Effects of Unilateral Swing Leg Resistance on Propulsion and Other Gait Characteristics During Treadmill Walking in Able-Bodied Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
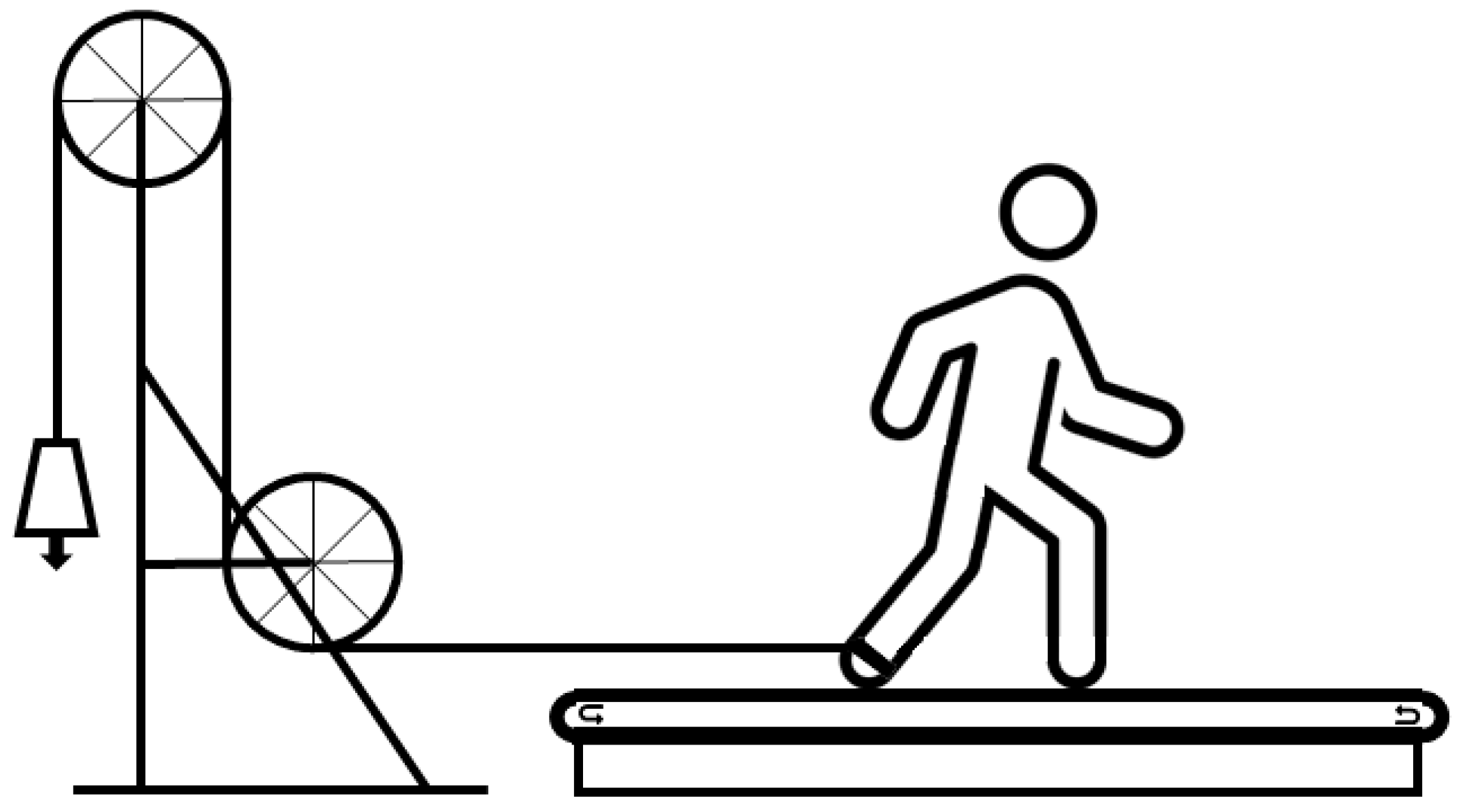
2.2. Experimental Protocol
2.3. Data Collection
2.4. Data Analysis
2.5. Statistical Analysis
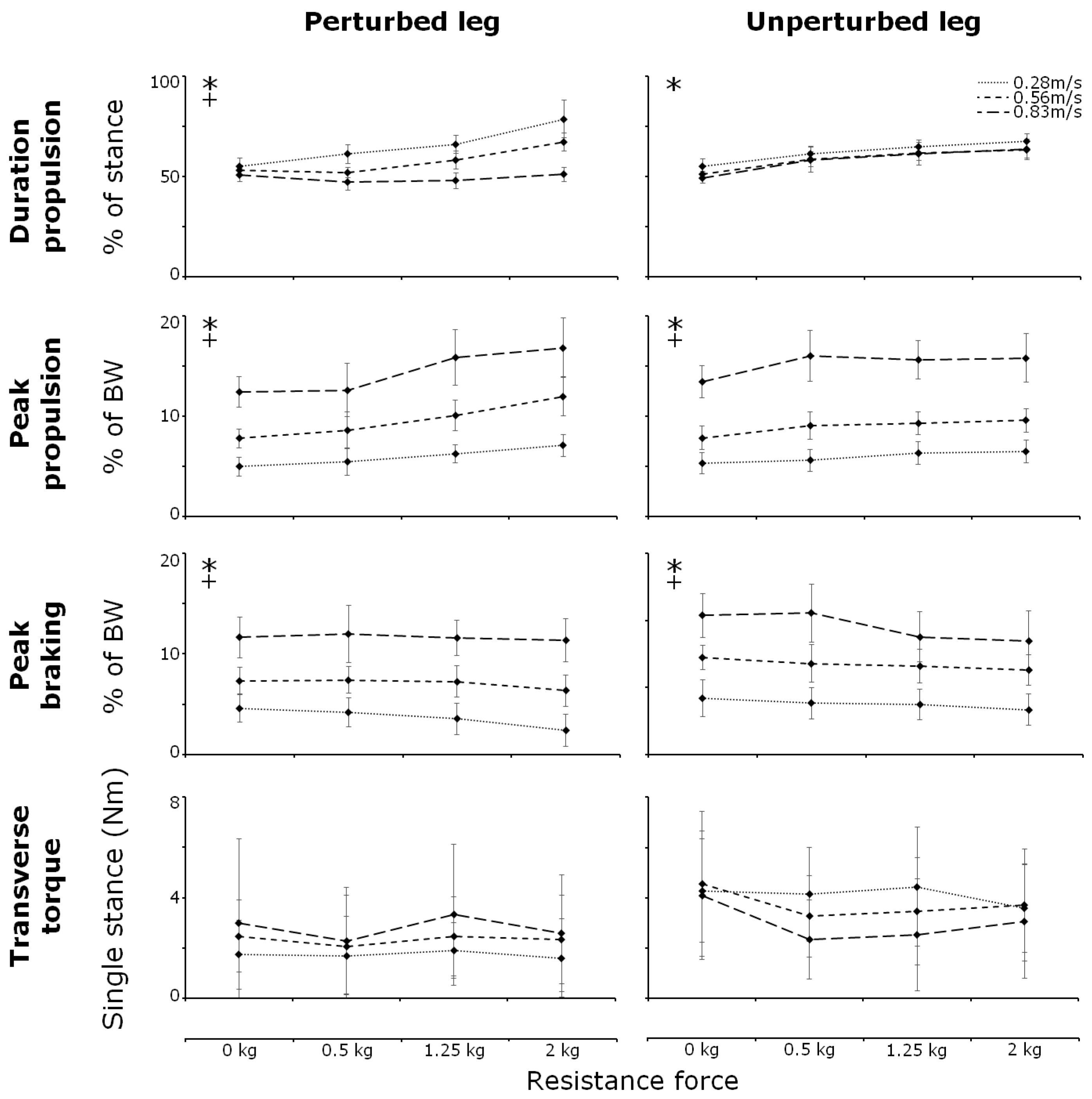
3. Results
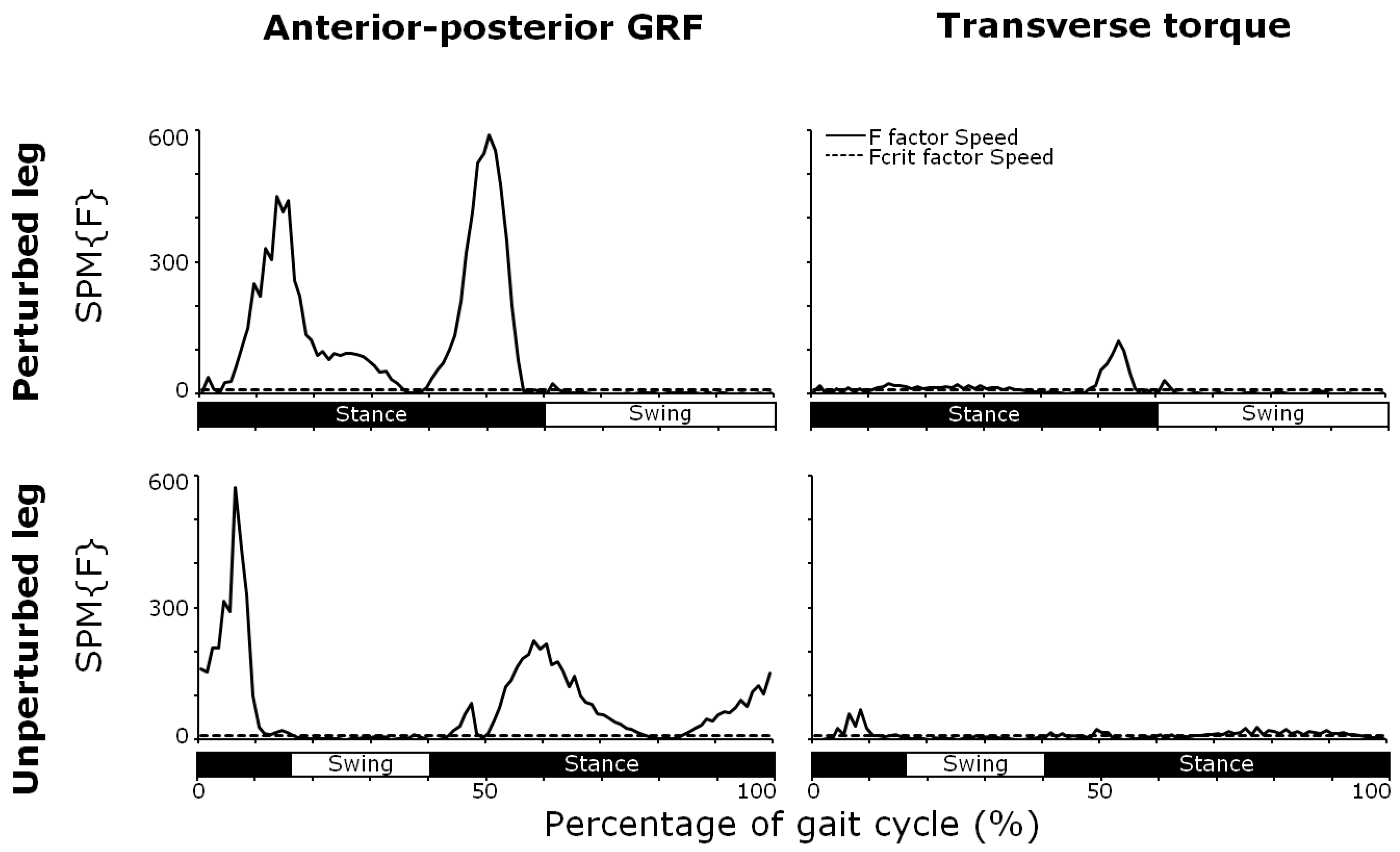
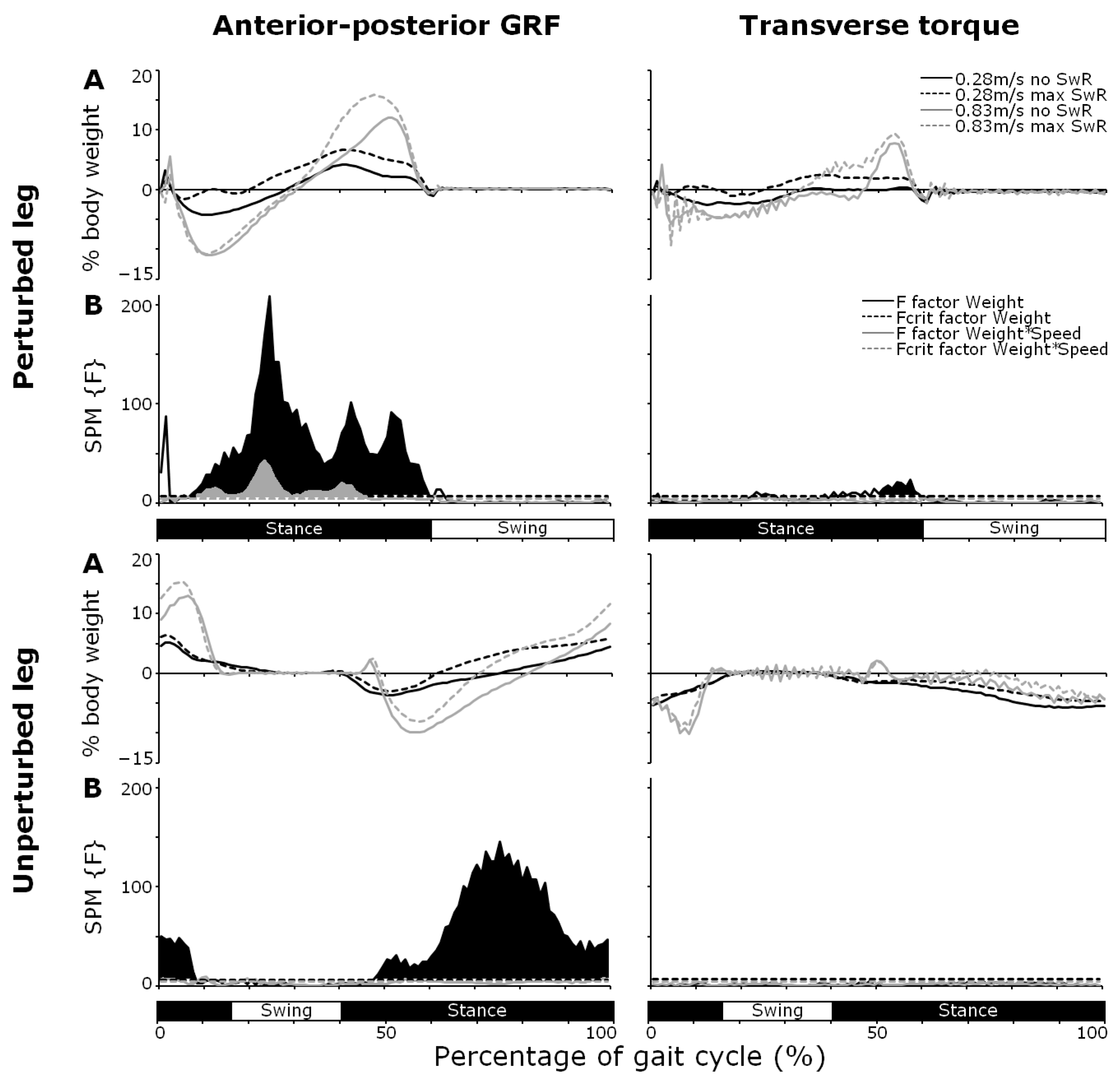
3.1. Anterior–Posterior GRF
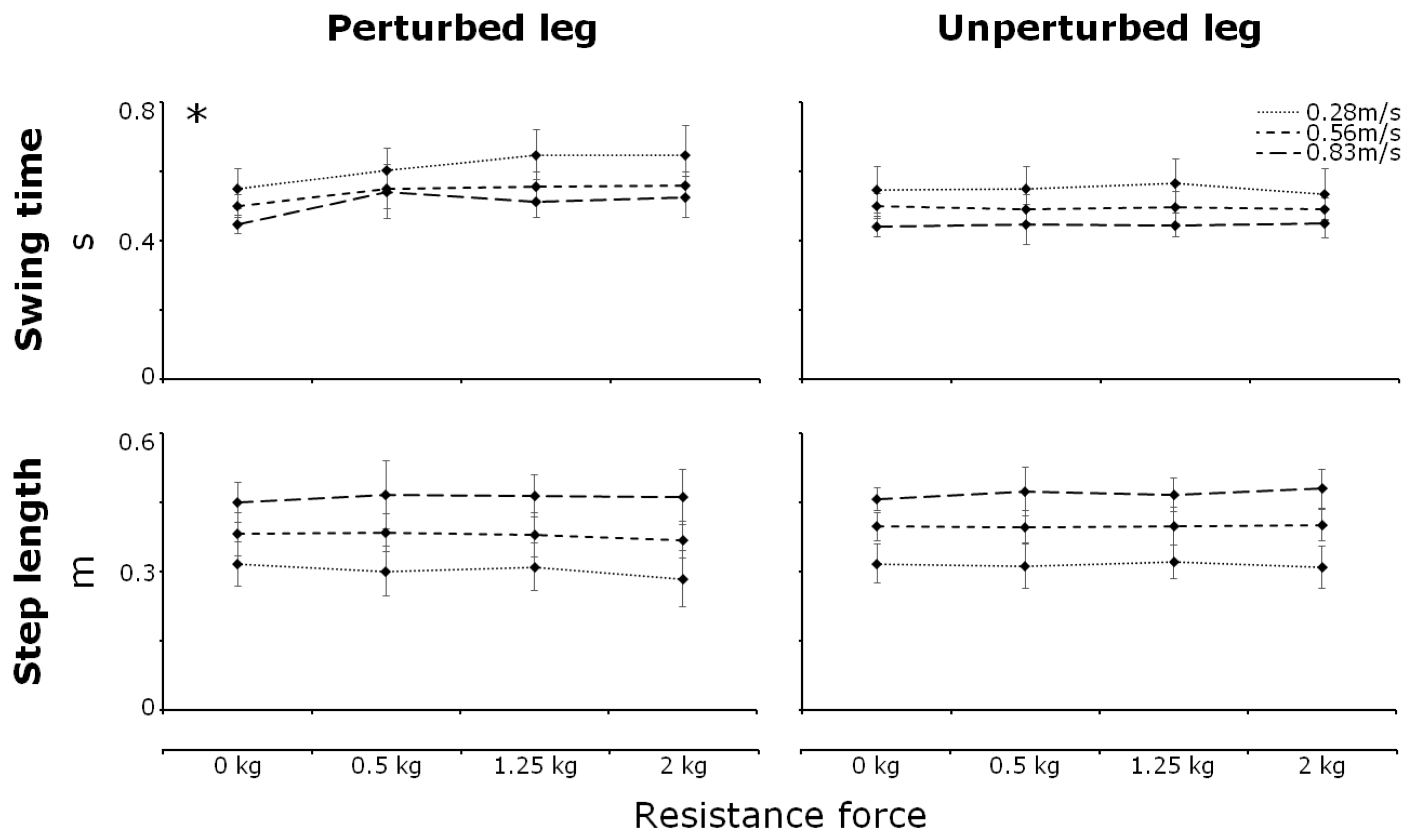
3.2. Step Parameters
3.3. Transverse Torques
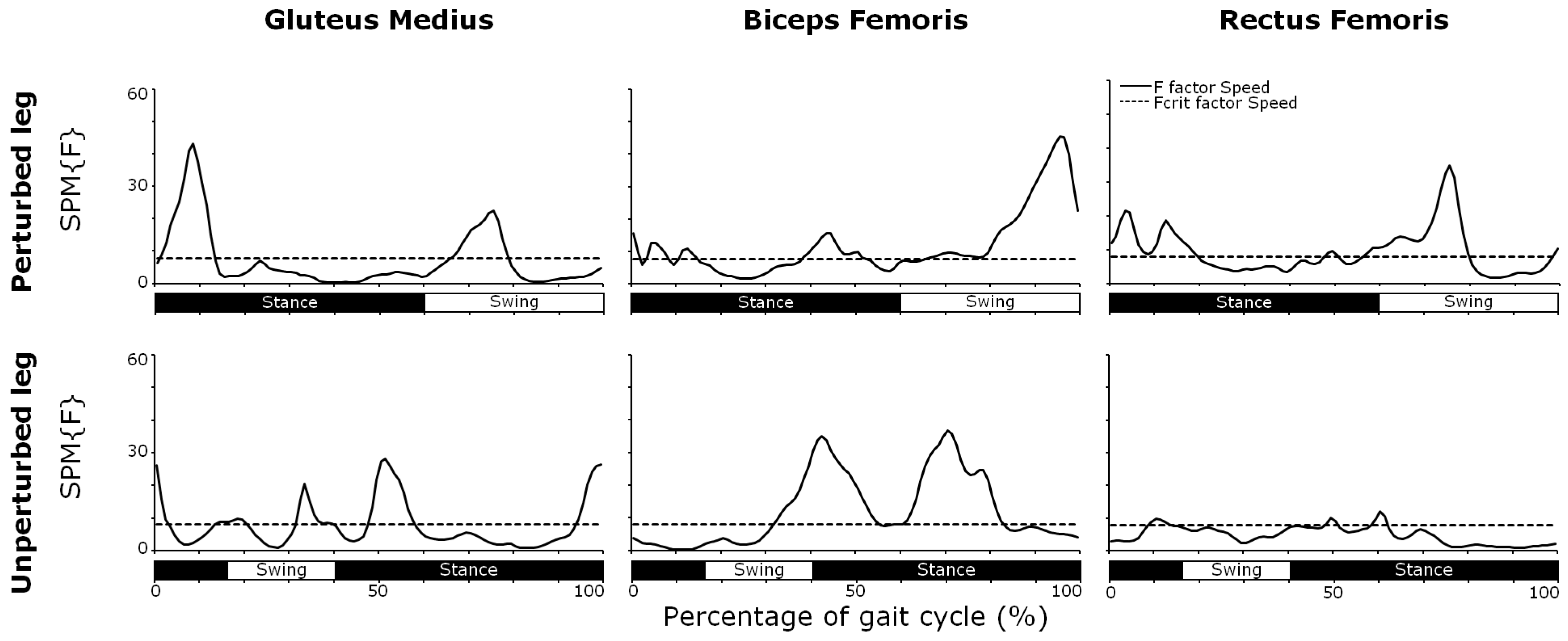
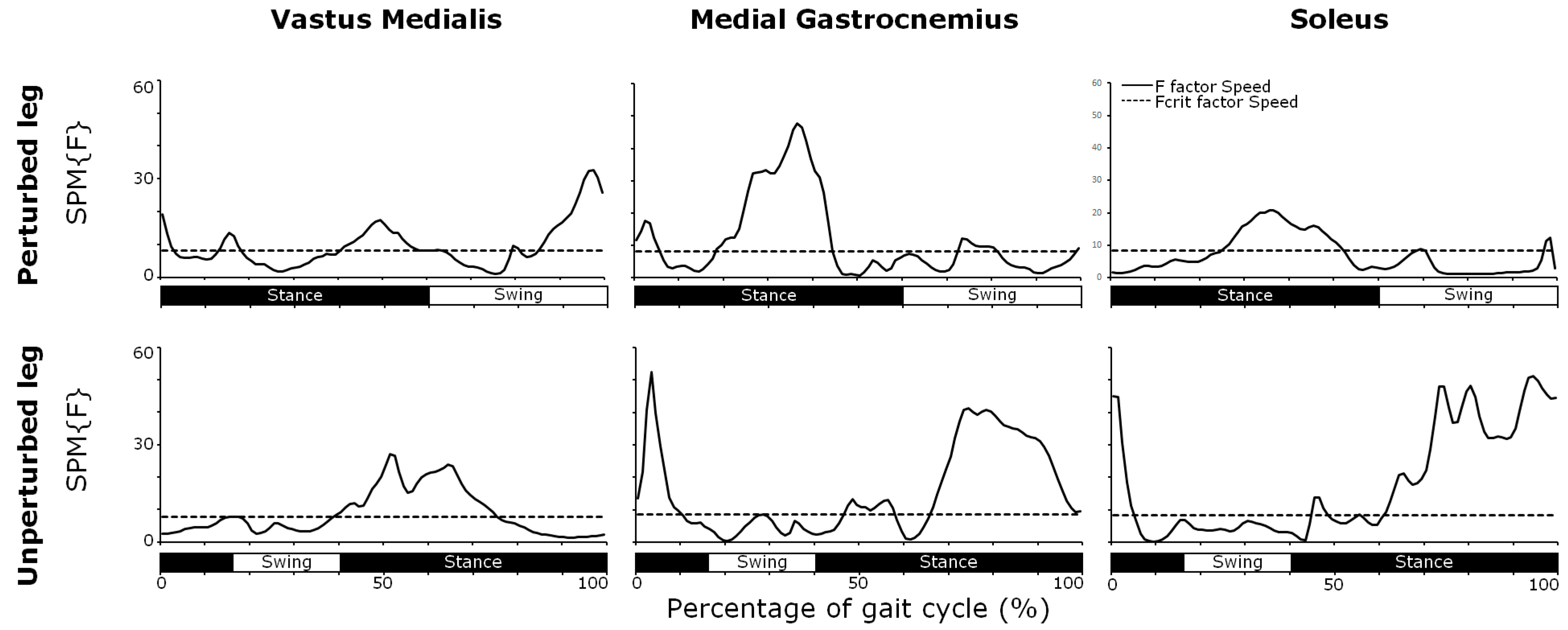
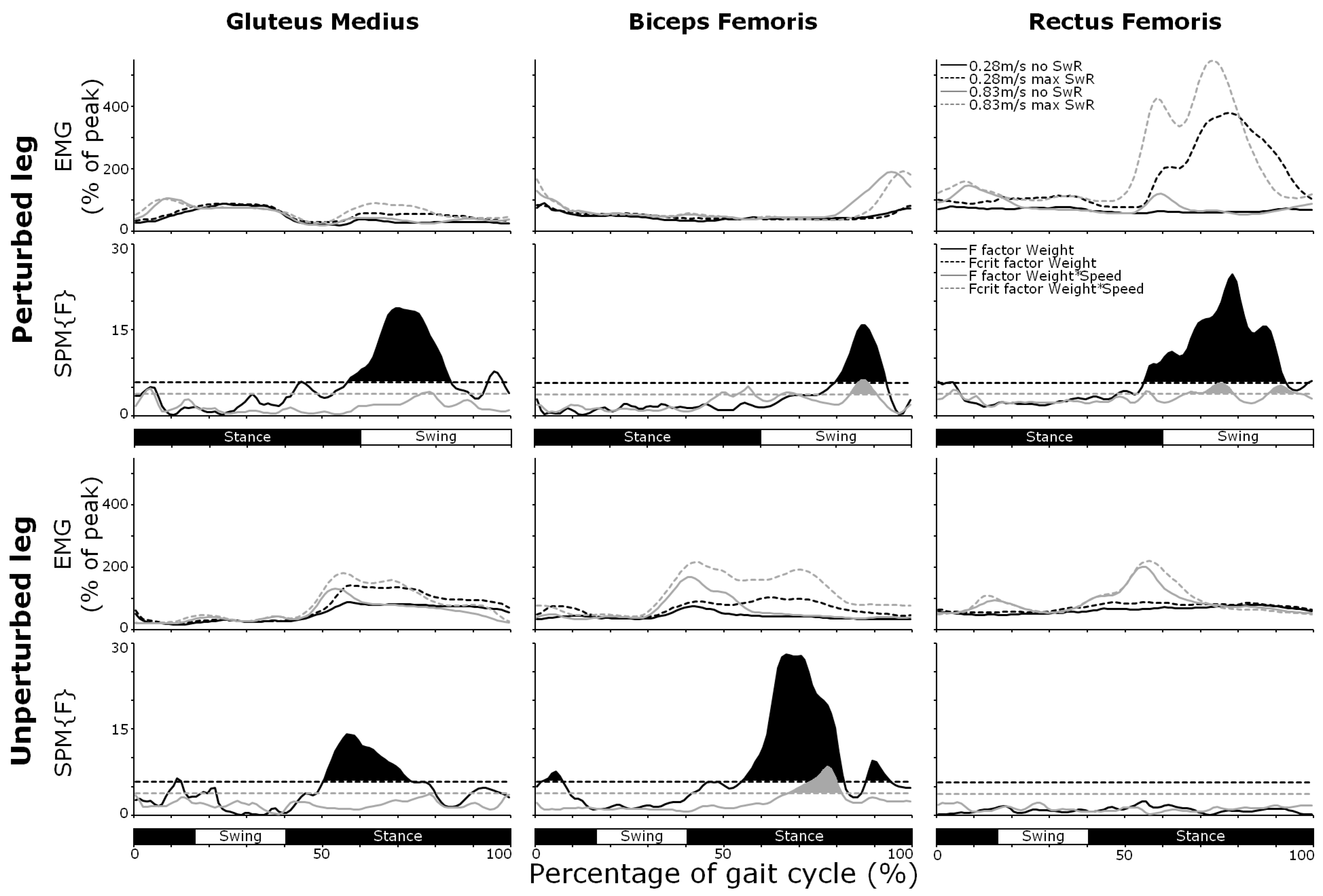
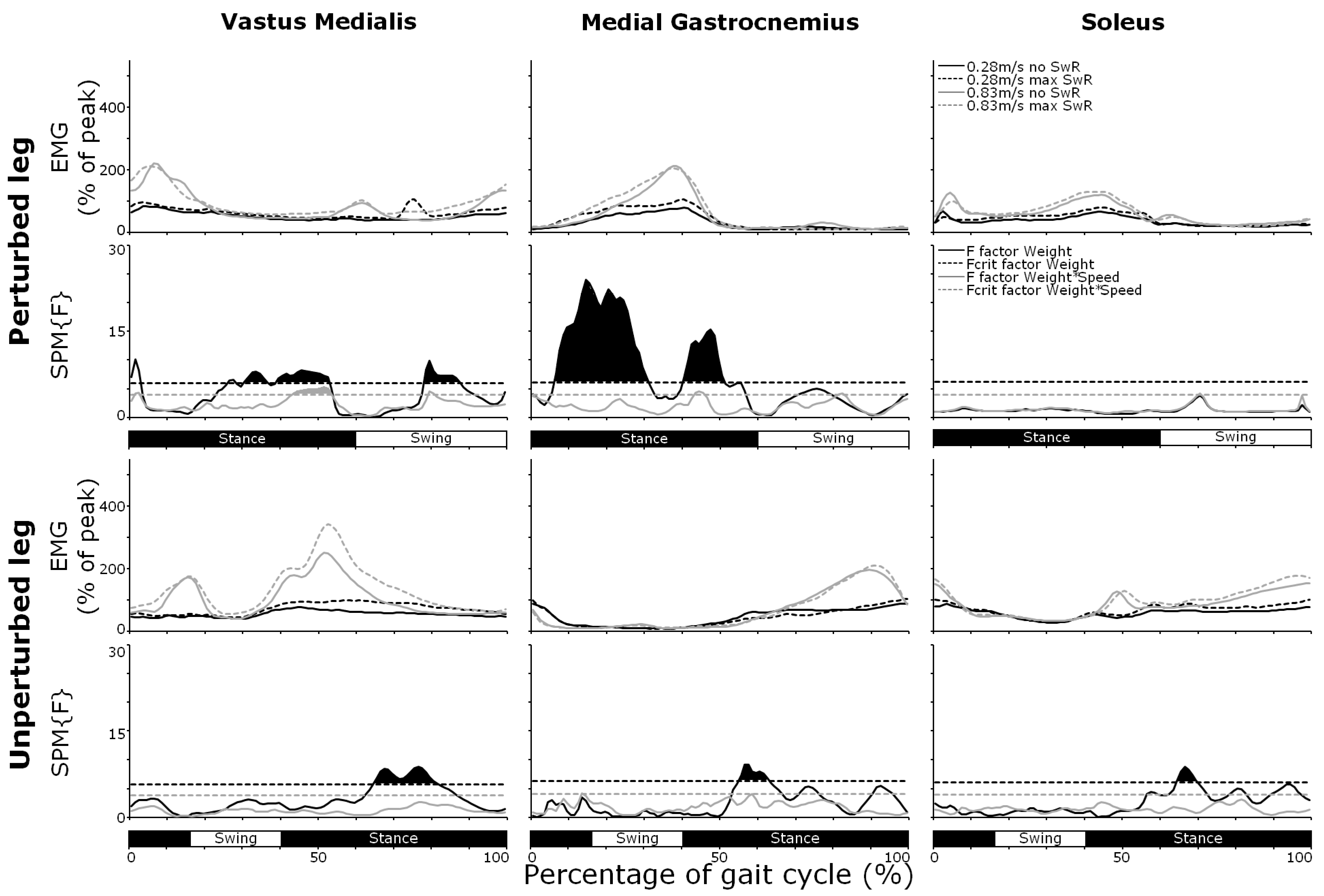
3.4. Muscle Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BF | Biceps Femoris |
| CoM | Center of Mass |
| EMG | Electromyography |
| GM | Gluteus Medius |
| GRF | Ground Reaction Force |
| MG | Medial Gastrocnemius |
| RF | Rectus Femoris |
| RM | Repeated measures |
| SOL | Soleus |
| VM | Vastus Medialis |
Appendix A
| Perturbed Leg | Unperturbed Leg | |||||||
|---|---|---|---|---|---|---|---|---|
| Fcrit | Weight (3,39) | Speed (2,26) | Weight*Speed (6,78) | Fcrit | Weight (3,39) | Speed (2,26) | Weight*Speed (6,78) | |
| A-P GRF | Weight: 6.8 Speed: 9.4 Weight*Speed: 4.3 | 0–1.9 * | 0.2–2.2 * | 1.4–2.3 * | Weight: 6.4 Speed: 8.8 Weight*Speed: 4.1 | 0.0–7.7 ** | 0.0–16.2 ** | 0.0–5.1 * |
| 4.7–5.4 * | 3.3–35.0 ** | 3.6–46.3 ** | 47.1–99.0 ** | 36.7–37.6 * | 7.6–10.9 * | |||
| 6.3–59.5 ** | 38.5–56.0 ** | 49.5–54.8 ** | 43.0–48.5 ** | 16.7–21.6 * | ||||
| 60.4–62.8 * | 57.9–58.1 * | 59.3–61.5 * | 49.6–77.5 ** | 51.6–52.8 * | ||||
| 60.4–62.0 * | 83.8–99.0 ** | 53.0–62.7 ** | ||||||
| 80.7–87.5 ** | ||||||||
| 88.9–90.2 * | ||||||||
| 91.6–92.8 * | ||||||||
| 94.2–99.0 * | ||||||||
| Transverse torque | Weight: 7.0 Speed: 9.7 Weight*Speed: 4.4 | 0.7–1.3 * | 0.3–1.5 * | 1.6–3.0 * | Weight: 6.5 Speed: 8.9 Weight*Speed: 4.2 | 3.3–10.6 ** | 9.8–10.2 * | |
| 22.1–27.2 ** | 3.9–4.0 * | 23.2–24.5 * | 13.3–14.3 * | |||||
| 28.8–29.1 * | 5.6–6.4 * | 25.4–26.6 * | 40.3–41.8 * | |||||
| 37.7–38.4 * | 7.9–8.1 * | 28.0–28.0 * | 42.3–43.6 * | |||||
| 39.4–40.8 * | 10.0–34.8 ** | 59.7–60.4 * | 48.3–51.6 ** | |||||
| 41.2–48.0 ** | 36.0–36.0 * | 59.8–60.1 * | ||||||
| 48.0–59.4 ** | 47.9–56.1 ** | 61.7–62.3 * | ||||||
| 60.9–61.0 * | 60.1–62.4 ** | 67.2–95.3 ** | ||||||
| Perturbed Leg | Unperturbed Leg | |||||||
|---|---|---|---|---|---|---|---|---|
| Fcrit | Weight (3,39) | Speed (2,26) | Weight*Speed (6,78) | Fcrit | Weight (3,39) | Speed (2,26) | Weight*Speed (6,78) | |
| GM | Weight: 5.8 Speed: 7.8 Weight*Speed: 3.8 | 43.8–44.3 * | 0.7–12.9 ** | 2.1–4.9 * | Weight: 5.9 Speed: 8.0 Weight*Speed: 3.9 | 10.6–12.0 * | 0–2.7 * | |
| 56.1–83.8 ** | 65.6–78.4 ** | 75.7–78.9 * | 49.7–73.1 ** | 12.9–20.2 * | ||||
| 93.4–97.5 * | 31.0–39.6 * | |||||||
| 47.1–57.6 ** | ||||||||
| 93.5–99.0 * | ||||||||
| BF | Weight: 5.7 Speed: 7.7 Weight*Speed: 3.8 | 78.8–92.7 ** | 0.0–1.5 * | 48.4–50.6 * | Weight: 5.9 Speed: 7.9 Weight*Speed: 3.8 | 1.2–6.8 * | 31.2–54.9 ** | 66.7–80.8 ** |
| 2.8–7.8 * | 53.2–57.6 * | 54.2–81.5 ** | 59.3–82.6 ** | |||||
| 10.0–14.3 * | 64.7–68.7 * | 87.2–93.9 * | ||||||
| 37.6–52.0 ** | 83.0–90.1 * | |||||||
| 65.0–99.0 ** | ||||||||
| RF | Weight: 5.8 Speed: 7.8 Weight*Speed: 3.8 | 0.0–0.5 * | 0.0–19.3 ** | 2.7–5.7 * | Weight: 5.7 Speed: 7.7 Weight*Speed: 3.8 | 8.2–14.0 * | ||
| 2.2–4.1 * | 47.1–50.4 * | 54.5–56.5 * | 47.4–50.7 * | |||||
| 54.4–92.2 ** | 55.7–79.3 ** | 65.3–78.4 ** | 58.0–61.8 * | |||||
| 98.0–99.0 * | 97.8–99.0 * | 87.4–96.1 * | ||||||
| VM | Weight: 5.9 Speed: 8.0 Weight*Speed: 3.9 | 0.0–2.5 * | 0.0–2.6 * | 0.8–2.3 * | Weight: 5.7 Speed: 7.7 Weight*Speed: 3.8 | 63.9–81.3 ** | 14.9–15.2 * | |
| 25.5–27.8 * | 12.6–17.7 * | 42.4–53.2 ** | 38.3–75.1 ** | |||||
| 29.7–36.2 * | 40.0–58.5 ** | 78.6–80.4 * | ||||||
| 38.1–52.5 ** | 59.3–63.1 * | |||||||
| 77.5–87.2 ** | 78.6–80.4 * | |||||||
| 84.6–99.0 ** | ||||||||
| MG | Weight: 6.0 Speed: 8.2 Weight*Speed: 3.9 | 5.5–30.7 ** | 0.0–5.2 ** | 42.6–45.4 * | Weight: 6.3 Speed: 8.6 Weight*Speed: 4.0 | 54.3–62.7 ** | 0.0–9.8 ** | 12.9–13.2 * |
| 39.6–50.9 ** | 17.8–44.0 ** | 80.6–82.4 * | 46.0–57.5 ** | |||||
| 72.0–80.9 ** | 65.4–99.0 ** | |||||||
| 98.5–99.0 * | ||||||||
| SOL | Weight: 6.2 Speed: 8.4 Weight*Speed: 4.0 | 24.4–51.8 ** | 69.6–70.3 * | Weight: 6.1 Speed: 8.2 Weight*Speed: 3.9 | 63.8–69.4 * | 0.0–4.7 * | ||
| 68.0–70.0 * | 44.3–48.1 * | |||||||
| 96.5–98.4 * | 54.6–55.4 * | |||||||
| 60.5–99.0 ** | ||||||||
References
- Awad, L.N.; Reisman, D.S.; Kesar, T.M.; Binder-Macleod, S.A. Targeting paretic propulsion to improve poststroke walking function: A preliminary study. Arch. Phys. Med. Rehabil. 2014, 95, 840–848. [Google Scholar] [CrossRef]
- Hsiao, H.; Knarr, B.A.; Pohlig, R.T.; Higginson, J.S.; Binder-Macleod, S.A. Mechanisms used to increase peak propulsive force following 12-weeks of gait training in individuals poststroke. J. Biomech. 2016, 49, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, S.; Gravel, D.; Arsenault, A.B.; Bourbonnais, D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin. Biomech. 1999, 14, 125–135. [Google Scholar] [CrossRef]
- Mauritz, K.H. Gait training in hemiplegia. Eur. J. Neurol. 2002, 9, 23–29. [Google Scholar] [CrossRef]
- Roelker, S.A.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. Paretic propulsion as a measure of walking performance and functional motor recovery post-stroke: A review. Gait Posture 2019, 68, 6–14. [Google Scholar] [CrossRef]
- Awad, L.N.; Lewek, M.D.; Kesar, T.M.; Franz, J.R.; Bowden, M.G. These legs were made for propulsion: Advancing the diagnosis and treatment of post-stroke propulsion deficits. J. Neuroeng. Rehabil. 2020, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Hof, A.L.; Nauta, J.; van der Knaap, E.R.; Schallig, M.A.; Struwe, D.P. Calf muscle work and segment energy changes in human treadmill walking. J. Electromyogr. Kinesiol. 1992, 2, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Meinders, M.; Gitter, A.; Czerniecki, J.M. The role of ankle plantar flexor muscle work during walking. Scand. J. Rehabil. Med. 1998, 30, 39–46. [Google Scholar] [CrossRef]
- Savin, D.N.; Tseng, S.C.; Morton, S.M. Bilateral adaptation during locomotion following a unilaterally applied resistance to swing in nondisabled adults. J. Neurophysiol. 2010, 104, 3600–3611. [Google Scholar] [CrossRef]
- Savin, D.N.; Morton, S.M.; Whitall, J. Generalization of improved step length symmetry from treadmill to overground walking in persons with stroke and hemiparesis. Clin. Neurophysiol. 2014, 125, 1012–1020. [Google Scholar] [CrossRef]
- Jonkers, I.; Delp, S.; Patten, C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture 2009, 29, 129–137. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373. [Google Scholar]
- Veeger, D.H.E.J.; Van der Woude, L.H.; Rozendal, R.H. The effect of rear wheel camber in manual wheelchair propulsion. J. Rehabil. Res. Dev. 1989, 26, 37–46. [Google Scholar]
- Blokland, I.; Gravesteijn, A.; Busse, M.; Groot, F.; van Bennekom, C.; van Dieen, J.; de Koning, J.; Houdijk, H. The relationship between relative aerobic load, energy cost, and speed of walking in individuals post-stroke. Gait Posture 2021, 89, 193–199. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C. European recommendations for surface electromyography. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Swart, S.B.; den Otter, R.; Lamoth, C.J. Anticipatory control of human gait following simulated slip exposure. Sci. Rep. 2020, 10, 9599. [Google Scholar] [CrossRef]
- Donno, L.; Monoli, C.; Frigo, C.A.; Galli, M. Forward and backward walking: Multifactorial characterization of gait parameters. Sensors 2023, 23, 4671. [Google Scholar] [CrossRef]
- Turns, L.J.; Neptune, R.R.; Kautz, S.A. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch. Phys. Med. Rehabil. 2007, 88, 1127–1135. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pataky, T.C. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J. Biomech. 2010, 43, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Neptune, R.R.; Kautz, S.A.; Zajac, F.E. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J. Biomech. 2001, 34, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, M.L.; Hubert, J.; Houdijk, H.; Bruijn, S.M. Influence of arm swing on cost of transport during walking. Biol. Open 2019, 8, bio039263. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.C.; Bowden, M.G.; Kelly, A.L.; Kautz, S.A. Altered post-stroke propulsion is related to paretic swing phase kinematics. Clin. Biomech. 2020, 72, 24–30. [Google Scholar] [CrossRef]
- Oh, K.; Baek, J.; Park, S. Gait strategy changes with acceleration to accommodate the biomechanical constraint on push-off propulsion. J. Biomech. 2012, 45, 2920–2926. [Google Scholar] [CrossRef]
- Awad, L.N.; Palmer, J.A.; Pohlig, R.T.; Binder-Macleod, S.A.; Reisman, D.S. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabilit. Neural Repair 2015, 29, 416–423. [Google Scholar] [CrossRef]
| Perturbed Leg | Unperturbed Leg | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight | Speed | Weight*Speed | Weight | Speed | Weight*Speed | |||||||
| F(3,39) | ηp2 | F(2,26) | ηp2 | F(6,78) | ηp2 | F(3,39) | ηp2 | F(2,26) | ηp2 | F(6,78) | ηp2 | |
| Peak braking | 14.3 ** | 0.5 | 211.4 ** | 0.9 | 2.7 * | 0.2 | 18.9 ** | 0.6 | 179.5 ** | 0.9 | 4.4 ** | 0.3 |
| Peak propulsion | 52.1 ** | 0.8 | 310.3 ** | 1.0 | 3.3 * | 0.2 | 46.6 ** | 0.8 | 305.1 ** | 1.0 | 4.7 ** | 0.3 |
| Duration propulsion | 90.8 ** | 0.9 | 82.6 ** | 0.9 | 33.0 ** | 0.7 | 128.9 ** | 0.9 | 22.9 ** | 0.6 | 1.0 | 0.1 |
| Swing time | 40.1 ** | 0.8 | 38.8 ** | 0.7 | 3.8 | 0.2 | 1.7 | 0.1 | 39.3 ** | 0.7 | 1.6 | 0.2 |
| Step length | 3.4 | 0.2 | 220.5 ** | 0.9 | 1.6 | 0.1 | 0.8 | 0.1 | 228.4 ** | 0.9 | 1.7 | 0.1 |
| Transverse ground reaction torque | 0.8 | 0.1 | 3.3 | 0.2 | 0.3 | 0.0 | 3.6 | 0.2 | 6.9 * | 0.3 | 2.5 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minkes-Weiland, S.; Houdijk, H.; Reinders-Messelink, H.A.; van der Woude, L.H.V.; Hartman, P.P.; den Otter, R. Effects of Unilateral Swing Leg Resistance on Propulsion and Other Gait Characteristics During Treadmill Walking in Able-Bodied Individuals. Biomechanics 2025, 5, 71. https://doi.org/10.3390/biomechanics5040071
Minkes-Weiland S, Houdijk H, Reinders-Messelink HA, van der Woude LHV, Hartman PP, den Otter R. Effects of Unilateral Swing Leg Resistance on Propulsion and Other Gait Characteristics During Treadmill Walking in Able-Bodied Individuals. Biomechanics. 2025; 5(4):71. https://doi.org/10.3390/biomechanics5040071
Chicago/Turabian StyleMinkes-Weiland, Sylvana, Han Houdijk, Heleen A. Reinders-Messelink, Luc H. V. van der Woude, Paul P. Hartman, and Rob den Otter. 2025. "Effects of Unilateral Swing Leg Resistance on Propulsion and Other Gait Characteristics During Treadmill Walking in Able-Bodied Individuals" Biomechanics 5, no. 4: 71. https://doi.org/10.3390/biomechanics5040071
APA StyleMinkes-Weiland, S., Houdijk, H., Reinders-Messelink, H. A., van der Woude, L. H. V., Hartman, P. P., & den Otter, R. (2025). Effects of Unilateral Swing Leg Resistance on Propulsion and Other Gait Characteristics During Treadmill Walking in Able-Bodied Individuals. Biomechanics, 5(4), 71. https://doi.org/10.3390/biomechanics5040071






