Abstract
Cadmium (Cd) is an environmental toxicant with serious public health consequences due to its persistence within arable soils, and the ease with which it enters food chains and then, accumulates in human tissues to induce a broad range of adverse health effects. The present review focuses on the role of zinc (Zn), a nutritionally essential metal, to protect against the cytotoxicity and carcinogenicity of Cd in urinary bladder epithelial cells. The stress responses and defense mechanisms involving the low-molecular-weight metal binding protein, metallothionein (MT), are highlighted. The efflux and influx transporters of the ZnT and Zrt-/Irt-like protein (ZIP) gene families are discussed with respect to their putative role in retaining cellular Zn homeostasis. Among fourteen ZIP family members, ZIP8 and ZIP14 mediate Cd uptake by cells, while ZnT1 is among ten ZnT family members solely responsible for efflux of Zn (Cd), representing cellular defense against toxicity from excessively high Zn (Cd) intake. In theory, upregulation of the efflux transporter ZnT1 concomitant with the downregulation of influx transporters such as ZIP8 and ZIP14 can prevent Cd accumulation by cells, thereby increasing tolerance to Cd toxicity. To link the perturbation of Zn homeostasis, reflected by the aberrant expression of ZnT1, ZIP1, ZIP6, and ZIP10, with malignancy, tolerance to Cd toxicity acquired during Cd-induced transformation of a cell model of human urothelium, UROtsa, is discussed as a particular example.
Keywords:
bladder cancer; cadmium; metal homeostasis; metallothionein; tolerance; urothelium; UROtsa; zinc; zinc transporters 1. Introduction
Cadmium (Cd) is a redox inert divalent metal that has no known biological role in humans [1,2,3,4]. Diet is a primary exposure source for non-smoking populations [1,2,3,4], whilst cigarette smoke is an additional source of Cd among those who smoked. Cd exists in cigarette smoke as a non-volatile oxide form (CdO), and a volatile metallic form with high transmission rates [5]. Of further concern, the electronegativity of Cd is similar to that of zinc (Zn), a nutritionally essential metal, whereas its ionic radius is similar to calcium (Ca) [3,4]. Hence, Cd can enter the body from the gut and lungs through the metal transporter systems and pathways evolved for acquisition and storage of Zn, Ca, and other nutritionally essential metals such as iron (Fe) and manganese (Mn) [3,4,6,7,8].
Metal transporters of the Zrt- and Irt-related protein (ZIP) family, such as ZIP8 and ZIP14, mediate Cd uptake by various cell types [9,10,11]. High blood Cd has been associated with certain variants of the ZIP8 and ZIP14 genes [12]. Of concern, Cd exhibits higher affinity than Zn for sulfur-containing ligands that include all amino acids, peptides and proteins with functional thiol (-SH) groups, notably cysteine, glutathione (GSH), metal binding protein metallothionein (MT), and zinc-finger transcription factors [13,14]. The perturbation of cellular Zn homeostasis may account, in large part, for the wide range of diversity of the toxic effects of Cd because of the key role of Zn in the regulation of cell growth, differentiation, apoptosis, and defense mechanisms. The Zn mimicking effects of Cd may also account for its carcinogenicity in multiple organs such as the lungs, prostate, breast, pancreas and urinary bladder [1,2,3,4].
This review focuses on the interplay of Zn and its transporters, contributing to the carcinogenicity of Cd in the urinary bladder epithelium. Early experimental studies showing the contribution of Zn and MT to Cd tolerance are briefly discussed. Emergent evidence for tolerance to Cd toxicity through the upregulation of metal efflux transporters concomitant with the downregulation of influx transporters is emphasized together with data from in vitro studies, demonstrating that long-term exposure to low-level environmental Cd, producing blood and urinary Cd at concentrations no greater than 1 µM, could cause cells to undergo malignant transformation.
2. Zinc, Zinc Transporters, and Metallothionein
Zinc (Zn), a redox inert divalent metal, is the second most abundant metal in the human body after iron (Fe) [15,16,17,18]. Zn is considered as a type-2 nutrient which is required for normal cellular metabolism [17]. Most of the body Zn (95%) is found within cells where it is the integral component of many metalloproteins involved in the regulation of cell growth, differentiation, apoptosis, and defense mechanisms [16,17,18]. The antioxidant enzyme, superoxide dismutase 1 (SOD1), is an example of Zn involvement in cellular defense mechanisms against oxidative stress [19]. Because metals cannot be synthesized nor degraded by the body, they must be acquired from the diet. To assimilate Zn from the diet, transporter systems and pathways specific to Zn have evolved [20] together with mechanisms to store and maintain the homeostasis of Zn in cells and to safeguard against deficiency or toxicity from excessively high Zn intake [21,22].
2.1. Zinc Transporters, Deranged Zinc Homeostasis and Cancer
To retain normal cellular metabolism and its tissue-specific, specialized functions, intracellular Zn concentrations are kept within a narrow range [15,16,17,18]. Accordingly, influx, efflux, subcellular compartmentalization (storage) and trafficking of Zn are coordinately regulated. These processes are mediated by metal transporters, encoded by two gene families—the Solute-Linked Carrier 30A (SLC30A) and the Solute-Linked Carrier 39A (SLC39A) [23,24]. The SLC39A family, also known as the Zrt-/Irt-like protein (ZIP) family, comprises 14 members, designated as ZIP1 to ZIP14 [23]. The SLC30A family, also known as Cation Diffusion Facilitators (CDF), comprises 10 members, designated as ZnT1 to ZnT10 [24]. Among ten SLC30A (ZnT) members, ZnT1 is solely responsible for Zn efflux, and overexpression of ZnT1 results in low intracellular Zn levels [25]. Of note, dysregulated cellular Zn homeostasis and aberrant expression of ZnT1, ZIP1, ZIP4, ZIP6, ZIP7, and ZIP10 have increasingly been observed in various types of cancer, including urinary bladder, prostate, pancreatic and breast cancers [25,26,27,28,29,30].
2.2. Putative Zinc Transporters Involved in Tolerance to Cadmium Toxicity
Among fourteen ZIP family members, ZIP8 and ZIP14 have been shown to mediate Cd uptake by cells [9,10,11]. Among ten ZnT family members, only ZnT1 has been shown to be solely responsible for an efflux of Cd [22,31]. In theory, upregulation of the efflux transporter ZnT1 concomitant with downregulation of influx transporters such as ZIP8 and ZIP14 can prevent the accumulation of Cd in cells, leading to Cd tolerance. To date, there are a few studies that link individual Zn transporters to resistance/sensitivity to Cd toxicity. The results of these studies are summarized as follows. Treatment of rat liver epithelial cells, TRL1215, with cyproterone, a synthetic steroidal antiandrogen with a structure related to progesterone, reduced Cd accumulation and decreased sensitivity to Cd toxicity [32]. However, the molecular basis for the reduction in Cd accumulation in TRL1215 cells treated with cyproterone was not investigated. In another study, silencing of ZnT1 expression increased Cd accumulation and enhanced Cd toxicity [31]. In yet another study, a reduction in Cd accumulation and Cd resistance was attributed to a decrease in ZIP8 expression, assessed by ZIP8 mRNA and protein levels [33]. Downregulation of ZIP8 was suggested to be a common feature of cells resistant to Cd [34]. It was determined that expression of the ZIP8 gene is regulated by an epigenetic mechanism; expression of the ZIP8 gene is diminished in a DNA hypermethylation state [34]. In a different report, downregulation of ZIP8 expression was linked to resistance to Cd toxicity following an increment of intracellular GSH concentration [35].
2.3. Metallothionein-Metal Complexes
MTs, a group of low-molecular-weight (6–7 kDa) metal binding proteins, are highly conserved and ubiquitously expressed at varying levels [34,35,36]. This group of proteins contains an unusually high molar content of cysteine whose sulfur atoms participate in the sequestration of nutritionally essential metals, Zn and copper (Cu), for storage purposes [37,38]. In theory, up to 7 atoms of Zn2+ (Cd2+) or 12 atoms of Cu2+ can be sequestered per molecule of MT, and metal–MT complexes are designated as Zn7MT (Cd7MT) or Cu12MT [37,38]. However, several species of mixed metal complexes such as Cd3Cu3ZnMT, Cd4CuZn2MT and Cd6CuMT are formed under in vivo conditions with different Cd exposure levels [38].
There are at least 16 MT isoforms in humans that belong to four major isoforms: MT-1–MT-4 [36]. Among these, MT-1 and MT-2 are expressed in most tissues, kidney tubular epithelial cells and leucocytes included [36,39,40,41]. Each isoform exhibits preferential binding affinity for Zn and Cu; MT-3, expressed abundantly in kidneys and neurons, has a higher binding affinity for Cu than MT-1 and MT-2 [42,43,44]. The genetic variants of MT are associated with variability in the urinary excretion of Cd, Zn and Cu [45]. Expression of MT-1 and MT-2 is inducible through the metal responsive transcription factor (MTF) and the metal response element (MRE) located in the promoters of the MT genes [36].
2.4. Zinc (Cadmium) as a Concomitant Inducer of MT and ZnT1
A study in rats, published in 1984, showed that MT expression was not detectable in the livers of Zn-deficient rats [46]. This finding indicates that MT synthesis is induced by Zn [46]. A study in humans, published in 1998, showed increased expression of MT in erythrocytes and monocytes from men, aged 19–35 years, who received Zn supplements at a dose of 50 mg per day for 18 days [47]. Several studies have then examined levels of MT and Zn transporters expressed by various blood cell types (peripheral blood mononuclear cells, T lymphocytes, monocytes, and erythrocytes) in an attempt to determine if MT and/or Zn transporter reflects Zn status better than plasma Zn levels [41]. The levels of ZIP1 and ZnT1 expressed by subtypes of white blood cells in response to Zn supplementation or depletion varied largely among studies, while changes in MT expression were consistent across studies [41]. It is likely that the inconsistent responses to Zn supplementation or depletion, reflected by changes in the variable expression levels of ZIP1 and ZnT1, could be related to differences in Cd exposure among study subjects, given that Cd is a potent inducer of MT [48,49]. Furthermore, Cd and Zn are both found to be inducers of ZnT1 [22,50,51]. Overexpression of ZnT1 decreased intracellular zinc concentrations [25]. Conversely, silencing of ZnT1 expression increased Cd accumulation and enhanced Cd toxicity [31].
2.5. Metallothionein as a ‘Double-Edged Sword’
It is widely believed that MTs are involved in the homeostasis of Zn and Cu and protection against oxidative damage and heavy metal toxicity. Because Cd exerts toxicity in the unbound state, i.e., as free Cd2+ ions, complexes of Cd and MT (CdMT) are often viewed as a detoxified form. However, although the expression of MT in hepatocytes prevents acute toxicity, studies using a normal rat liver epithelial cell line, TRL1215, demonstrated that Zn2+ ions or Cd2+ ions that were bound to MT, for example as Cd4CuZn2MT, could be substituted and released by nitric oxide (NO) [49,52]. The NO-induced release of Cd2+ ions previously sequestered in MT implies that hepatotoxicity may occur long after exposure [53]. Furthermore, hepatic CdMT can serve as an endogenous source of Cd in a similar way to bone being a reservoir of lead [4,54,55]. CdMT can be released into the circulation and delivered to kidneys, inducing nephrotoxicity long after exposure cessation [4,56,57]. In a Thai study, elevated MT transcript levels expressed by leucocytes were associated with reduced nephrotoxicity, assessed with urinary excretion levels of albumin and β2-microglobulin [39]. In light of new knowledge, the finding of this Thai study could be reinterpreted to suggest a lower Cd accumulation in those with higher MT expression due to concomitant ZnT1 induction by Cd, leading to increased efflux of Cd. Simultaneous upregulation of ZnT1 and MT in response to Cd is a cellular adaptive mechanism to resist Cd-induced cytotoxicity.
It was shown in another study that Cd did not substitute Zn in the antioxidant enzyme, SOD1, but it reduced the activity of SOD1 in human embryonic kidney cells (HEK293T) through MT induction and perturbation of Zn homeostasis [19]. In another study in mice, an increased susceptibility to lung injury after a prolonged exposure to cigarette smoke was attributable to a disturbed Zn homeostasis resulting from an insufficient Zn intake or overexpression of ZIP8 [58]. Of relevance, evidence for Cd-induced derangement of cellular homeostasis of Zn and Cu has been reported in Australian [59], Thai [60,61], and Korean [62] studies.
3. Cadmium as a Risk Factor for Bladder Cancer
Urothelial carcinoma ranks the ninth most common cancer worldwide, and bladder cancer is a common type of urothelial cancer [63,64,65,66,67,68]. Owing to its high recurrence rates (30–70%), frequent cystoscopy and urine cytology are required [64,65,66]. Consequently, bladder cancer is the fifth highest cancer treatment and the highest care cost per patient in the U.S., although it ranks the sixth most common cancer in the U.S. [64]. In most cases, the cancer originates from the transitional cells of bladder mucosal epithelium, and it is named transitional cell carcinoma (TCC) [63]. One-third of bladder cancer cases manifested as non-papillary tumors with high invasive and metastasis potential, while two-thirds manifested as non-invasive, resettable papillary tumors [63].
In the past, the majority of bladder cancer was associated with workplace exposure to aromatic amines, polycyclic hydrocarbons and heavy metals [63,64]. In recent times, workplace exposure accounted for 5–15% of bladder cancer cases in Europe with cigarette smoking arising as a predominant risk factor [69]. Blood Cd levels in cigarette smokers were higher than non-smokers by 2- to 6-fold [2]. In Italy, workplace exposure contributed to 4–10% of bladder cancer cases [70].
In a Spanish study of 1219 newly diagnosed bladder cancer cases and 1271 controls, cigarette smoking accounted for nearly all excess bladder cancer risk in men [69]. In a Belgian study of 172 bladder cancer cases and 395 non-cancer controls, the mean blood Cd in bladder cancer cases was 1.6-fold higher than the mean blood Cd in controls of 0.7 µg/L. The risk of bladder cancer was increased by 5.7-fold as blood Cd rose from the lowest tertile to the highest tertile [71] after adjustment for gender, age, smoking habits, and workplace exposure [71]. In a German study, the median urinary Cd in bladder cancer cases was 2.25-fold higher than controls of 0.8 µg/L [72].
3.1. Cadmium-Induced Cell Transformation: An In Vitro Carcinogenicity Test
The carcinogenicity of Cd has been tested in vitro using human cells, including RWPE-1 prostate epithelium [73], UROtsa urothelium [74,75], MCF-10A breast epithelium [76,77,78], BEAS bronchial epithelium [79], and HPDE pancreatic ductal epithelium [80]. It is notable that Cd concentrations used to induce cell transformation in vitro varied by 10-fold. Cd concentration, as high as 10 µM, was required to transform prostate epithelial cells [73], whereas 2.5 µM Cd was needed to cause malignant transformation of breast epithelium [76]. In contrast, urothelial, bronchial and pancreatic ductal epithelium became malignant cells after prolonged exposure to Cd as low as 1 µM [80]. These data suggest that variability among cell types in their capacities to tolerate Cd could be related to the levels of metal transporters involved in the influx and efflux of Cd. In addition, it could be related to expression levels of other stress response proteins that are induced by Cd such as MT and heme oxygenase-1 (HO-1) [39,40].
A 10-fold increase in the expression levels of the oncogenes, c-myc and c-jun, together with a change in DNA methylation state was observed in transformed TRL1215 rat liver epithelial cells [81,82]. Global DNA hypomethylation and overexpression of c-myc and Kras were observed in transformed MCF-10 cells [76]. In transformed RWPE-1 cells, the expression of the tumor suppressor genes (RASSF1A and p16) were diminished, but the DNA (cytosine-5-)-methyltransferase 3b (DNMT3b) gene was overexpressed, leading to a generalized DNA hypermethylation state [76]. Cd seemingly affected the expression of oncogenes through epigenetic mechanisms rather than oxidative stress-induced mechanisms [81,82].
3.2. UROtsa Cell Line as a Cell Model to Dissect the Carcinogenicity of Cadmium
Prolonged exposure to 1 µM Cd caused UROtsa cells to transform to cancer cells [71], and the tumors generated in nude mice from injected transformed UROtsa cells (a heterotransplant experiment) displayed gene expression profiles similar to the basal subtype of muscle invasive bladder cancer [72]. Microarray (the Affymetrix 133 Plus 2.0 chip) data indicated that at least 285 genes are upregulated and 215 genes are downregulated during Cd-induced cellular transformation [83]. The UROtsa cell line is non-tumorigenic, derived from the ureter epithelium of a 12-year-old female donor, immortalized with SV40 large T-antigen [84]. The UROtsa cells display the phenotypic and morphologic characteristics of primary transitional epithelial cells of the bladder [85].
Several lines of evidence have established the UROtsa cell line as a suitable cell model of urinary bladder epithelium. Expression of MT-1 and MT-2 isoforms in this cell line is induced by Cd [86]. These results have been replicated in a study using normal human urothelial (NHU) cells [50], in which the specific MT isoforms induced by Cd have been identified. However, expression of MT-3 in NHU was not detectable [50], although MT-3 isoform was expressed by parental UROtsa cells and transformed UROtsa cells [87,88,89]. Of interest, Cd-induced expression of the ZnT1 gene was observed in non-differentiated and differentiated NHU cells [50], and such ZnT1 induction by Cd has been replicated in UROtsa cells [51].
3.3. Zinc Transporters Expressed by Parental UROtsa Cells
In this section, data from three publications by Sens et al. [71] and Satarug et al. [30,51] recapitulate the evidence of a perturbation of cellular zinc homeostasis following low environmental exposure to Cd, producing blood Cd concentrations no greater than 1 µg/L. Published data showing the effects of Cd2+ on expression levels of 19 zinc transporters in UROtsa cells are reproduced (Table 1).

Table 1.
Expression levels of zinc transporter genes in a cell culture model of human urothelial (UROtsa) cells at basal state and 24 h after exposure to 1, 2 and 4 µM cadmium.
Expression levels of 19 zinc transporters in two batches of UROtsa cell cultures were qualitatively similar. The Golgi zinc transporter ZnT7 was expressed in the highest abundance: 734 and 758 transcripts per 1000 copies of β-actin gene transcripts in batch I and II, respectively. ZIP8, ZIP14, ZnT1 and ZnT5 genes expressed by batch I cells were 0.1, 83, 181, and 150 transcripts per 1000 copies of β-actin gene transcripts, and the corresponding ZIP8, ZIP14, ZnT1 and ZnT5 expressed by batch II cells were 2.1, 146, 365 and 510 copies per 1000 copies of β-actin gene, respectively. Based on the putative role of ZnT1 as the sole Cd efflux transporter, and ZIP8 and ZIP14 as Cd influx transporters, lower expression levels of ZIP8 than ZIP14 suggested that ZIP14 and ZnT1 could contribute to Cd accumulation by UROtsa cells. Despite low levels of transcripts, ZIP8 protein expression in UROtsa cells was detectable by Western blot analysis [90].
3.4. Upregulation of ZnT1 and Acquired Resistance to Cadmium
It is increasingly apparent that epigenetic changes, DNA methylation included, are especially frequent in urothelial carcinoma [91]. In this section, published data showing that Cd in low concentrations (0.01–1 µM) induced expression of ZnT1 in UROtsa cells are reproduced and discussed together with those showing Cd resistance acquired after treatment with an inhibitor of DNA methylation, 5-aza-2′-deoxycytidine (aza-dC), producing a DNA hypomethylation state [30,51].
Among three Cd concentrations tested, 1 µM Cd induced ZnT1 (Figure 1A). An increment of Cd2+ concentrations from 0.1 to 1.5 µM produced progressive increases in expression levels of ZnT1 (Figure 1B). The highest fold induction within 24 h was 8.8, achieved with 1.5 µM Cd concentration. Exposure to 0.25 µM Cd for 48 h induced ZnT1 expression to the same extent achieved with 24-h exposure to 1.5 µM Cd. Of note, 24-h exposure to 1 µM Cd resulted in a 7.5-fold increase in ZnT1 expression. However, ZnT1 fold induction declined from 7.5 at 24 h to 1.4 at 48 h, suggesting cytotoxicity of 1 µM Cd when exposure was continued for a further 24 h.
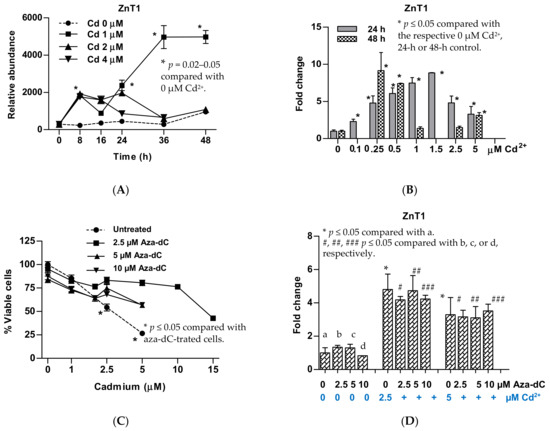
Figure 1.
Change in expression of the ZnT1 gene in response to cadmium and inhibition of DNA methylation. (A) Expression levels of the ZnT1 gene as a function of exposure durations and Cd2+ concentrations; (B) expression levels of the ZnT1 gene 24 h and 48 h after exposure to various Cd2+ concentrations; (C) resistance to Cd toxicity in aza-dC-treated cells; (D) changes in ZnT1 expression in aza-dC-treated cells. Fold change or a ratio was defined as the number of transcripts of a given gene relative to β-actin in treated cells divided by number of transcripts of the same gene relative to β-actin control cells (0 µM Cd2+). ZnT1 expressions in (A,B) were from different batches of cells. The effect of 48-h exposure was not assessed for 0.1 and 1.5 µM Cd2+ concentrations (B).
UROtsa cells treated with aza-dC 24 h prior to Cd exposure exhibited tolerance to Cd toxicity (Figure 1C). The concentration of Cd causing 50% loss of cell viability was increased from 2.5 to 5 µM Cd. Treatment with aza-dC did not affect ZnT1 expression nor did the extent of Cd-induced ZnT1 expression (Figure 1D). Furthermore, the resistance to Cd toxicity was observed 96 h after aza-dCd treatment [48]. This may suggest the rate of Cd efflux may have exceeded or equaled to the rates of Cd influx. In theory, however, intracellular Cd-to-Zn ratio would be an important contributor to an efflux of Cd, given that ZnT1 mediates the efflux of both Zn and Cd [22,25,31].
4. Insights from Transformed UROtsa Cells
Seven transformed UROtsa clones have been produced from independent UROtsa cell culture batches and they are named as UTCd1–UTCd7 [68]. The average doubling times of UTCd2, 3, 4, 5, and 6 ranged between 16.4 and 20.7 h. These doubling times were shorter than those of the parent UROtsa cells, with an average doubling time of 33.2 h. UTCd1 and UTCd7 had average doubling time lengths in the same range as the parental UROtsa cells. By subcutaneous injection, all seven transformed UROtsa clones formed tumors characteristic of TCC in nude mice. Of seven transformed clones, UTCd1 formed tumors in all five nude mice. UTCd3 and UTCd 7 formed tumors in four mice, while UTCd5 formed tumors in three mice, and the remaining clones (UTCd2, 4, 6) formed tumors in two mice. Transcript levels of ZnT and ZIP Zn transporters expressed by UTCd1-UTCd7 relative to parental UROtsa cells are reproduced in Table 2.

Table 2.
Expression of zinc transporter genes in seven transformed UROtsa clones relative to non-tumorigenic parent UROtsa cells.
By intraperitoneal injection, only the UTCd1 clone showed the propensity to invade other tissues. UTCd6 had a doubling time 40% shorter than UTCd1 (16.9 h vs. 27.8 h) but it had lower malignancy potential as this clone formed tumors in only two mice, while UTCd1 formed tumors in all five nude mice. Herein, expression profiles of ZnT and ZIP classes in this UTCd1 clone are recapitulated, together with those of UCdT6 that had shorter doubling time but were lacking invasive potential (Figure 2).
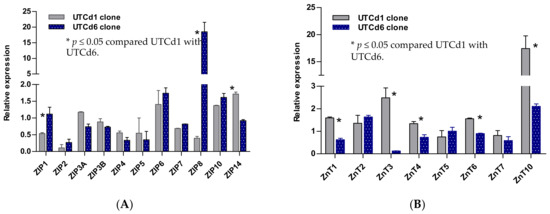
Figure 2.
Expression profiles for zinc transporter genes in UTCd1 vs. UTCd6. Relative expression levels of ZIP zinc transporters (A). Expression levels of ZnT zinc transporters (B). The ratio was defined as number of transcripts of a given gene in each clone relative to β–actin/number of transcripts of the same gene in the parental UROtsa cell line relative to β-actin. A ratio of 1, less than 1, or greater than 1 indicates no change, a decrease, or an increase in expression levels, respectively, compared with the parent UROtsa cells. Expression levels of Zn transporters are as reported by Satarug et al. [30].
UTCd1 and UTCd6 both showed elevated expression levels of ZIP6 and ZIP10, known to regulate cell migration [92,93,94], but only UTCd1 exhibited invasiveness. These data suggest that ZIP1 downregulation, together with ZIP14 upregulation, may also be required to gain the invasive propensities that UTCd1 exhibited. Decreased ZIP1 expression and zinc depletion were associated with the onset of prostate cancer, whereas ZIP1 overexpression decreased malignancy potential [95].
Expression of the ZIP6 and ZIP10 genes was increased in breast cancers with highly invasive properties [91], suggesting the function of ZIP6 and ZIP10 to be analogous to their homologous Drosophila fear of intimacy (foi) gene, involved in the control of cell migration [93]. Likewise, increased ZIP6 expression in human cervical cancer cells was associated with invasive properties through the MAPK/Snail-Slug signaling pathway [96]. Increased expression of ZIP4, ZIP11, ZnT1, or ZnT6 has been associated with poor prognosis in patients with pancreatic adenocarcinoma [29].
The UTCd1 had a doubling time similar to parental UROtsa cells and it was the only clone in which ZIP8 expression was reduced concomitantly with an increased ZnT1. This UTCd1 clone may thus have acquired tolerance to Cd2+ toxicity, and the increased ZnT1 gene expression potentially reflected perturbation of cellular zinc homeostasis. The acquired Cd tolerance may have increased the probability of surviving in the continuing presence of Cd, during which cells were induced to adapt. Furthermore, suppression of ZnT1 expression by miR-411 resulted in the inhibition of growth and metastasis of bladder cancer [97].
Several mechanisms have been postulated to explain how chronic exposure to Cd increases cancer risk. These include oxidative stress, apoptosis resistance, defective DNA damage repair, and altered gene expression [98]. Data from UROtsa cells reviewed herein suggest that chronic exposure to low-level Cd induces cellular adaptive responses involving MT, and Zn (Cd) transporters, notably ZnT1 efflux transporter, to acquire Cd tolerance. This adaptive survival mechanism was also seen in rodent lung epithelial cells, in which the ZIP8 gene was downregulated after chronic Cd exposure [99]. However, the diminished ZIP8 expression also caused a reduction in cellular Zn uptake and Zn deficiency [99]. Thus, immediate cell survival at the cost of a high intracellular Cd-to-Zn ratio elevating risk of carcinogenicity could be a double-edged sword in cell response to Cd exposures.
In another study, a continuous exposure to 5 μM Cd2+ for 20 wks transformed the HPL-1D, human peripheral lung epithelium, to cancer cells, displaying cancer cell characteristics; decreased expression of the tumor suppressor genes p16 and SLC38A3 increased expression of the oncoproteins KRAS and NRAS and vimentin, the epithelial-to-mesenchymal transition marker [100]. Most importantly, transformed HPL-1D cells exhibited Cd tolerance, evident from a diminished Cd accumulation, and increased expression of MT-1A, MT-2A, HO-1, ZnT1, ZnT5 and ZIP8. Therefore, we argue that perturbation of Zn homeostasis through changes in the expression of Zn2+ (Cd2+) transporter genes is a universal mechanism by which Cd induces malignant cell transformation.
5. Conclusions
The carcinogenicity of Cd in a cell culture model of human urothelium (UROtsa) could be attributed, at least in part, to Cd-induced dysregulation of cellular zinc homeostasis, reflected by the abnormal expression of multiple ZIP and ZnT Zn transporter genes. Distinctively increased expression of ZnT1, ZnT4, ZnT6 and ZnT10 concomitant with a reduction in expression of ZIP1, ZIP8 and ZIP14 were seen in one transformed UROtsa clone exhibiting a high invasiveness propensity. The high ZnT1 levels expressed by this particular transformed UROtsa clone may suggest that tolerance to Cd could accompany the process of cell transformation in the urothelium. The hypermethylation observed in bladder cancer may also be involved, as it is linked to a downregulation of ZIP8 gene expression. Because Cd is a weak mutagen, it is plausible that the effects of Cd on the expression of zinc transporter genes seen in transformed UROtsa cells are through epigenetic mechanisms, DNA methylation included. This conclusion is consistent with data from cancer genomics showing that epigenetic changes are especially frequent in urothelial carcinoma.
Author Contributions
Conceptualization, S.S., D.A.V. and G.C.G.; methodology, S.S., D.A.V. and G.C.G.; formal analysis, S.S.; investigation, S.S.; resources, D.A.V. and G.C.G.; original draft preparation, S.S.; review and editing, D.A.V. and G.C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by grant number R01 ES015100 from the National Institute of Environmental Health Sciences (NIEHS), NIH, USA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The Commission for Higher Education, Thailand Ministry of Education in partnership with the National Research Centre for Environmental Toxicology, and The University of Queensland provided S.S. overseas travel support. Opinions expressed in this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH, USA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health risk assessment of dietary cadmium intake: Do current guidelines indicate how much is safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Phelps, K.R. Cadmium Exposure and Toxicity. In Metal Toxicology Handbook; Bagchi, D., Bagchi, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 219–274. [Google Scholar]
- Pappas, R.S.; Fresquez, M.R.; Watson, C.H. Cigarette Smoke Cadmium Breakthrough from Traditional Filters: Implications for Exposure. J. Anal. Toxicol. 2015, 39, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F.; Fels, J.; Lee, W.-K.; Zarbock, R. Channels, transporters and receptors for cadmium and cadmium complexes in eukaryotic cells: Myths and facts. BioMetals 2019, 32, 469–489. [Google Scholar] [CrossRef]
- Flanagan, P.R.; McLellan, J.S.; Haist, J.; Cherian, M.G.; Chamberlain, M.J.; Valberg, L.S. Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterology 1978, 46, 609–623. [Google Scholar] [CrossRef]
- Finley, J.W. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am. J. Clin. Nutr. 1999, 70, 37–43. [Google Scholar] [CrossRef]
- Fujishiro, H.; Hamao, S.; Tanaka, R.; Kambe, T.; Himeno, S. Concentration-dependent roles of DMT1 and ZIP14 in cadmium absorption in Caco-2 cells. J. Toxicol. Sci. 2017, 42, 559–567. [Google Scholar] [CrossRef]
- Aydemir, T.B.; Cousins, R.J. The Multiple Faces of the Metal Transporter ZIP14 (SLC39A14). J. Nutr. 2018, 148, 174–184. [Google Scholar] [CrossRef]
- Jenkitkasemwong, S.; Wang, C.-Y.; MacKenzie, B.; Knutson, M.D. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. BioMetals 2012, 25, 643–655. [Google Scholar] [CrossRef]
- Rentschler, G.; Kippler, M.; Axmon, A.; Raqib, R.; Skerfving, S.; Vahter, M.; Broberg, K. Cadmium concentrations in human blood and urine are associated with polymorphisms in zinc transporter genes. Metallomics 2014, 6, 885–891. [Google Scholar] [CrossRef]
- Moulis, J.M.; Bourguinon, J.; Catty, P. Chapter 23 Cadmium. In RSC Metallobiology Series No. 2, Binding, Transport and Storage of Metal. Ions in Biological Cells; Wolfgang, M., Anthony, W., Eds.; The Royal Society of Chemistry: London, UK, 2014; pp. 695–746. [Google Scholar]
- Carpenter, M.C.; Shah, A.S.; DeSilva, S.; Gleaton, A.; Su, A.; Goundie, B.; Croteau, M.L.; Stevenson, M.J.; Wilcox, D.E.; Austin, R.N. Thermodynamics of Pb(ii) and Zn(ii) binding to MT-3, a neurologically important metallothionein. Metallomics 2016, 8, 605–617. [Google Scholar] [CrossRef]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc Homeostasis in Humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [PubMed]
- Lichten, L.A.; Cousins, R.J. Mammalian Zinc Transporters: Nutritional and Physiologic Regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Polykretis, P.; Cencetti, F.; Donati, C.; Luchinat, E.; Banci, L. Cadmium effects on superoxide dismutase 1 in human cells revealed by NMR. Redox Biol. 2019, 21, 101102. [Google Scholar] [CrossRef] [PubMed]
- Nishito, Y.; Kambe, T. Absorption Mechanisms of Iron, Copper, and Zinc: An Overview. J. Nutr. Sci. Vitaminol. 2018, 64, 1–7. [Google Scholar] [CrossRef]
- Kambe, T.; Taylor, K.M.; Fu, D. Zinc transporters and their functional integration in mammalian cells. J. Biol. Chem. 2021, 296, 100320. [Google Scholar] [CrossRef]
- Nishito, Y.; Kambe, T. Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 2019, 294, 15686–15697. [Google Scholar] [CrossRef]
- Huang, L.; Tepaamorndech, S. The SLC30 family of zinc transporters—A review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 2013, 34, 548–560. [Google Scholar] [CrossRef]
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Lehvy, A.I.; Horev, G.; Golan, Y.; Glaser, F.; Shammai, Y.; Assaraf, Y.G. Alterations in ZnT1 expression and function lead to impaired intracellular zinc homeostasis in cancer. Cell Death Discov. 2019, 5, 144. [Google Scholar] [CrossRef]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target. Ther. 2017, 2, 17029. [Google Scholar] [CrossRef]
- Pan, Z.; Choi, S.; Ouadid-Ahidouch, H.; Yang, J.-M.; Beattie, J.H.; Korichneva, I. Zinc transporters and dysregulated channels in cancers. Front. Biosci. 2017, 22, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Takatani-Nakase, T. Zinc Transporters and the Progression of Breast Cancers. Biol. Pharm. Bull. 2018, 41, 1517–1522. [Google Scholar] [CrossRef]
- Zhu, B.; Huo, R.; Zhi, Q.; Zhan, M.; Chen, X.; Hua, Z.-C. Increased expression of zinc transporter ZIP4, ZIP11, ZnT1, and ZnT6 predicts poor prognosis in pancreatic cancer. J. Trace Elem. Med. Biol. 2021, 65, 126734. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.; Somji, S.; Sens, M.; Sens, D. Aberrant Expression of ZIP and ZnT Zinc Transporters in UROtsa Cells Transformed to Malignant Cells by Cadmium. Stresses 2021, 1, 78–89. [Google Scholar] [CrossRef]
- Ohana, E.; Sekler, I.; Kaisman, T.; Kahn, N.; Cove, J.; Silverman, W.F.; Amsterdam, A.; Hershfinkel, M. Silencing of ZnT-1 expression enhances heavy metal influx and toxicity. J. Mol. Med. 2006, 84, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, M.; Cherrington, N.J.; Hartley, D.P.; Klaassen, C.D.; Waalkes, M.P. Cyproterone acetate induces a cellular tolerance to cadmium in rat liver epithelial cells involving reduced cadmium accumulation. Toxicology 2001, 165, 13–25. [Google Scholar] [CrossRef]
- Fujishiro, H.; Okugaki, S.; Yasumitsu, S.; Enomoto, S.; Himeno, S. Involvement of DNA hypermethylation in down-regulation of the zinc transporter ZIP8 in cadmium-resistant metallothionein-null cells. Toxicol. Appl. Pharmacol. 2009, 241, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Ohashi, T.; Takuma, M.; Himeno, S. Suppression of ZIP8 expression is a common feature of cadmium-resistant and manganese-resistant RBL-2H3 cells. Metallomics 2013, 5, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Aiba, I.; Hossain, A.; Kuo, M.T. Elevated GSH Level Increases Cadmium Resistance through Down-Regulation of Sp1-Dependent Expression of the Cadmium Transporter ZIP8. Mol. Pharmacol. 2008, 74, 823–833. [Google Scholar] [CrossRef]
- Vašák, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. JBIC J. Biol. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef]
- Petering, D.H. Reactions of the Zn Proteome with Cd2+ and Other Xenobiotics: Trafficking and Toxicity. Chem. Res. Toxicol. 2016, 30, 189–202. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism. Int. J. Mol. Sci. 2017, 18, 1237. [Google Scholar] [CrossRef]
- Boonprasert, K.; Satarug, S.; Morais, C.; Gobe, G.C.; Johnson, D.W.; Na-Bangchang, K.; Vesey, D.A. The stress response of human proximal tubule cells to cadmium involves up-regulation of haemoxygenase 1 and metallothionein but not cytochrome P450 enzymes. Toxicol. Lett. 2016, 249, 5–14. [Google Scholar] [CrossRef]
- Boonprasert, K.; Ruengweerayut, R.; Aunpad, R.; Satarug, S.; Na-Bangchang, K. Expression of metallothionein isoforms in peripheral blood leukocytes from Thai population residing in cadmium-contaminated areas. Environ. Toxicol. Pharmacol. 2012, 34, 935–940. [Google Scholar] [CrossRef]
- Hennigar, S.R.; Kelley, A.M.; McClung, J.P. Metallothionein and Zinc Transporter Expression in Circulating Human Blood Cells as Biomarkers of Zinc Status: A Systematic Review. Adv. Nutr. 2016, 7, 735–746. [Google Scholar] [CrossRef]
- Garrett, S.H.; Sens, M.A.; Todd, J.H.; Somji, S.; Sens, D.A. Expression of MT-3 protein in the human kidney. Toxicol. Lett. 1999, 105, 207–214. [Google Scholar] [CrossRef]
- Vašák, M.; Meloni, G. Mammalian Metallothionein-3: New Functional and Structural Insights. Int. J. Mol. Sci. 2017, 18, 1117. [Google Scholar] [CrossRef]
- Sabolić, I.; Skarica, M.; Ljubojevic, M.; Breljak, D.; Herak-Kramberger, C.M.; Crljen, V.; Ljubešić, N. Expression and immunolocalization of metallothioneins MT1, MT2 and MT3 in rat nephron. J. Trace Elem. Med. Biol. 2018, 46, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.V.; Barrick, B.; Christopher, E.P.; Shafer, M.M.; Makar, K.W.; Song, X.; Lampe, J.W.; Vilchis, H.; Ulery, A.; Newcomb, P.A. Genetic variation in metallothionein and metal-regulatory transcription factor 1 in relation to urinary cadmium, copper, and zinc. Toxicol. Appl. Pharmacol. 2015, 289, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Mehra, R.K.; Bremner, I. Measurement of Plasma Metallothionein-I in the Assessment of the Zinc Status of Zinc-Deficient and Stressed Rats. J. Nutr. 1984, 114, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, V.K.; Burnett, F.R.; Cousins, R.J. Metallothionein Expression Is Increased in Monocytes and Erythrocytes of Young Men during Zinc Supplementation. J. Nutr. 1998, 128, 707–713. [Google Scholar] [CrossRef]
- Takeda, K.; Fujita, H.; Shibahara, S. Differential Control of the Metal-Mediated Activation of the Human Heme Oxygenase-1 and Metallothionein IIa Genes. Biochem. Biophys. Res. Commun. 1995, 207, 160–167. [Google Scholar] [CrossRef]
- Katakai, K.; Liu, J.; Nakajima, K.; Keefer, L.K.; Waalkes, M.P. Nitric oxide induces metallothionein (MT) gene expression apparently by displacing zinc bound to MT. Toxicol. Lett. 2001, 119, 103–108. [Google Scholar] [CrossRef]
- McNeill, R.V.; Mason, A.S.; Hodson, M.E.; Catto, J.W.F.; Southgate, J. Specificity of the metallothionein-1 response by cadmium-exposed normal human urothelial cells. Int. J. Mol. Sci. 2019, 20, 1344. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.; Somji, S.; Sens, M.; Sens, D. Zinc, Zinc Transporters, and Cadmium Cytotoxicity in a Cell Culture Model of Human Urothelium. Toxics 2021, 9, 94. [Google Scholar] [CrossRef]
- Misra, R.R.; Hochadel, J.F.; Smith, G.T.; Cook, J.C.; Waalkes, M.P.; Wink, D.A. Evidence That Nitric Oxide Enhances Cadmium Toxicity by Displacing the Metal from Metallothionein. Chem. Res. Toxicol. 1996, 9, 326–332. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Reilly, P.E.; Esumi, H.; Moore, M.R. Evidence for a Synergistic Interaction between Cadmium and Endotoxin Toxicity and for Nitric Oxide and Cadmium Displacement of Metals in the Kidney. Nitric Oxide 2000, 4, 431–440. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef]
- Sabolić, I.; Breljak, D.; Skarica, M.; Herak-Kramberger, C.M. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 2010, 23, 897–926. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The source and pathophysiologic significance of excreted cadmium. Toxics 2019, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.; Phelps, K. The Effect of Cadmium on GFR Is Clarified by Normalization of Excretion Rates to Creatinine Clearance. Int. J. Mol. Sci. 2021, 22, 1762. [Google Scholar] [CrossRef]
- Knoell, D.L.; Smith, D.; Bao, S.; Sapkota, M.; Wyatt, T.A.; Zweier, J.L.; Flury, J.; Borchers, M.T.; Knutson, M. Imbalance in zinc homeostasis enhances lung Tissue Loss following cigarette smoke exposure. J. Trace Elem. Med. Biol. 2020, 60, 126483. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Baker, J.R.; Reilly, P.E.; Moore, M.R.; Williams, D.J. Changes in zinc and copper homeostasis in human livers and kidneys associated with exposure to environmental cadmium. Hum. Exp. Toxicol. 2001, 20, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Nishijo, M.; Ujjin, P.; Moore, M.R. Chronic exposure to low-level cadmium induced zinc-copper dysregulation. J. Trace Elem. Med. Biol. 2018, 46, 32–38. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Ujjin, P.; Vesey, D.A. Gender differences in zinc and copper excretion in response to co-exposure to low environmental concentrations of cadmium and dead. Stresses 2021, 1, 3–15. [Google Scholar] [CrossRef]
- Eom, S.Y.; Yim, D.H.; Huang, M.; Park, C.H.; Kim, G.B.; Yu, S.D.; Choi, B.S.; Park, J.D.; Kim, Y.D.; Kim, H. Copper-zinc imbalance induces kidney tubule damage and oxidative stress in a population exposed to chronic environmental cadmium. Int. Arch. Occup. Environ. Health 2020, 93, 337–344. [Google Scholar] [CrossRef]
- Pasin, E.; Josephson, D.Y.; Mitra, A.P.; Cote, R.J.; Stein, J.P. Superficial Bladder Cancer: An Update on Etiology, Molecular Development, Classification, and Natural History. Rev. Urol. 2008, 10, 31–43. [Google Scholar]
- Hong, Y.M.; Loughlin, K.R. Economic Impact of Tumor Markers in Bladder Cancer Surveillance. Urology 2008, 71, 131–135. [Google Scholar] [CrossRef]
- Cumberbatch, M.G.; Cox, A.; Teare, D.; Catto, J.W. Contemporary occupational carcinogen exposure and bladder cancer: A systematic review and meta-analysis. JAMA Oncol. 2015, 1, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Garren, B.; Nielsen, M.E.; Tang, L. Lifestyle and nutritional modifiable factors in the prevention and treatment of bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Dy, G.W.; Gore, J.L.; Forouzanfar, M.H.; Naghavi, M.; Fitzmaurice, C. Global Burden of Urologic Cancers, 1990–2013. Eur. Urol. 2017, 71, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Nishiyama, H. Epidemiology of urothelial carcinoma. Int. J. Urol. 2017, 24, 730–734. [Google Scholar] [CrossRef]
- Samanic, C.; Kogevinas, M.; Dosemeci, M.; Malats, N.; Real, F.X.; Garcia-Closas, M.; Real, F.X.; Garcia-Closas, M.; Serra, C.; Carrato, A.; et al. Smoking and bladder cancer in Spain: Effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol. Prev. Biomark. 2006, 15, 1348–1354. [Google Scholar] [CrossRef]
- Sciannameo, V.; Carta, A.; D’Errico, A.; Giraudo, M.T.; Fasanelli, F.; Arici, C.; Maule, M.; Carnà, P.; Destefanis, P.; Rolle, L.; et al. New insights on occupational exposure and bladder cancer risk: A pooled analysis of two Italian case-control studies. Int. Arch. Occup. Environ. Health 2018, 92, 347–359. [Google Scholar] [CrossRef]
- Kellen, E.; Zeegers, M.P.; Hond, E.D.; Buntinx, F. Blood cadmium may be associated with bladder carcinogenesis: The Belgian case-control study on bladder cancer. Cancer Detect. Prev. 2007, 31, 77–82. [Google Scholar] [CrossRef]
- Wolf, C.; Strenziok, R.; Kyriakopoulos, A. Elevated metallothionein-bound cadmium concentrations in urine from bladder carcinoma patients, investigated by size exclusion chromatography-inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2009, 631, 218–222. [Google Scholar] [CrossRef]
- Achanzar, W.E.; Diwan, B.A.; Liu, J.; Quader, S.T.; Webber, M.M.; Waalkes, M.P. Cadmium-induced malignant transformation of human prostate epithelial cells. Cancer Res. 2001, 61, 455–458. [Google Scholar] [PubMed]
- Sens, D.A.; Park, S.; Gurel, V.; Sens, M.A.; Garrett, S.H.; Somji, S. Inorganic cadmium- and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol. Sci. 2004, 79, 56–63. [Google Scholar] [CrossRef]
- Hoggarth, Z.E.; Osowski, D.B.; Freeberg, B.A.; Garrett, S.H.; Sens, D.A.; Sens, M.A.; Zhou, X.D.; Zhang, K.K.; Somji, S. The urothelial cell line UROtsa transformed by arsenite and cadmium display basal characteristics associated with muscle invasive urothelial cancers. PLoS ONE 2018, 13, e0207877. [Google Scholar] [CrossRef]
- Benbrahim-Tallaa, L.; Tokar, E.J.; Diwan, B.A.; Dill, A.L.; Coppin, J.-F.; Waalkes, M.P. Cadmium Malignantly Transforms Normal Human Breast Epithelial Cells into a Basal-like Phenotype. Environ. Health Perspect. 2009, 117, 1847–1852. [Google Scholar] [CrossRef]
- Soh, M.A.; Garrett, S.H.; Somji, S.; Dunlevy, J.R.; Zhou, X.D.; Sens, M.A.; Bathula, C.S.; Allen, C.; Sens, D.A. Arsenic, cadmium and neuron specific enolase (ENO2, γ-enolase) expression in breast cancer. Cancer Cell Int. 2011, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Blommel, K.; Knudsen, C.S.; Wegner, K.; Shrestha, S.; Singhal, S.K.; Mehus, A.A.; Garrett, S.H.; Singhal, S.; Zhou, X.; Voels, B.; et al. Meta-analysis of gene expression profiling reveals novel basal gene signatures in MCF-10A cells transformed with cadmium. Oncotarget 2020, 11, 3601–3617. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, L.Z.; Jiang, Y.; Zhu, Y.; Guo, N.L.; Barnett, J.; Rojanasakul, Y.; Agani, F.; Jiang, B.H. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol. Sci. 2012, 125, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Tokar, E.J.; Kim, A.J.; Bell, M.W.; Waalkes, M.P. Chronic Cadmium Exposure In Vitro Causes Acquisition of Multiple Tumor Cell Characteristics in Human Pancreatic Epithelial Cells. Environ. Health Perspect. 2012, 120, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, M.; Achanzar, W.E.; Qu, W.; Li, G.; Waalkes, M.P. Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp. Cell Res. 2003, 286, 355–365. [Google Scholar] [CrossRef]
- Qu, W.; Diwan, B.A.; Reece, J.M.; Bortner, C.D.; Pi, J.; Liu, J.; Waalkes, M.P. Cadmium-induced malignant transformation in rat liver cells: Role of aberrant oncogene expression and minimal role of oxidative stress. Int. J. Cancer 2005, 114, 346–355. [Google Scholar] [CrossRef]
- Garrett, S.H.; Somji, S.; Sens, N.A.; Zhang, K.K. Prediction of the Number of Activated Genes in Multiple Independent Cd+2- and As+3-Induced Malignant Transformations of Human Urothelial Cells (UROtsa). PLoS ONE 2014, 9, e85614. [Google Scholar] [CrossRef] [PubMed]
- Petzoldt, J.L.; Leigh, I.M.; Duffy, P.G.; Sexton, C.; Masters, J.R.W. Immortalisation of human urothelial cells. Urol. Res. 1995, 23, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.R.; Masters, J.R.W.; Park, S.; Todd, J.H.; Garrett, S.H.; Sens, M.A.; Somji, S.; Nath, J.; Sens, D.A. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ. Health Perspect. 2001, 109, 801–808. [Google Scholar] [CrossRef]
- Sens, D.; Rossi, M.; Park, S.; Gurel, V.; Nath, J.; Garrett, S.; Sens, M.A.; Somji, S. Metallothionein isoform 1 and 2 gene expression in a human urothelial cell line (UROtsa) exposed to CdCl2 and NaAsO2. J. Toxicol. Environ. Health A 2003, 66, 2031–2046. [Google Scholar] [CrossRef]
- Sens, M.A.; Somji, S.; Lamm, D.L.; Garrett, S.H.; Slovinsky, F.; Todd, J.H.; Sens, D.A. Metallothionein isoform 3 as a potential biomarker for human bladder cancer. Environ. Health Perspect. 2000, 108, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.D.; Sens, M.A.; Garrett, S.H.; Somji, S.; Park, S.; Gurel, V.; Sens, D.A. Enhanced expression of metallothionein isoform 3 protein in tumor heterotransplants derived from As+3- and Cd+2-transformed human urothelial cells. Toxicol. Sci. 2006, 93, 322–330. [Google Scholar] [CrossRef]
- Somji, S.; Garrett, S.H.; Toni, C.; Zhou, X.D.; Zheng, Y.; Ajjimaporn, A.; Sens, M.A.; Sens, D.A. Differences in the epigenetic regulation of MT-3 gene expression between parental and Cd+2 or As+3 transformed human urothelial cells. Cancer Cell Int. 2011, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Ajjimaporn, A.; Botsford, T.; Garrett, S.H.; Sens, M.A.; Zhou, X.D.; Dunlevy, J.R.; Sens, D.A.; Somji, S. ZIP8 expression in human proximal tubule cells, human urothelial cells transformed by Cd+2 and As+3 and in specimens of normal human urothelium and urothelial cancer. Cancer Cell Int. 2012, 12, 16. [Google Scholar] [CrossRef]
- Audenet, F.; Attalla, K.; Sfakianos, J.P. The evolution of bladder cancer genomics: What have we learned and how can we use it? Urol. Oncol. Semin. Orig. Investig. 2018, 36, 313–320. [Google Scholar] [CrossRef]
- Kagara, N.; Tanaka, N.; Noguchi, S.; Hirano, T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007, 98, 692–697. [Google Scholar] [CrossRef]
- Mathews, W.R.; Wang, F.; Eide, D.J.; Van Doren, M. Drosophila fear of intimacy Encodes a Zrt/IRT-like Protein (ZIP) Family Zinc Transporter Functionally Related to Mammalian ZIP Proteins. J. Biol. Chem. 2005, 280, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc Transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef]
- Golovine, K.; Makhov, P.; Uzzo, R.G.; Shaw, T.; Kunkle, D.; Kolenko, V.M. Overexpression of the zinc uptake transporter hZIP1 inhibits nuclear factor-κB and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin. Cancer Res. 2008, 14, 5376–5384. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, W.; Taylor, K.M.; Cai, B.; Li, X. LIV-1 suppression inhibits HeLa cell invasion by targeting ERK1/2-Snail/Slug pathway. Biochem. Biophys. Res. Commun. 2007, 363, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, T.; Jin, H.; Yin, L.; Yu, H.; Bi, J. MiR-411 suppresses the development of bladder cancer by regulating ZnT1. OncoTargets Ther. 2018, 11, 8695–8704. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Desmarais, T.; Costa, M. Metals and Mechanisms of Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 537–554. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Gao, Y.-M.; Wu, D.-D.; Yu, F.-Y.; Zang, Z.-S.; Yang, L.; Yao, Y.; Cai, N.-L.; Zhou, Y.; Chiu, J.-F.; et al. Aberrant cytokine secretion and zinc uptake in chronic cadmium-exposed lung epithelial cells. Proteom. Clin. Appl. 2017, 11, 1600059. [Google Scholar] [CrossRef] [PubMed]
- Person, R.J.; Tokar, E.J.; Xu, Y.; Orihuela, R.; Ngalame, N.N.O.; Waalkes, M.P. Chronic cadmium exposure in vitro induces cancer cell characteristics in human lung cells. Toxicol. Appl. Pharmacol. 2013, 273, 281–288. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).