1. Introduction
Expansive clays are often associated with low bearing capacity, high compressibility, along with swelling and shrinkage behavior. Volumetric changes, owing to moisture variation, cause damage to the lightly loaded structures, including pavements, retaining walls, and residential houses. The change in moisture could be due to seasonal or climatic variations and evapotranspiration of vegetation. Due to this problem, damage to lightly loaded structures built on these soils is more than any other natural disaster such as earthquakes and floods [
1]. The annual cost of damage due to this type of soil increased from
$2.2 billion/year in 1973 [
2] to
$15 billion/year in 2012 [
3].
Soil stabilization techniques have been used over the decades to mitigate this problem. Petry and Little [
4] discussed several stabilization methods, including mechanical compaction, chemical stabilization, pre-wetting, moisture barriers, lime injections, and deep soil mixing. Chemical stabilization using additives such as lime and cement is the most widely used treatment method in the United States and around the world [
5]. However, there are environmental concerns associated with these methods, including the generation of greenhouse gases and the adverse impact on plants due to elevated pH levels. Lime and cement production contribute to greenhouse gases. Cement manufacturing is both energy- and emissions-intensive because it requires 60–130 kg of fuel and 110 kWh of electricity leading to produce one ton of cement which leads to CO
2 emissions [
6]. Therefore, it is important to identify an alternative stabilization method that can be both environmentally friendly and cost-effective.
Microbial-induced calcium carbonate precipitation (MICP) is an environment-friendly technique that could be an alternative to conventional stabilization methods. It was shown that microorganisms could greatly help in immobilizing carbon dioxide in mineral precipitates and sequester carbon [
7,
8]. In recent years, the use of the MICP technique is gaining attention as a versatile and green method of soil improvement. Biostimulation is a type of MICP process where indigenous microbes are stimulated to precipitate calcium carbonate. However, to stimulate the ureolytic bacteria present in the soil, substrate solutions must pass through the soil and reach the microbes. Using percolating or flushing system under gravity to pass the substrate solutions in clayey soils is challenging due to the low permeability of these soils. Chittoori et al. [
9] studied biostimulation in clayey soils by injecting substrate solutions under high pressures. They noted that calcium carbonate precipitation was possible by injecting treatment solutions at high pressures. However, for shallow treatments, including pavement applications, injecting at high pressures could be counterproductive because higher pressures can fracture the soil or heave pavement. Clogging at the injection location could be of concern as well.
In this study, a new MICP application approach is evaluated by mixing substrate solutions with soil, similar to mixing lime or cement in case of chemical stabilization. In this novel approach, soil samples were first mixed with enrichment solutions to stimulate the bacteria, followed by cementation solutions to precipitate calcium carbonate. The protocol consists of mixing the enrichment solutions at optimum moisture content and allowing them to stimulate bacteria for different time periods, termed mellowing periods. During the mellowing periods, samples were left on the countertop, and moisture loss (enrichment solution loss) was allowed. At the end of the mellowing period, the lost moisture was replaced with cementation solutions that contained calcium chloride, and the soil sample was compacted at optimum moisture content (OMC) and maximum dry unit weight (MDUW). The compacted samples were sealed and cured for different time periods at 100% humidity conditions to ensure no moisture loss during the curing process. Three soils with varying plasticity and clay characteristics were used to evaluate the approach. Five different mellowing periods and three different curing periods were evaluated to determine the optimal time periods for both steps (mellowing and curing). Two types of cementation solutions whose calcium chloride concentrations varied were also studied to test the effect of cementation solutions on MICP effectiveness. The performance of treatments was evaluated using unconfined compression strength, free swell index, and percentage calcium carbonate precipitated. The results obtained from these studies are presented in this paper.
2. Microbial-Induced Calcite Precipitation (MICP)
MICP process is based on the comprehension of microbiology, geochemistry, and geotechnical engineering [
10]. In this process, ureolytic bacteria hydrolyze urea to produce ammonium and carbonate ions (Equation (1)). After the addition of Ca
2+ ions, calcium carbonate crystals (Equation (2)) are precipitated on the cell wall of the bacteria [
11].
Four main factors affect the MICP process: calcium ion concentration, dissolved inorganic carbon (DIC) concentration, pH, and availability of nucleation sites [
12]. In addition to this, the ability to metabolize, grow, and reproduce, affect the survivability of microbe [
13].
Microbial growth, metabolic activity, and cell-surface charge are dependent on the change in pH [
13]. The ammonia produced with urea hydrolysis is the reason for increasing the pH of the medium. Stocks-Fischer et al. [
14] stated that the urease activity increased mostly from pH 6.0 to 8.0 for
Sporosarcina Pasteurii. However, urease activity was highest at pH 8.0 and decreased with higher pH, although there was some urease activity noted at pH 9.0. In addition, it can be noted that the pH for optimal urease activity may differ in mixed cultures or with other individual species of ureolytic microbes. However, if there is sufficient chemical reagent, the rate of urea hydrolysis has a direct relationship with the bacterial cell concentration. More bacteria produce more urease per unit volume to start the urea hydrolysis. Stocks-Fischer et al. [
14] observed that the bacterial cells could serve as nucleation sites for calcium carbonate to precipitate. Lian et al. [
15] identified from SEM images that the nucleation of calcium carbonate takes place at bacteria cell walls. High salinity can cause inhibition and stop microbial activity [
16]. The salinity of cementation fluid is dependent on calcium salt. Microbial activity can be obstructed by high salinity, which can limit the urease production from ureolytic bacteria [
17]. Burbank et al. [
18] optimized the concentration of calcium to be used in biomineralization using stimulated indigenous bacteria.
2.1. Applications of MICP
Calcium carbonate precipitation in the MICP process bridges adjacent soil particles, cementing soil particles together [
19,
20]. The precipitation of calcium carbonate reduces permeability and compressibility while increasing soil strength [
10]. MICP has several applications in diverse fields, including an increase in concrete strength and durability [
20], mitigation of sand liquefaction [
21], the permeability of sands [
22], soil strength [
23,
24,
25], and brick durability [
26].
There are very few studies that explored the application of MICP in expansive soils. To test the hypothesis that the low pore space in these soils is not compatible for MICP application, Chittoori et al. [
27] performed a mercury intrusion porosimetry (MIP) study on two expansive soils to observe the pore size and pore volume at different compaction levels. It was found that 30% to 50% of the pore volume was larger than 1.5 μm (the average cell size of typical urease-producing soil bacteria) at the MDUW [
27]. It is evident that there is space available for bacterial growth and mobilization. There have been some studies regarding biotreatment on expansive soils. Bing [
28] conducted biotreatment on kaolin, marine clay, and bentonite and observed that strength increased by around 150% and 400% for both treated kaolin and treated marine clay, respectively. Cheng and Shahin [
29] attempted three different MICP methods, including injection, premixing, and diffusion for clayey sands to investigate the variation of strength and amount of calcium carbonate precipitation. They noted that soils with 5% clay content worked best in the injection method. Cardoso et al. [
30] investigated the compressibility and pore-clogging of the biocemented sand–kaolin mixture and found that the osmotic consolidation effect might be a contributing factor for high compressibility along with the bacterial activity.
2.2. MICP Methods
There are two methods to apply MICP:
Bioaugmentation and
Biostimulation. In
bioaugmentation, exogenous bacteria are introduced into the soil to precipitate calcium carbonate. Most studies have applied the bioaugmentation method on silty and sandy soils [
20,
31,
32,
33,
34]. Bioaugmentation processes have seen successful implementation in the improvement in concrete strength and durability [
35], mitigation of sand liquefaction [
21], and reducing the permeability of sand [
22].
Chittoori and Neupane [
36] studied the application of bioaugmentation to mitigate expansive soil swelling. They studied two different protocols on three selected soils having low, medium, and high plasticity characteristics. Different concentrations of bacteria and substrate were mixed with soil and cured for 7 days in one protocol. In the other protocol, different concentrations of bacteria were mixed into the soil, and compacted. Substrate solutions were then injected into the compacted sample. It was reported that low to medium plastic soils could be effectively treated using MICP via bioaugmentation. However, in this method, augmented exogenous bacteria have to adjust to the new environment and compete with native microorganisms, which can affect the survival rate and metabolic potential of the augmented bacteria [
37]. It was observed that the survival rate of exogenous microorganisms in a new environment, tends to decline rapidly and the organisms rarely propagate [
38]. In addition, the uneven distribution of bacteria and clogging near the inlet were other issues associated with this method [
14]. The requirement of injecting non-native bacterial strains into soil has restricted the technology from becoming an economical method [
39].
On the other hand, biostimulation uses indigenous bacteria for calcium carbonate precipitation [
18], and is becoming a popular method of application for MICP. This approach does not require expensive non-native monoclonal bacterial cultivation and injection into natural soil ecosystems, which have made it economically and environmentally beneficial. These ureolytic microbes are more resilient than the injected microbes, which resulted in a uniform distribution of calcium carbonate and sustained enzymatic capabilities [
40]. Usually, the microbe population is 10
6 to 10
12 per gram in soil [
41,
42]. Boquet et al. [
41] believed that nearly all soil bacteria could precipitate calcium carbonate. With the biostimulation processes, it is possible to enrich and increase the number of native ureolytic bacteria in a variety of soils [
18]. It was first demonstrated by Burbank et al. [
18] who showed that indigenous microorganisms capable of hydrolyzing urea could be enriched to induce calcium carbonate precipitation in potentially liquefiable saturated soils both in the laboratory and in situ. Gomez et al. [
42] also conducted a field test for calcium carbonate precipitation in granular soils, using one-dimensional column specimens which resulted in significant improvement in geotechnical properties, including unconfined compressive strength and permeability. Chittoori et al. [
9] studied a method using biostimulation to treat natural expansive soils through an injection system. A significant reduction in swelling strain and increase in unconfined compression strength after one treatment cycle were observed. In the current research, a new application protocol was studied for applying MICP via biostimulation to treat expansive clays. This research is an initial step to establish an alternative treatment protocol for shallow stabilization of expansive soils.
3. Materials and Methods
3.1. Soils
Three soils with varying plasticity characteristics were chosen to evaluate the proposed method of MICP application. Of the three soils, one is a naturally occurring expansive soil while the other two soils were prepared by mixing different percentages of the natural soil and medium-fine sand (D
60 = 0.68 mm, D
10 = 0.24 mm, and C
u = 2.83). This was intended to reflect the roles of clay content and plasticity characteristics in this method. The natural soil was collected along the US 95 highway close to Marsing, Idaho. This soil contained about 70% clay and is denoted as C-70. This clay content was adjusted to 30 and 40% by adding the sand, and these soils are denoted as C-30 and C-40, respectively. All three soils were tested for various geotechnical engineering properties, including Atterberg limits, maximum dry density, 1-D swell strain, 1-D swell pressure, and free swell index. Apart from the free swell index test, which was conducted as per Holtz and Gibbs (1956), the rest of the tests were conducted as per the American Standards and Testing Method (ASTM). The standard test number indentifiers for each of these tests is provided in
Table 1. The results in
Table 2 indicate that the maximum dry unit weight (MDUW) ranged from 11.0 to 15.6 kN/m
3 and the optimum moisture content (OMC) ranged from 32.6% to 21.5% for the three soils tested here. Please note the increase in MDUW and decrease in OMC as the clay content is reduced. Moreover, the increase in clay particles from C-30 to C-70 soil contributed to the gradual increase in unconfined compressive strength in these soils. The UCS values ranged from 69.6 to 155.1 kPa. The gradual improvement in strength could be due to the inner bonding of fine particles. It should be noted here that soils with higher sand content showed lower UCS values. This is because of the reduction in cohesion and the inability of sand particles to adhere to one another, and does not mean that these samples are weak. In 1-D swell, a specimen was enclosed and inundated. Different loads were applied to develop different stress levels on the two identical specimens for each type of soil. The 1-D swell strain ranged from 17.9 % to 2.6%, and the swell pressure ranged from 287 kPa to 70 kPa for the three soils tested.
3.2. Treatment Solutions
Two types of treatment solutions were used in this research to stimulate bacteria and achieve calcium carbonate precipitation, namely,
enrichment and
cementation solutions.
Enrichment solutions contained both a carbon source as acetate and a nitrogen source in the form of urea. The composition of enrichment solutions was 100 mM of sodium acetate, 333 mM of urea, and 0.5 g/L of corn steep liquor (CSL). Corn steep liquor comprises amino acids, vitamins, and minerals and is used to stimulate the initial activity within the soil profile [
18]. The presence of urea works as a nitrogen source, and the increase in the pH level as a result of the presence of ammonium from urea hydrolysis creates an environment for bacteria that can survive in a high-pH environment and use urea or ammonia as a nitrogen source [
14]. As the number of ureolytic bacteria increases, the rate of hydrolysis increases, which increases the rate of precipitation [
27].
Cementation solutions contained all the enrichment solutions with the addition of calcium chloride. In this research, two types of cementation solutions were used. In the cementation solution, the concentration of calcium chloride is 250 mM, and in another cementation composition, the concentration of calcium chloride is 500 mM [
27]. Two concentrations of calcium chloride were used to observe the effect of the concentration of calcium chloride in calcium carbonate precipitation. The concentrations of all the components are detailed in
Table 3.
3.3. Treatment Protocol
The treatment consisted of mixing soil with a volume of enrichment solutions corresponding to the optimum moisture content of the soil from the standard Proctor test. After mixing, the indigenous bacteria, the samples were allowed to hydrolyze urea for different periods of time (1, 2, 3, 4, and 7 days). In chemical stabilization protocols, mellowing period is the time between mixing (soil with chemicals and water) and sample compaction (for curing). Unlike shallow stabilization protocols, moisture loss was permitted during this time in this research. The lost moisture was replaced with the cementation solution. Cementation solution was mixed into the soil samples to bring back the moisture content to OMC, and soils were mixed thoroughly for even distribution. The soil samples were compacted into a cylinder of 7.1 cm diameter and 14.2 cm height at their corresponding maximum dry unit weight. After that, samples were cured with controlled 100% humidity and room temperature for 0, 3, and 7 days of curing time. The curing periods are denoted as CP. The mellowing periods are denoted as MP.
Figure 1 presents a pictorial representation of the treatment protocol.
3.4. Evaluation Tests
Unconfined compression strength (UCS), calcium carbonate, and free swelling index (FSI) tests were conducted to evaluate the effect of biostimulated MICP. The following sections briefly describe the procedures followed in conducting these tests.
3.4.1. Unconfined Compression Strength (UCS)
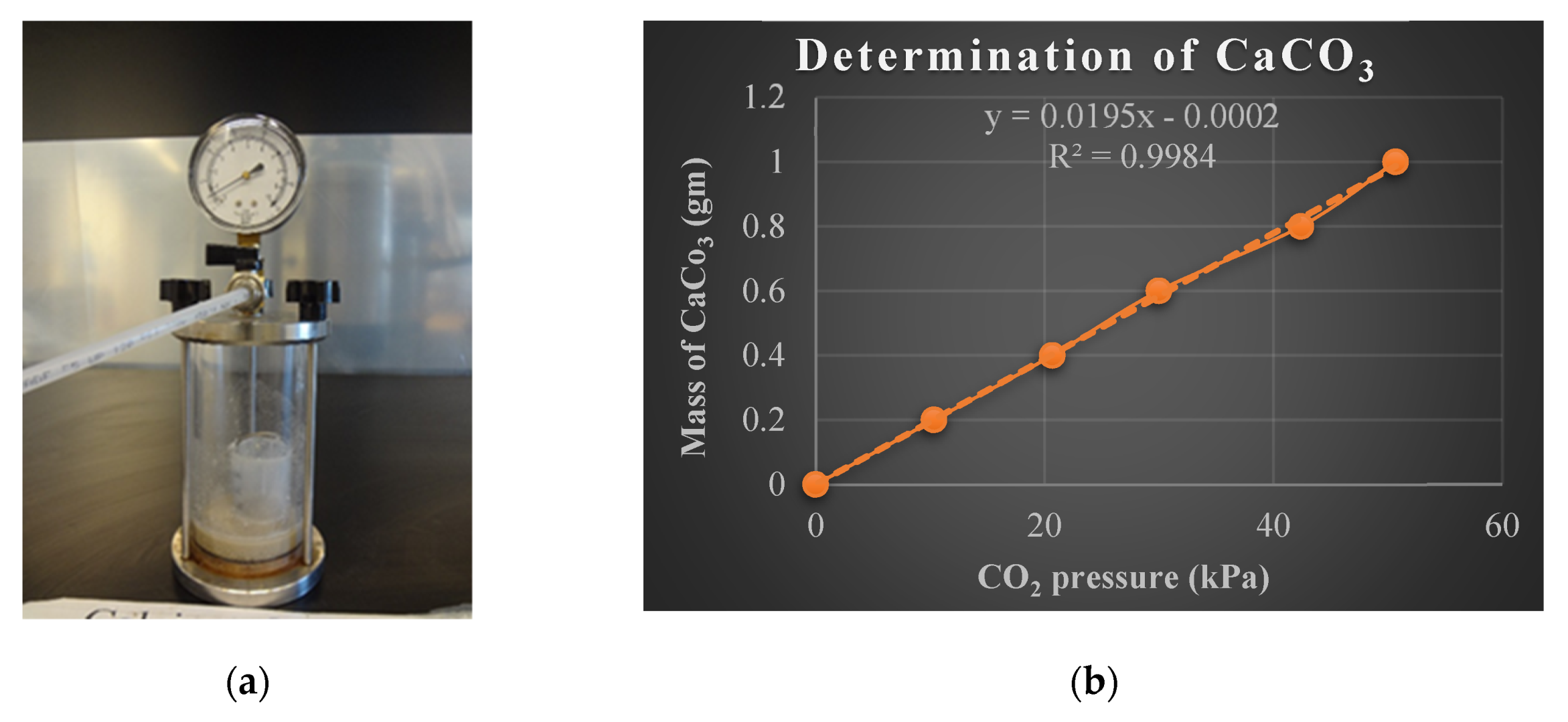
The purpose of this test is to determine the compressive strength of the soil. UCS test is an unconsolidated undrained test where the lateral confining pressure is equal to zero. This test was conducted as per ASTM D2166. Soil samples of 7.1 cm diameter and 14.2 cm height were compacted to their corresponding MDUW and OMC from the standard Proctor test. The compression was strain-controlled, and the rate of strain was 2.5 mm/min. The equipment used for this test was shown
Figure 2.
3.4.2. Calcium Carbonate Test
After UCS tests, the samples were oven-dried and tested for carbonate content as per ASTM D 4373. A simple portable device was used to carry out this gasometric method. This device consisted of a reaction cylinder, a cup filled with 1M hydrochloric acid (HCl), and a pressure gauge (
Figure 3a). Initially, 10 g of soil sample was poured into the reaction chamber, and a small cup with 40 mL of HCl was placed inside the chamber. The reaction chamber was closed tightly, and the small cup was tilted to create a reaction between the HCl and soil samples which released carbon dioxide and pressurized the chamber. The pressure inside the chamber was recorded using the pressure gauge mounted on the chamber. This pressure is related to an equivalent amount of calcium carbonate (CaCO
3) using a calibration curve (See
Figure 3b) prepared with known amounts of reagent grade CaCO
3.
3.4.3. Free Swell Index (FSI)
The free swell index is a simple experimental procedure performed to estimate the expansion potential of a given soil [
43]. It is defined as the ratio between the increase in the volume of soil (without any external constraints) after submergence in water (polar fluid) and the volume of soil after submergence in kerosene (nonpolar fluid). In this test, two representative oven-dried soil samples (passing a #40 sieve) weighing 10 grams each were poured into two graduated cylinders of 100 mL capacity with the help of a funnel. One cylinder was filled with distilled water, while the other was filled with kerosene up to 100 mL mark. Entrapped air was removed by mild shaking and stirring with a glass rod. Soil samples are allowed to attain an equilibrium state of volume without any further change in the volume of the soils in 24 h [
44]. The final volumes of the soil samples in both cylinders were recorded, and the FSI was calculated using the following equation:
where, V
d = volume of the soil sample from the graduated cylinder containing distilled water and V
k = volume of the soil sample from the graduated cylinder containing kerosene.
4. Results
This section presents the results obtained from the testing described above. A summary of the results is presented first, followed by a discussion on the reasons for the observed changes. The test data was also examined to study the effect of parameters such as mellowing and curing periods, type of cementation solution, and the type of soil on the effectiveness of MICP in this application.
UCS, calcium carbonate content, and FSI tests were performed on treated and untreated soils to evaluate strength changes, calcium carbonate precipitation, and swell changes. The results obtained from UCS, calcium carbonate content, and FSI tests are presented in
Table 4,
Table 5 and
Table 6, respectively. The tables include test data from both cementation compositions (CS-1 and CS-2) for all curing and mellowing periods studied in this research.
The
UCS values for the untreated C-30, C-40, and C-70 soils were 69, 88, and 155 kPa, respectively. It can be noted from
Table 4 that the UCS values after treatment ranged from 63 kPa to 267 kPa with different curing and mellowing periods for CS-1, while those for CS-2 ranged from 45 to 182 kPa. It is evident that the treatments had a positive impact on the UCS, which shows promise for this approach. It should be noted that the highest percentage change in UCS after treatment was achieved in C-30 soil, which showed a 287% change, while the lowest was observed in C-70 soil with only a 53% increase. The increase in C-40 soil was 188%. The increase in the UCS values after treatments is attributed to the precipitated calcium carbonate as a result of the bacterial stimulation via the addition of enrichment and cementation solutions. The higher amount of clay in the C-70 soil could be adversely impacting the calcite formation, thereby hindering UCS increase.
The
calcium carbonate values for the untreated C-30, C-40, and C-70 soils were determined to be 0%. It can be observed from
Table 5 that the calcium carbonate values ranged from 0% to 1.36% with different curing and mellowing periods for CS-1, while those for CS-2 ranged from 0 % to 0.88%. It was observed that C-70 soil precipitated the highest amount of calcite (1.36%), followed by C-70 soil (1.27%), and finally, by C-30 soil (1.17%). This observation does not support the earlier statement that the C-30 soil showed the highest UCS increase due to the greatest calcite precipitation. This indicates that higher CaCO3 precipitation does not automatically cause greater strength change. Further examination of this aspect will be made in the following sections.
The
FSI values for the untreated C-30, C-40, and C-70 soils were 108%, 123%, and 162%, respectively.
Table 6 shows that the FSI values ranged from 8% to 190% for CS-1, while those for CS-2 ranged from 33% to 266%. The largest reduction in FSI values was observed in C-40 soil (93%), while the lowest was observed in C-70 soil (72%). It should be noted here that the FSI values increased beyond the untreated values for some of the curing and mellowing periods. Further examination of these test results is provided in the next section, where the effects of parameters such as mellowing period, curing period, type of cementation solutions, and the soil type are examined.
5. Discussion
This section contains a discussion on the reasons for the observed changes discussed in the previous section. The test data was also examined to study the effects of mellowing and curing periods, type of cementation solution, and the type of soil on the effectiveness of MICP in this application.
5.1. Effect of Mellowing and Curing Periods
Figure 4,
Figure 5 and
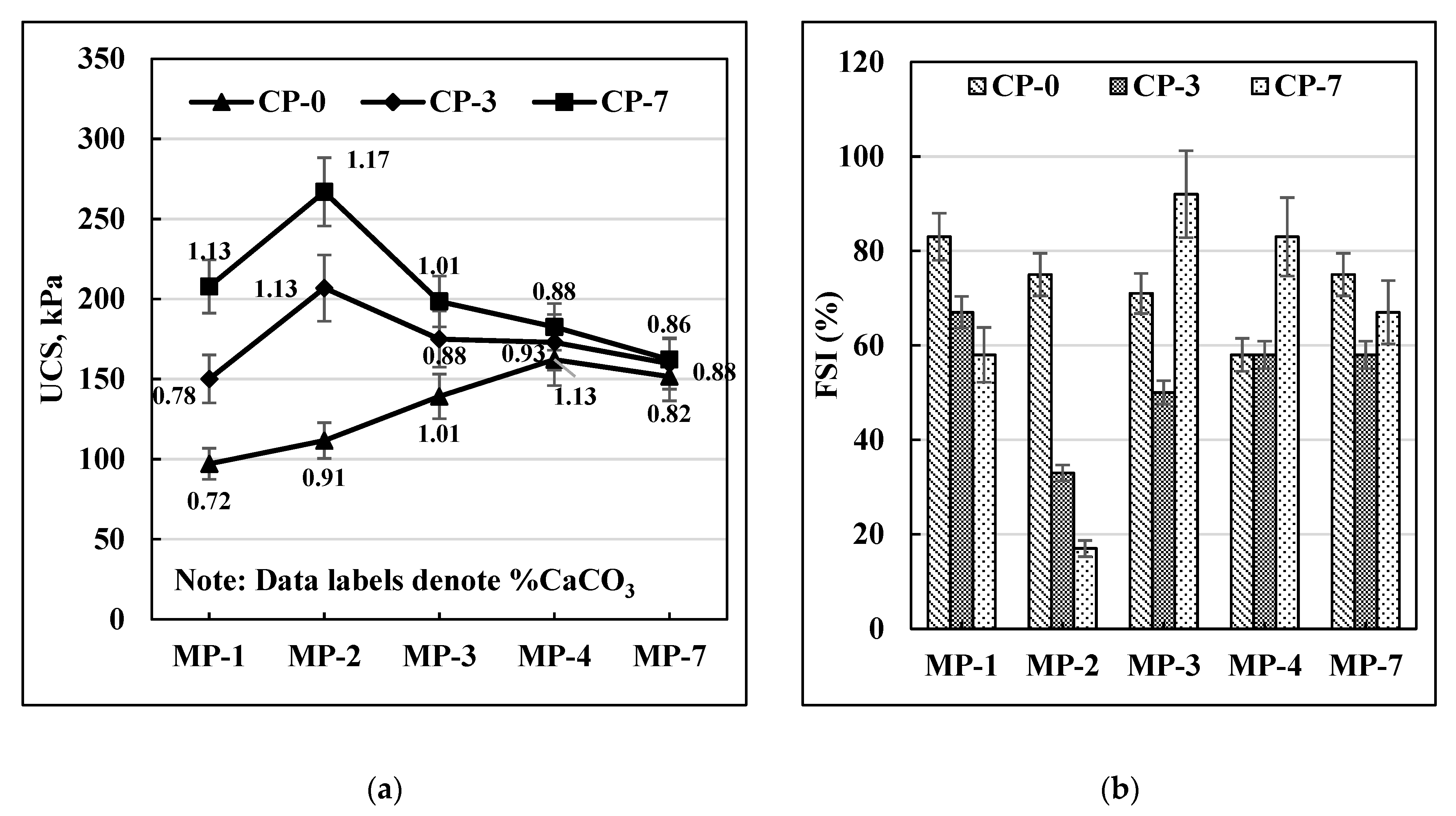
Figure 6 present the variation of UCS and FSI data with mellowing and curing periods for soils C-30, C-40, and C-70. These soils were treated with cementation solution CS-1. It can be noted from
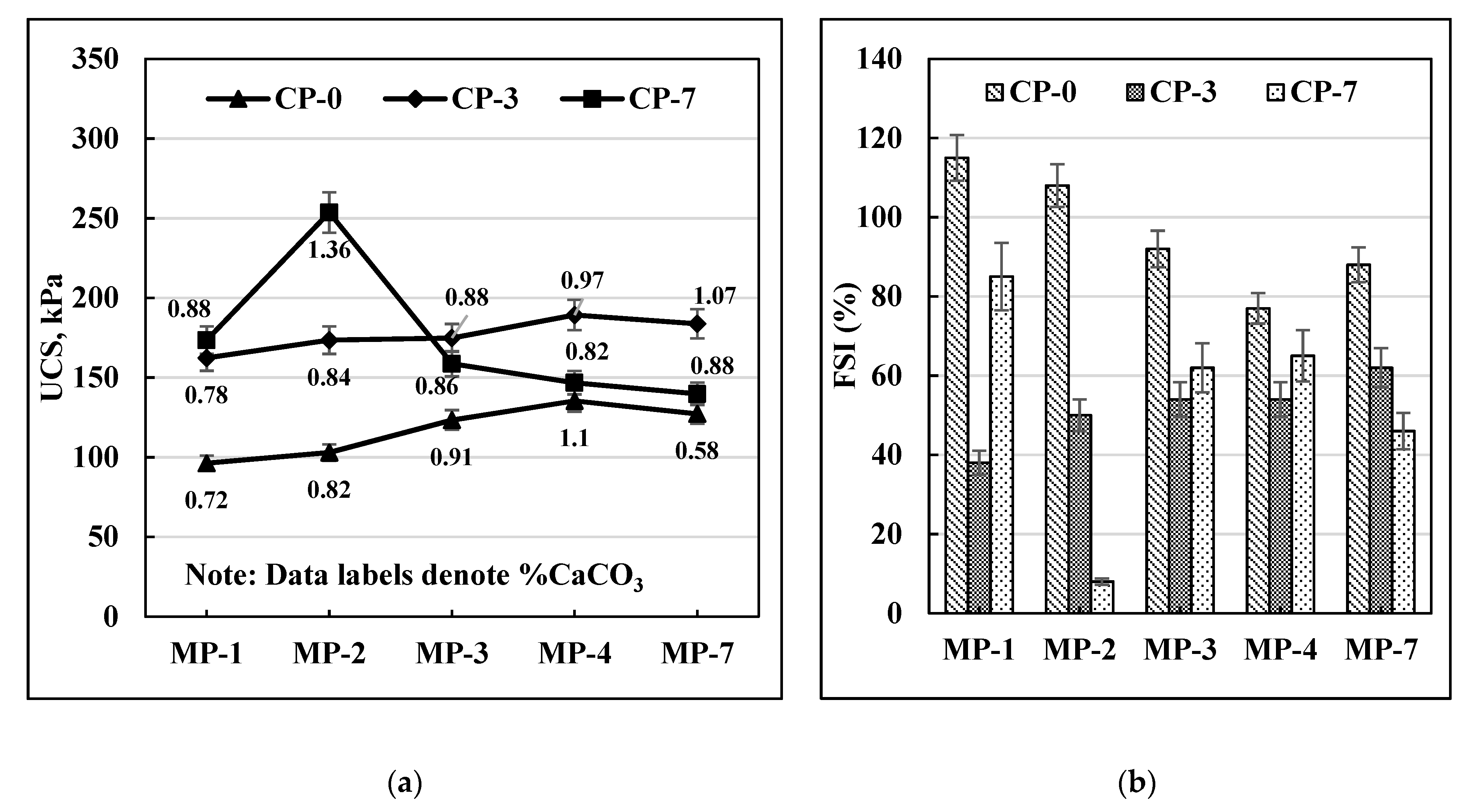
Figure 4a that the UCS values for C-30 soil increased with mellowing periods until MP-4 and then dropped for MP-7 for zero-day cured (CP-0) samples. However, in the case of CP-3 and CP-7 curing periods, the UCS values dropped after two days of mellowing. It can also be observed that CP-7 at MP-2 gave the highest UCS value. A similar trend was observed for C-40 soil (
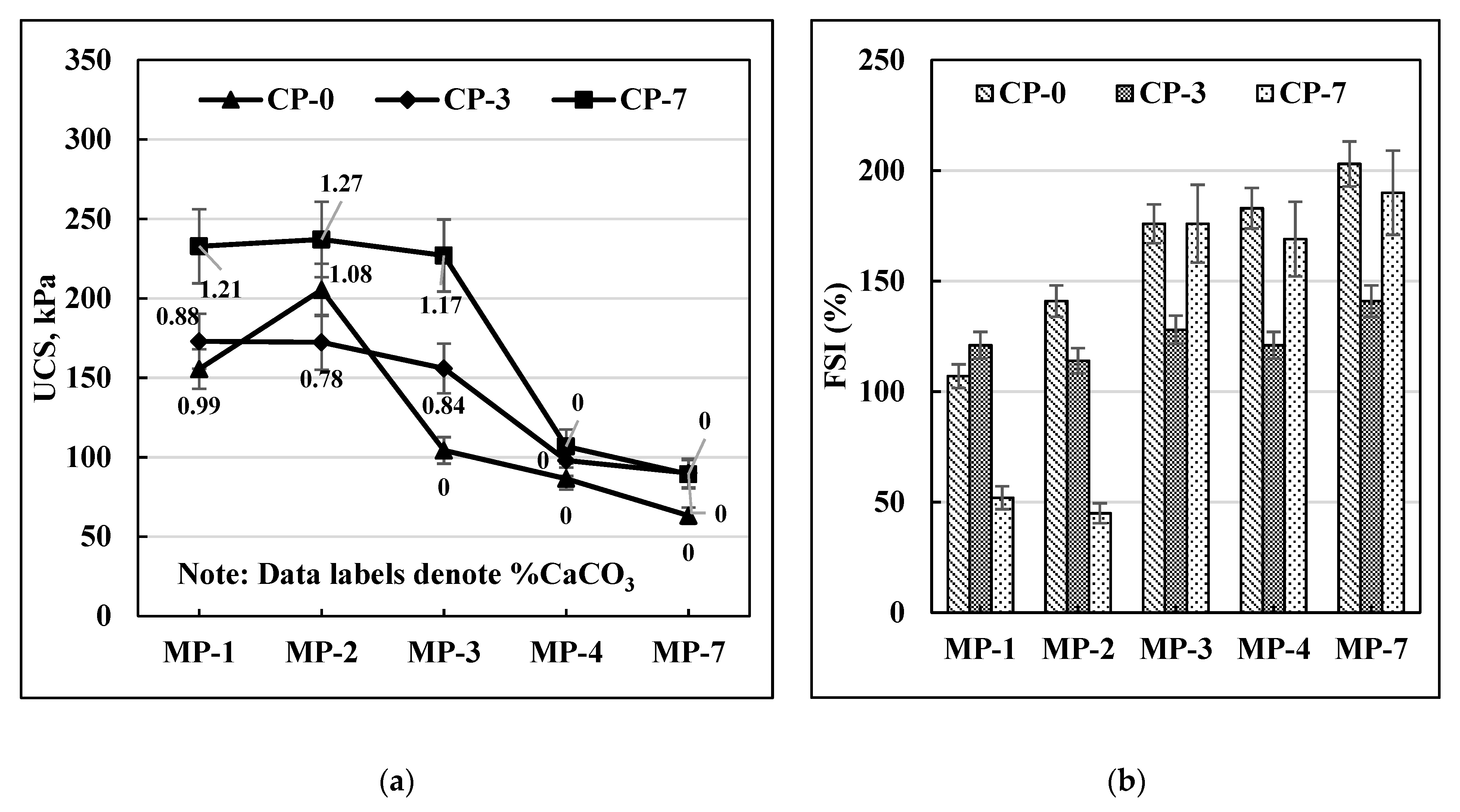
Figure 5a), where the maximum increase in UCS value was observed at CP-7 and MP-2. In the case of C-70 soil (
Figure 6a), the UCS values increased with mellowing periods, MP-1 and MP-2, and decreased for all remaining mellowing periods. A maximum increase in UCS value was observed at CP-7 and MP-2 for C-70 soil as well. Based on these observations, it can be concluded that the best treatment period for all soils was two days of mellowing followed by seven days of curing. As the clay content in the soils increased from C-30 to C-70, it appears that an increase in the mellowing period beyond two days was not beneficial for any of the soils. This could be due to the loss in moisture during the mellowing period reducing bacterial activity. When cementation solutions were added at the end of mellowing periods, the presence of calcium in these solutions may be hindering bacteria growth, as was observed in earlier research [
17,
18]. The increase in strength can be attributed to the precipitation of calcite, as can be seen in the plots as data labels in
Figure 4a,
Figure 5a, and
Figure 6a.
A similar trend can be observed for the FSI data as well as presented in
Figure 4b,
Figure 5b, and
Figure 6b for C-30, C40, and C-70 soils. All three soils had the lowest FSI values for MP-2 after 7 days of curing.
Overall, it can be concluded that MP-2 and CP-7 are the optimal mellowing and curing times for achieving the most effective MICP response on these soils.
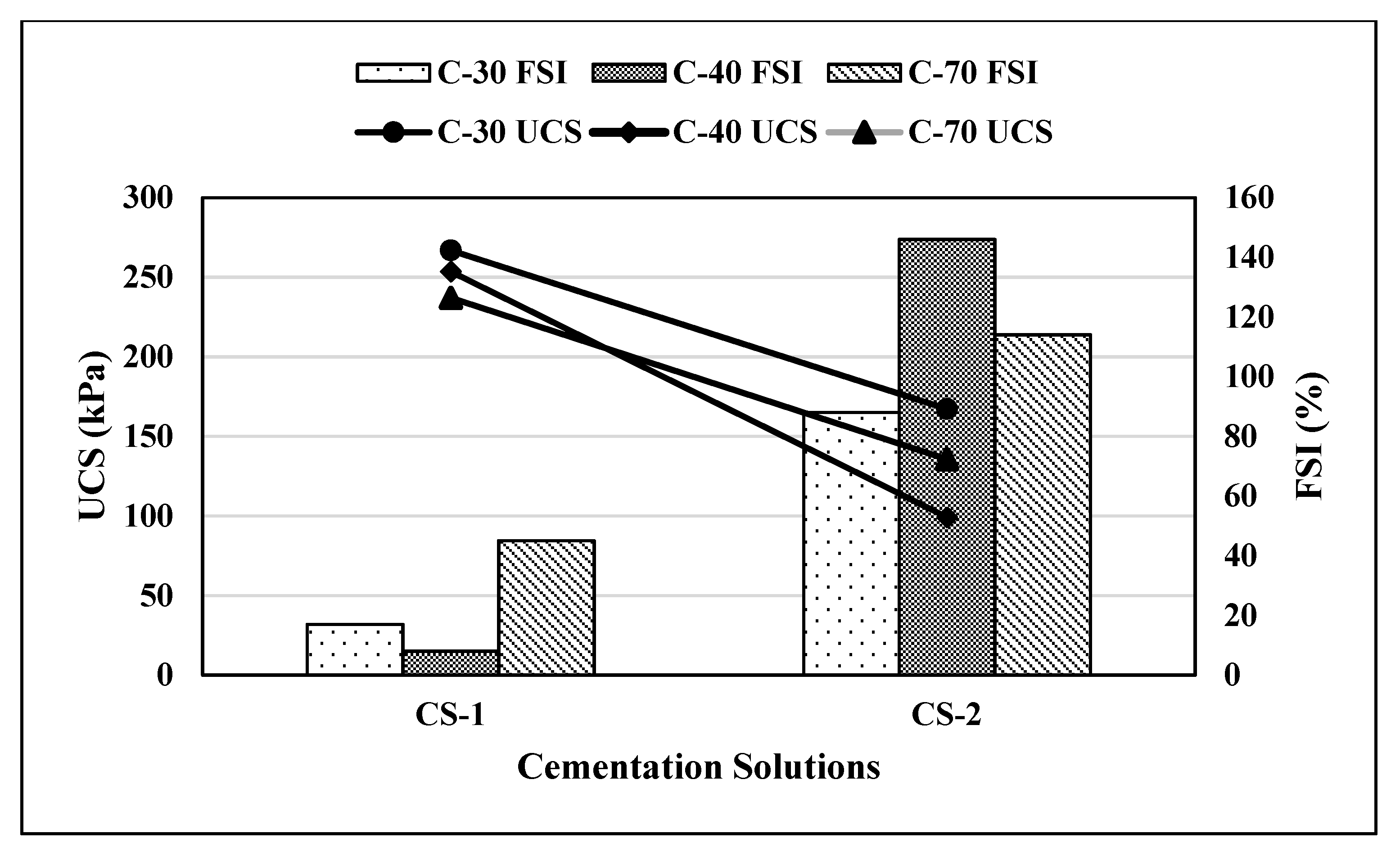
5.2. Effect of Type of Cementation Solution
To observe the effect of calcium chloride concentration in the cementation solutions, two concentrations of CaCl
2 were used in this study (
Figure 7). CS-1 contained 250 mM of CaCl
2 while CS-2 contained 500 mM CaCl
2. It can be observed from
Figure 7 that using CS-2, UCS values decreased by 37%, 60%, and 39.13% for C-30, C-40, and C-70 soils, respectively, compared to CS-1. In addition, FSI values increased by 200%, 1400%, and 43.8% for C-30, C-40, and C-70 soils, respectively, with CS-2, compared to CS-1. There was an overall improvement in UCS and FSI values for CS-1 in comparison to CS-2, which could be due to the inhibition effect on a microbial activity which can limit the urease production from ureolytic bacteria with a higher concentration of calcium chloride. Additionally, it was observed [
18,
36] that a lower concentration of CaCl
2 leads to more homogeneous CaCO
3 crystal formation at the particle contact points which contributes to the strength improvement with minimum soil disturbance and permeability reduction.
5.3. Effect of Soil Type
Since there are three different soils with varying clay contents, the effect of soil type was also ascertained. The percentage change in UCS and FSI compared to the untreated values is presented in
Figure 8. It should be noted here that the data presented in
Figure 8 represents soils treated with CS-1 for MP-2 and CP-7. The maximum change in UCS value was observed for C-30 soil while the same for FSI was observed in C-40 soil. The improvement in UCS is very well correlated with the clay content, where the improvement went down with an increase in clay content. The untreated soils with low clay contents had lower UCS values, and the bonding from the CaCO3 due to MICP treatments increased the UCS value and hence showed better improvement in UCS compared to soils with higher clay contents. The FSI data did not show any meaningful trends. It can also be noted from the figure that the highest amount of CaCO
3 precipitation (1.36%) was observed in C-40 soil, while the lowest amount was observed for C-30 soil (1.17%). Hence the precipitation of higher amounts of CaCO
3 may not always result in improved engineering performance as the type of CaCO
3 formed plays an important role along with gradation, density, and other geotechnical characteristics, which need further examination beyond this study.
6. Summary and Findings
A new method for the application of a biostimulated MICP technique was developed in this research. Experiments were conducted to study the effectiveness of mixing protocols to stimulate indigenous bacteria to stabilize expansive soils. Three soils with varying plasticity characteristics were studied, and their performance was evaluated using UCS, calcium carbonate precipitation, and FSI tests. It was observed that the improvement in strength was proportional to calcium carbonate precipitation in these soils. Additionally, free swell index test results were inversely proportional to calcium carbonate precipitation and UCS.
Findings from this research study are summarized as follows:
- 1
MICP was successfully used to alter the behavior of three clayey soils with varying plasticity characteristics.
- 2
It was observed that two days of mellowing time and seven days of curing produced optimal results for the three soils tested in this research.
- 3
It was noted that an increase in the mellowing period beyond two days caused heavy moisture loss which could have contributed to the low strength of the soil samples.
- 4
Between the two types of treatment solutions, treatments with CS-1 resulted in better overall performance than that with CS-2.
- 5
It was observed that C-30 and C-40 soils showed better improvement in strength than C-70 soil.
Author Contributions
Conceptualization by B.C.S.C. and M.B.; methodology, B.C.S.C., T.R. and M.B.; software, T.R.; validation, B.C.S.C., T.R. and M.B.; formal analysis, T.R., investigation, T.R.; resources, B.C.S.C.; data curation, B.C.S.C., T.R. and M.B.; writing—original draft preparation, T.R.; writing—review and editing, B.C.S.C. and T.R.; visualization, T.R.; supervision, B.C.S.C.; project administration, B.C.S.C.; funding acquisition, B.C.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
The authors acknowledge the assistance of the Department of Civil Engineering at Boise State University which assisted the research team in various aspects including partial support to the student working on this research and material support for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nelson, J.; Miller, D.J. Expansive Soils: Problems and Practice in Foundation and Pavement Engineering; John Wiley & Sons Inc: New York, NY, USA, 1992. [Google Scholar]
- Jones, D.E., Jr.; Holts, W.G. Expansive soils—The hidden disaster. Am. Soc. Civ. Eng. 1973, 43, 49–51. [Google Scholar]
- Jones, L.D.; Jefferson, I. Expansive soils. In ICE Manual of Geotechnical Engineering, Geotechnical Engineering Principles, Problematic Soils and Site Investigation; ICE Publishing: London, UK, 2012; Volume 1, pp. 413–441. ISBN 0727757075. [Google Scholar]
- Petry, T.M.; Little, D.N. Review of stabilization of clays and expansive soils in pavements and lightly loaded structures—history, Practice, and Future. J. Mater. Civ. Eng. 2002, 14, 447–460. [Google Scholar] [CrossRef]
- Sherwood, P.T. Soil Stabilization with Cement and Lime: State-of-the-Art Review; ScienceOpen: Boston, MA, USA, 1993; ISBN 0-11-551190-3. [Google Scholar]
- UN Climate Change News, World Cement Association Urges Climate Action. Available online: https://unfccc.int/news/world-cement-association-urges-climate-action (accessed on 12 August 2021).
- Avirneni, D.; Peddinti, P.R.T.; Saride, S. Durability and long term performance of geopolymer stabilized reclaimed asphalt pavement base courses. Constr. Build. Mater. 2016, 121, 198–209. [Google Scholar] [CrossRef]
- Okyay, T.O.; Nguyen, H.N.; Castro, S.L.; Rodrigues, D.F. CO2 sequestration by ureolytic microbial consortia through microbially-induced calcite precipitation. Sci. Total. Environ. 2016, 572, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Chittoori, B.C.S.; Burbank, M.; Islam, M.T. Evaluating the effectiveness of soil-native bacteria in precipitating calcite to stabilize expansive soils. In Proceedings of the International Foundations Congress and Equipment Expo., Orlando, FL, USA, 1 January 2018; pp. 59–68. [Google Scholar]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Burne, R.A.; Chen, Y.Y.M. Bacterial ureases in infectious diseases. Microbes Infect. 2000, 2, 533–542. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key roles of PH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Rebata-Landa, V. Microbial activity in sediments: Effects on soil behavior. Available online: https://smartech.gatech.edu/handle/1853/19720 (accessed on 10 October 2021).
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Upscaling effects of soil improvement by microbially induced calcite precipitation by surface percolation. Geomicrobiol. J. 2014, 31, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Rivadeneyra, M.A.; Delgado, G.; Ramos-cormenzana, A.; Delgado, R. Biornineralization of carbonates by halomonas eurihalina in solid and liquid media with different SaHr Fies: Crystal formation sequence. Res. Microbiol. 1998, 149, 277–287. [Google Scholar] [CrossRef]
- Nemati, M.; Greene, E.A.; Voordouw, G. Permeability profile modification using bacterially formed calcium carbonate: Comparison with enzymic option. Process. Biochem. 2005, 40, 925–933. [Google Scholar] [CrossRef]
- Burbank, M.B.; Weaver, T.J.; Green, T.L.; Williams, B.C.; Crawford, R.L. Precipitation of calcite by indigenous microorganisms to strengthen liquefiable soils. Geomicrobiol. J. 2011, 28, 301–312. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Montoya, B.M.; DeJong, J.T.; Boulanger, R.; Gerhard, R.; Ganchenko, A.; Chou, J.-C. Liquefaction mitigation using microbial induced calcite precipitation. In Proceedings of the GeoCongress, Reston, VA, USA, 1 October 2012. [Google Scholar]
- Nemati, M.; Voordouw, G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzym. Microb. Technol. 2003, 33, 635–642. [Google Scholar] [CrossRef]
- Van Der Ruyt, M.; Van Der Zon, W. Biological in situ reinforcement of sand in near-shore areas. Proc. Inst. Civ. Eng. Geotech. Eng. 2009, 162, 81–83. [Google Scholar] [CrossRef]
- Lu, W.; Qian, C.; Wang, R. Study on soil solidification based on microbiological precipitation of CaCO3. Sci. China Technol. Sci. 2010, 53, 2372–2377. [Google Scholar] [CrossRef]
- Burbank, M.; Weaver, T.; Lewis, R.; Williams, T.; Williams, B.; Crawford, R. Geotechnical tests of sands following bio-induced calcite precipitation catalyzed by indigenous bacteria. J. Geotech. Geoenviron. Eng. 2012, 139, 928–936. [Google Scholar] [CrossRef]
- Sarda, D.; Choonia, H.S.; Sarode, D.D.; Lele, S.S. Biocalcification by bacillus pasteurii urease: A novel application. J. Ind. Microbiol. Biotechnol. 2009, 36, 1111–1115. [Google Scholar] [CrossRef]
- Chittoori, B.C.S.; Moghal, A.A.B.; Pedarla, A.; Al-Mahbashi, A.M. Effect of unit weight on porosity and consolidation characteristics of expansive clays. J. Test. Eval. 2017, 45, 94–104. [Google Scholar] [CrossRef]
- Bing, L. Geotechnical Porperties of Biocement Treated Sand and Clay. Available online: https://dr.ntu.edu.sg/handle/10356/62560 (accessed on 10 October 2021).
- Cheng, L.; Shahin, M.A. Assessment of different treatment methods by microbial-induced calcite precipitation for clayey soil improvement. In Proceedings of the 68th Canadian Geotechnical Conference, Quebec, QC, Canada, 21 September 2015. [Google Scholar]
- Cardoso, R.; Pires, I.; Duarte, S.O.D.; Monteiro, G.A. Effect of clay’s chemical interaction on biocementation. Appl. Clay Sci. 2018, 156, 96–103. [Google Scholar] [CrossRef]
- van Paassen, L.A.; Harkes, M.P.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C.M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Improvements in engineering properties of soils through microbial-induced calcite precipitation. KSCE J. Civ. Eng. 2013, 17, 718–728. [Google Scholar] [CrossRef]
- DeJong, J.T.; Martinez, B.C.; Mortensen, B.M.; Nelson, D.C.; Waller, J.T.; Weil, M.H.; Ginn, T.R.; Weathers, T.; Barkouki, T.; Fujita, Y.; et al. Upscaling of bio-mediated soil improvement. In Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering (Volumes 1, 2, 3 and 4); IOS Press: Amsterdam, The Netherlands, 2009; pp. 2300–2303. [Google Scholar]
- Mortensen, B.M.; Haber, M.J.; Dejong, J.T.; Caslake, L.F.; Nelson, D.C. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 2011, 111, 338–349. [Google Scholar] [CrossRef]
- De Muynck, W.; Debrouwer, D.; De Belie, N.; Verstraete, W. Bacterial carbonate precipitation improves the durability of cementitious materials. Cem. Concr. Res. 2008, 38, 1005–1014. [Google Scholar] [CrossRef]
- Chittoori, B.; Neupane, S. Evaluating the application of microbial induced calcite precipitation technique to stabilize expansive soils. In Proceedings of the Tunneling in Soft Ground, Ground Conditioning and Modification Techniques, HangZhou, China, 23–25 July 2018; Cheng, W.-C., Yang, J., Wang, J., Eds.; Springer International Publishing: Cham, Swizerland, 2019; pp. 10–19. [Google Scholar]
- Wenderoth, D.F.; Rosenbrock, P.; Abraham, W.R.; Pieper, D.H.; Höfle, M.G. Bacterial community dynamics during biostimulation and bioaugmentation experiments aiming at chlorobenzene degradation in groundwater. Microb. Ecol. 2003, 46, 161–176. [Google Scholar] [CrossRef]
- van Veen, J.A.; van Overbeek, L.S.; van Elsas, J.D. Fate and activity of microorganisms introduced into soil. Microbiol. Mol. Biol. Rev. MMBR 1997, 61, 121–135. [Google Scholar]
- Gomez, M.G.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C.; Tsesarsky, M. Stimulation of native microorganisms for biocementation in samples recovered from field scale treatment depths. J. Geotech. Geoenviron. Eng. 2018, 144, 04017098. [Google Scholar] [CrossRef]
- Torsvik, V.; Goksoyr, J.; Daae, F.L. High diversity in DNA of soil bacteria. Appl. Environ. Microbiol. 1990, 56, 782–787. [Google Scholar] [CrossRef] [Green Version]
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Gomez, M.G.; Martinez, B.C.; DeJong, J.T.; Hunt, C.E.; DeVlaming, L.A.; Major, D.W.; Dworatzek, S.M. Field-scale bio-cementation tests to improve sands. Proc. Inst. Civ. Eng.—Ground Improv. 2015, 168, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Holtz, W.G.; Gibbs, H.J. Triaxial Shear Tests on Pervious Gravelly Soils BT—American Society of Civil Engineers—Proceedings. Am. Soc. Civ. Eng.—Proc.—J. Soil Mech. Found. Div. 1956, 82, 867. [Google Scholar]
- Sridharan, A.; Prakash, K. Classification procedures for expansive soils. Proc. Inst. Civ. Eng.—Geotech. Eng. 2000, 143, 235–240. [Google Scholar] [CrossRef] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).