Characterization of Carbonated and Raw Ferronickel Slags as Cementing Materials
Abstract
1. Introduction
- Capture and stable storage of carbon dioxide inside a material.
- Separation of magnesium from silica in two distinct minerals: magnesite (MgCO3) and silica (SiO2). This might lead to a higher reactivity of the silica fraction with calcium in cementitious materials.
2. Materials and Methods
2.1. Feedstock and Carbonation Process
2.1.1. Feedstock Origins
2.1.2. Direct Aqueous Carbonation of the Ferronickel Slag Using an Attrition-Leaching Process
2.2. Analytical Techniques
2.2.1. Determination of the Elemental Composition
2.2.2. Characterization of the Mineralogy
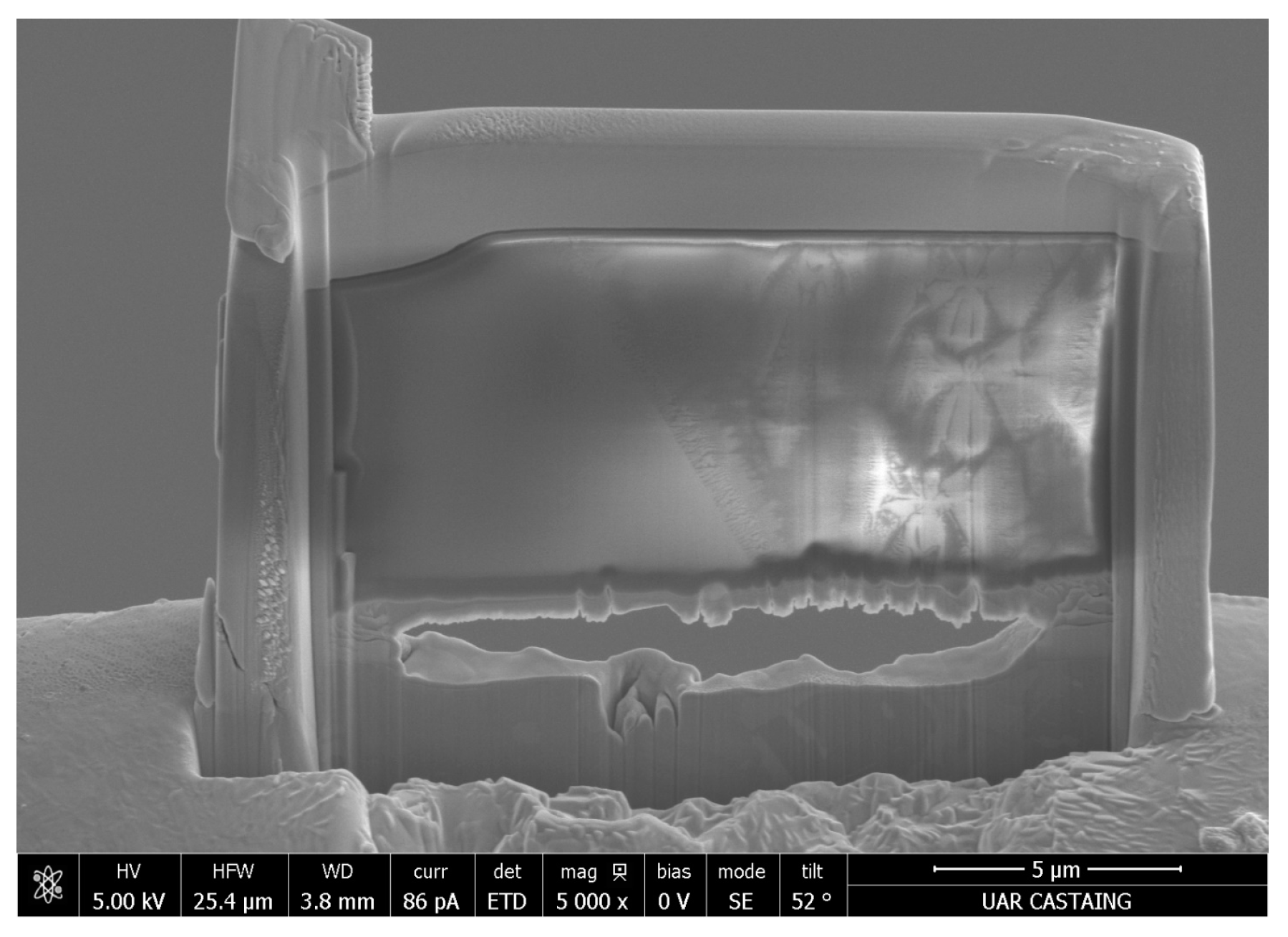
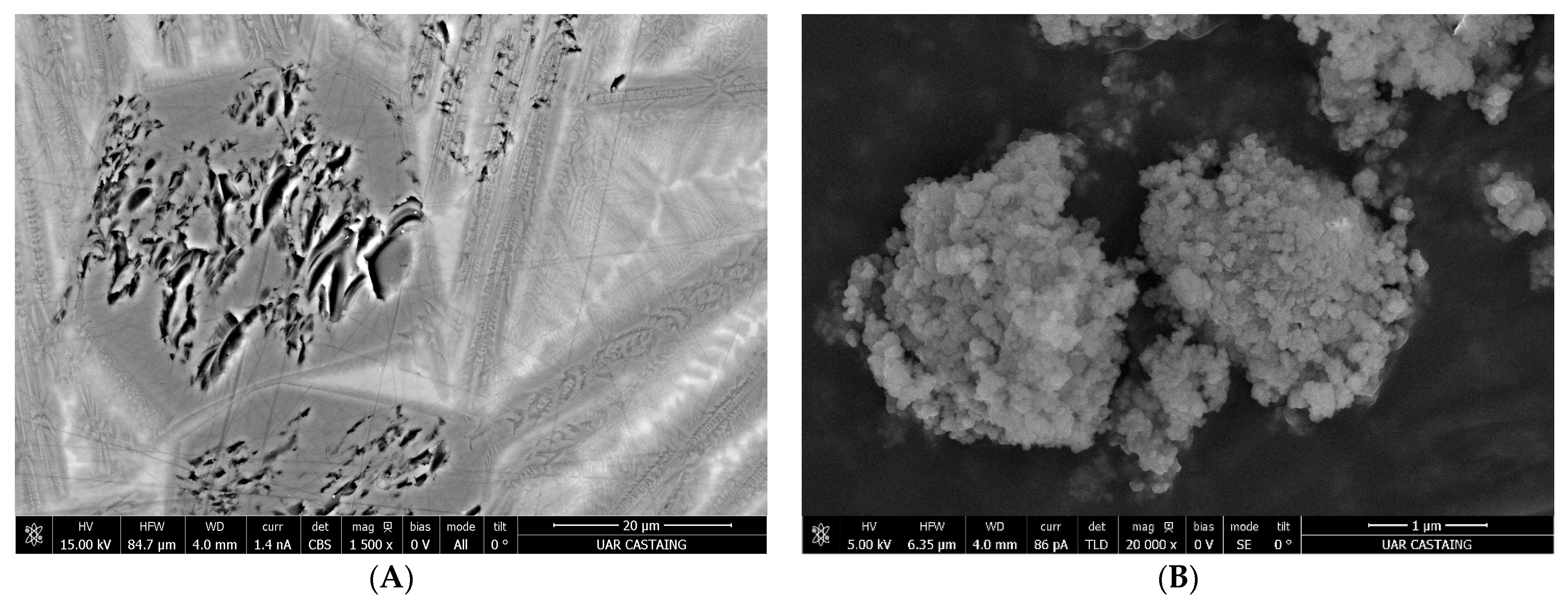
2.2.3. Microstructure
3. Results
3.1. Chemical Analysis
3.2. Mineralogy
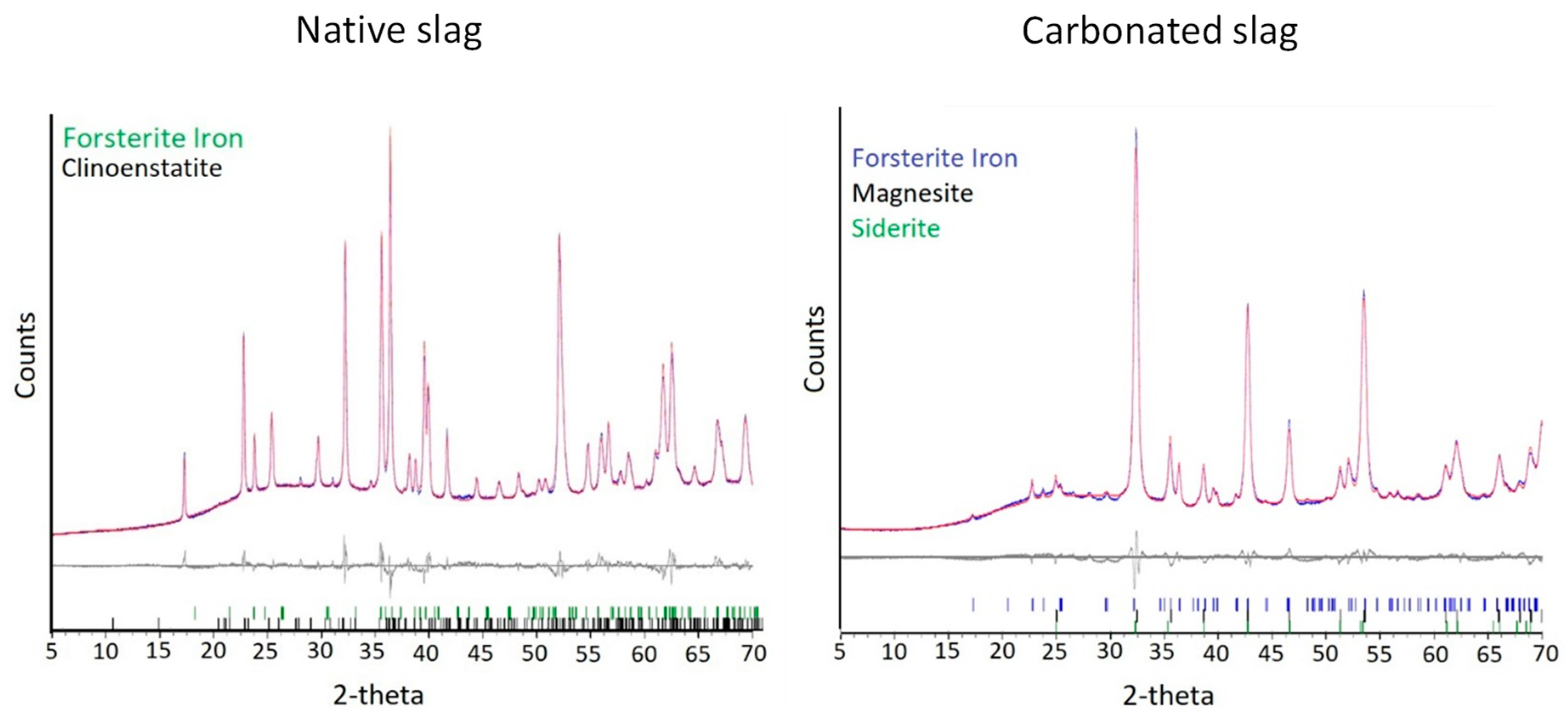
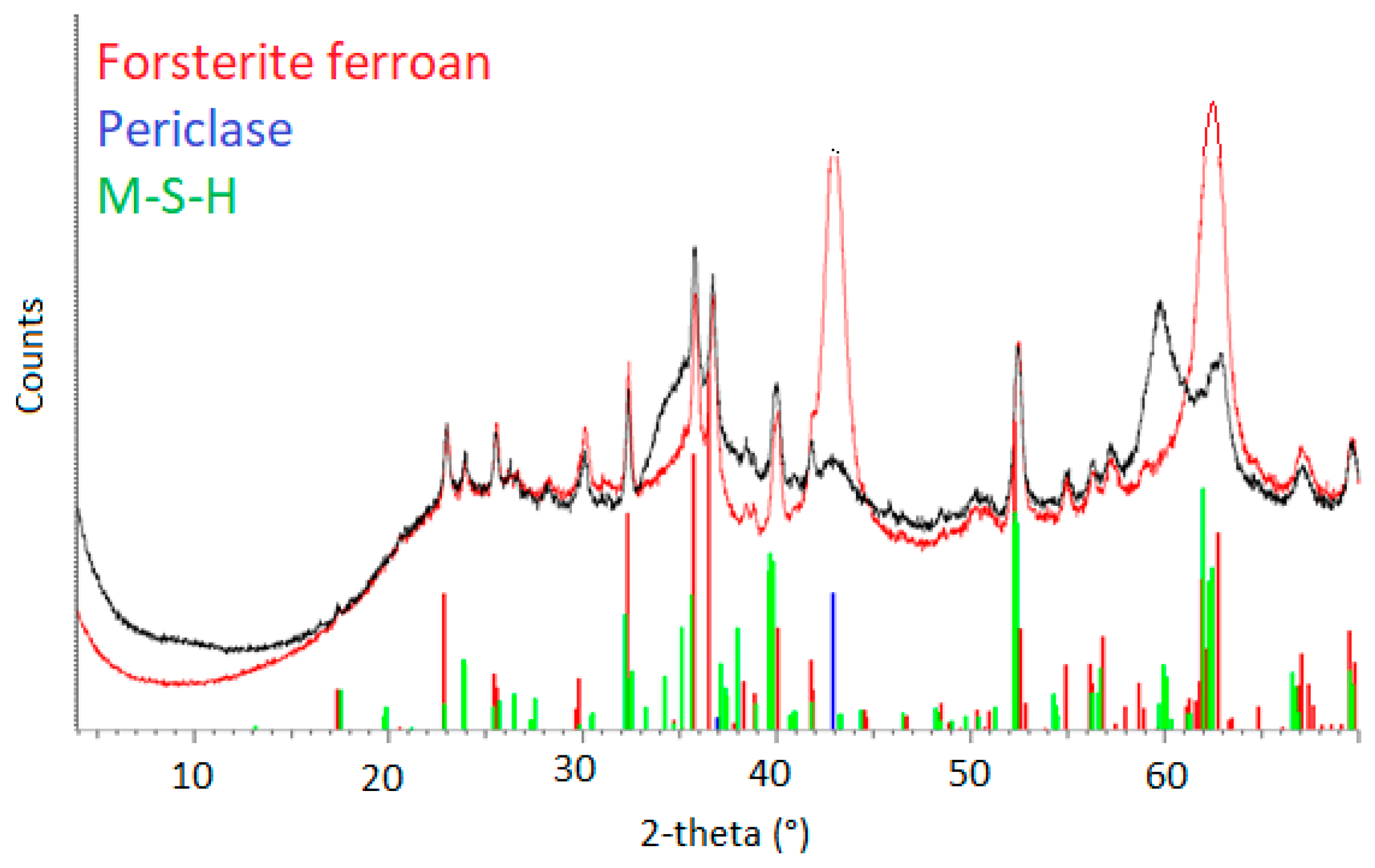
3.2.1. XRD and Rietveld Analysis
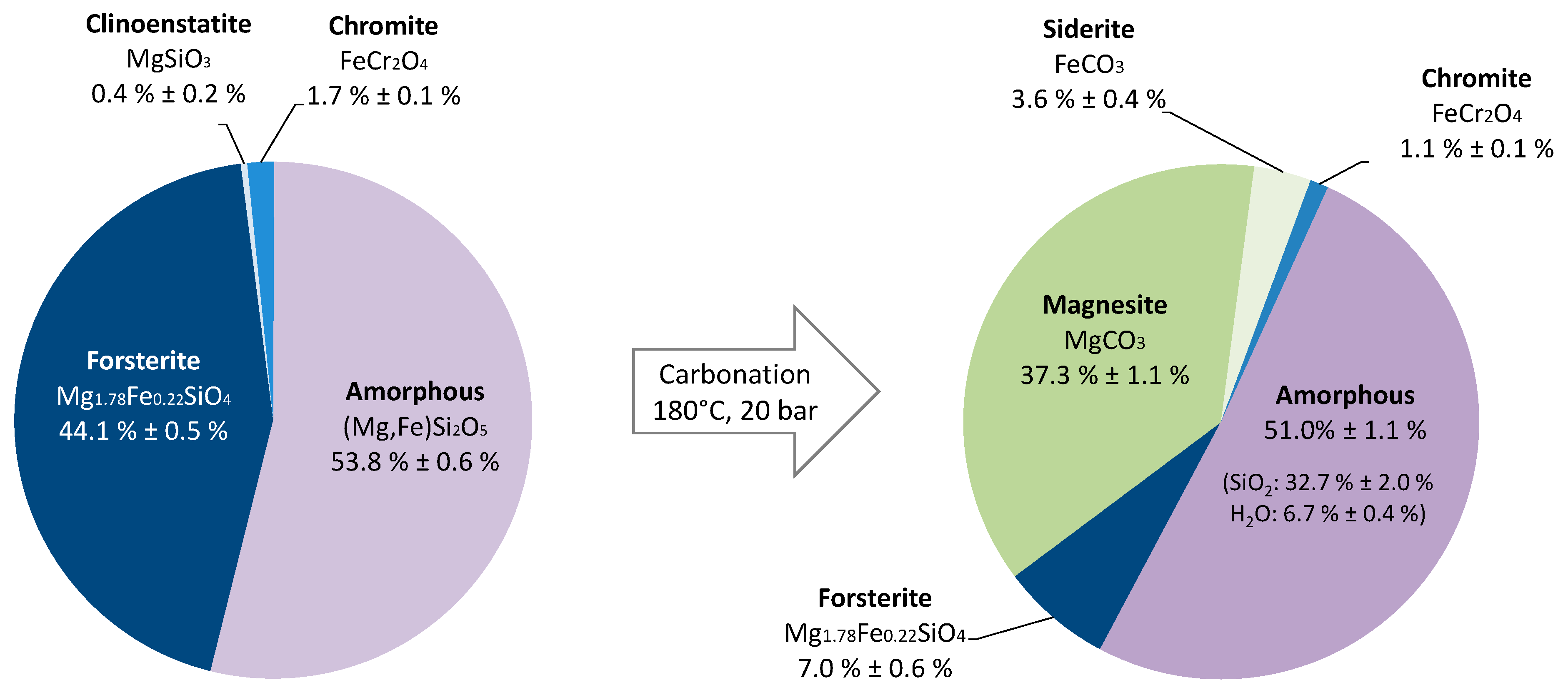
3.2.2. Mass Balances
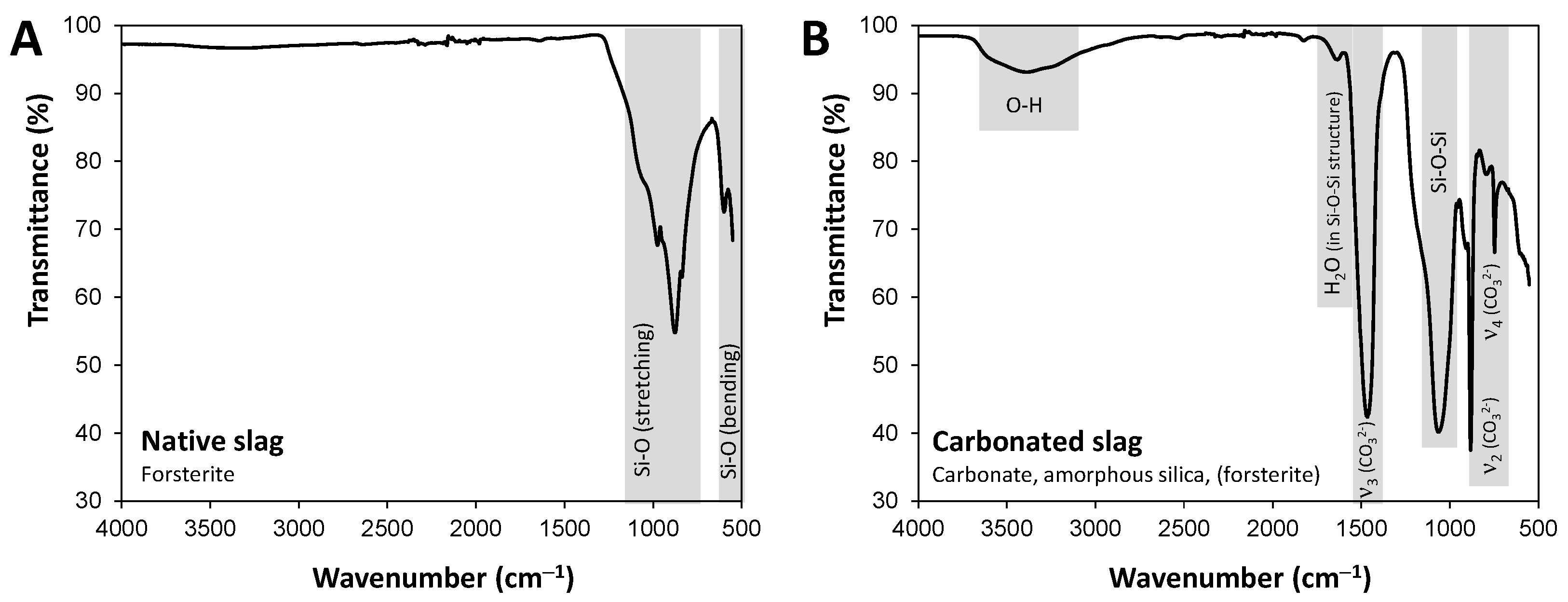
3.2.3. FT-IR
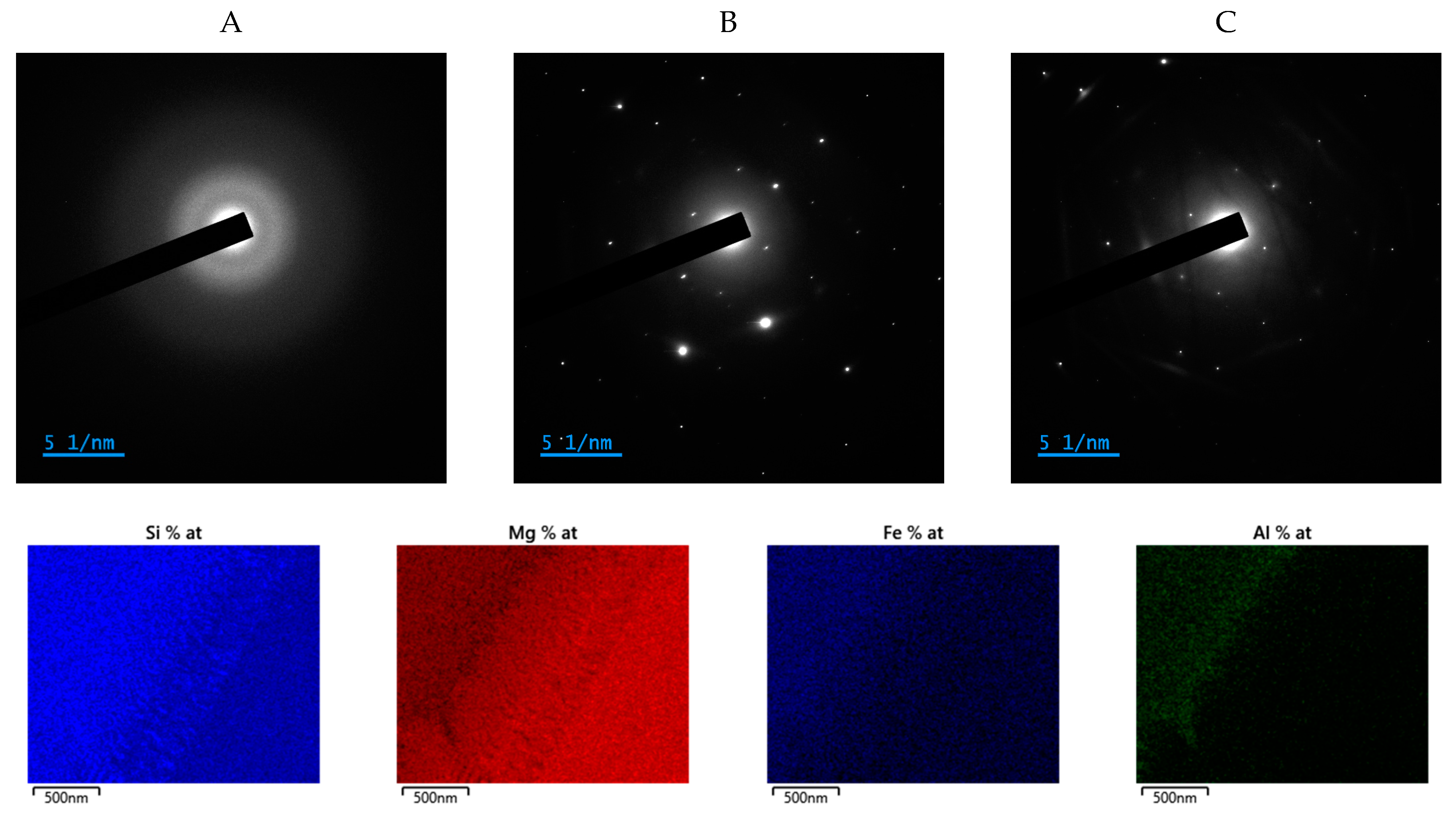
3.2.4. Electron Microscopy Coupled to Elemental Analysis (SEM- and TEM-EDS)
- To confirm the composition of the forsterite (1.8 MgO/0.2 FeO);
- To analyze the amorphous material in terms of elemental composition and variability;
- To observe the carbonates in the carbonated slag, either as separate magnesite and siderite or as a (Mg,Fe)CO3 solid solution.
- Area A, amorphous: Mg/Si = 0.41; Fe/Si = 0.21; Al/Si = 0.13. The Mg/Si ratio is very close to the one calculated with the mass balance (Section 3.2.2); the other two ratios are slightly higher with this technique. However, it confirms that aluminum is only detected in the amorphous phase.
- Area B, crystalline: Mg/Si = 1.8; Fe/Si = 0.2; (Mg + Fe)/Si = 2 and Mg/Fe = 10, which matches with the composition of forsterite determined by the Rietveld refinement: Mg1.8Fe0.2SiO4 (Section 3.2.1).
- Area C, crystalline: Mg/Si = 1.3; Fe/Si = 0.1; (Mg + Fe)/Si = 1.4 and Mg/Fe = 10, which corresponds to no existing magnesium silicate and is probably a mixture of enstatite and forsterite.
- Area A, crystalline, corresponds to carbonates and is mainly composed of magnesium, iron, carbon, and oxygen. As all EDS analyses revealed the presence of Mg and Fe in A, it was deduced that carbonates form a single mixed phase and do not separate magnesite from siderite. This is consistent with thermodynamic calculations, which indicate that the solid solution is the most stable [31]. However, the electron beam could not be correctly focused, and, therefore, EDS did not make it possible to verify the Mg/Fe ratio.
- Area B, crystalline, is mostly composed of magnesium, iron, and silica and, thus, probably corresponds to the residual forsterite.
- Area C, amorphous, is mainly composed of silica and alumina.
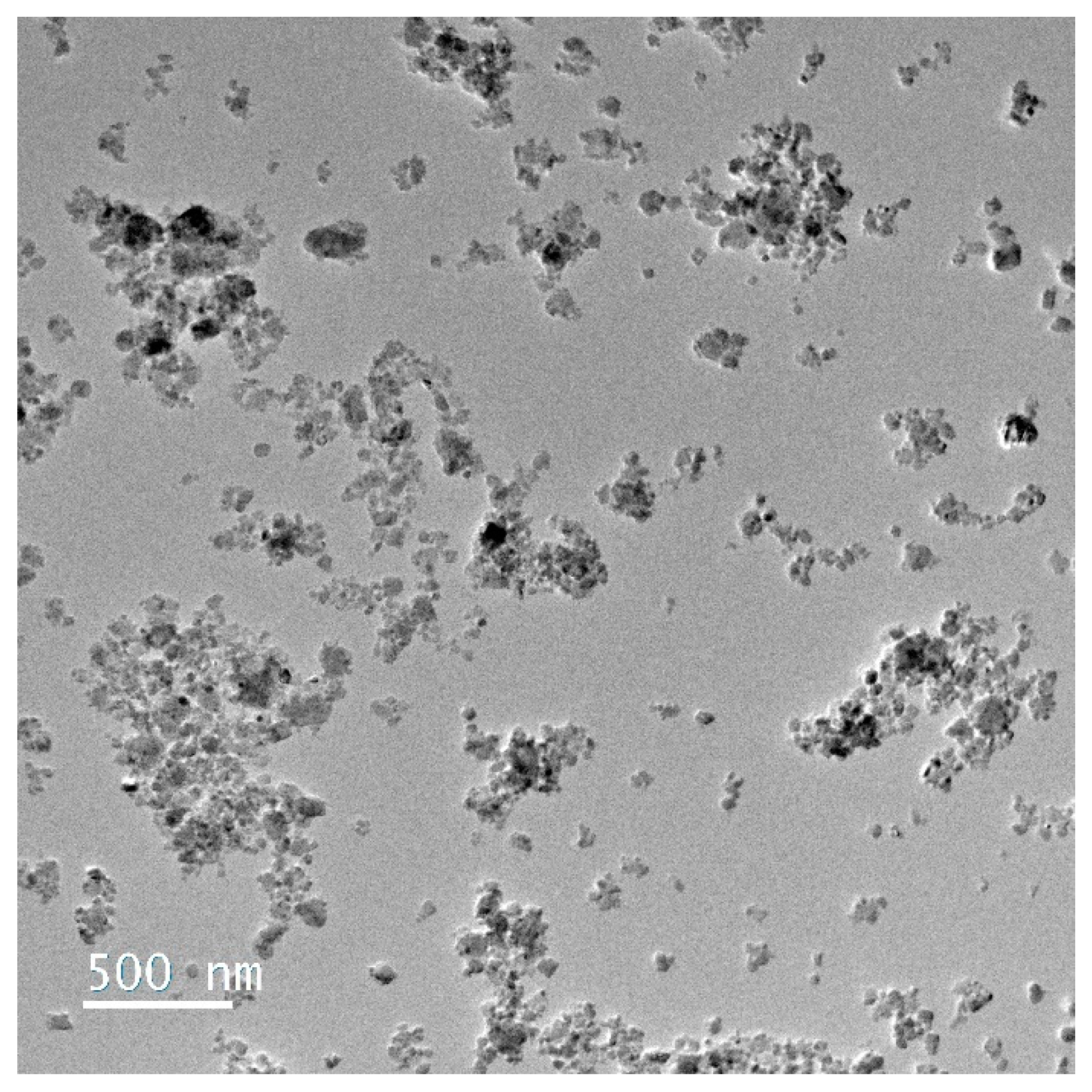
3.3. Morphology
4. Discussion
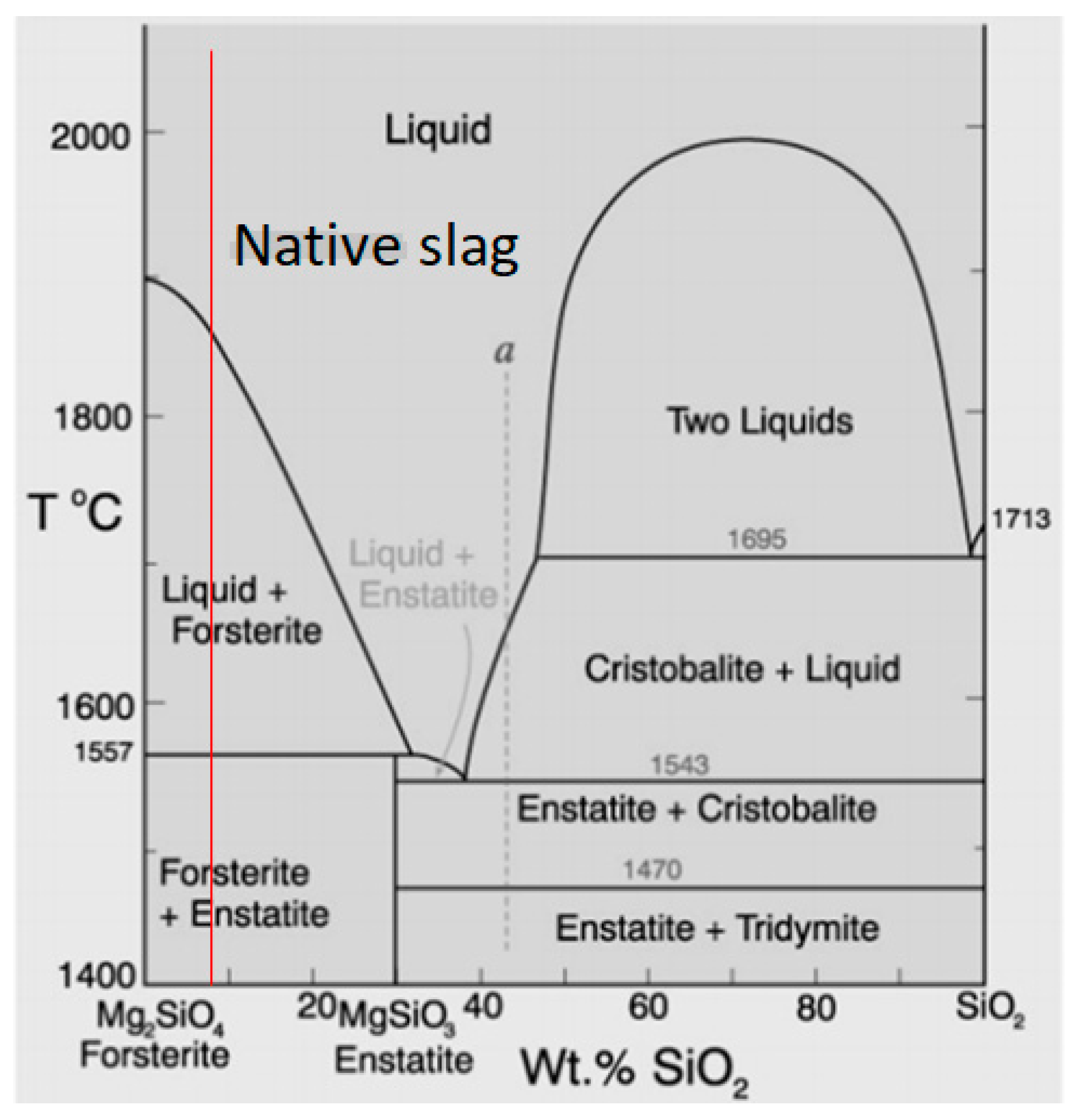
4.1. Understanding the Carbonation Process
4.2. The Reuse of Carbonated Slag
4.3. Carbonated Slag as Pozzolanic Material
4.4. Carbonated Slag as Magnesium-Silicate Binder
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA. Technology Roadmap—Low-Carbon Transition in the Cement Industry; IEA: Paris, France, 2018; Available online: https://www.iea.org/reports/technology-roadmap-low-carbon-transition-in-the-cement-industry (accessed on 3 May 2019).
- Ben Haha, M.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Lavergne, F.; Belhadi, R.; Carriat, J.; Fraj, A.B. Effect of nano-silica particles on the hydration, the rheology and the strength development of a blended cement paste. Cem. Concr. Compos. 2019, 95, 42–55. [Google Scholar] [CrossRef]
- Maragkos, I.; Giannopoulou, I.P.; Panias, D. Synthesis of ferronickel slag-based geopolymers. Miner. Eng. 2009, 22, 196–203. [Google Scholar] [CrossRef]
- Bouasria, M.; Babouri, L.; Khadraoui, F.; Chateigner, D.; Gascoin, S.; Pralong, V.; Benzaama, M.H.; Orberger, B.; El Mendili, Y. Insights into the partial replacement of cement by ferronickel slags from New Caledonia. Eur. J. Environ. Civ. Eng. 2022, 26, 3662–3680. [Google Scholar] [CrossRef]
- Yang, T.; Yao, X.; Zhang, Z. Geopolymer prepared with high-magnesium nickel slag: Characterization of properties and microstructure. Constr. Build. Mater. 2014, 59, 188–194. [Google Scholar] [CrossRef]
- Balomenos, E.; Panias, D.; Mud, R. Iron recovery and production of high added value products from the metallurgical by-products of primary aluminum and ferro-nickel industries. In Proceedings of the 3rd International Slag Valorisation Symposium, Belgium, Leuven, 19–20 March 2013; pp. 161–172. [Google Scholar]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J. Hazard. Mater. 2009, 161, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Le Nickel—SNL. FNS: A Promising Construction Material for the Pacific Region. 2017. Available online: http://sln.nc/sites/default/files/flippingbook/slnslg/fichiers/assets/common/downloads/publicati622on.pdf (accessed on 3 May 2019).
- Yang, T.; Wu, Q.; Zhu, H.; Zhang, Z. Geopolymer with improved thermal stability by incorporating high-magnesium nickel slag. Constr. Build. Mater. 2017, 155, 475–484. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Yang, T.; Li, L.; Zhu, H.; Wang, H. Conversion of local industrial wastes into greener cement through geopolymer technology: A case study of high-magnesium nickel slag. J. Clean. Prod. 2017, 141, 463–471. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, C.; Yang, J.; Chong, L.; Xu, X.; Wu, Q. Influence of nickel slag powders on properties of magnesium potassium phosphate cement paste. Constr. Build. Mater. 2019, 205, 668–678. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, Y.; Tong, W.; Ma, H. Utilization of nickel slag as raw material in the production of Portland cement for road construction. Constr. Build. Mater. 2018, 193, 426–434. [Google Scholar] [CrossRef]
- Shoya, M.; Aba, M.; Tsukinaga, Y.; Tokuhashi, K. Frost resistance and air void system of self-compacting concrete incorporating slag as a fine aggregate. ACI Spec. Publ. 2003, 212, 1093–1108. [Google Scholar]
- Sakoi, Y.; Aba, M.; Tsukinaga, Y.; Nagataki, S. Properties of concrete used in ferronickel slag aggregate. In Proceedings of the 3rd International Conference on Sustainable Construction Materials and Technologies, Japan, Tokyo, 18–21 August 2013; pp. 1–6. [Google Scholar]
- Yang, T.; Zhang, Z.; Wang, Q.; Wu, Q. ASR potential of nickel slag fine aggregate in blast furnace slag-fly ash geopolymer and Portland cement mortars. Constr. Build. Mater. 2020, 262, 119990. [Google Scholar] [CrossRef]
- Bourgeois, F.; Laniesse, P.; Cyr, M.; Julcour, C. Definition and exploration of the integrated CO2 mineralization technological cycle. Front. Energy Res. 2020, 8, 113. [Google Scholar] [CrossRef]
- Julcour-Lebigue, C.; Bourgeois, F.; Bonfils, B.; Benhamed, I.; Guyyot, F.; Bodénan, F.; Petito, C.; Gaucher, E.C. Development of an attrition leaching hybrid process for direct aqueous mineral carbonation. Chem. Eng. J. 2015, 262, 716–726. [Google Scholar] [CrossRef]
- Seetharaman, S. (Ed.) Treatise on Process Metallurgy, Vol. 3: Industrial Processes; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Shi, M. Characteristics and reactivity of ferronickel slag powder. Constr. Build. Mater. 2017, 156, 773–789. [Google Scholar] [CrossRef]
- Julcour, C.; Cassayre, L.; Benhamed, I.; Diouani, J.; Bourgeois, F. Insights Into Nickel Slag Carbonation in a Stirred Bead Mill. Front. Chem. Eng. 2020, 2, 588579. [Google Scholar] [CrossRef]
- Dufourny, A.; Julcour, C.; Esvan, J.; Cassayre, L.; Laniesse, P.; Bourgeois, F. Observation of the depassivation effect of attrition on magnesium silicates direct aqueous carbonation products, Sec. Negative Emission Technologies. Front. Clim. 2022, 4, 946735. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. Physical Measure Laboratory, X-ray form Factor. Attenuation and Scattering Tables. Available online: https://physics.nist.gov/PhysRefData/FFast/html/form.html (accessed on 11 April 2022).
- Toby, B.H. R factor in Rietveld analysis: How good is good enough? Powder Diffr. 2016, 21, 67–70. [Google Scholar] [CrossRef]
- Choudhary, R.; Venkatraman, S.K.; Bulygina, I.; Chatterjee, A.; Abraham, J.; Senatov, F.; Kaloshkin, S.; Ilyasov, A.; Abakumov, M.; Knyazeva, M.; et al. Impact of forsterite addition on mechanical and biological properties of composites. J. Asian Ceram. Soc. 2020, 8, 1051–1065. [Google Scholar] [CrossRef]
- Nojehdehi, A.M.; Moghaddam, F.; Hamedani, M.T. Mechanical properties of glass ionomer cement incorporating forsterite nanoparticles synthesized by the sol-gel method. J. Sol-Gel Sci. Technol. 2023, 107, 161–169. [Google Scholar] [CrossRef]
- Oh, K.D.; Morikawa, H.; Iwai, S.; Aoki, H. The crystal structure of magnesite. Am. Mineral. 1973, 58, 1029–1033. [Google Scholar]
- Dubrawski, J.V.; Channon, A.L.; Warne, S.S.J. Examination of the siderite-magnesite mineral series by Fourier transform infrared spectroscopy. Am. Mineral. 1989, 74, 187–190. [Google Scholar]
- Santillán, J.; Catalli, K.; Williams, Q. An infrared study of carbon-oxygen bonding in magnesite to 60 GPa. Am. Mineral. 2005, 90, 1669–1673. [Google Scholar] [CrossRef]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef] [PubMed]
- Cassayre, L.; Bourgeois, F.; Julcour-Lebigue, C.; Benhamed, I.; Diouani, J.; Nahdi, K. Defining the operating conditions of the attrition-leaching process using thermodynamic process modelling. In Proceedings of the International Mineral Processing Congress IMPC 2016: XXVII, Québec, QC, Canada, 11–15 September 2016. Ref. GHGT14. [Google Scholar]
- Gore, A.Y.; Banker, G.S. Surface chemistry of colloidal silica and possible application to stabilize aspirin in solid matrixes. J. Pharm. Sci. 1979, 62, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Bowen, N.L.; Anderson, O. The binary system: MgO-SiO2. Am. J. Sci. 1914, 37, 487–500. [Google Scholar] [CrossRef]
- Umemoto, K. Phase transitions in MgSiO3 post-perovskite in super-Earth mantles. Earth Planet. Sci. Lett. 2017, 478, 40–45. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Fidjestøl, P.; Lewis, R. Microsilica as an Addition, Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 679–712. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]






| MgO | SiO2 | Al2O3 | CaO | Fe2O3 | Cr2O3 | MnO | ZrO2 | H2O | CO2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| MAC (m2/kg) | 29.0 | 36.4 | 32.1 | 125.9 | 217.4 | 175.6 | 214.4 | 103.3 | 10.3 | 9.6 |
| Quartz (SiO2) | Corundum (Al2O3) | Rutile/Anatase (TiO2) | Zincite (ZnO) | Fluorite (CaF2) | |
|---|---|---|---|---|---|
| MAC | 36.0 | 30.9 | 124.2 | 49.3 | 89.8 |
| MgO | SiO2 | Al2O3 | CaO | Fe2O3 | Cr2O3 | MnO | ZrO2 | L.O.I | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ferronickel slag | 31.2 (1.6) | 52.6 (2.6) | 2.7 (0.1) | 0.70 (0.03) | 12.4 (0.6) | 1.10 (0.05) | 0.20 (0.02) | <D.L. | <D.L. | 101.2 (5.0) |
| Carbonated slag | 20.3 (1.0) | 35.6 (1.8) | 2.8 (0.1) | 1.21 (0.06) | 10.4 (0.5) | 0.86 (0.04) | 0.35 (0.02) | 1.24 (0.06) | 30.3 (1.5) | 103.5 (5.1) |
| Temperature Range (°C) | Mass Loss (wt. %) | Detected Gas |
|---|---|---|
| 30–1100 °C | 27.4% | H2O + CO2 |
| 30–300 °C | 6.7% | H2O (CO2) |
| 300–600 °C | 20.7% | CO2 |
| Native Slag | Carbonated Slag | |
|---|---|---|
| % Forsterite iron | 44.1 ± 0.6 | 7.0 ± 0.6 |
| % Clinoenstatite | 0.4 ± 0.3 | - |
| % Magnesite | - | 37.3 ± 0.7 |
| % Siderite | - | 3.6 ± 0.3 |
| % Residual: amorphous and undetected crystalline phases | 55.4 ± 0.6 | 52.1 ± 1.0 |
| Mineral | Chemical Formula | Proportion | Oxide Formula | Oxide (wt. %) | Oxide (mol. %) |
|---|---|---|---|---|---|
| Forsterite | Mg1.8Fe0.2SiO4 | 44.1 | MgO | 21.4 | 44.2 |
| Fe2O3 | 5.2 | 2.7 | |||
| SiO2 | 18.0 | 24.8 | |||
| Clinoenstatite | MgSiO3 | 0.4 | MgO | 0.2 | 0.3 |
| SiO2 | 0.2 | 0.3 | |||
| Chromite | FeCr2O4 | 1.7 | Fe2O3 | 0.6 | 0.3 |
| Cr2O3 | 1.1 | 0.7 | |||
| Amorphous | Unknown | 53.8 | MgO | 9.6 | 20.0 |
| Fe2O3 | 6.5 | 3.4 | |||
| SiO2 | 34.3 | 47.3 | |||
| Al2O3 | 2.7 | 2.1 | |||
| MnO | 0.2 | 0.2 | |||
| CaO | 0.7 | 1.0 |
| Mineral | Chemical Formula | Proportion | Oxide Formula | Oxide (wt. %) | Oxide (mol. %) |
|---|---|---|---|---|---|
| Forsterite | Mg1.8Fe0.2SiO4 | 7.0 | MgO | 3.6 | 5.6 |
| Fe2O3 | 0.5 | 0.2 | |||
| SiO2 | 2.9 | 2.9 | |||
| Chromite | FeCr2O4 | 1.1 | Fe2O3 | 0.4 | 0.2 |
| Cr2O3 | 0.9 | 0.3 | |||
| Magnesite | MgCO3 | 37.3 | MgO | 17.8 | 27.3 |
| CO2 | 19.5 | 27.3 | |||
| Siderite | FeCO3 | 3.6 | Fe2O3 | 2.5 | 1.0 |
| CO2 | 1.4 | 1.9 | |||
| Amorphous | ? | 51.0 | SiO2 | 32.7 | 33.5 |
| Fe2O3 | 7.0 | 2.7 | |||
| Al2O3 | 2.8 | 1.7 | |||
| MnO | 0.2 | 0.1 | |||
| CaO | 1.2 | 1.3 | |||
| H2O | 6.7 | 29.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laniesse, P.; Dufourny, A.; Bourgeois, F.; Julcour, C.; Cyr, M. Characterization of Carbonated and Raw Ferronickel Slags as Cementing Materials. Constr. Mater. 2024, 4, 524-542. https://doi.org/10.3390/constrmater4030028
Laniesse P, Dufourny A, Bourgeois F, Julcour C, Cyr M. Characterization of Carbonated and Raw Ferronickel Slags as Cementing Materials. Construction Materials. 2024; 4(3):524-542. https://doi.org/10.3390/constrmater4030028
Chicago/Turabian StyleLaniesse, Priscillia, Adrien Dufourny, Florent Bourgeois, Carine Julcour, and Martin Cyr. 2024. "Characterization of Carbonated and Raw Ferronickel Slags as Cementing Materials" Construction Materials 4, no. 3: 524-542. https://doi.org/10.3390/constrmater4030028
APA StyleLaniesse, P., Dufourny, A., Bourgeois, F., Julcour, C., & Cyr, M. (2024). Characterization of Carbonated and Raw Ferronickel Slags as Cementing Materials. Construction Materials, 4(3), 524-542. https://doi.org/10.3390/constrmater4030028






