Abstract
Background: In spinal fusion surgery, intersomatic compression force is currently applied subjectively by the operating surgeon, despite its critical role on implant stability and risk of subsidence. No standardized measurement or guideline exists to control or quantify the amount of force applied. Methods: In a two-phase exploratory study, we evaluated whether proprioceptive muscle memory allows reliable reproduction of applied manual compression forces. In Phase 1, 30 participants applied force to a compression clamp equipped with a strain gauge, simulating spinal interbody compression on a 3D-printed vertebral model. They were then asked to reproduce this force using a hand dynamometer at defined time intervals. In Phase 2, intraoperative compression forces applied during spinal fusion procedures were retrospectively assessed by having the operating surgeon reproduce the force on a dynamometer. Results: Participants were able to reproduce their initial manual compression force within a 15% deviation, even 15 min after the initial application. In 116 clinical cases, an average compression force of 146.3 ± 18.5 N was recorded. No significant differences were observed across different spinal segments. Conclusions: These findings provide initial data toward defining a reproducible reference range for indirect intraoperative compression assessment. Standardization of applied force may help improve biomechanical outcomes and reduce complications such as implant migration, pseudarthrosis, or cage subsidence.
1. Introduction
Increasing life expectancy and the associated demographic shift have led to a rise in age-related degenerative spine diseases [1,2,3]. In addition to compression of the spinal structures, these changes also lead to instabilities and deformities in the coronal and sagittal plane, which—depending on their severity—can be associated with considerable restrictions in quality of life [4,5].
While conservative therapy is effective for many patients, surgical intervention becomes necessary in cases of persistent symptoms or structural instability. Over the past three decades, lumbar interbody fusion has become a standard technique, offering high primary stability and fusion rates exceeding 90% when combined with interbody implants (cages) and pedicle screw–rod systems. In these studies, fusion rates of 90% and higher were demonstrated [6,7,8]. The combination of a screw–rod system and a cage may require intersomatic compression and the correction of deformities.
A critical and often underestimated aspect is the compression force applied by the surgeon. Too little compression may compromise primary stability, while excessive force increases the risk of cage subsidence, particularly in osteoporotic patients [9,10]. From a biomechanical perspective, controlled interbody compression promotes intimate contact between the cage and the vertebral endplates, which is essential for primary mechanical stability and successful osseous integration. Proper load transfer encourages bone remodeling and fusion, while excessive force can damage endplate integrity and hinder the biological healing process. Thus, the applied compression force is not only a technical parameter, but also a determinant of long-term construct success.
Despite its importance, there are no standardized values or validated methods to quantify compression force intraoperatively. The exact amount of compression is therefore a central, but so far insufficiently investigated element of the surgical technique—with a potential influence on the functional outcome, the need for revision surgery, and the long-term prognosis.
Only very limited information is available in the literature regarding compression forces applied during spinal fusion surgery. A brief search of PubMed confirmed that no clinical studies have systematically documented intraoperative compression magnitudes, with existing research focusing primarily on alignment parameters or in vitro implant stability. Instead, the focus has typically been on sagittal balance and alignment [11,12,13]. Other in vitro studies have investigated the effect of compression force on the stability of standalone cages and transpedicular instrumentation and found an increase in stability with higher preload [14], but no clinical data exist on applied compression magnitudes. Implant manufacturers also do not provide information on the recommended compression forces. Instead, compression is applied in everyday clinical practice based on the surgeon’s experience.
Parallel to the evolution of surgical techniques, significant advancements have been made in implant design, imaging, and navigation technologies, aiming to improve the safety and precision of spinal fusion procedures. Despite these innovations, the application of compression force remains largely unstandardized and surgeon-dependent. While alignment parameters can be verified radiographically, there is currently no method to objectively quantify the force applied during cage insertion or final construct assembly.
This lack of quantifiable intraoperative data becomes particularly relevant in the context of increasing automation and robotic assistance in spine surgery [15]. Robotic systems, which are already used to improve screw placement accuracy, rely on predefined algorithms and parameters. To enable robotic systems to support or eventually automate procedural steps such as interbody compression, a reliable empirical foundation is required. In the absence of standardized force metrics, robotic platforms and AI-based planning tools cannot emulate the nuanced manual techniques employed by experienced surgeons. Therefore, establishing objective intraoperative force data is essential—not only for improving surgical consistency but also as a prerequisite for the safe integration of robotic and autonomous systems [15].
Given the lack of approved intraoperative force-measuring tools, this study investigates whether manual compression force can be reproduced via proprioceptive memory and measured with a dynamometer. Proprioceptive mechanisms, including muscle spindle function and kinaesthetic sensing, provide the sensory basis for movement and force perception [16,17,18]. These studies provide physiological background on how proprioceptive feedback contributes to sensing muscle length, position, and effort. Phase 1 aimed to test the feasibility of this approach in a simulated setting, assessing whether applied compression forces can be reproduced reliably. Phase 2 then served as an exploratory investigation to document the actual range of compression forces applied intraoperatively.
2. Materials and Methods
2.1. Sample of Participants
This study was conducted in two consecutive phases. In Phase 1, a controlled experimental setup was used to evaluate how reliably manual compression forces can be reproduced under standardized laboratory conditions. A total of 30 participants (19 males, 11 females) took part to evaluate how reliably manual compression forces can be reproduced under controlled laboratory conditions. The mean age of male participants was 39.9 ± 9.5 years, the mean age of female participants was 35.6 ± 10.0 years, and the overall mean age was 38.3 ± 10.3 years.
Phase 2 (intraoperative data collection):
Data were obtained from 116 surgical cases of lumbar interbody fusion procedures. For this phase, no personal demographic data such as age or sex were recorded, as the focus was exclusively on the quantification of intraoperative compression forces rather than patient-specific outcomes.
The inclusion criteria were as follows: age ≥ 18 years, voluntary participation, and for Phase 2, indication for lumbar interbody fusion with informed consent. The exclusion criteria were as follows: refusal to participate.
2.2. Study Design
The study was conducted in two phases. Phase 1 evaluated the reproducibility of applied compression force using proprioception on a simulated spine model. Phase 2 applied the same method intraoperatively to collect quantitative compression force data during spinal fusion procedures. Ethical approval for the study was obtained from the local ethics committee (approval number 355/20-ek; September 2020).
2.3. Phase 1: Simulation Testing
A compression clamp (DePuySynthes, Norderstedt, Germany) was fitted with a strain gauge near the hinge to achieve linear correlation between force and deformation (Figure 1a). The device was calibrated with a load cell. Data acquisition was carried out using the DAQ-measuring amplifier from ME-Meßsysteme (GmbH, Hennigsdorf, Germany). The strain gauge was designed as a Poisson half-bridge, so that temperature expansions are automatically compensated. Six compression cycles were performed for calibration. For this purpose, the clamp was pressed directly onto the force sensor. Due to friction effects within the parallel guide mechanism, the clamp tended to jam on the non-compliant force sensor surface, resulting in measurable hysteresis. Therefore, only the compression phase was used for calibration. A linear regression was calculated using Matlab 2020a (MathWorks, Natick, MA, USA), yielding a coefficient of determination of R2 = 0.985. The standard deviation of the procedure was calculated in accordance with DIN 32645, resulting in Sxy = 4.93 N. At the target measurement range of 200 N, this corresponds to a measurement uncertainty of approximately 2.5%, which is considered acceptable.
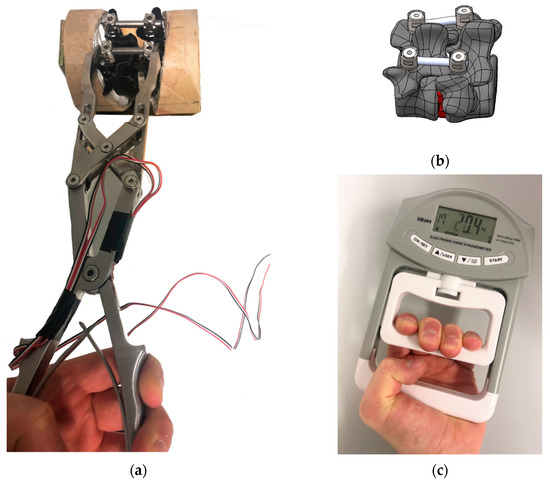
Figure 1.
Phase 1 test setup showing (a) compression clamp on 3D-printed spinal segment, (b) schematic representation of the segment, and (c) measurement with the hand dynamometer.
A total of 30 participants (19 male, 11 female) were included in the first phase of this study, with a mean age of 38.3 years (SD = 10.25). The cohort consisted of 2 chief surgeons, 9 senior consultants, 2 board-certified specialists, 8 surgical residents, and 9 biomedical engineers, representing a balanced mix of clinical and technical backgrounds. This diversity allowed for the assessment of force reproducibility across varying levels of surgical experience and mechanical familiarity. They were instructed to apply a moderate target force (~200 N) using their dominant hand while standing and leaning forward over a table. The clamp was positioned on a 3D-printed L4/L5 spine segment as shown in Figure 1b and securely stabilized in a vice to prevent any movement. This setup was designed to realistically simulate the ergonomic and mechanical conditions of intraoperative compression during spinal fusion surgery. After one minute, they reproduced this force on a hand dynamometer (Model EH101, Camry Industries Company Ltd., Hong Kong, China, Figure 1c). Repeat measurements were taken at 5, 10, and 15 min. A statistical analysis of the collected data was carried out using Microsoft Excel.
2.4. Phase 2: Intraoperative Assessment
During the second phase of the study, a total of 116 spinal fusion procedures were performed by four board-certified spine surgeons at a single spine center. Immediately after completing the interbody compression maneuver during routine instrumentation, each surgeon was asked to reproduce the applied manual compression force on a sterilized hand dynamometer. The dynamometer was handed to the surgeon by the scrub nurse to ensure sterility and workflow integrity. Importantly, the display of the device was concealed from the surgeon to avoid any visual feedback. The measured values were documented by a member of the surgical nursing team and anonymized for subsequent analysis.
2.5. Statistical Analysis
The analysis was restricted to descriptive statistics, as the study was conceived as an exploratory proof-of-concept investigation. No hypothesis-driven testing or power calculations were performed. Measured force values were analyzed by calculating absolute and relative deviations between the target and reproduced forces. For all measurements, mean values, standard deviations, and ranges were determined to assess central tendency and variability. Time-dependent trends in reproducibility were examined by comparing deviations across repeated measurements (1, 5, 10, and 15 min). Additionally, boxplots were generated to visualize the distribution and spread of the applied forces. All data were compiled, processed, and graphically displayed using Microsoft Excel (Office 2016, Microsoft Corporation, Redmond, WA, USA).
3. Results
3.1. Phase 1
Participants applied an average compression force of 198 N (SD = 80 N) on the 3D-printed vertebral model. The complete dataset is shown in Figure 2. Due to a technical failure during data acquisition, one participant’s measurement at the T5 time point could not be recorded and was excluded from the analysis.
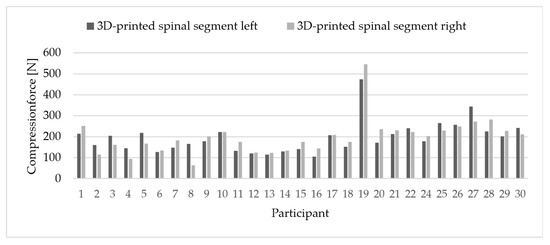
Figure 2.
Comparison of applied force per participant on the dummy.
Left–right deviations averaged 32 N (SD = 24 N). On average, participants showed an individual deviation of 35 N (SD = 38 N) between the clamp and the dynamometer measurements (Figure 3).
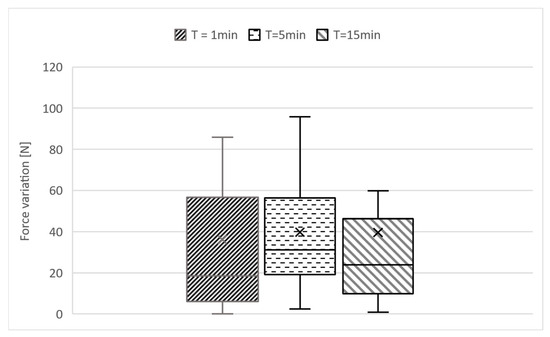
Figure 3.
The deviation in the values measured on the force dynamometer at 3 different points in time is presented in relation to the individual force applied to the dummy.
As shown in Figure 4, after 15 min, 24 out of 30 participants showed individual deviations within ±15%. An exploratory subgroup comparison (including eight spine surgeons) revealed overlapping deviations, with most participants in both groups within this range. Since individual variation exceeded subgroup differences, these were not further analyzed.
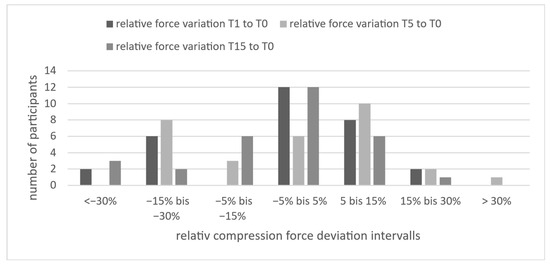
Figure 4.
Distribution of participants according to relative deviation in reproduced compression force at T1, T5, and T15 compared to the initial force (T0), categorized in seven deviation intervals. Most values lie within ±15%, indicating high reproducibility of proprioceptively recalled force over time.
3.2. Phase 2
Across 116 procedures, the mean compression force was 146.3 N (SD = 18.5 N). Segment-specific results were as follows (see Table 1):

Table 1.
Mean compression forces (in Newtons) applied during spinal fusion surgery by segment level (L1–S1).
The Spearman correlation between spinal segment and applied force was low (r = 0.091, p = 0.338), indicating no significant association between segment level and the amount of applied compression force. The mean forces applied by individual surgeons ranged from approximately 139 N to 148 N. Standard deviations ranged from 14 N to 21 N, suggesting moderate intra-operator variability. Surgeon 2 showed the highest variability, while Surgeon 5 demonstrated the most consistent force application.
A comparison of the results is shown in the boxplot (Figure 5). It shows a slightly lower average force during actual surgical procedures.
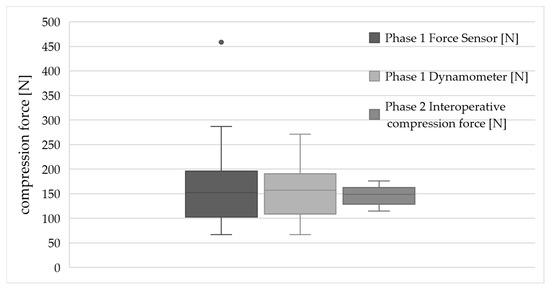
Figure 5.
Boxplot comparison of compression forces measured in Phase 1 (via force sensor and dynamometer) and Phase 2 (intraoperative application). The grey dot indicates an outlier value outside 1.5 times the interquartile range.
Additional data supporting these results are provided in the Supplementary Materials (Figures S1 and S2, Table S1).
4. Discussion
4.1. Summary of Main Findings
The primary purpose of this study was to evaluate whether manual compression forces applied during spinal fusion procedures can be measured and standardized using proprioceptive feedback. Phase 1 demonstrated that participants were able to reproduce previously applied compression forces with satisfactory consistency under controlled conditions, confirming the feasibility of the proposed proprioceptive measurement approach. In Phase 2, the same method was successfully implemented intraoperatively, providing the first quantitative insight into the range of compression forces typically applied during spinal fusion surgery. These findings support our initial hypothesis that proprioceptive muscle memory enables reproducible application of manual compression forces and that this method can be transferred to the operating room. Comparable results have been reported in studies [19] examining the reliability of proprioceptive force control in manual tasks, which similarly describe stable intraindividual reproduction of applied forces under standardized conditions.
This study demonstrates that proprioceptive muscle memory allows consistent reproduction of applied compression forces. Even 15 min after initial application, most participants maintained deviations under 15%, justifying the principle of this method to use it in Phase 2. It should be noted that the Phase 1 cohort included both clinical and non-clinical participants. The study was designed to validate the reproducibility of proprioceptive force reproduction independent of surgical experience. As such, it primarily reflects the psychomotor feasibility of proprioceptive transfer, not the operative training level.
4.2. Interpretation and Biomechanical Context
Intraoperatively, an average force of ~146 N was applied. Compression forces are relevant factors on primary stability, fusion rates, and the risk of implant failure avoiding pseudarthrosis and subsidence [9,20]. In addition to mechanical stability, applied compressive forces influence biological healing processes at the implant–bone interface. Mechanotransduction thresholds are known to regulate bone formation, and both under- and overloading can disrupt osteointegration. Future studies should investigate whether typical intraoperative compression forces fall within the optimal range for promoting osseous fusion. Subsidence rates reported in the literature vary widely depending on the fusion technique, implant type, and patient-specific characteristics. A systematic review documented subsidence rates ranging from 6% to 51%, with TLIF and LLIF procedures being particularly affected [9]. In addition to bone mineral density (BMD), factors such as cage design (material, height, footprint) and the precise positioning of the implant within the endplate are considered critical [10,21]. Biomechanical weakness in the central region of the endplate significantly increases the risk of implant subsidence [9,22]. In a retrospective cohort study, Zhou et al. demonstrated that irregular endplate morphologies are significantly associated with higher subsidence rates following TLIF procedures [23]. Further relevant factors include patient age, cage position (anterior vs. central), cage height, and the integrity of the cortical endplate region [9,24]. Additionally, bone mineral density and regional endplate characteristics—such as cortical thickness and surface contour—may alter the tolerance to applied force. Integrating preoperative CT-based Hounsfield unit analysis or DEXA scores could enable force-adapted strategies tailored to patient-specific bone quality. Such strategies might involve upper force limits for osteoporotic patients and defined compression corridors based on HU thresholds or T-scores.
4.3. Methodological Considerations
Our study presents, for the first time, a reproducible method for indirect quantification of the applied compression force, thereby opening the possibility for standardized force application. This indirect estimation of intraoperative compression force is a methodological surrogate. Due to current regulatory constraints (e.g., MDR classification of sensor-equipped devices), direct force measurements at the implant–bone interface were not feasible. At present, no approved device for direct intraoperative force measurement at the implant–endplate interface is available. Developing and certifying such sensor-equipped instruments in collaboration with a medical device manufacturer represents a key future goal. Once available, these tools will allow validation of the surrogate method presented here against true intraoperative force profiles. Future studies should aim to incorporate sensorized instruments to obtain in situ force profiles. Defining an optimal compression range may help avoid both under- and overcompression. Both extremes are associated with clinical risks: overcompression can lead to endplate violation and to early subsidence, while undercompression may result in impaired implant migration, instability, and persistent postoperative pain [9,24]. In addition to the absolute compression magnitude, the rate at which the force is applied may influence endplate preservation. Rapid application of high forces can induce localized structural failure, whereas gradual compression may better preserve trabecular integrity. Furthermore, the pressure per unit area is determined not only by the applied force but also by the surface area of the cage endplate. Narrow cages or designs with reduced footprint may concentrate forces and increase subsidence risk, even under moderate load. While all measurements in this study were performed using a standardized compression clamp, the mechanical characteristics of different clamp designs (e.g., handle leverage, gear ratio, force amplification) may influence force transmission. Future work should assess whether variability in instrument design affects the accuracy of proprioceptive force reproduction.
Although some studies have not found a significant association between subsidence and clinical outcomes such as pain or function [25], the biomechanical implications remain undisputed—particularly with respect to pseudarthrosis formation and secondary loss of correction [9,11]. It is also worth distinguishing between immediate mechanical endplate failure and delayed subsidence due to microfracture-induced remodeling. Whether intraoperative compression contributes more to primary or secondary forms of subsidence remains to be elucidated. It is worth noting that anterior or lateral fusion techniques (e.g., ALIF, OLIF, LLIF) may involve different load transmission mechanics. The generalizability of proprioceptive force reproduction in these approaches has yet to be evaluated. While a previous study has investigated the compressive forces acting on interbody cages [26], the reported data were obtained from cadaver experiments, in which the measured forces resulted from a combination of implant compression and the gravitational load (external compression) of the overlying anatomical structures. To date, the initial intraoperative compression force itself has not been systematically investigated. Therefore, our findings provide a clinically and biomechanically relevant foundation for developing evidence-based compression guidelines. Integrating such recommendations into future implant usage instructions or clinical guidelines represents a promising approach to mitigating the associated complications. This cost-effective and modular approach to measuring forces applied during surgery can be extended to other surgical instruments, enabling the assessment of intraoperative process parameters. In the long term, this could contribute to advances in the standardization of surgical protocols and provide a data foundation for developing standard operating procedures (SOPs) for both human surgeons and surgical robots [15]. However, for robotic systems to safely execute compression maneuvers, predefined and validated force targets are required. The absence of such benchmarks currently limits full automation of this procedural step. The potential for real-time intraoperative feedback—whether via analog indicators, haptic resistance, or digital readouts—could support surgeons in maintaining compression within an optimal therapeutic window. Establishing such feedback systems would not only enhance consistency but also facilitate training and pave the way for robotic execution of compression maneuvers. While our findings suggest that intraoperative compression force can be reliably reproduced using proprioceptive memory, this study did not assess clinical endpoints such as fusion rates, implant subsidence, or patient-reported outcome measures (e.g., Oswestry Disability Index). As such, no direct correlation between applied force and postoperative success can be inferred from the current data. A prospective clinical trial including radiological follow-up and validated functional scores is currently being prepared to investigate whether standardized compression force application translates into improved biomechanical and clinical outcomes. Moreover, if reliable force data become available, they could also be integrated into the training of young surgeons—for example, in simulation workshops or structured educational programs.
4.4. Strengths, Limitations, and Practical Applications
The proposed method for intraoperative compression force estimation is simple, low-cost, and fully compatible with existing surgical workflows, providing a foundation for future studies aiming to establish objective compression thresholds. Furthermore, it offers a reproducible framework that can be implemented in both experimental and intraoperative contexts, serving as a proof of concept for standardized force assessment in spinal fusion surgery.
Several limitations should be acknowledged. First, the results of Phase 1 are based on a controlled experimental setup using a 3D-printed spine model, which does not replicate the viscoelastic and anisotropic properties of native vertebral endplates. Therefore, the absolute force thresholds derived from this model may not fully translate to in vivo biomechanics. However, Phase 1 was deliberately not designed to replicate the biomechanical properties of native endplates but rather to serve as a proof of concept for proprioceptive reproducibility. The 3D-printed model provided a standardized and reproducible setup. Second, the cohort of 30 participants included both clinical and non-clinical individuals. While this allowed assessment of general proprioceptive ability, the findings may not fully reflect the behavior of highly trained surgeons under real operative conditions. Third, intraoperative forces were not directly measured at the implant interface due to regulatory constraints. Instead, we used a proprioceptive method involving a hand dynamometer, which, while practical and reproducible, remains an indirect surrogate. Fourth, only descriptive statistics were applied, and no hypothesis-driven group comparisons or power calculations were performed. The use of descriptive statistics only reflects the exploratory character of this work. Inferential testing was intentionally omitted, as the limited sample size would not have allowed robust subgroup comparisons. Future prospective trials with larger patient cohorts will incorporate hypothesis-driven analyses to address inter-surgeon variability, time-dependent reproducibility, and segment-specific effects. Lastly, no clinical outcomes such as subsidence, fusion rate, or patient-reported metrics were assessed. A follow-up prospective trial including imaging and clinical endpoints is currently planned. Practically, this work provides a methodological foundation for future research and clinical translation. The approach can support surgical training by improving awareness of manual force application, guide implant development toward force-adaptive designs, and inform the creation of intraoperative feedback systems or robotic assistance protocols.
5. Conclusions
This study demonstrates that proprioceptive muscle memory enables reproducible estimation of manually applied compression forces during spinal fusion surgery, both in a controlled experimental and intraoperative setting. However, this exploratory dataset provides initial evidence toward defining intraoperative target ranges for compression, which may ultimately help reduce implant-related complications such as cage migration, pseudarthrosis, or endplate failure. Integrating this practical and low-cost approach into existing surgical workflows represents a scalable first step toward standardizing compression in spine surgery. Future studies should investigate whether force standardization improves fusion rates and patient outcomes. They should also explore how such standardization may support intraoperative feedback systems and robotic execution protocols. In addition, investigations should correlate intraoperative compression forces with radiographic outcomes such as fusion, subsidence, and implant migration, as well as with patient-reported measures like ODI or VAS. Incorporating bone quality parameters (e.g., Hounsfield units or DEXA) may help to define patient-specific compression corridors and establish clinically meaningful benchmarks.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomechanics5040091/s1, Figure S1: Boxplot of the measured force by segment; Figure S2: Boxplot of the measured force by operator; Table S1: Spearman correlation by segment and force.
Author Contributions
Conceptualization, N.H.v.d.H. and R.H.; methodology, R.H. and S.S.; software, R.H.; validation, S.S., R.H. and N.H.v.d.H.; formal analysis, R.H. and N.H.v.d.H.; investigation, R.H., S.S. and P.M.; resources, S.S.; data curation, R.H. and P.M.; writing—original draft preparation, R.H.; writing—review and editing, N.H.v.d.H., S.S. and C.-E.H.; visualization, R.H.; supervision, N.H.v.d.H.; project administration, C.-E.H.; funding acquisition, C.-E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Deutsche Arthrose-Hilfe”, grant number “P491-A585-Heyde-EP2-von 1-wirbelsäule-op-III-9k-2020-21”; Supported by the Open Access Publishing Fund of Leipzig University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Leipzig (protocol code 355/20-ck, approved in September 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Acknowledgments
The authors used ChatGPT (OpenAI, version GPT4o) exclusively to refine linguistic style and improve the readability of the manuscript. No content generation, data analysis, reference handling, or interpretation of results was performed by the tool. The authors have carefully reviewed and edited all AI-assisted text and take full responsibility for the scientific content of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| 3D | Three-Dimensional |
| BMD | Bone Mineral Density |
| CT | Computed Tomography |
| DAQ | Data Acquisition |
| DEXA | Dual-Energy X-ray Absorptiometry |
| DIN | Deutsches Institut für Normung (German Institute for Standardization) |
| HU | Hounsfield Units |
| L1–S1 | Lumbar Vertebra 1 to Sacral Vertebra 1 |
| MA | Massachusetts (State abbreviation—MathWorks location) |
| MDR | Medical Device Regulation (EU) |
| N | Newton (Unit of Force) |
| N.T. | New Territories (Region in Hong Kong) |
| R2 | Coefficient of Determination (Statistical Measure) |
| SD | Standard Deviation |
| SOP | Standard Operating Procedure |
| T0, T1, T5, T15 | Time points in the study (e.g., immediately, after 1, 5, 15 min) |
| TLIF | Transforaminal Lumbar Interbody Fusion |
| LLIF | Lateral Lumbar Interbody Fusion |
| OLIF | Oblique Lumbar Interbody Fusion |
| ALIF | Anterior Lumbar Interbody Fusion |
References
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future Life Expectancy in 35 Industrialised Countries: Projections with a Bayesian Model Ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef]
- Mavrych, V.; Bolgova, O.; Ganguly, P.; Kashchenko, S. Age-Related Changes of Lumbar Vertebral Body Morphometry. Austin J. Anat. 2014, 1, 1–7. [Google Scholar]
- Weiler, C.; Schietzsch, M.; Kirchner, T.; Nerlich, A.G.; Boos, N.; Wuertz, K. Age-Related Changes in Human Cervical, Thoracal and Lumbar Intervertebral Disc Exhibit a Strong Intra-Individual Correlation. Eur. Spine J. 2012, 21, 810–818. [Google Scholar] [CrossRef]
- Akbar, M.; Bullmann, V.; Granitzka, M.; Diebo, B.G.; Shah, N.V.; Stroud, S.G.; Paulino, C.B.; Schwab, F.J.; Lafage, V.; Birkenmaier, C.; et al. Komplikationen bei der Behandlung adulter spinaler Deformitäten. Der Orthopäde 2018, 47, 274–275. [Google Scholar] [CrossRef]
- Dall, T.M.; Gallo, P.D.; Chakrabarti, R.; West, T.; Semilla, A.P.; Storm, M.V. An Aging Population and Growing Disease Burden Will Require a Large and Specialized Health Care Workforce by 2025. Health Aff. 2013, 32, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Lauber, S.; Schulte, T.L.; Liljenqvist, U.; Halm, H.; Hackenberg, L. Clinical and Radiologic 2—4-Year Results of Transforaminal Lumbar Interbody Fusion in Degenerative and Isthmic Spondylolisthesis Grades 1 and 2. Spine 2006, 31, 1693–1698. [Google Scholar] [CrossRef]
- Hackenberg, L.; Halm, H.; Bullmann, V.; Vieth, V.; Schneider, M.; Liljenqvist, U. Transforaminal Lumbar Interbody Fusion: A Safe Technique with Satisfactory Three to Five Year Results. Eur. Spine J. 2005, 14, 551–558. [Google Scholar] [CrossRef]
- Nemoto, O.; Asazuma, T.; Yato, Y.; Imabayashi, H.; Yasuoka, H.; Fujikawa, A. Comparison of Fusion Rates Following Transforaminal Lumbar Interbody Fusion Using Polyetheretherketone Cages or Titanium Cages with Transpedicular Instrumentation. Eur. Spine J. 2014, 23, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Parisien, A.; Wai, E.K.; ElSayed, M.S.A.; Frei, H. Subsidence of Spinal Fusion Cages: A Systematic Review. Int. J. Spine Surg. 2022, 16, 1103–1118. [Google Scholar] [CrossRef]
- Hu, Z.; He, D.; Gao, J.; Zeng, Z.; Jiang, C.; Ni, W.; Yik, J.H.N.; Zhao, X.; Fan, S. The Influence of Endplate Morphology on Cage Subsidence in Patients With Stand-Alone Oblique Lateral Lumbar Interbody Fusion (OLIF). Glob. Spine J. 2023, 13, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Obermueller, T.; Wagner, A.; Kogler, L.; Joerger, A.-K.; Lange, N.; Lehmberg, J.; Meyer, B.; Shiban, E. Radiographic Measurements of Cervical Alignment, Fusion and Subsidence after ACDF Surgery and Their Impact on Clinical Outcome. Acta Neurochir. 2020, 162, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.-J.; Volkheimer, D.; Robie, B.; Christensen, F.B. Two-Piece ALIF Cage Optimizes the Bone–Implant Interface in a 360° Setting. Eur. Spine J. 2017, 26, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, Q.; Chen, W.; Xu, K.; Wu, Q.; Chen, G. Posterolateral Lumbar Fusion versus Transforaminal Lumbar Interbody Fusion for the Treatment of Degenerative Lumbar Scoliosis. J. Clin. Neurosci. 2013, 20, 1241–1245. [Google Scholar] [CrossRef]
- Patwardhan, A.G.; Carandang, G.; Ghanayem, A.J.; Havey, R.M.; Cunningham, B.; Voronov, L.I.; Phillips, F.M. Compressive Preload Improves the Stability of Anterior Lumbar Interbody Fusion Cage Constructs. J. Bone Jt. Surg. 2003, 85, 1749–1756. [Google Scholar] [CrossRef]
- Maier-Hein, L.; Vedula, S.; Speidel, S.; Navab, N.; Kikinis, R.; Park, A.; Eisenmann, M.; Feussner, H.; Forestier, G.; Hashizume, M.; et al. Surgical Data Science: Enabling next-Generation Surgery. arXiv 2017, arXiv:1701.06482. [Google Scholar] [CrossRef]
- Moon, K.M.; Kim, J.; Seong, Y.; Suh, B.-C.; Kang, K.; Choe, H.K.; Kim, K. Proprioception, the Regulator of Motor Function. BMB Rep. 2021, 54, 393–402. [Google Scholar] [CrossRef]
- Kröger, S. Proprioception 2.0: Novel Functions for Muscle Spindles. Curr. Opin. Neurol. 2018, 31, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Gandevia, S.C. The Kinaesthetic Senses. J. Physiol. 2009, 587, 4139–4146. [Google Scholar] [CrossRef]
- Weerakkody, N.; Percival, P.; Morgan, D.L.; Gregory, J.E.; Proske, U. Matching Different Levels of Isometric Torque in Elbow Flexor Muscles after Eccentric Exercise. Exp. Brain Res. 2003, 149, 141–150. [Google Scholar] [CrossRef]
- Heyde, C.-E.; Robinson, Y.; Jeszenszky, D. Ätiologie und Pathogenese der Spondylodiszitis. Die Wirbelsäule 2017, 1, 237–244. [Google Scholar] [CrossRef]
- Grant, J.P.; Oxland, T.R.; Dvorak, M.F. Mapping the Structural Properties of the Lumbosacral Vertebral Endplates. Spine 2001, 26, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Okano, I.; Salzmann, S.N.; Reisener, M.; Chiapparelli, E.; Shue, J.; Sama, A.A.; Cammisa, F.P.; Girardi, F.P.; Hughes, A.P. Endplate Volumetric Bone Mineral Density Is a Predictor for Cage Subsidence Following Lateral Lumbar Interbody Fusion: A Risk Factor Analysis. Spine J. 2021, 21, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, X.; Xu, L.; Li, S.; Du, C.; Sun, X.; Wang, B.; Zhu, Z.; Qiu, Y. Does Vertebral End Plate Morphology Affect Cage Subsidence After Transforaminal Lumbar Interbody Fusion? World Neurosurg. 2019, 130, e694–e701. [Google Scholar] [CrossRef]
- Rickert, M.; Fennema, P.; Wehner, D.; Rahim, T.; Hölper, B.; Eichler, M.; Makowski, M.; Meurer, A.; Brenneis, M. Postoperative Cage Migration and Subsidence Following TLIF Surgery Is Not Associated with Bony Fusion. Sci. Rep. 2023, 13, 12597. [Google Scholar] [CrossRef]
- Tempel, Z.J.; McDowell, M.M.; Panczykowski, D.M.; Gandhoke, G.S.; Hamilton, D.K.; Okonkwo, D.O.; Kanter, A.S. Graft Subsidence as a Predictor of Revision Surgery Following Stand-Alone Lateral Lumbar Interbody Fusion. J. Neurosurg. Spine 2018, 28, 50–56. [Google Scholar] [CrossRef]
- Calek, A.-K.; Cornaz, F.; Suter, M.; Fasser, M.-R.; Baumgartner, S.; Sager, P.; Farshad, M.; Widmer, J. Load Distribution on Intervertebral Cages with and without Posterior Instrumentation. Spine J. 2024, 24, 889–898. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).