Conceptualizing Multiple Stressors and Their Consequences in Agroforestry Systems
Abstract
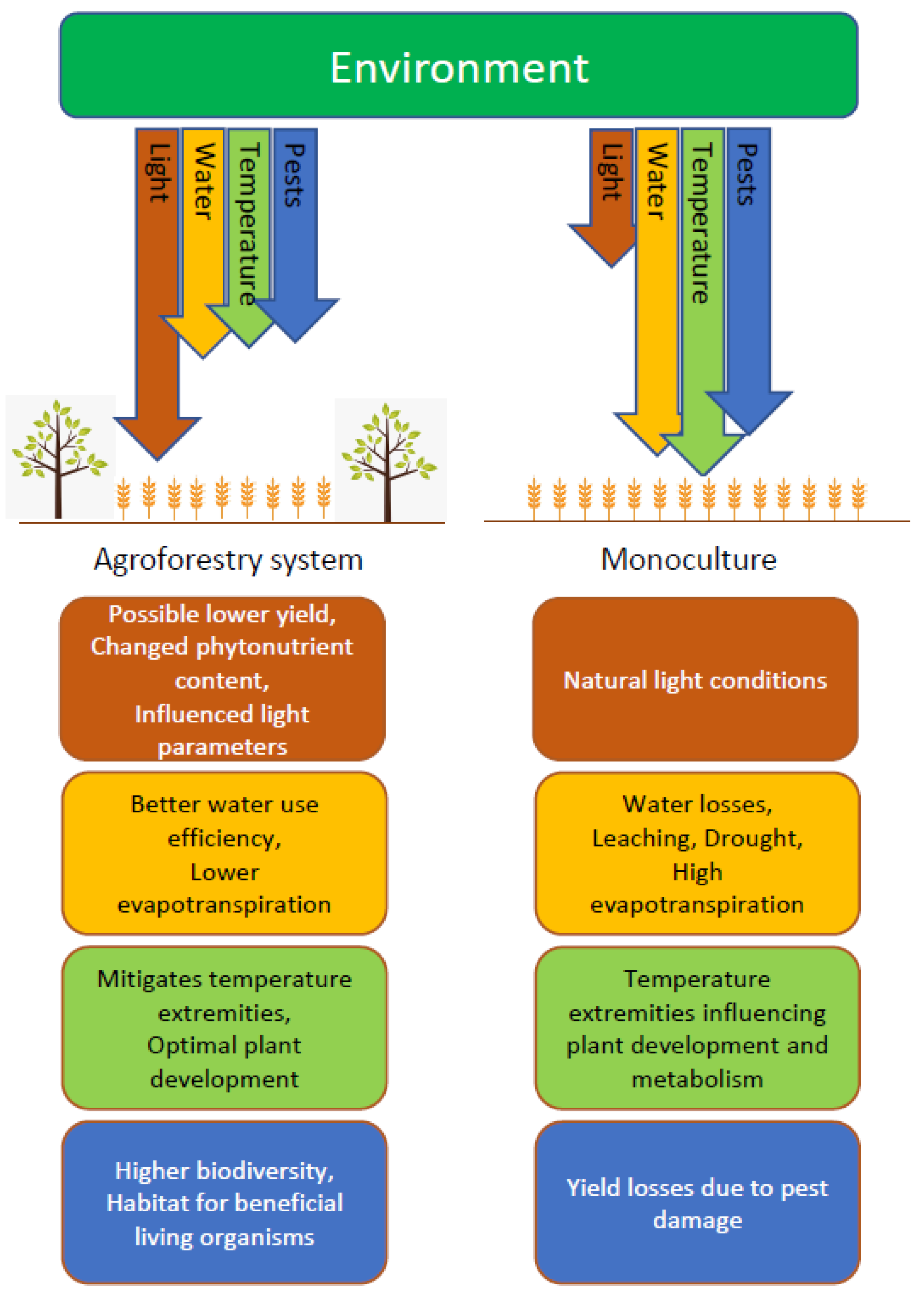
1. Introduction
2. Materials and Methods
3. Light Stress Consequences
4. Temperature Stress Consequences
5. Soil Stress Consequences
6. Water Stress Consequences
7. Salt Stress Consequences
8. Pest and Disease Stress Consequences
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Boisvenue, C.; Running, S.W. Impacts of climate change on natural forest productivity-evidence since the middle of the 20th century. Glob Change Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Karvatte, N.; Miyagi, E.S.; de Oliveira, C.C.; Barreto, C.D.; Mastelaro, A.P.; Bungenstab, D.J.; Alves, F.V. Infrared thermography for microclimate assessment in agroforestry systems. Sci. Total Environ. 2020, 731, 139252. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Rocchini, D.; Coomes, D. Advances in Microclimate Ecology Arising from Remote Sensing. Trends Ecol. Evol. 2019, 34, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Bramer, I.; Anderson, B.J.; Bennie, J.; Bladon, A.J.; De Frenne, P.; Hemming, D.; Hill, R.A.; Kearney, M.R.; Körner, C.; Korstjens, A.H.; et al. Advances in Monitoring and Modelling Climate at Ecologically Relevant Scales. Adv. Ecol. Res. 2018, 58, 101–161. [Google Scholar] [CrossRef]
- Menzel, C.M. Effect of Temperature on Soluble Solids Content in Strawberry in Queensland, Australia. Horticulturae 2022, 8, 367. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 2008, 133, 682–691. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Reynolds, P.E.; Simpson, J.A.; Thevathasan, N.V.; Gordon, A.M. Effects of tree competition on corn and soybean photosynthesis, growth, and yield in a temperate tree-based agroforestry intercropping system in southern Ontario, Canada. Ecol. Eng. 2007, 29, 362–371. [Google Scholar] [CrossRef]
- Gao, L.; Xu, H.; Bi, H.; Xi, W.; Bao, B.; Wang, X.; Bi, C.; Chang, Y. Intercropping Competition between Apple Trees and Crops in Agroforestry Systems on the Loess Plateau of China. PLoS ONE 2013, 8, e70739. [Google Scholar] [CrossRef]
- Sanna, F.; Re, G.A.; Piluzza, G.; Campesi, G.; Sulas, L. Forage yield, nutritive value and N-fixation ability of legume based swards are affected by light intensity in a Mediterranean agroforestry system. Agrofor. Syst. 2019, 93, 2151–2161. [Google Scholar] [CrossRef]
- Jia, J.; Xu, M.; Bei, S.; Zhang, H.; Xiao, L.; Gao, Y.; Zhang, Y.; Sai, L.; Xue, L.; Lei, J.; et al. Impact of reduced light intensity on wheat yield and quality: Implications for agroforestry systems. Agrofor. Syst. 2021, 95, 1689–1701. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.J.; Gan, Y.W.; Duan, Z.P.; Hao, X.D.; Xu, W.L.; Li, L.H. Different tree age affects light competition and yield in wheat grown as a companion crop in jujube-wheat agroforestry. Agrofor. Syst. 2019, 93, 653–664. [Google Scholar] [CrossRef]
- Zhang, D.; Du, G.; Sun, Z.; Bai, W.; Wang, Q.; Feng, L.; Zheng, J.; Zhang, Z.; Liu, Y.; Yang, S.; et al. Agroforestry enables high efficiency of light capture, photosynthesis and dry matter production in a semi-arid climate. Eur. J. Agron. 2018, 94, 1–11. [Google Scholar] [CrossRef]
- Lu, D.; Liu, B.; Ren, M.; Wu, C.; Ma, J.; Shen, Y. Light Deficiency Inhibits Growth by Affecting Photosynthesis Efficiency as well as JA and Ethylene Signaling in Endangered Plant Magnolia sinostellata. Plants 2021, 10, 2261. [Google Scholar] [CrossRef]
- Piato, K.; Subía, C.; Lefort, F.; Pico, J.; Calderón, D.; Norgrove, L. No Reduction in Yield of Young Robusta Coffee When Grown under Shade Trees in Ecuadorian Amazonia. Life 2022, 12, 807. [Google Scholar] [CrossRef]
- Gomes, L.; Bianchi, F.; Cardoso, I.; Fernandes, R.; Filho, E.F.; Schulte, R. Agroforestry systems can mitigate the impacts of climate change on coffee production: A spatially explicit assessment in Brazil. Agric. Ecosyst. Environ. 2020, 294, 106858. [Google Scholar] [CrossRef]
- Magalhães, C.A.; Zolin, C.A.; Lulu, J.; Lopes, L.B.; Furtini, I.V.; Vendrusculo, L.G.; Zaiatz, A.P.; Pedreira, B.C.; Pezzopane, J. Improvement of thermal comfort indices in agroforestry systems in the southern Brazilian Amazon. J. Therm. Biol. 2020, 91, 102636. [Google Scholar] [CrossRef]
- Swieter, A.; Langhof, M.; Lamerre, J. Competition, stress and benefits: Trees and crops in the transition zone of a temperate short rotation alley cropping agroforestry system. J. Agron. Crop Sci. 2021, 208, 209–224. [Google Scholar] [CrossRef]
- Dufour, L.; Dupraz, C.; Lauri, P.É.; Gosme, M. Effect of Agroforestry on phenology and components of yield of different varieties of durum wheat. In Proceedings of the 3rd European Agroforestry Conference, Montpellier, France, 23–25 May 2016; p. 317. [Google Scholar]
- Wang, Q.; Xu, Z.; Hu, T.; Rehman, H.U.; Chen, H.; Li, Z.; Ding, B.; Hu, H. Allelopathic activity and chemical constituents of walnut (Juglans regia) leaf litter in walnut–winter vegetable agroforestry system. Nat. Prod. Res. 2014, 28, 2017–2020. [Google Scholar] [CrossRef]
- D’hervilly, C.; Marsden, C.; Capowiez, Y.; Béral, C.; Delapré-Cosset, L.; Bertrand, I. Trees and herbaceous vegetation strips both contribute to changes in soil fertility and soil organism communities in an agroforestry system. Plant Soil 2021, 463, 537–553. [Google Scholar] [CrossRef]
- Ledesma, N.A.; Kawabata, S. Responses of two strawberry cultivars to severe high temperature stress at different flower development stages. Sci. Hortic. 2016, 211, 319–327. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, S.R.; Singh, G. Soil carbon, microbial activity and nitrogen availability in agroforestry systems on moderately alkaline soils in northern India. Appl. Soil. Ecol. 2000, 15, 283–294. [Google Scholar] [CrossRef]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Clivot, H.; Petitjean, C.; Marron, N.; Dallé, E.; Genestier, J.; Blaszczyk, N.; Santenoise, P.; Laflotte, A.; Piutti, S. Early effects of tem-perate agroforestry practices on soil organic matter and microbial enzyme activity. Plant Soil. 2020, 453, 189–207. [Google Scholar] [CrossRef]
- Guo, W.; Li, B.; Zhang, X.; Wang, R. Effects of water stress on water use efficiency and water balance components of Hippophae rhamnoides and Caragana intermedia in the soil–plant–atmosphere continuum. Agrofor. Syst. 2010, 80, 423–435. [Google Scholar] [CrossRef]
- Panozzo, A.; Huang, H.-Y.; Bernazeau, B.; Meunier, F.; Turc, O.; Duponnois, R.; Prin, Y.; Vamerali, T.; Desclaux, D. Impact of Olive Trees on the Microclimatic and Edaphic Environment of the Understorey Durum Wheat in an Alley Orchard of the Mediterranean Area. Agronomy 2022, 12, 527. [Google Scholar] [CrossRef]
- Dagar, J.C.; Minhas, P.S. Saline Irrigation for Productive Agroforestry Systems. Agroforestry for the Management of Water-Logged Saline Soils and Poor-Quality Waters; Springer: New Delhi, India, 2016; pp. 145–161. [Google Scholar]
- Singh, D.; Kumar, A. Multivariate screening approach indicated adaptive tolerance to salt stress in the seedlings of an agroforestry tree, Eucalyptus tereticornis Sm. Plant Cell Tissue Organ Cult. 2021, 145, 545–560. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Garrett, H.E.; Kallenbach, R.L. Agroforestry and grass buffer effects on water quality in grazed pastures. Agrofor. Syst. 2010, 79, 81–87. [Google Scholar] [CrossRef]
- Ehret, M.; Gras, R.; Wachendorf, M. The effect of shade and shade material on white clover/perennial ryegrass mixtures for temperate agroforestry systems. Agrofor. Syst. 2015, 89, 557–570. [Google Scholar] [CrossRef]
- Schroth, G.; Krauss, U.; Gasparotto, L.; Aguilar, J.A.D.; Vohland, K. Pests and diseases in agroforestry systems of the humid tropics. Agrofor. Syst. 2000, 50, 199–241. [Google Scholar] [CrossRef]
- Sipos, L.; Boros, I.F.; Csambalik, L.; Székely, G.; Jung, A.; Balázs, L. Horticultural lighting system optimalization: A review. Sci. Hortic. 2020, 273, 109631. [Google Scholar] [CrossRef]
- Boardman, N.K. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Björkman, O.; Holmgren, P. Adaptability of the photosynthetic apparatus to light intensity in ecotypes from exposed and shaded habitats. Physiol. Plant 1963, 16, 889–914. [Google Scholar] [CrossRef]
- Kephart, K.D.; Buxton, D.R.; Taylor, S.E. Growth of C3 and C4 perennial grasses in reduced irradiance. Crop Sci. 1992, 32, 1033–1038. [Google Scholar] [CrossRef]
- Lin, C.H.; McGraw, R.L.; George, M.F.; Garrett, H.E. Shade effects on forage crops with potential in temperate agroforestry practices. Agrofor. Syst. 1998, 44, 109–119. [Google Scholar] [CrossRef]
- Mbow, C.; Smith, P.; Skole, D.; Duguma, L.; Bustamante, M. Achieving mitigation and adaptation to climate change through sustainable agroforestry practices in Africa. Curr. Opin. Environ. Sustain. 2013, 6, 8–14. [Google Scholar] [CrossRef]
- Wang, Q.; Han, S.; Zhang, L.; Zhang, D.; van der Werf, W.; Evers, J.B.; Sun, H.; Su, Z.; Zhang, S. Density responses and spatial distribution of cotton yield and yield components in jujube (Zizyphus jujube)/cotton (Gossypium hirsutum) agroforestry. Eur. J. Agron. 2014, 79, 58–65. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Liu, J.; Han, S.; Wang, Q.; Evers, J.; Liu, J.; van der Werf, W.; Li, L. Plant density affects light interception and yield in cotton grown as companion crop in young jujube plantations. Field Crop. Res. 2014, 169, 132–139. [Google Scholar] [CrossRef]
- Gong, W.; Qi, P.; Du, J.; Sun, X.; Wu, X.; Song, C.; Liu, W.; Wu, Y.; Yu, X.; Yong, T.; et al. Transcriptome Analysis of Shade-Induced Inhibition on Leaf Size in Relay Intercropped Soybean. PLoS ONE 2014, 9, e98465. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.; Zhao, B.; Liu, P.; Zhang, J.-W. Physiological and comparative proteomic analysis provides new insights into the effects of shade stress in maize (Zea mays L.). BMC Plant Biol. 2020, 20, 60. [Google Scholar] [CrossRef]
- Franklin, K.A.; Praekelt, U.; Stoddart, W.M.; Billingham, O.E.; Halliday, K.; Whitelam, G.C. Phytochromes B, D, and E Act Redundantly to Control Multiple Physiological Responses in Arabidopsis. Plant Physiol. 2003, 131, 1340–1346. [Google Scholar] [CrossRef]
- Alarcon, V.J.; Sassenrath, G.F. Optimizing canopy photosynthetic rate through PAR modeling in cotton (Gossypium spp.) crops. Comput. Electron. Agric. 2015, 119, 142–152. [Google Scholar] [CrossRef]
- Powell, G.W.; Bork, E.W. Aspen canopy removal and root trenching effects on understory vegetation. Ecol. Manag. 2006, 230, 76–90. [Google Scholar] [CrossRef]
- Schmidt, M.; Lischeid, G.; Nendel, C. Microclimate and matter dynamics in transition zones of forest to arable land. Agric. For. Meteorol. 2019, 268, 1–10. [Google Scholar] [CrossRef]
- Lin, C.H.; McGraw, R.L.; George, M.F.; Garrett, H.E. Nutritive quality and morphological development under the partial shade of some forage species with agroforestry potential. Agrofor. Syst. 2001, 53, 269–281. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V. High-temperature stress. In Plant Genetic Resources and Climate Change; Jackson, M., Ford-Lloyd, B., Parry, M., Eds.; CAB eBooks: Wallingford, UK, 2014; pp. 201–220. [Google Scholar] [CrossRef]
- Dreesen, F.E.; De Boeck, H.J.; Janssens, I.A.; Nijs, I. Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ. Exp. Bot. 2012, 79, 21–30. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Mittler, R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002, 130, 1143–1151. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef]
- Prasad, P.V.; Pisipati, S.R.; Mutava, R.N.; Tuinstra, M.R. Sensitivity of Grain Sorghum to High Temperature Stress during Reproductive Development. Crop Sci. 2008, 48, 1911–1917. [Google Scholar] [CrossRef]
- García, G.A.; Dreccer, M.F.; Miralles, D.J.; Serrago, R.A. High night temperatures during grain number determination reduce wheat and barley grain yield: A field study. Glob. Chang. Biol. 2015, 21, 4153–4164. [Google Scholar] [CrossRef]
- Gosme, M.; Dufour, L.; Inurreta-Aguirre, H.; Dupraz, C. Microclimatic effect of agroforestry on diurnal temperature cycle. In Proceedings of the 3rd European Agroforestry Conference, Montpellier, France, 25–26 May 2016; pp. 183–186. [Google Scholar]
- Sharma, P.; Rai, S.C.; Sharma, R.; Sharma, E. Effects of land-use change on soil microbial C, N and P in a Himalayan watershed. Pedobiologia 2004, 48, 83–92. [Google Scholar] [CrossRef]
- Dinesh, R.; Chaudhuri, S.G.; Ganeshamurthy, A.N.; Dey, C. Changes in soil microbial indices and their relationships following deforestation and cultivation in a wet tropical forest. Appl. Soil. Ecol. 2003, 24, 17–26. [Google Scholar] [CrossRef]
- Ndaw, S.M.; Gama-Rodrigues, A.; Sales, K.R.; Rosado, A.S. Relationships between bacterial diversity, microbial biomass, and litter quality in soils under different plant covers in northern Rio de Janeiro State, Brazil. Can. J. Microbiol. 2009, 55, 1089–1095. [Google Scholar] [CrossRef]
- Mead, D.J. The role of agroforestry in industrialized nations: The southern hemisphere perspective with special emphasis on Australia and New Zealand. Agrofor. Syst. 1995, 31, 143–156. [Google Scholar] [CrossRef]
- Repo, T.; Kalliokoski, T.; Domisch, T.; Lehto, T.; Mannerkoski, H.; Sutinen, S.; Finér, L. Effects of timing of soil frost thawing on Scots pine. Tree Physiol. 2005, 25, 1053–1062. [Google Scholar] [CrossRef]
- Repo, T.; Lehto, T.; Finér, L. Delayed soil thawing affects root and shoot functioning and growth in Scots pine. Tree Physiol. 2008, 28, 1583–1591. [Google Scholar] [CrossRef]
- Rivest, D.; Lorente, M.; Olivier, A.; Messier, C. Soil biochemical properties and microbial resilience in agroforestry systems: Effects on wheat growth under controlled drought and flooding conditions. Sci. Total Environ. 2013, 463–464, 51–60. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Bainard, L.D.; Klironomos, J.N.; Gordon, A.M. Arbuscular mycorrhizal fungi in tree-based intercropping systems: A review of their abundance and diversity. Pedobiologia 2011, 54, 57–61. [Google Scholar] [CrossRef]
- Orwin, K.H.; Wardle, D.A. Plant species composition affects belowground properties and the resistance and resilience of the soil microflora to a drying disturbance. Plant Soil 2005, 278, 205–221. [Google Scholar] [CrossRef]
- Banning, N.C.; Murphy, D. Effect of heat-induced disturbance on microbial biomass and activity in forest soil and the relationship between disturbance effects and microbial community structure. Appl. Soil Ecol. 2008, 40, 109–119. [Google Scholar] [CrossRef]
- Unger, I.M.; Kennedy, A.C.; Muzika, R.-M. Flooding effects on soil microbial communities. Appl. Soil Ecol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S.; Abdoli, S. How can biochar-based metal oxide nanocomposites counter salt toxicity in plants? Environ. Geochem. Health 2020, 43, 2007–2023. [Google Scholar] [CrossRef]
- Pessarakli, M.H.M.; Sheibanirad, A. Plant Responses under Environmental Stress Conditions. Adv. Plants Agric. Res. 2015, 2, 73. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Li, F.L.; Bao, W.K.; Wu, N. Effects of water stress on growth, dry matter allocation and water-use efficiency of a leguminous species, Sophora davidii. Agrofor. Syst. 2009, 77, 193–201. [Google Scholar] [CrossRef]
- Anderson, S.H.; Udawatta, R.P.; Seobi, T.; Garrett, H.E. Soil water content and infiltration in agroforestry buffer strips. Agrofor. Syst. 2008, 75, 5–16. [Google Scholar] [CrossRef]
- Jactel, H.; Petit, J.; Desprez-Loustau, M.L.; Delzon, S.; Piou, D.; Battisti Akoricheva, J. Drought effects on damage by forest insects and pathogens: A meta-analysis. Glob. Change Biol. 2012, 18, 267–276. [Google Scholar] [CrossRef]
- Rouault, G.; Candau, J.-N.; Lieutier, F.; Nageleisen, L.-M.; Martin, J.-C.; Warzée, N. Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Ann. For. Sci. 2006, 63, 613–624. [Google Scholar] [CrossRef]
- Ngugi, M.R.; Doley, D.H.; Mark, A.; Dart, P.; Ryan, P. Leaf water relations of Eucalyptus cloeziana and Eucalyptus argophloia in response to water deficit. Tree Physiol. 2003, 23, 335–343. [Google Scholar] [CrossRef][Green Version]
- Singh, B.; Singh, G. Effects of controlled irrigation on water potential, nitrogen uptake and biomass production in Dalbergia sissoo seedlings. Environ. Exp. Bot. 2006, 55, 209–219. [Google Scholar] [CrossRef]
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. N. Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef]
- Kato, T.; Kimura, R.; Kamichika, M. Estimation of evapotranspiration, transpiration ratio and water-use efficiency from a sparse canopy using a compartment model. Agric. Water Manag. 2004, 65, 173–191. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Ayaz, A.; Saqib, S.; Huang, H.; Zaman, W.; Lü, S.; Zhao, H. Genome-wide comparative analysis of long-chain acyl-CoA synthetases (LACSs) gene family: A focus on identification, evolution and expression profiling related to lipid synthesis. Plant Physiol. Biochem. 2021, 161, 1–11. [Google Scholar] [CrossRef]
- Lawlor, D.W. Limitation to Photosynthesis in Water-stressed Leaves: Stomata vs. Metabolism and the Role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant Productivity and Environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Ashraf, M. Breeding for salinity tolerance proteins in plants. Crit. Rev. Plant Sci. 1994, 13, 17–42. [Google Scholar] [CrossRef]
- Meloni, D.; Gulotta, M.; Martinez, C.; Oliva, M. The effects of salt stress on growth, nitrate reduction and proline and glycine betaine accumulation in Prosopis alba. Braz. J. Plant Physiol. 2004, 16, 39–46. [Google Scholar] [CrossRef]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. In Plant Stress Physiology; Shabala, S., Ed.; Oxford: London, UK, 2012; pp. 59–93. [Google Scholar]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Sgherri, C.; Muñoz-Rueda, A.; Navari-Izzo, F.; Mena-Petite, A. The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol. Plant. 2009, 135, 29–42. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth underwater/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Afrin, S.; Bashar, K.K.; Afrin, T.; Mahamud, A.G.M.S.U.; Polash, M.A.S.; Hossain, M.T.; Sohel, M.A.T.; Brestic, M.; et al. Differential Response of Sugar Beet to Long-Term Mild to Severe Salinity in a Soil–Pot Culture. Agriculture 2019, 9, 223. [Google Scholar] [CrossRef]
- Awan, A.R.; Mahmood, K. Tree plantation in problem soils. In Text Book of Appl Forestry; Tahir Siddiqui, M., Nawaz, F., Eds.; University of Agriculture: Faisalabad, Pakistan, 2017; pp. 140–159. [Google Scholar]
- Dagar, J.C.; Tewari, V.P. (Eds.) Agroforestry: Anecdotal to Modern Science; Switzerland AG Springer Nature: Cham, Switzerland, 2018; p. 879. [Google Scholar]
- Bin Yousaf, M.T.; Nawaz, M.F.; Rehman, M.Z.U.; Gul, S.; Yasin, G.; Rizwan, M.; Ali, S. Effect of three different types of biochars on eco-physiological response of important agroforestry tree species under salt stress. Int. J. Phytoremediation 2021, 23, 1412–1422. [Google Scholar] [CrossRef]
- Gordon, T.R.; Reynolds, G.J. Plasticity in plant-microbe interactions: A perspective based on the pitch canker pathosystem. Phytoparasitica 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Barón, M.; Flexas, J.; Delucia, E.H. Photosynthetic responses to biotic stress. In Terrestrial Photosynthesis in a Changing Environment—A Molecular, Physiological and Ecological Approach; Flexas, J., Loreto, F., Medrano, H., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 331–350. [Google Scholar]
- Dittrich-Schroder, G.; Wingfield, M.J.; Hurley, B.P.; Slippers, B. Diversity in Eucalyptus susceptibility to the gall-forming wasp Leptocybe invasa. Agric. For. Èntomol. 2012, 14, 419–427. [Google Scholar] [CrossRef]
- Das, T.; Majumdar, M.H.D.; Devi, R.T.; Rajesh, T. Climate change impacts on plant diseases. SAARC J. Agric. 2017, 14, 200–209. [Google Scholar] [CrossRef]
- Abramovitch, R.; Anderson, J.C.; Martin, G.B. Bacterial elicitation and evasion of plant innate immunity. Nat. Rev. Mol. Cell Biol. 2006, 7, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Pumariño, L.; Sileshi, G.W.; Gripenberg, S.; Kaartinen, R.; Barrios, E.; Muchane, M.N.; Midega, C.; Jonsson, M. Effects of agroforestry on pest, disease and weed control: A meta-analysis. Basic Appl. Ecol. 2015, 16, 573–582. [Google Scholar] [CrossRef]
- Horbach, R.; Navarro-Quesada, A.R.; Knogge, W.; Deising, H.B. When and how to kill a plant cell: Infection strategies of plant pathogenic fungi. J. Plant Physiol. 2011, 168, 51–62. [Google Scholar] [CrossRef]
- Busey, P. Cultural management of weeds in turfgrass: A review. Crop Sci. 2003, 43, 1899–1911. [Google Scholar] [CrossRef]
- Turgeon, A.J.; McCarty, L.B.; Christians, N. Weed Control in Turf and Ornamentals; Prentice-Hall: Hoboken, NJ, USA, 2009. [Google Scholar]
- Datnoff, L.E. Silicon in the Life and Performance of Turfgrass. Appl. Turfgrass Sci. 2005, 2, 1–6. [Google Scholar] [CrossRef][Green Version]
- Nair, P.K.R.; Gordon, A.M.; Mosquera-Losada, M.-R. Agroforestry. In Encyclopedia of Ecology; Jorgensen, S.E., Faith, B.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, p. 101. [Google Scholar]
- Garrett, H.E. (Ed.) North American Agroforestry: An Integrated Science and Practice, 2nd ed.; ASA: Madison, WI, USA, 2009. [Google Scholar]
- Gordon, A.; Newman, S.; Coleman, B. Temperate Agroforestry Systems, 2nd ed.; CABI: Wallingford, UK, 2018. [Google Scholar]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Weiguo, F.; Pingping, L.; Yanyou, W.; Jianjian, T. Effects of different light intensities on anti-oxidative enzyme activity, quality and biomass in lettuce. Hortic. Sci. 2012, 39, 129–134. [Google Scholar] [CrossRef]

| Type of Stress | Crop Species | Tree Species | Effect | Impact of AF on Crop(s) | Reference |
|---|---|---|---|---|---|
| Light stress | Soybean (Glycine max) | Silver maple (Acer sacharrinum) | Positive | Soybean yield and development | [11] |
| Apple (Malus domestica) | Black locust (Robinia pseudoacacia). | Positive | Crop yield components | [12] | |
| Palisade grass (Urochloa brizantha) | Oak (Quercus sp.) | Negative | N rates derived from atmosphere | [13] | |
| Wheat (Triticum aestivum) | Walnut(Juglans regia) | Neutral | Wheat yield | [14] | |
| Wheat (Triticum aestivum). | Jujube (Zizyphus jujuba) | Positive | Wheat yield and development | [15] | |
| Sweet potato (Ipomoea batatas) | Apricot (Prunus armeniaca) | Positive | Productivity | [16] | |
| Cotton (Gossypium hirsutum) | Jujube (Zizyphus jujuba) | Neutral | Yield | [17] | |
| Robusta coffee (Coffea canephora) | Erythrina (Erythrina spp.) and Santos mahogany (Myroxylon balsamum) | Neutral | Yield | [18] | |
| Temperature stress | Coffea arabica (Coffea arabica) | Pau-abóbora (Erythrina verna) | Positive | Shifts in areas suitable for coffee production in 2050 | [19] |
| Palisade grass (Urochloa brizantha) | Hybrid eucalyptus (Eucalyptus urograndis) | Positive | Microclimate | [20] | |
| Sunflower (Helianthus annuus) | Tree strips (Populus nigra x P. maximowiczii, P. maximowiczii x P. trichocarpa, P. koreana x P. trichocarpa) | Neutral | Yield and growth | [21] | |
| Wheat (Triticum aestivum) | Aspen (Populus sp.) | Positive | Yield components | [22] | |
| Lettuce (Lactuca sativa var. angustata) | Walnut (Juglans nigra) | Neutral | Yield | [23] | |
| Soil Stress | Carrot (Daucus carota subsp. sativus) | Walnut(Juglans nigra x Juglans regia) | Positive | Soil fertility and soil life | [24] |
| Alfalfa (Medicago sativa) | Aspen (Populus sp.) | Negative | Soil water content | [24] | |
| Barley (Hordeum vulgare) | Walnut (Juglans regia × nigra cv. NG23) | Negative | Soil nutrient content | [25] | |
| Berseem clover (Trifolium alexandrium) | Red irongum (Eucalyptus tereticornis) | Positive | Utilization of moderately alkaline soils | [26] | |
| Red gum (Corymbia calophylla) | Marri (Corymbia calophylla) | Positive | Crop productivity | [27] | |
| Water stress | Sea-buckthorn (Hippophae rhamnoides) | Siberian pea tree(Caragana arborescens) | Negative | Drought tolerance | [28] |
| Red clover (Trifolium pretense) | Eastern cottonwood trees (Populus deltoides) | Neutral | Water quality | [29] | |
| Durum Wheat (Triticum durum) | Olive (Olea europaea) | Negative | Soil water conservation | [30] | |
| Banana (Musa x paradisiaca). | Cacao (Theobroma cacao) | Positive | Yield | [31] | |
| Salt stress | - | Forest red gum (Eucalyptus tereticornis) | Positive | Salt tolerance | [32] |
| Guinea grass (Panicum maximum) | Samphire (Tecticornia pergranulata) | Positive | Farming system components | ||
| White clover (Trifolium repens) | - | Negative | Productivity and clover ratio | [33] | |
| Pest and Disease stress | Papaya (Carica papaya) | Cassava (Manihot esculenta) | Positive | Yield | [34] |
| Pea (Tephrosia spp.) | African locust bean (Parkia biglobosa) | Positive | Pest management | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, M.; Szalai, Z.; Divéky-Ertsey, A.; Gál, I.; Csambalik, L. Conceptualizing Multiple Stressors and Their Consequences in Agroforestry Systems. Stresses 2022, 2, 242-255. https://doi.org/10.3390/stresses2030018
Mustafa M, Szalai Z, Divéky-Ertsey A, Gál I, Csambalik L. Conceptualizing Multiple Stressors and Their Consequences in Agroforestry Systems. Stresses. 2022; 2(3):242-255. https://doi.org/10.3390/stresses2030018
Chicago/Turabian StyleMustafa, Mohammed, Zita Szalai, Anna Divéky-Ertsey, Izóra Gál, and László Csambalik. 2022. "Conceptualizing Multiple Stressors and Their Consequences in Agroforestry Systems" Stresses 2, no. 3: 242-255. https://doi.org/10.3390/stresses2030018
APA StyleMustafa, M., Szalai, Z., Divéky-Ertsey, A., Gál, I., & Csambalik, L. (2022). Conceptualizing Multiple Stressors and Their Consequences in Agroforestry Systems. Stresses, 2(3), 242-255. https://doi.org/10.3390/stresses2030018










