Morphology and Knee Joint Kinetics in National Football League Draft Prep Players: Implications for Osteoarthritis Development †
Abstract
1. Introduction
2. Materials and Methods
2.1. Body Morphology and Anthropometric Measurements
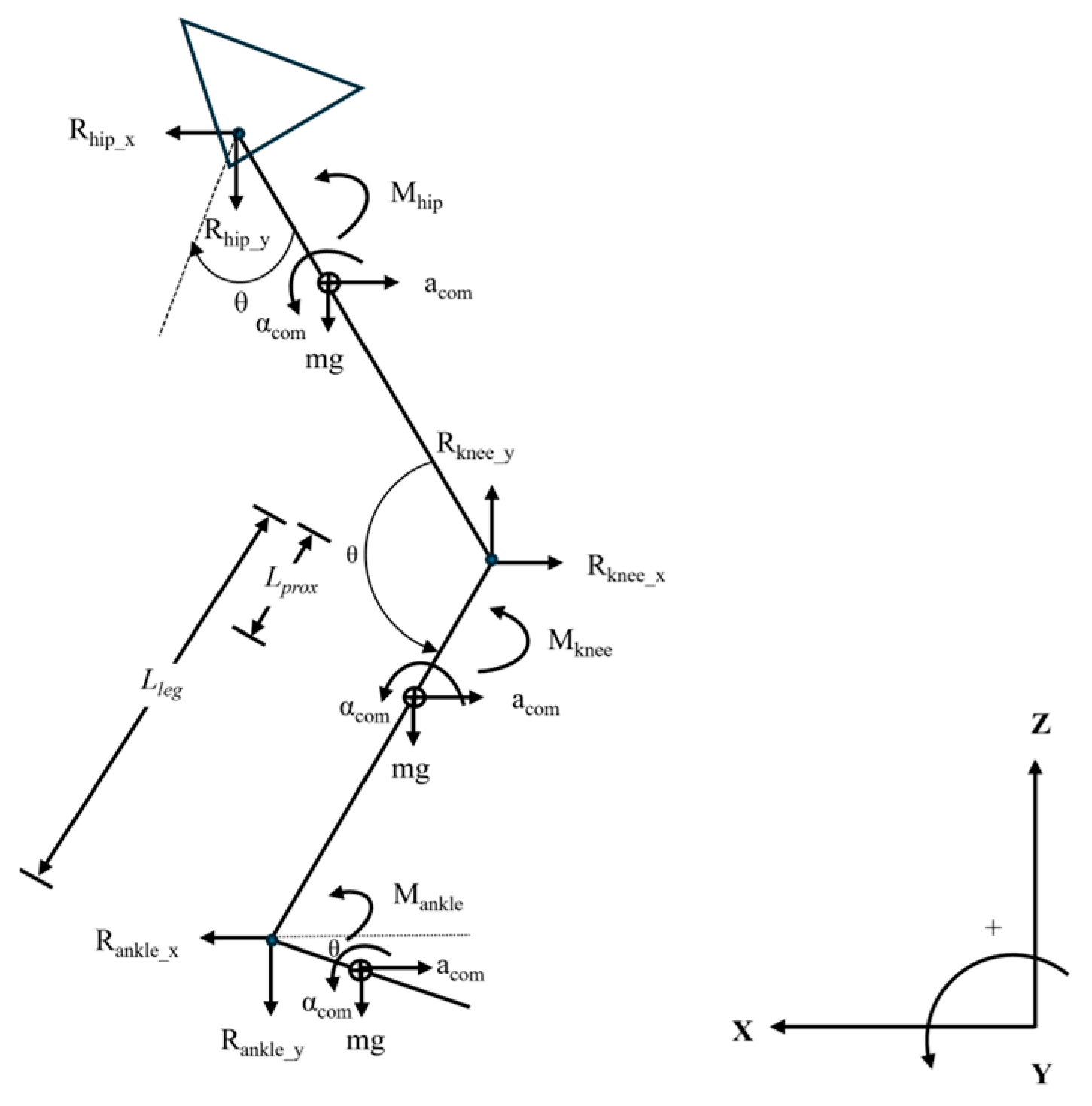
2.2. Running Mechanics
Data Processing
2.3. Statistical Analysis
3. Results
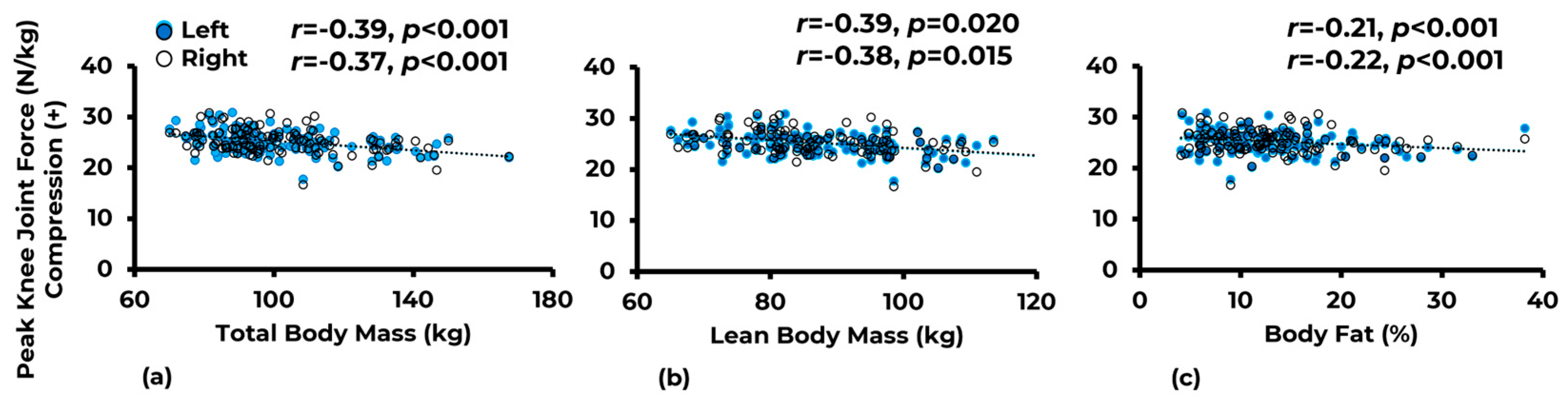
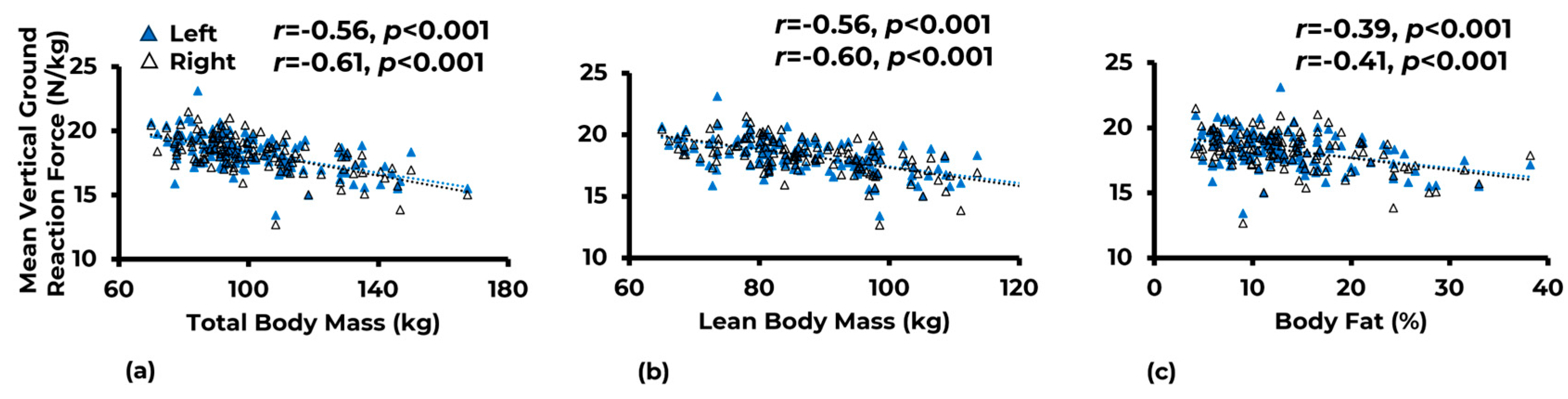
3.1. Associations
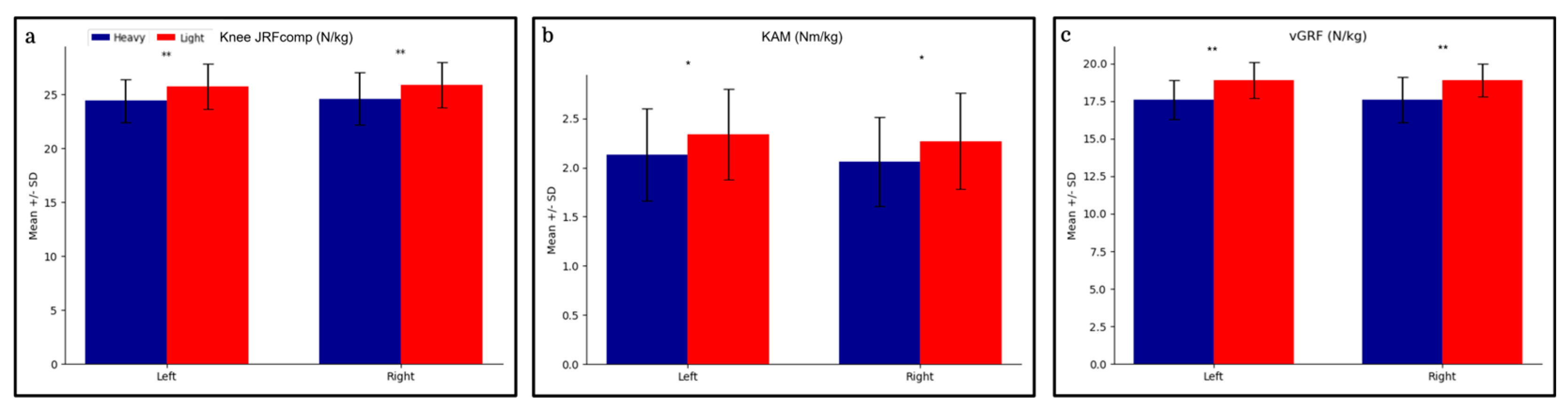
3.2. Differences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BF% | Body fat percentage |
| BIA | Bioelectrical impedance analysis |
| BMI | Body mass index |
| KAM | Knee adductor moment |
| kg | Kilogram |
| JRFcomp | Compression joint reaction force |
| LBM | Lean body mass |
| N/kg | Newton per kilogram |
| Nm/kg | Newton-meter per kilogram |
| TBM | Total body mass |
References
- Deshpande, B.R.; Katz, J.N.; Solomon, D.H.; Yelin, E.H.; Hunter, D.J.; Messier, S.P.; Suter, L.G.; Losina, E. Number of persons with symptomatic knee osteoarthritis in the US: Impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. 2016, 68, 1743–1750. [Google Scholar] [CrossRef]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef]
- Griffin, T.M.; Guilak, F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc. Sport Sci. Rev. 2005, 33, 195–200. [Google Scholar] [CrossRef]
- Lo, G.H.; McAlindon, T.E.; Kriska, A.M.; Price, L.L.; Rockette-Wagner, B.J.; Mandl, L.A.; Eaton, C.B.; Hochberg, M.C.; Jackson, R.D.; Kwoh, C.K.; et al. Football increases future risk of symptomatic radiographic knee osteoarthritis. Med. Sci. Sports Exerc. 2020, 52, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Golightly, Y.M.; Marshall, S.W.; Callahan, L.F.; Guskiewicz, K. Early-onset arthritis in retired National Football League players. J. Phys. Act. Health 2009, 6, 638–643. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Cortez, B.; Baker, J.; Borg-Stein, J.; Wasfy, M.; Baggish, A.L.; Zafonte, R. Prevalence of total hip and knee arthroplasty in former National Football League players: Comparison with the general US population and other populations of professional athletes. BMJ Open Sport Exerc. Med. 2020, 6, e000833. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.R.; Harmatz, J.S.; Zhao, Y.; Greenblatt, D.J. Body size changes among National Collegiate Athletic Association New England Division III football players, 1956–2014: Comparison with age-matched population controls. J. Athl. Train. 2016, 51, 373–381. [Google Scholar] [CrossRef]
- Anzell, A.R.; Potteiger, J.A.; Kraemer, W.J.; Otieno, S. Changes in height, body weight, and body composition in American style football players from 1942 to 2011. J. Strength Cond. Res. 2013, 27, 77–284. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, B.H. Anthropometric cross-sectional comparisons of college football players and potential health implications. J. Strength Cond. Res. 2012, 26, 3358–3364. [Google Scholar] [CrossRef]
- Jacobson, B.H.; Dawes, J.; Smith, D.; Johnson, Q. Kinanthropometric characteristic comparisons of NCAA Division I offensive and defensive linemen spanning eight decades. J. Strength Cond. Res. 2022, 36, 3404–3408. [Google Scholar] [CrossRef]
- Robbins, D.W. Positional physical characteristics of players drafted into the National Football League. J. Strength Cond. Res. 2011, 25, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Secora, C.A.; Latin, R.W.; Berg, K.E.; Noble, J.M. Comparison of physical and performance characteristics of NCAA Division I football players: 1987 and 2000. J. Strength Cond. Res. 2004, 18, 286–291. [Google Scholar] [CrossRef]
- Yamamoto, J.B.; Yamamoto, B.E.; Yamamoto, P.P.; Yamamoto, L.G. Epidemiology of college athlete sizes, 1950s to current. Res. Sports Med. 2008, 16, 111–127. [Google Scholar] [CrossRef]
- Provencher, M.T.; Chahla, J.; Sanchez, G.; Cinque, M.E.; Kennedy, N.I.; Whalen, J.; Price, M.D.; Moatshe, G.; LaPrade, R.F. Body mass index versus body fat percentage in prospective National Football League athletes: Overestimation of obesity rate in athletes at the National Football League scouting combine. J. Strength Cond. Res. 2018, 32, 1013–1019. [Google Scholar] [CrossRef]
- Harp, J.B.; Hecht, L. Obesity in the National Football League. JAMA 2005, 293, 1058–1062. [Google Scholar] [CrossRef]
- Murphy, L.; Helmick, C.G. The impact of osteoarthritis in the United States: A population-health perspective—A population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthop. Nurs. 2012, 31, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Bowles, K.A.; Wang, Y.; Cicuttini, F.; Davies-Tuck, M.; Hinman, R.S. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann. Rheum. Dis. 2011, 70, 1770–1774. [Google Scholar] [CrossRef]
- Chang, A.H.; Moisio, K.C.; Chmiel, J.S.; Eckstein, F.; Guermazi, A.; Prasad, P.V.; Zhang, Y.; Almagor, O.; Belisle, L.; Hayes, K.; et al. External knee adduction and flexion moments during gait and medial tibiofemoral disease progression in knee osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1099–1106. [Google Scholar] [CrossRef]
- Chehab, E.F.; Favre, J.; Erhart-Hledik, J.C.; Andriacchi, T.P. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1833–1839. [Google Scholar] [CrossRef]
- Huang, C.; Chan, P.K.; Chiu, K.Y.; Yan, C.H.; Yeung, S.S.; Fu, S.N. Exploring the relationship between pain intensity and knee moments in participants with medial knee osteoarthritis: A cross-sectional study. BMC Musculoskelet. Disord. 2021, 22, 685. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Hurwitz, D.E.; Thonar, E.J.; Sum, J.A.; Lenz, M.E.; Dunlop, D.D.; Andriacchi, T.P. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998, 41, 1233–1240. [Google Scholar] [CrossRef]
- D’Lima, D.D.; Fregly, B.J.; Patil, S.; Steklov, N.; Colwell, C.W., Jr. Knee joint forces: Prediction, measurement, and significance. Proc. Inst. Mech. Eng. Part H 2012, 226, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Harding, G.T.; Dunbar, M.J.; Hubley-Kozey, C.L.; Stanish, W.D.; Astephen Wilson, J.L. Obesity is associated with higher absolute tibiofemoral contact and muscle forces during gait with and without knee osteoarthritis. Clin. Biomech. 2016, 31, 79–86. [Google Scholar] [CrossRef]
- Messier, S.P.; Pater, M.; Beavers, D.P.; Legault, C.; Loeser, R.F.; Hunter, D.J.; DeVita, P. Influences of alignment and obesity on knee joint loading in osteoarthritic gait. Osteoarthr. Cartil. 2014, 22, 912–917. [Google Scholar] [CrossRef]
- Miyazaki, T.; Wada, M.; Kawahara, H.; Sato, M.; Baba, H.; Shimada, S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann. Rheum. Dis. 2002, 61, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Reimann, I. Experimental osteoarthritis of the knee in rabbits induced by alteration of the load-bearing. Acta Orthop. Scand. 1973, 44, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Saxby, D.J.; Modenese, L.; Bryant, A.L.; Gerus, P.; Killen, B.; Fortin, K.; Wrigley, T.V.; Bennell, K.L.; Cicuttini, F.M.; Lloyd, D.G. Tibiofemoral contact forces during walking, running and sidestepping. Gait Posture 2016, 49, 78–85. [Google Scholar] [CrossRef]
- Chen, C.T.; Burton-Wurster, N.; Lust, G.; Bank, R.A.; Tekoppele, J.M. Compositional and metabolic changes in damaged cartilage are peak-stress, stress-rate, and loading-duration dependent. J. Orthop. Res. 1999, 17, 870–879. [Google Scholar] [CrossRef]
- Kerin, A.J.; Coleman, A.; Wisnom, M.R.; Adams, M.A. Propagation of surface fissures in articular cartilage in response to cyclic loading in vitro. Clin. Biomech. 2003, 18, 960–968. [Google Scholar] [CrossRef]
- Souza, R.B.; Kumar, D.; Calixto, N.; Singh, J.; Schooler, J.; Subburaj, K.; Li, X.; Link, T.M.; Majumdar, S. Response of knee cartilage T1rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1367–1376. [Google Scholar] [CrossRef]
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp. J. Intern. Med. 2011, 2, 205–212. [Google Scholar]
- Kutzner, I.; Trepczynski, A.; Heller, M.O.; Bergmann, G. Knee adduction moment and medial contact force--facts about their correlation during gait. PLoS ONE 2013, 8, e81036. [Google Scholar] [CrossRef]
- Mündermann, A.; Dyrby, C.O.; Andriacchi, T.P. Secondary gait changes in patients with medial compartment knee osteoarthritis: Increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005, 52, 2835–2844. [Google Scholar] [CrossRef]
- Mokha, M.; McBride, S.; Haberer, J.; Polletta, J.; Branum, N.; Bonsangue, M.; Schafer, A.; Stensland, J.; Hojeij, N.; Christoforides, E. Association between morphology and hip and knee joint reaction forces during running in American football players: Implications for osteoarthritis development. In Proceedings of the 42nd International Society of Biomechanics in Sports Conference, Salzburg, Austria, 15–19 July 2024. [Google Scholar]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003. [Google Scholar]
- Geatano, J. Holm–Bonferroni Sequential Correction: An EXCEL Calculator–Ver. 1.2; Microsoft Excel: Redmond, WA, USA, 2013. [Google Scholar]
- Ciacci, S.; Di Michele, R.; Fantozzi, S.; Merni, F. Assessment of kinematic asymmetry for reduction of hamstring injury risk. Int. J. Athl. Train. 2013, 18, 18–23. [Google Scholar] [CrossRef][Green Version]
- Vincent, H.K.; Kilgore, J.E., III; Chen, C.; Bruner, M.; Horodyski, M.; Vincent, K.R. Impact of body mass index on biomechanics of recreational runners. Phys. Med. Rehabil. 2020, 12, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Uhlrich, S.D.; Mazzoli, V.; Silder, A.; Finlay, A.K.; Kogan, F.; Gold, G.E.; Delp, S.L.; Beaupre, G.S.; Kolesar, J.A. Personalised gait retraining for medial compartment knee osteoarthritis: A randomised controlled trial. Lancet Rheumatol. 2025, 7, e708–e718. [Google Scholar] [CrossRef]
- Driban, J.B.; Hootman, J.M.; Sitler, M.R.; Harris, K.P.; Cattano, N.M. Is participation in certain sports associated with knee osteoarthritis? A systematic review. J. Athl. Train. 2017, 52, 497–506. [Google Scholar] [CrossRef]
- Becroft, L.; Ooi, G.; Forsyth, A.; King, S.; Tierney, A. Validity of multi-frequency bioelectric impedance methods to measure body composition in obese patients: A systematic review. Int. J. Obes. 2019, 43, 1497–1507. [Google Scholar] [CrossRef] [PubMed]

| Group | Total Body Mass (kg) | Lean Body Mass (kg) | Body Fat (%) |
|---|---|---|---|
| Total (N = 125) | 101.4 ± 19.3 | 87.2 ± 11.6 | 13.3 ± 6.5 |
| (97.9, 104.8) | (85.1, 89.2) | (12.2, 14.5) | |
| Lighter (n1 = 64) | 86.8 ± 6.7 | 78.3 ± 6.0 | 9.9 ± 3.6 |
| (85.2, 88.5) | (76.8, 79.8) | (9.0, 10.8) | |
| Heavier (n2 = 61) | 116.6 ± 16.1 | 96.6 ± 8.1 | 16.9 ± 7.0 |
| (112.4, 120.7) | (94.5, 98.6) | (15.1, 18.5) |
| Group | Limb | Peak Joint Reaction Force (N/kg) | Peak Knee Adductor Moment (Nm/kg) | Mean Vertical Ground Reaction Force (N/kg) |
|---|---|---|---|---|
| Total (N = 125) | Left | 25.1 ± 2.2 | 2.2 ± 0.5 | 18.3 ± 1.4 |
| (24.7, 25.5) | (2.1, 2.3) | (18.0, 18.5) | ||
| Right | 25.2 ± 2.3 | 2.2 ± 0.5 | 18.3 ± 1.4 | |
| (24.8, 25.6) | (2.1, 2.3) | (18.0, 18.5) | ||
| Lighter (n1 = 64) | Left | 25.7 ± 2.1 | 2.3 ± 0.5 | 18.8 ± 1.2 |
| (25.2, 26.3) | (2.2, 2.5) | (18.6, 19.2) | ||
| Right | 25.8 ± 2.1 | 2.3 ± 0.5 | 18.9 ± 1.1 | |
| (25.3, 26.3) | (2.1, 2.4) | (18.7, 19.2) | ||
| Heavier (n2 = 61) | Left | 24.4 ± 2.0 | 2.1 ± 0.5 | 17.6 ± 1.3 |
| (23.9, 24.9) | (2.0, 2.3) | (17.3, 18.0) | ||
| Right | 24.6 ± 2.4 | 2.1 ± 0.5 | 17.6 ± 1.5 | |
| (24.0, 25.2) | (2.0, 2.2) | (17.2, 18.0) |
| Variable | Limb | Total Body Mass (kg) | Lean Body Mass (kg) | Body Fat (%) |
|---|---|---|---|---|
| Joint reaction force | Left | −0.39 ** | −0.39 * | −0.21 ** |
| Right | −0.37 ** | −0.38 * | −0.22 ** | |
| Knee adductor moment | Left | −0.21 * | −0.20 * | −0.14 |
| Right | −0.28 ** | −0.30 ** | −0.09 | |
| Mean vertical ground reaction force | Left | −0.56 ** | −0.56 ** | −0.39 ** |
| Right | −0.61 ** | −0.60 ** | −0.41 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokha, M.; Stensland, J.; Schafer, A.; McBride, S. Morphology and Knee Joint Kinetics in National Football League Draft Prep Players: Implications for Osteoarthritis Development. Biomechanics 2025, 5, 77. https://doi.org/10.3390/biomechanics5040077
Mokha M, Stensland J, Schafer A, McBride S. Morphology and Knee Joint Kinetics in National Football League Draft Prep Players: Implications for Osteoarthritis Development. Biomechanics. 2025; 5(4):77. https://doi.org/10.3390/biomechanics5040077
Chicago/Turabian StyleMokha, Monique, Jack Stensland, Andrew Schafer, and Sean McBride. 2025. "Morphology and Knee Joint Kinetics in National Football League Draft Prep Players: Implications for Osteoarthritis Development" Biomechanics 5, no. 4: 77. https://doi.org/10.3390/biomechanics5040077
APA StyleMokha, M., Stensland, J., Schafer, A., & McBride, S. (2025). Morphology and Knee Joint Kinetics in National Football League Draft Prep Players: Implications for Osteoarthritis Development. Biomechanics, 5(4), 77. https://doi.org/10.3390/biomechanics5040077






