; Liu, Y.; Zhao, H.; Su, H.; Wang, M.; Zhao, Y. The influence of duckweed species diversity on biomass productivity and nutrient removal efficiency in swine wastewater. Bioresour. Technol. 2014, 167, 383–389. [Google Scholar] [CrossRef]
- Sońta, M.; Rekiel, A.; Batorska, M. Use of Duckweed (Lemna L.) in Sustainable Livestock Production and Aquaculture—A Review. Ann. Anim. Sci. 2019, 19, 257–271. [Google Scholar] [CrossRef]
- Al-Khafaji, M.S.; Al-Ani, F.H.; Ibrahim, A.F. Removal of Some Heavy Metals from Industrial Wastewater by Lemmna minor. KSCE J. Civ. Eng. 2018, 22, 1077–1082. [Google Scholar] [CrossRef]
- Torbati, S. Toxicological risks of Acid Bordeaux B on duckweed and the plant potential for effective remediation of dye-polluted waters. Environ. Sci. Pollut. Res. 2019, 26, 27699–27711. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S. Synchronized urban wastewater treatment and biomass production using duckweed Lemna gibba L. Ecol. Eng. 2014, 64, 337–343. [Google Scholar] [CrossRef]
- Saha, P.; Banerjee, A.; Sarkar, S. Phytoremediation Potential of Duckweed (Lemna minor L.) On Steel Wastewater. Int. J. Phytoremediat. 2015, 17, 589–596. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, M.; Tian, Y.; Zhao, M.; Zhang, B.; Zhao, M.; Zeng, K.; Yin, B. Duckweed (Spirodela polyrhiza) as green manure for increasing yield and reducing nitrogen loss in rice production. Field Crops Res. 2017, 214, 273–282. [Google Scholar] [CrossRef]
- Kittiwongwattana, C.; Thawai, C. 2014 Rhizobium lemnae sp. nov., a bacterial endophyte of Lemna aequinoctialis. Int. J. Syst. Evol. Microbiol. 2014, 64, 2455–2460. [Google Scholar] [CrossRef]
- Toyama, T.; Kuroda, M.; Ogata, Y.; Hachiya, Y.; Quach, A.; Tokura, K.; Tanaka, Y.; Mori, K.; Morikawa, M.; Ike, M. Enhanced biomass production of duckweeds by inoculating a plant growth-promoting bacterium, Acinetobacter calcoaceticus P23, in sterile medium and non-sterile environmental waters. Water Sci. Technol. 2017, 76, 1418–1428. [Google Scholar] [CrossRef]
- Yamaga, F.; Washio, K.; Morikawa, M. Sustainable Biodegradation of Phenol by Acinetobacter calcoaceticus P23 Isolated from the Rhizosphere of Duckweed Lemna aoukikusa. Environ. Sci. Technol. 2010, 44, 6470–6474. [Google Scholar] [CrossRef]
- Jäger, T.; Scherr, C.; Simon, M.; Heusser, P.; Baumgartner, S. Effects of Homeopathic Arsenicum Album, Nosode, and Gibberellic Acid Preparations on the Growth Rate of Arsenic-Impaired Duckweed (Lemna gibba L.). Sci. World J. 2010, 10, 2112–2129. [Google Scholar] [CrossRef]
- Iatrou, E.I.; Kora, E.; Stasinakis, A.S. Investigation of biomass production, crude protein and starch content in laboratory wastewater treatment systems planted with Lemna minor and Lemna gibba. Environ. Technol. 2019, 40, 2649–2656. [Google Scholar] [CrossRef]
- Ishizawa, H.; Ogata, Y.; Hachiya, Y.; Tokura, K.; Kuroda, M.; Inoue, D.; Toyama, T.; Tanaka, Y.; Mori, K.; Morikawa, M.; et al. Enhanced biomass production and nutrient removal capacity of duckweed via two-step cultivation process with a plant growth-promoting bacterium, Acinetobacter calcoaceticus P23. Chemosphere 2020, 238, 124682. [Google Scholar] [CrossRef]
- Rho, H.; Doty, S.L.; Kim, S.-H. Endophytes alleviate the elevated CO2-dependent decrease in photosynthesis in rice, particularly under nitrogen limitation. J. Exp. Bot. 2020, 71, 707–718. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, S.; Liu, F. Arbuscular mycorrhiza improve growth, nitrogen uptake, and nitrogen use efficiency in wheat grown under elevated CO2. Mycorrhiza 2016, 26, 133–140. [Google Scholar] [CrossRef]
- Burgner, S.E.; Nemali, K.; Massa, G.D.; Wheeler, R.M.; Morrow, R.C.; Mitchell, C.A. Growth and photosynthetic responses of Chinese cabbage (Brassica rapa L. cv. Tokyo Bekana) to continuously elevated carbon dioxide in a simulated Space Station “Veggie” crop-production environment. Life Sci. Space Res. 2020, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Khairina, Y.; Jog, R.; Boonmak, C.; Toyama, T.; Oyama, T.; Morikawa, M. Indigenous bacteria, an excellent reservoir of functional plant growth promoters for enhancing duckweed biomass yield on site. Chemosphere 2021, 268, 129247. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H. Effects of nitrogen: Phosphorus supply ratios on nitrogen fixation in agricultural and pastoral ecosystems. Biogeochemistry 1992, 18, 19–35. [Google Scholar] [CrossRef]
- Zheng, M.; Li, D.; Lu, X.; Zhu, X.; Zhang, W.; Huang, J.; Fu, S.; Lu, X.; Mo, J. Effects of phosphorus addition with and without nitrogen addition on biological nitrogen fixation in tropical legume and non-legume tree plantations. Biogeochemistry 2016, 131, 65–76. [Google Scholar] [CrossRef]
- O’Sullivan, J.B.; Jin, J.; Tang, C. White lupin (Lupinus albus L.) exposed to elevated atmospheric CO2 requires additional phosphorus for N2 fixation. Plant Soil 2022, 476, 477–490. [Google Scholar] [CrossRef]
- Moore, B.D.; Cheng, S.-H.; Sims, D.; Seemann, J.R. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ. 1999, 22, 567–582. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.A.; Morris, C.F.; Pinter, P.J., Jr.; Wall, G.W.; Hunsaker, D.J.; Adamsen, F.J.; LaMorte, R.L.; Leavitt, S.W.; Thompson, T.L.; Matthias, A.D. Elevated CO2, drought and soil nitrogen effects on wheat grain quality. New Phytol. 2001, 150, 295–303. [Google Scholar] [CrossRef]
- Fangmeier, A.; Grüters, U.; Högy, P.; Vermehren, B.; Jäger, H.-J. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat—II. Nutrients (N, P, K, S, Ca, Mg, Fe, Mn, Zn). Environ. Pollut. 1997, 96, 43–59. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Wheeler, R.M.; Fitzpatrick, A.H.; Tibbitts, T.W. Potatoes as a Crop for Space Life Support: Effect of CO2, Irradiance, and Photoperiod on Leaf Photosynthesis and Stomatal Conductance. Front. Plant Sci. 2019, 10, 1632. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-Microbe Interactions Facing Environmental Challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef]
- Landolt, E. Morphological differentiation and geographical distribution of the Lemna gibba-lemna minor group. Aquat. Bot. 1975, 1, 345–363. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams, W.W., III; López-Pozo, M.; Doherty Garcia, N.; McNamara, M.; Escobar, C.M.; Demmig-Adams, B. Features of the Duckweed Lemna That Support Rapid Growth under Extremes of Light Intensity. Cells 2021, 10, 1481. [Google Scholar] [CrossRef]
- Lindeboom, N.; Wanasundara, P.K.J.P.D. Interference of phenolic compounds in Brassica napus, Brassica rapa and Sinapis alba seed extracts with the Lowry protein assay. Food Chem. 2007, 104, 30–38. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein Measurement With the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Hunt, R. Relative growth rates. In Basic Growth Analysis: Plant Growth Analysis for Beginners; Hunt, R., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1990; pp. 25–34. ISBN 978-94-010-9117-6. [Google Scholar]
- Stewart, J.J.; Adams, W.W., III; Cohu, C.M.; Polutchko, S.K.; Lombardi, E.M.; Demmig-Adams, B. Differences in light-harvesting, acclimation to growth-light environment, and leaf structural development between Swedish and Italian ecotypes of Arabidopsis thaliana. Planta 2015, 242, 1277–1290. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B. Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident sunlight. Planta 1992, 186, 390–398. [Google Scholar] [CrossRef]
- Gilmore, A.M.; Yamamoto, H.Y. Resolution of lutein and zeaxanthin using a non-endcapped, lightly carbon-loaded C18 high-performance liquid chromatographic column. J. Chromatogr. A 1991, 543, 137–145. [Google Scholar] [CrossRef]
- Langley, J.A.; Megonigal, J.P. Ecosystem response to elevated CO2 levels limited by nitrogen-induced plant species shift. Nature 2010, 466, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Ju, W.; Chen, J.M.; Ciais, P.; Cescatti, A.; Sardans, J.; Janssens, I.A.; Wu, M.; Berry, J.A.; et al. Recent global decline of CO2 fertilization effects on vegetation photosynthesis. Science 2020, 370, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Mattupalli, C.; Eversole, K.; Leach, J.E. Enabling sustainable agriculture through understanding and enhancement of microbiomes. New Phytol. 2021, 230, 2129–2147. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenir, M.C.; López-Pozo, M.; Polutchko, S.K.; Stewart, J.J.; Adams, W.W., III; Escobar, A.; Demmig-Adams, B. Productivity and Nutrient Quality of Lemna minor as Affected by Microbiome, CO2 Level, and Nutrient Supply. Stresses 2023, 3, 69-85. https://doi.org/10.3390/stresses3010007
Zenir MC, López-Pozo M, Polutchko SK, Stewart JJ, Adams WW III, Escobar A, Demmig-Adams B. Productivity and Nutrient Quality of Lemna minor as Affected by Microbiome, CO2 Level, and Nutrient Supply. Stresses. 2023; 3(1):69-85. https://doi.org/10.3390/stresses3010007
Chicago/Turabian StyleZenir, Madeleine C., Marina López-Pozo, Stephanie K. Polutchko, Jared J. Stewart, William W. Adams, III, Adam Escobar, and Barbara Demmig-Adams. 2023. "Productivity and Nutrient Quality of Lemna minor as Affected by Microbiome, CO2 Level, and Nutrient Supply" Stresses 3, no. 1: 69-85. https://doi.org/10.3390/stresses3010007
APA StyleZenir, M. C., López-Pozo, M., Polutchko, S. K., Stewart, J. J., Adams, W. W., III, Escobar, A., & Demmig-Adams, B. (2023). Productivity and Nutrient Quality of Lemna minor as Affected by Microbiome, CO2 Level, and Nutrient Supply. Stresses, 3(1), 69-85. https://doi.org/10.3390/stresses3010007








