Nitration of Flavonoids and Tocopherols as Potential Modulators of Nitrosative Stress—A Study Based on Their Conformational Structures and Energy Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Models for the Energetic Study
2.2. Theoretical Analysis of Electronical Stability
2.3. Molecular Mechanics Calculations
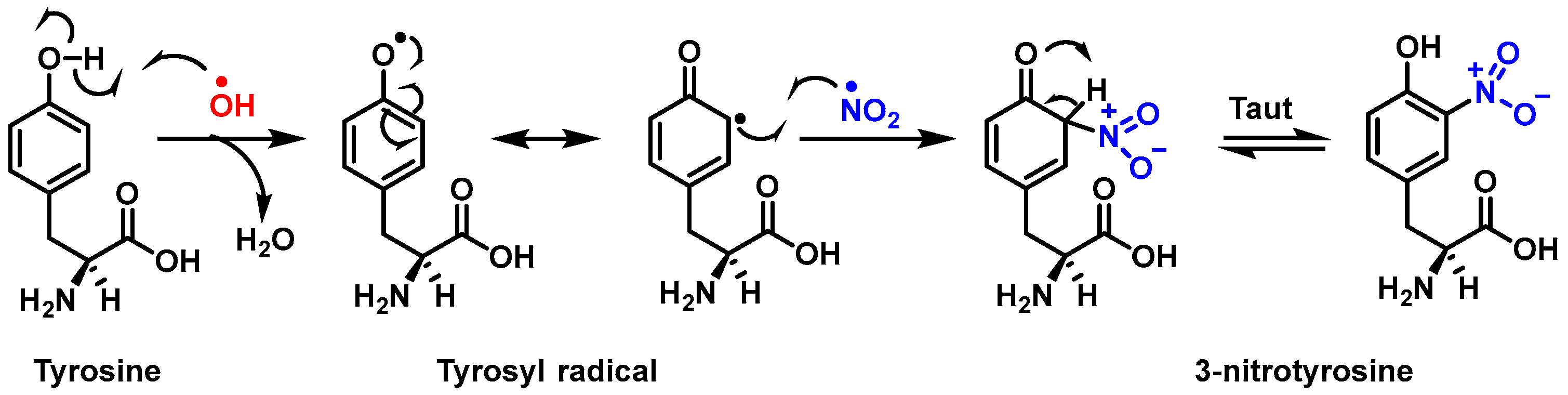
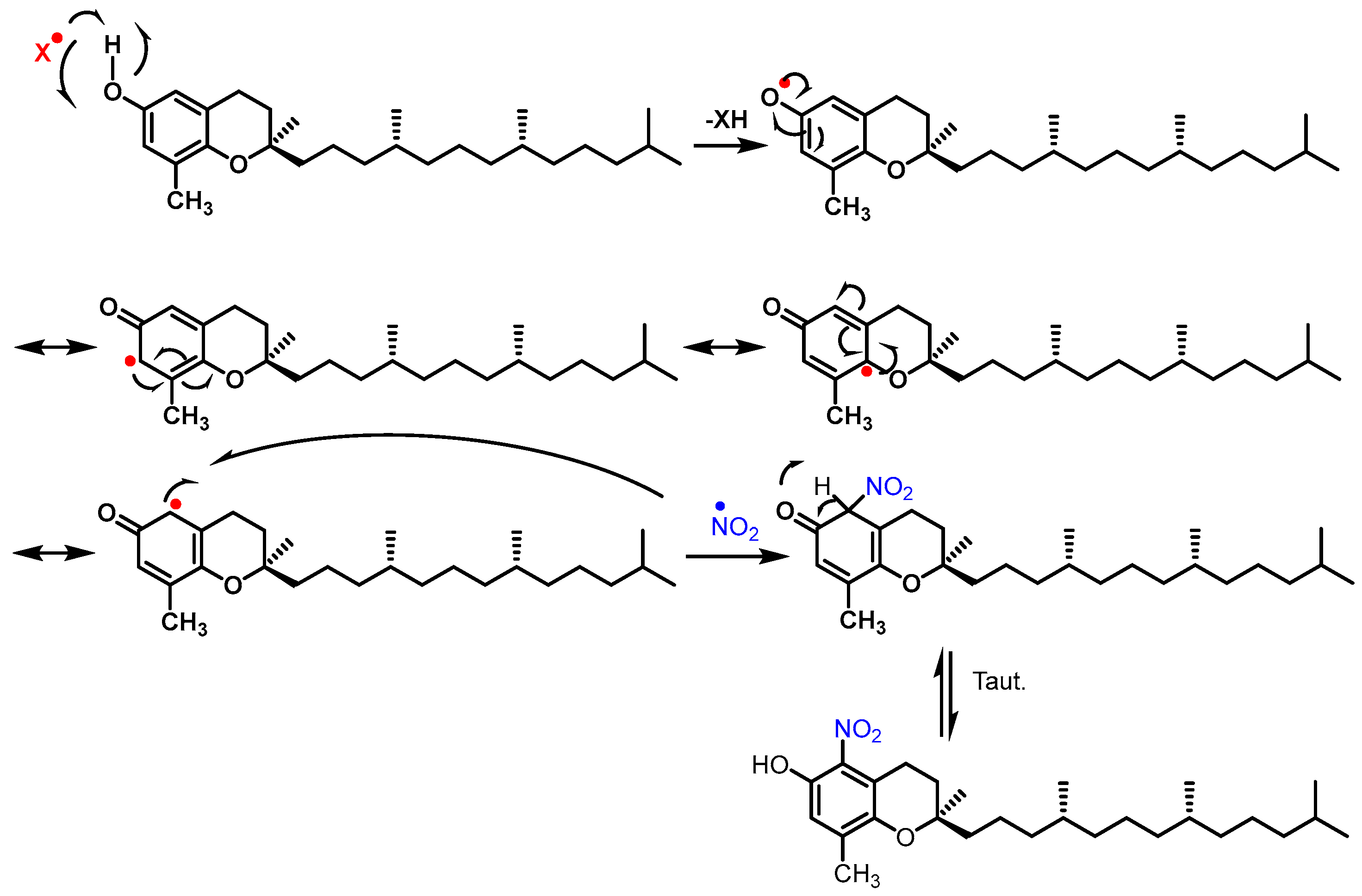
2.4. Considerations for Acting as Potential •NO2 Radical Scavengers
3. Results and Discussion
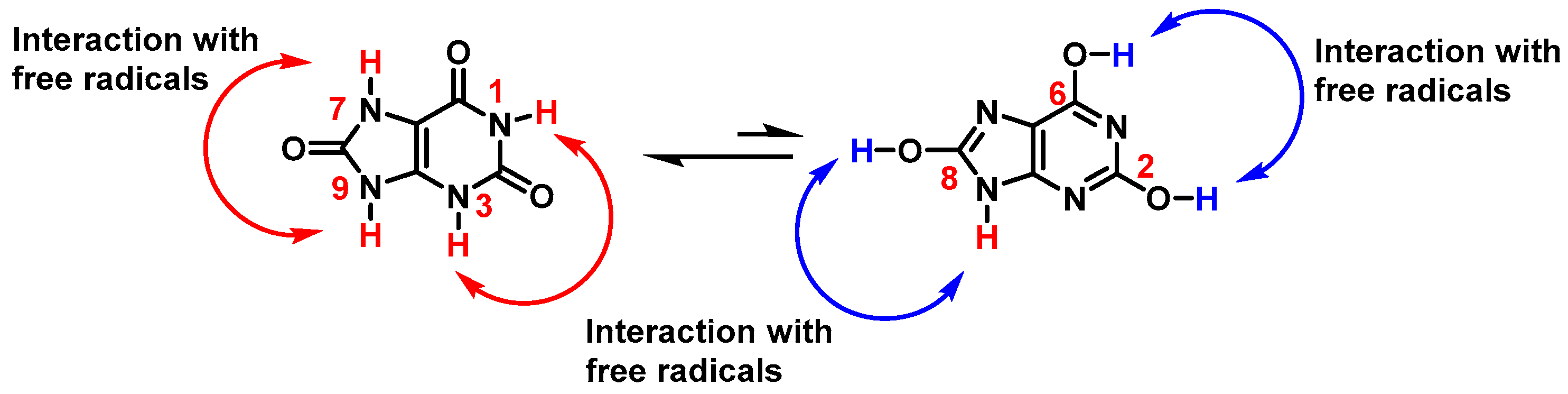
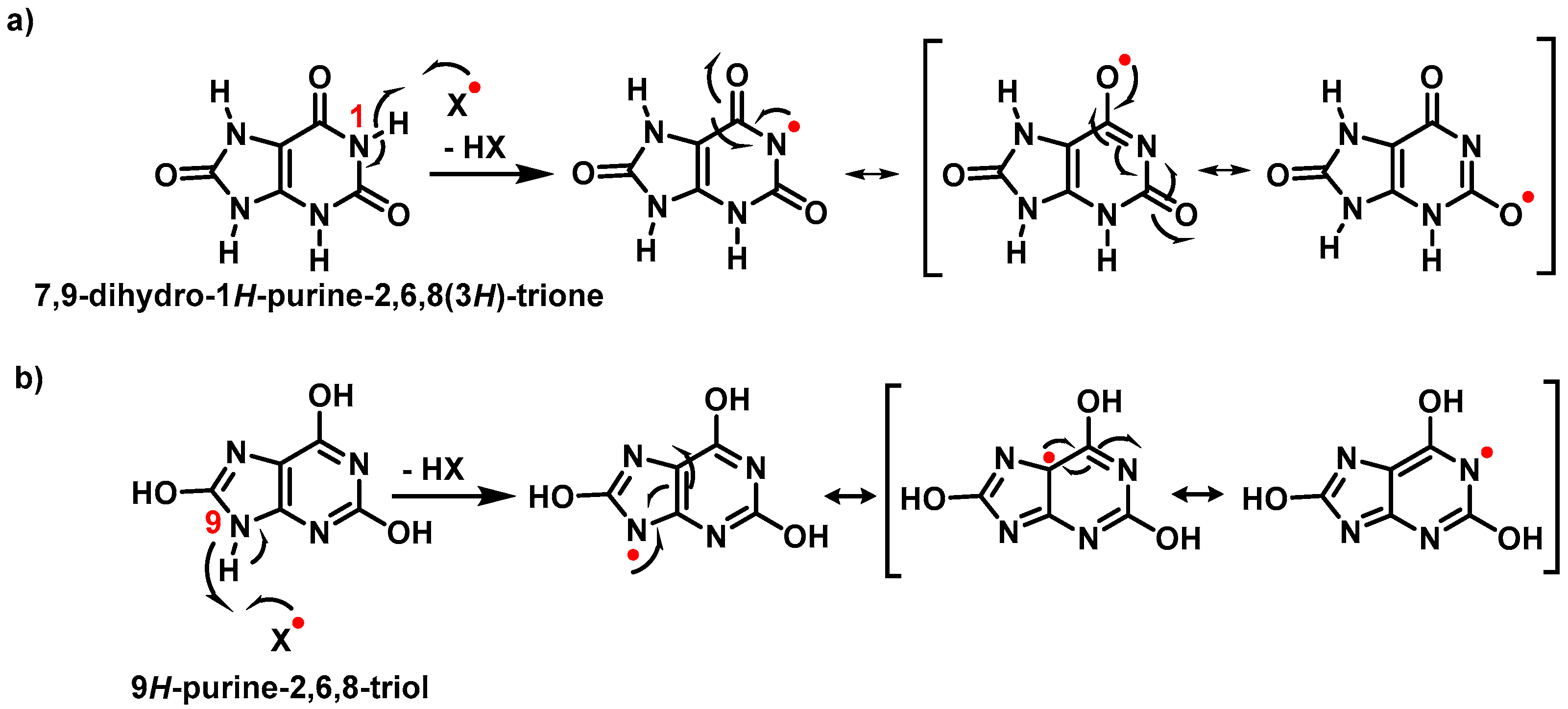
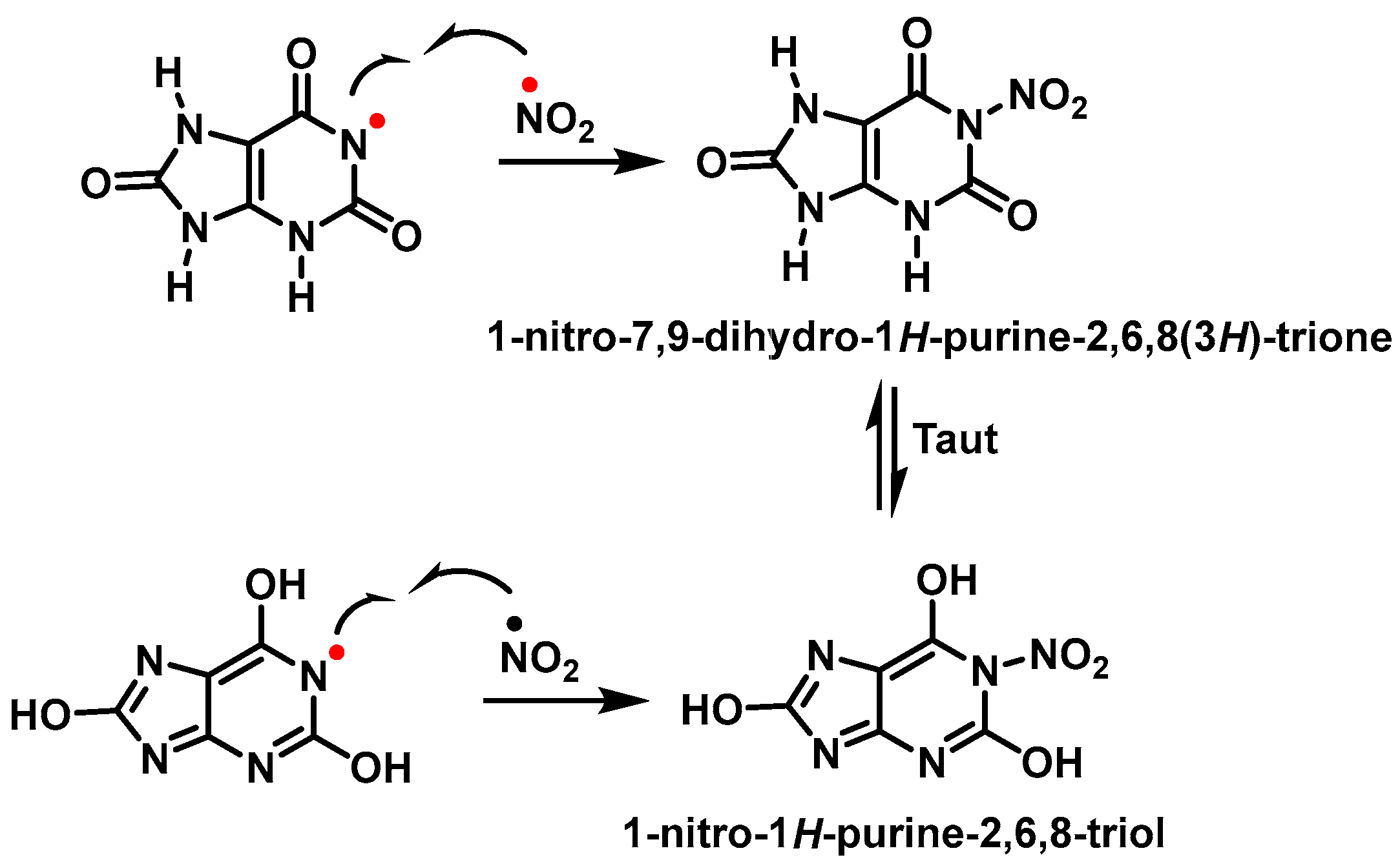
3.1. Uric Acid as Antioxidant and Scavenger of •NO2 Radical
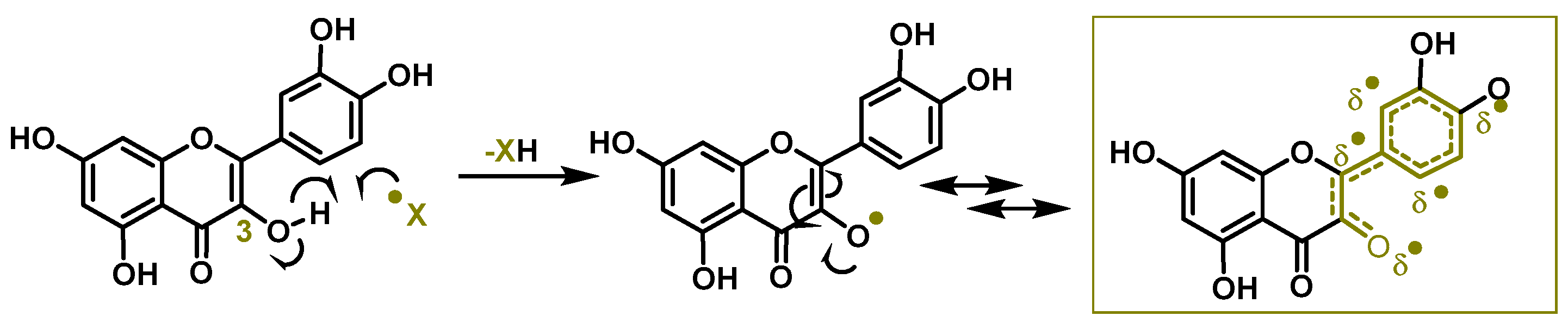
3.2. Chemical Analysis of the Antioxidant Capacity of Flavonoids and Tocopherols
3.3. Proposed Mechanism for the Nitration of Flavonoids and Tocopherols
3.4. Energetic Analysis of Flavonoids, Tocopherols and the Nitrated Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [PubMed]
- Martinez, M.C.; Andriantsitohaina, R. Reactive nitrogen species: Molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as Antioxidants: Determination of Radical-Scavenging Efficiencies. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 343–355. [Google Scholar]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Thompson, M.; Williams, C.R.; Elliot, G.E. Stability of flavonoid complexes of copper(II) and flavonoid antioxidant activity. Anal. Chim. Acta 1976, 85, 375–381. [Google Scholar] [CrossRef]
- Aziz, M.A.; Diab, A.S.; Mohammed, A.A. Antioxidant Categories and Mode of Action. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Halliwell, B. How to Characterize an Antioxidant: An Update. In Free Radicals and Oxidative Stress: Environment, Drugs and Food Additives (Biochemical Society Symposia, Vol 61); Portland Press: London, UK, 1995; pp. 73–101. [Google Scholar]
- Tucker, J.M.; Townsend, D.M. Alpha-tocopherol: Roles in prevention and therapy of human disease. Biomed. Pharmacother. 2005, 59, 380–387. [Google Scholar] [CrossRef]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and flavonols’ antiradical structure–activity relationship—A quantum chemical study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Hooper, D.C.; Spitsin, S.; Kean, R.B.; Champion, J.M.; Dickson, G.M.; Chaudhry, I.; Koprowski, H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA 1998, 95, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Scott, G.S.; Hooper, D.C. The role of uric acid in protection against peroxynitrite-mediated pathology. Med. Hypotheses 2001, 56, 95–100. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Cueto, R.; Splenser, A.E.; Valavanidis, A.; Zhang, H.; Uppu, R.M.; Pryor, W.A. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch. Biochem. Biophys. 2000, 376, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Ketsawatsakul, U.; Halliwell, B. A Reassessment of the peroxynitrite scavenging activity of uric acid. Ann. N. Y. Acad. Sci. 2002, 962, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Lastra, J.M.; Andrés-Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Theoretical three-dimensional zinc complexes with glutathione, amino acids and flavonoids. Stresses 2021, 1, 123–141. [Google Scholar] [CrossRef]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2019, 119, e25665. [Google Scholar] [CrossRef] [Green Version]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, V.; Alderete, J.B. Theoretical calculations on the tautomerism of uric acid in gas phase and aqueous solution. J. Mol. Struct. 2005, 755, 209–214. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS metabolism in cancer cells: The potential role of quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar] [CrossRef] [Green Version]
- Duncan, C.; Li, H.; Dykhuizen, R.; Frazer, R.; Johnston, P.; MacKnight, G.; Smith, L.; Lamza, K.; McKenzie, H.; Batt, L.; et al. Protection against oral and gastrointestinal diseases: Importance of dietary nitrate intake, oral nitrate reduction and enterosalivary nitrate circulation. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 939–948. [Google Scholar] [CrossRef]
- Takahama, U.; Hirota, S. Possible reactions of dietary phenolic compounds with salivary nitrite and thiocyanate in the stomach. Antioxidants 2017, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Niki, E. Lipid oxidation that is, and is not, inhibited by vitamin E: Consideration about physiological functions of vitamin E. Free Radic. Biol. Med. 2021, 176, 1–15. [Google Scholar] [CrossRef] [PubMed]






| Position | Energy Content (kcal/mol) | Developed Formula | Three-Dimensional Structure |
|---|---|---|---|
| N-3 | −35.5 | 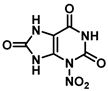 | 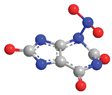 |
| N-1 | −77.1 | 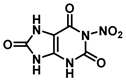 | 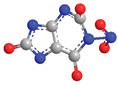 |
| N-7 | 10.9 | 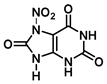 | 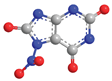 |
| N-9 | 4.2 | 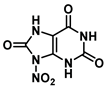 | 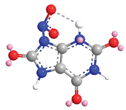 |
| O in C-2 | 15.4 | 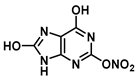 | 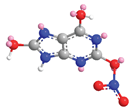 |
| O in C-6 | 10.8 | 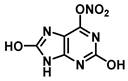 |  |
| O in C-8 | 24 | 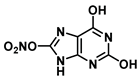 | 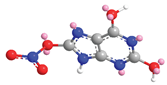 |
| Tocopherol Structure | R1 | R2 | R3 | Name |
|---|---|---|---|---|
 | CH3 | CH3 | CH3 | α- |
| CH3 | H | CH3 | β- | |
| H | CH3 | CH3 | γ- | |
| H | H | CH3 | δ- |
| Flavonoid Energy Content (kcal/mol) | Developed Formula | Spatial Structure |
|---|---|---|
| Quercetin 14.9 | 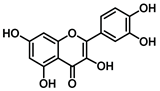 | 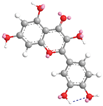 |
| Naringenin 6.5 | 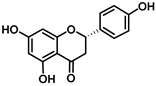 | 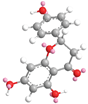 |
| Luteolin 7.7 |  | 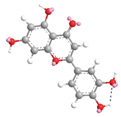 |
| Catechin −6.6 | 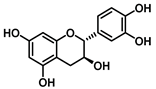 |  |
| Aurantinidin 7.6 | 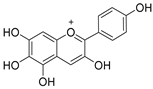 |  |
| C-Position | Energy Content (kcal/mol) | Developed Formula | Spatial Structure |
|---|---|---|---|
| C-6 | 14.2 | 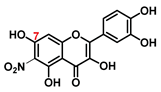 |  |
| C-8 | −11 |  |  |
| C-5′ | 5.5 | 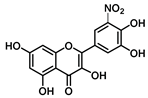 | 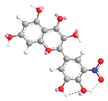 |
| C-6′ | −4.7 | 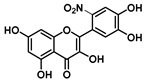 | 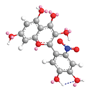 |
| C-2′ | −26 | 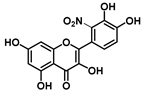 |  |
| C-Position | Energy Content (kcal/mol) | Developed Formula | Spatial Structure |
|---|---|---|---|
| C-6 | 4.7 |  |  |
| C-8 | −8.5 | 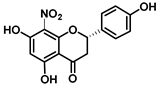 | 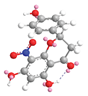 |
| C-3′ | −1.9 | 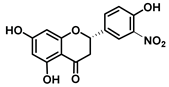 | 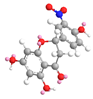 |
| C-Position | Energy Content (kcal/mol) | Developed Formula | Spatial Structure |
|---|---|---|---|
| C-6 | 8.5 | 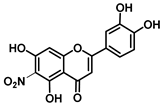 |  |
| C-8 | −12.6 | 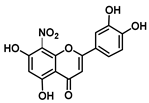 | 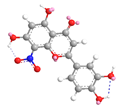 |
| C-5′ | 3.0 | 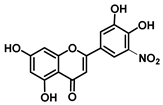 | 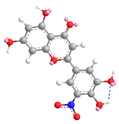 |
| C-6′ | −27.7 |  |  |
| C-2′ | −27.9 |  | 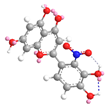 |
| C-Position | Energy Content (kcal/mol) | Developed Formula | Spatial Structure |
|---|---|---|---|
| C-6 | −59.1 |  |  |
| C-8 | −13.6 | 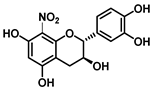 | 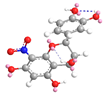 |
| C-5′ | −10.3 | 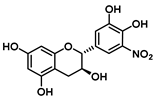 | 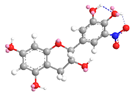 |
| C-6′ | −34.1 | 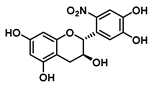 | 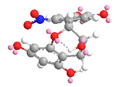 |
| C-2′ | −7.7 | 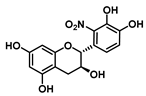 | 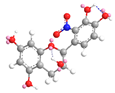 |
| C-Position | Energy Content (kcal/mol) | Developed Formula | Spatial Structure |
|---|---|---|---|
| C-8 | −64.1 | 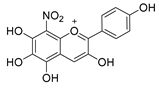 | 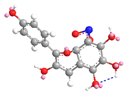 |
| C-3′ | 11.8 | 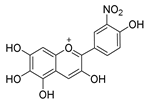 | 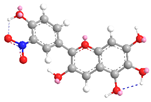 |
| Name Energy Content (kcal/mol) | Developed Formula | Spatial Structure |
|---|---|---|
| β-tocopherol23.7 | 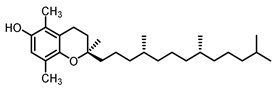 |  |
| γ- tocopherol21.9 | 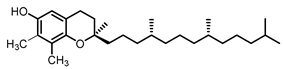 | 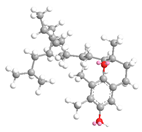 |
| δ- tocopherol22.6 | 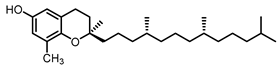 | 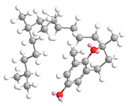 |
| Name Nitration Position | Energy Content (kcal/mol) | Developed Formula | Spatial Structure |
|---|---|---|---|
| β Tocopherol C-7 | 37.5 | 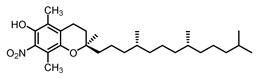 |  |
| γ Tocopherol C-5 | 23.9 | 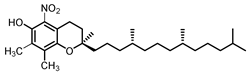 | 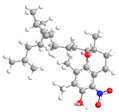 |
| δ-Tocopherol C-5 | 22.3 | 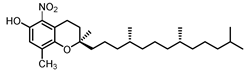 | 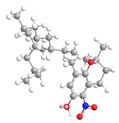 |
| δ-Tocopherol C-7 | 34.9 |  | 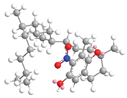 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Nitration of Flavonoids and Tocopherols as Potential Modulators of Nitrosative Stress—A Study Based on Their Conformational Structures and Energy Content. Stresses 2022, 2, 213-230. https://doi.org/10.3390/stresses2020015
Pérez de la Lastra JM, Juan CA, Plou FJ, Pérez-Lebeña E. Nitration of Flavonoids and Tocopherols as Potential Modulators of Nitrosative Stress—A Study Based on Their Conformational Structures and Energy Content. Stresses. 2022; 2(2):213-230. https://doi.org/10.3390/stresses2020015
Chicago/Turabian StylePérez de la Lastra, José Manuel, Celia Andrés Juan, Francisco J. Plou, and Eduardo Pérez-Lebeña. 2022. "Nitration of Flavonoids and Tocopherols as Potential Modulators of Nitrosative Stress—A Study Based on Their Conformational Structures and Energy Content" Stresses 2, no. 2: 213-230. https://doi.org/10.3390/stresses2020015
APA StylePérez de la Lastra, J. M., Juan, C. A., Plou, F. J., & Pérez-Lebeña, E. (2022). Nitration of Flavonoids and Tocopherols as Potential Modulators of Nitrosative Stress—A Study Based on Their Conformational Structures and Energy Content. Stresses, 2(2), 213-230. https://doi.org/10.3390/stresses2020015







