Gait Analysis in Multiple Sclerosis: A Scoping Review of Advanced Technologies for Adaptive Rehabilitation and Health Promotion
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Data Analysis
3. Results
3.1. Database Searches
3.2. Study Origin
3.3. Study Design
3.4. MS Patient Groups and Demographic Profiles
3.5. Reference Groups
3.6. Gait Parameters
3.7. Gait Measurement Tools
3.8. Study Setting for Gait Analysis
4. Discussion
4.1. Digital Gait Biomarkers: From Measurement to Meaning
4.2. Real-World Gait as a Diagnostic Lens
4.3. Cognition in Motion: Dual-Task Gait and Neural Efficiency
4.4. Redefining Fall Risk: Movement Disruptions at the Margins
4.5. When the Body and the Mind Disagree: The Perception–Performance Gap
4.6. Smart Dysfunction: Adaptive Strategies in MS Gait
4.7. Measuring Motor Resilience: What Gait Reveals Post-Intervention
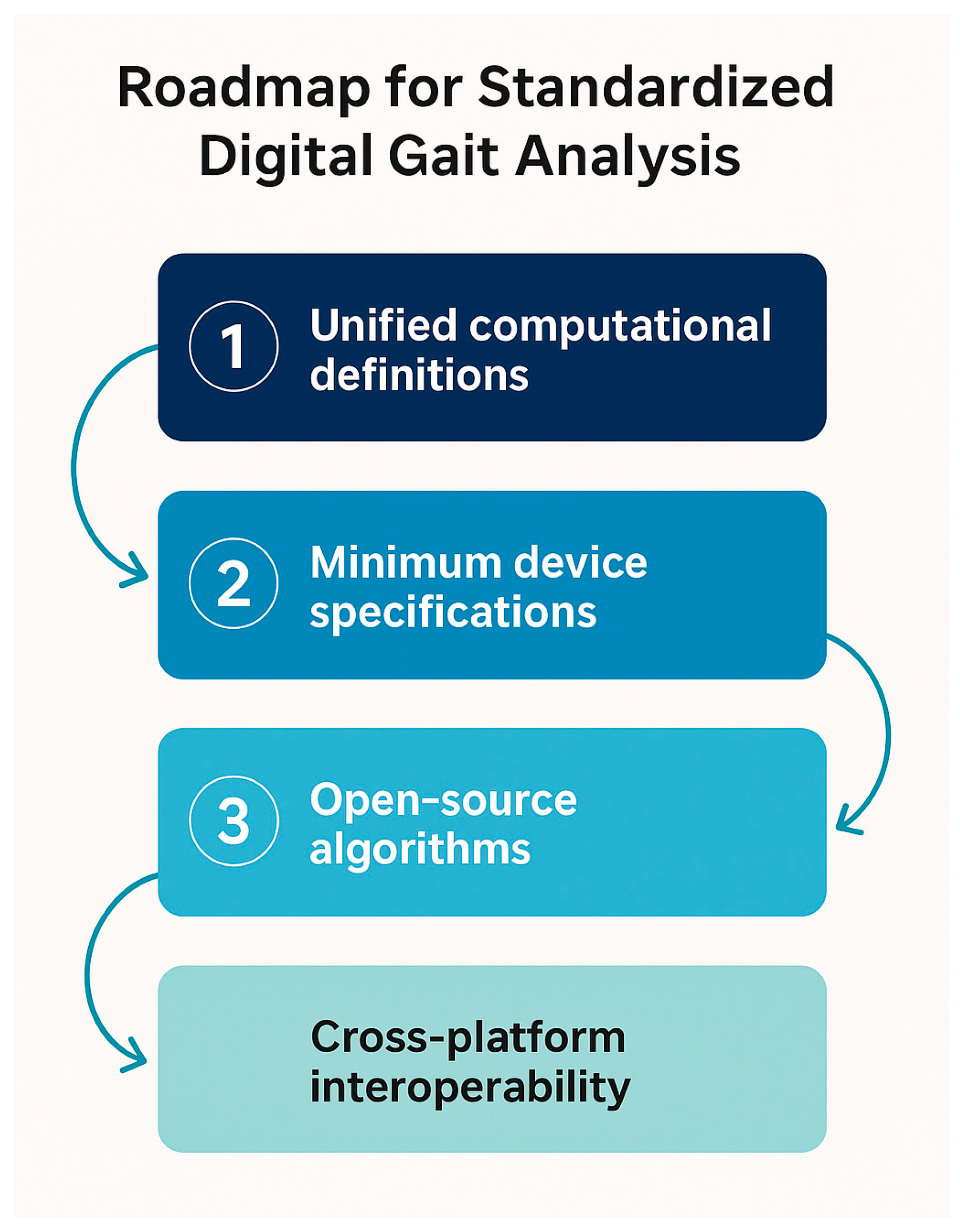
4.8. Toward Standardization of Gait Assessment Methods
4.9. Strenghts and Limitations of the Study
4.10. Clinical Implications and Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalron, A. Gait variability across the disability spectrum in people with multiple sclerosis. J. Neurol. Sci. 2016, 361, 1–6. [Google Scholar] [CrossRef]
- Müller, R.; Hamacher, D.; Hansen, S.; Oschmann, P.; Keune, P.M. Wearable inertial sensors are highly sensitive in the detection of gait disturbances and fatigue at early stages of multiple sclerosis. BMC Neurol. 2021, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Plakias, S.; Karakitsiou, G.; Nikova, A.; Christidi, F.; Kokkotis, C.; Giarmatzis, G.; Tsakni, G.; Katsouri, I.-G.; Dimitrios, S. Mapping the Landscape of Biomechanics Research in Stroke Neurorehabilitation: A Bibliometric Perspective. Biomechanics 2024, 4, 664–684. [Google Scholar] [CrossRef]
- Morel, E.; Allali, G.; Laidet, M.; Assal, F.; Lalive, P.H.; Armand, S. Gait Profile Score in multiple sclerosis patients with low disability. Gait Posture 2017, 51, 169–173. [Google Scholar] [CrossRef]
- Davies, B.L.; Hoffman, R.M.; Kurz, M.J. Individuals with multiple sclerosis redistribute positive mechanical work from the ankle to the hip during walking. Gait Posture 2016, 49, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, I.; Galli, M.; Kleiner, A.F.R.; Corona, F.; Coghe, G.; Marongiu, E.; Loi, A.; Crisafulli, A.; Cocco, E.; Marrosu, M.G. The Required Coefficient of Friction for evaluating gait alterations in people with Multiple Sclerosis during gait. Mult. Scler. Relat. Disord. 2016, 10, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Massot, C.; Bègue, J.; Simoneau-Buessinger, E.; Donze, C.; Caderby, T.; Leteneur, S. Patients with multiple sclerosis and low disability display cautious rotational behavior during gait initiation. Clin. Biomech. 2025, 122, 106431. [Google Scholar] [CrossRef]
- Zahn, A.; Koch, V.; Schreff, L.; Oschmann, P.; Winkler, J.; Gaßner, H.; Müller, R. Validity of an inertial sensor-based system for the assessment of spatio-temporal parameters in people with multiple sclerosis. Front. Neurol. 2023, 14, 1164001. [Google Scholar] [CrossRef]
- Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Solaro, C.; Rovaris, M.; Brichetto, G.; Confalonieri, P.; Tacchino, A.; Carpinella, I.; Cattaneo, D. Uncovering subtle gait deterioration in people with early-stage multiple sclerosis using inertial sensors: A 2-year multicenter longitudinal study. Sensors 2023, 23, 9249. [Google Scholar] [CrossRef]
- Zanotto, T.; Sosnoff, J.J.; Ofori, E.; Golan, D.; Zarif, M.; Bumstead, B.; Buhse, M.; Kaczmarek, O.; Wilken, J.; Muratori, L. Variability of objective gait measures across the expanded disability status scale in people living with multiple sclerosis: A cross-sectional retrospective analysis. Mult. Scler. Relat. Disord. 2022, 59, 103645. [Google Scholar] [CrossRef] [PubMed]
- Granja Domínguez, A.; Romero Sevilla, R.; Alemán, A.; Durán, C.; Hochsprung, A.; Navarro, G.; Páramo, C.; Venegas, A.; Lladonosa, A.; Ayuso, G.I. Study for the validation of the FeetMe® integrated sensor insole system compared to GAITRite® system to assess gait characteristics in patients with multiple sclerosis. PLoS ONE 2023, 18, e0272596. [Google Scholar] [CrossRef]
- Regev, K.; Eren, N.; Yekutieli, Z.; Karlinski, K.; Massri, A.; Vigiser, I.; Kolb, H.; Piura, Y.; Karni, A. Smartphone-based gait assessment for multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 82, 105394. [Google Scholar] [CrossRef] [PubMed]
- Ganzetti, M.; Graves, J.S.; Holm, S.P.; Dondelinger, F.; Midaglia, L.; Gaetano, L.; Craveiro, L.; Lipsmeier, F.; Bernasconi, C.; Montalban, X. Neural correlates of digital measures shown by structural MRI: A post-hoc analysis of a smartphone-based remote assessment feasibility study in multiple sclerosis. J. Neurol. 2023, 270, 1624–1636. [Google Scholar] [CrossRef]
- Christidi, F.; Orgianelis, I.; Merkouris, E.; Koutsokostas, C.; Tsiptsios, D.; Karavasilis, E.; Psatha, E.A.; Tsiakiri, A.; Serdari, A.; Aggelousis, N. A comprehensive review on the role of resting-state functional magnetic resonance imaging in predicting post-stroke motor and sensory outcomes. Neurol. Int. 2024, 16, 189–201. [Google Scholar] [CrossRef]
- Pau, M.; Leban, B.; Porta, M.; Frau, J.; Coghe, G.; Cocco, E. Cyclograms reveal alteration of inter-joint coordination during gait in people with multiple sclerosis minimally disabled. Biomechanics 2022, 2, 331–341. [Google Scholar] [CrossRef]
- Voisard, C.; de l’Escalopier, N.; Vienne-Jumeau, A.; Moreau, A.; Quijoux, F.; Bompaire, F.; Sallansonnet, M.; Brechemier, M.-L.; Taifas, I.; Tafani, C. Innovative multidimensional gait evaluation using IMU in multiple sclerosis: Introducing the semiogram. Front. Neurol. 2023, 14, 1237162. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Chen, Z.; Motl, R.; Hernandez, M.E.; Sowers, R. Predicting multiple sclerosis from gait dynamics using an instrumented treadmill: A machine learning approach. IEEE Trans. Biomed. Eng. 2020, 68, 2666–2677. [Google Scholar] [CrossRef]
- Trentzsch, K.; Schumann, P.; Śliwiński, G.; Bartscht, P.; Haase, R.; Schriefer, D.; Zink, A.; Heinke, A.; Jochim, T.; Malberg, H. Using machine learning algorithms for identifying gait parameters suitable to evaluate subtle changes in gait in people with multiple sclerosis. Brain Sci. 2021, 11, 1049. [Google Scholar] [CrossRef]
- Tsiara, A.A.; Plakias, S.; Kokkotis, C.; Veneri, A.; Mina, M.A.; Tsiakiri, A.; Kitmeridou, S.; Christidi, F.; Gourgoulis, E.; Doskas, T. Artificial Intelligence in the Diagnosis of Neurological Diseases Using Biomechanical and Gait Analysis Data: A Scopus-Based Bibliometric Analysis. Neurol. Int. 2025, 17, 45. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Ioannidis, P.; Vlotinou, P.; Kokkotis, C.; Megagianni, S.; Toumaian, M.; Terzoudi, K.; Koutzmpi, V.; Despoti, A.; Megari, K.; et al. The role of a computerized cognitive intervention program on the neuropsychiatric symptoms in mild cognitive impairment. Aging Med. Healthc. 2024, 15, 122–128. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Plakias, S.; Vlotinou, P.; Athanasouli, P.; Terzoudi, A.; Kyriazidou, S.; Serdari, A.; Karakitsiou, G.; Megari, K.; Aggelousis, N. Innovative health promotion strategies: A 6-month longitudinal study on computerized cognitive training for older adults with minor neurocognitive disorders. Eur. J. Investig. Health Psychol. Educ. 2025, 15, 34. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Angelini, L.; Hodgkinson, W.; Smith, C.; Dodd, J.M.; Sharrack, B.; Mazzà, C.; Paling, D. Wearable sensors can reliably quantify gait alterations associated with disability in people with progressive multiple sclerosis in a clinical setting. J. Neurol. 2020, 267, 2897–2909. [Google Scholar] [CrossRef] [PubMed]
- Storm, F.A.; Nair, K.; Clarke, A.J.; Van der Meulen, J.M.; Mazzà, C. Free-living and laboratory gait characteristics in patients with multiple sclerosis. PLoS ONE 2018, 13, e0196463. [Google Scholar] [CrossRef] [PubMed]
- Prigent, G.; Aminian, K.; Gonzenbach, R.R.; April, R.; Paraschiv-Ionescu, A. Effects of multidisciplinary inpatient rehabilitation on everyday life physical activity and gait in patients with multiple sclerosis. J. Neuroeng. Rehabil. 2024, 21, 88. [Google Scholar] [CrossRef]
- Galli, M.; Coghe, G.; Sanna, P.; Cocco, E.; Marrosu, M.G.; Pau, M. Relationship between gait initiation and disability in individuals affected by multiple sclerosis. Mult. Scler. Relat. Disord. 2015, 4, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Moumdjian, L.; Maes, P.-J.; Dalla Bella, S.; Decker, L.M.; Moens, B.; Feys, P.; Leman, M. Detrended fluctuation analysis of gait dynamics when entraining to music and metronomes at different tempi in persons with multiple sclerosis. Sci. Rep. 2020, 10, 12934. [Google Scholar] [CrossRef]
- Dujmovic, I.; Radovanovic, S.; Martinovic, V.; Dackovic, J.; Maric, G.; Mesaros, S.; Pekmezovic, T.; Kostic, V.; Drulovic, J. Gait pattern in patients with different multiple sclerosis phenotypes. Mult. Scler. Relat. Disord. 2017, 13, 13–20. [Google Scholar] [CrossRef]
- Castiglia, S.F.; Dal Farra, F.; Trabassi, D.; Turolla, A.; Serrao, M.; Nocentini, U.; Brasiliano, P.; Bergamini, E.; Tramontano, M. Discriminative ability, responsiveness, and interpretability of smoothness index of gait in people with multiple sclerosis. Arch. Physiother. 2025, 15, 9. [Google Scholar] [CrossRef]
- Escudero-Uribe, S.; Hochsprung, A.; Heredia-Camacho, B.; Izquierdo-Ayuso, G. Effect of training exercises incorporating mechanical devices on fatigue and gait pattern in persons with relapsing-remitting multiple sclerosis. Physiother. Can. 2017, 69, 292–302. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Flachenecker, F.; Gaßner, H.; Rothhammer, V.; Klucken, J.; Eskofier, B.M.; Kluge, F. Short inertial sensor-based gait tests reflect perceived state fatigue in multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 58, 103519. [Google Scholar] [CrossRef]
- Cantu, A.; Carsin, A.E.; Caruso, M.; Caulfield, B.; Cereatti, A.; Chiari, L.; D’Ascanio, I.; Eskofier, B.; Fernstad, S.; Froehlich, M.; et al. Assessing real-world gait with digital technology? Validation, insights and recommendations from the Mobilise-D consortium. J. Neuroeng. Rehabil. 2023, 20, 78. [Google Scholar] [CrossRef]
- Hochsprung, A.; Domínguez, A.G.; Magni, E.; Uribe, S.E.; García, A.M. Efectos del entrenamiento en bicicleta con retroalimentación visual sobre la marcha en pacientes con esclerosis múltiple. Neurología 2020, 35, 89–95. [Google Scholar] [CrossRef]
- Kirk, C.; Küderle, A.; Micó-Amigo, M.E.; Bonci, T.; Paraschiv-Ionescu, A.; Ullrich, M.; Soltani, A.; Gazit, E.; Salis, F.; Alcock, L. Mobilise-D insights to estimate real-world walking speed in multiple conditions with a wearable device. Sci. Rep. 2024, 14, 1754. [Google Scholar]
- Kluge, F.; Brand, Y.E.; Micó-Amigo, M.E.; Bertuletti, S.; D’Ascanio, I.; Gazit, E.; Bonci, T.; Kirk, C.; Küderle, A.; Palmerini, L.; et al. Real-world gait detection using a wrist-worn inertial sensor: Validation study. JMIR Form. Res. 2024, 8, e50035. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, Y.; Guilhendou, C.; Moulin, T.; Soares, A.V.; Decavel, P. Neuro-orthopaedic check-up and walking in people with multiple sclerosis: Toward a more specific assessment to improve rehabilitation results. J. Exerc. Rehabil. 2024, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.G.; Page, J.C.M.; Ruiz, F.J.R. Effect of dual-task-induced uncertainty on gait biomechanics in patients with multiple sclerosis with 2–6.5 EDSS grade. Gait Posture 2016, 49, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Franzén, E.; Bezuidenhout, L.; Ekman, U.; Piehl, F.; Johansson, S. Cognitive-motor interference in people with mild to moderate multiple sclerosis, in comparison with healthy controls. Mult. Scler. Relat. Disord. 2022, 67, 104181. [Google Scholar] [CrossRef]
- Coghe, G.; Corona, F.; Pilloni, G.; Porta, M.; Frau, J.; Lorefice, L.; Fenu, G.; Cocco, E.; Pau, M. Is there any relationship between upper and lower limb impairments in people with multiple sclerosis? A kinematic quantitative analysis. Mult. Scler. Int. 2019, 2019, 9149201. [Google Scholar] [CrossRef]
- Andreopoulou, G.; Mercer, T.H.; Enriquez, J.G.; Justin, M.; MacLeod, N.; Harrison, E.; Mahad, D.J.; van der Linden, M.L. Exercise-induced changes in gait kinematics in multiple sclerosis with minimal neurological disability. Mult. Scler. Relat. Disord. 2021, 47, 102630. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Porta, M.; Coghe, G.; Cocco, E. What gait features influence the amount and intensity of physical activity in people with multiple sclerosis? Medicine 2021, 100, e24931. [Google Scholar] [CrossRef]
- Carratalá-Tejada, M.; Cuesta-Gómez, A.; Ortiz-Gutiérrez, R.; Molina-Rueda, F.; Luna-Oliva, L.; Miangolarra-Page, J.C. Reflex locomotion therapy for balance, gait, and fatigue rehabilitation in subjects with multiple sclerosis. J. Clin. Med. 2022, 11, 567. [Google Scholar] [CrossRef]
- Mestanza Mattos, F.G.; Luciano, F.; Lencioni, T.; Gervasoni, E.; Jonsdottir, J.; Anastasi, D.; Pavei, G.; Clerici, M.; Cattaneo, D. Complementary use of statistical parametric mapping and gait profile score to describe walking alterations in multiple sclerosis: A cross-sectional study. Sci. Rep. 2023, 13, 10465. [Google Scholar] [CrossRef]
- Huang, S.-C.; Dalla Costa, G.; Pisa, M.; Gregoris, L.; Leccabue, G.; Congiu, M.; Comi, G.; Leocani, L. The danger of walking with socks: Evidence from kinematic analysis in people with progressive multiple sclerosis. Sensors 2020, 20, 6160. [Google Scholar] [CrossRef]
- Huang, S.-C.; Guerrieri, S.; Dalla Costa, G.; Pisa, M.; Leccabue, G.; Gregoris, L.; Comi, G.; Leocani, L. Intensive neurorehabilitation and gait improvement in progressive multiple sclerosis: Clinical, kinematic and electromyographic analysis. Brain Sci. 2022, 12, 258. [Google Scholar] [CrossRef]
- Berg-Hansen, P.; Moen, S.M.; Austeng, A.; Gonzales, V.; Klyve, T.D.; Negård, H.; Seeberg, T.M.; Celius, E.G.; Meyer, F. Sensor-based gait analyses of the six-minute walk test identify qualitative improvement in gait parameters of people with multiple sclerosis after rehabilitation. J. Neurol. 2022, 269, 3723–3734. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, C.; Ziegler, J.; Zentek, T.; Rashid, A.; Strauss, S.; Tallner, A.; Grothe, M. Smartphone-based gait analysis in the assessment of fatigue and fatigability in people with multiple sclerosis: A supervised cohort study. J. Neurol. 2025, 272, 217. [Google Scholar] [CrossRef] [PubMed]
- Santinelli, F.B.; Abasıyanık, Z.; Ramari, C.; Gysemberg, G.; Kos, D.; Pau, M.; Kalron, A.; Meyns, P.; Ozakbas, S.; Feys, P. Manifestations of walking fatigability in people with multiple sclerosis based on gait quality and distance walked during the six minutes walking test. Mult. Scler. Relat. Disord. 2024, 91, 105909. [Google Scholar] [CrossRef] [PubMed]
- Schumann, P.; Scholz, M.; Trentzsch, K.; Jochim, T.; Śliwiński, G.; Malberg, H.; Ziemssen, T. Detection of fall risk in multiple sclerosis by gait analysis—An innovative approach using feature selection ensemble and machine learning algorithms. Brain Sci. 2022, 12, 1477. [Google Scholar] [CrossRef]
- Massot, C.; Simoneau, E.; Peron, D.; Barbier, F.; Kwiatkowski, A.; Donze, C.; Leteneur, S. Simplified stance limb kinetics patterns revealed during gait initiation in early stage of multiple sclerosis. Clin. Biomech. 2022, 91, 105549. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Küderle, A.; Gaßner, H.; Klucken, J.; Eskofier, B.M.; Kluge, F. Inertial sensor-based gait parameters reflect patient-reported fatigue in multiple sclerosis. J. Neuroeng. Rehabil. 2020, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Motti Ader, L.G.; Greene, B.R.; McManus, K.; Tubridy, N.; Caulfield, B. Short bouts of gait data and body-worn inertial sensors can provide reliable measures of spatiotemporal gait parameters from bilateral gait data for persons with multiple sclerosis. Biosensors 2020, 10, 128. [Google Scholar] [CrossRef]
- Zörner, B.; Hostettler, P.; Meyer, C.; Killeen, T.; Gut, P.; Linnebank, M.; Weller, M.; Straumann, D.; Filli, L. Prognosis of walking function in multiple sclerosis supported by gait pattern analysis. Mult. Scler. Relat. Disord. 2022, 63, 103802. [Google Scholar] [CrossRef]
- Carpinella, I.; Bertoni, R.; Anastasi, D.; Cardini, R.; Lencioni, T.; Ferrarin, M.; Cattaneo, D.; Gervasoni, E. Walk Longer! Using Wearable Inertial Sensors to Uncover Which Gait Aspects Should Be Treated to Increase Walking Endurance in People with Multiple Sclerosis. Sensors 2024, 24, 7284. [Google Scholar] [CrossRef]
- Broscheid, K.-C.; Behrens, M.; Bilgin-Egner, P.; Peters, A.; Dettmers, C.; Jöbges, M.; Schega, L. Instrumented assessment of motor performance fatigability during the 6-min walk test in mildly affected people with multiple sclerosis. Front. Neurol. 2022, 13, 802516. [Google Scholar] [CrossRef] [PubMed]
- Bourke, A.K.; Scotland, A.; Lipsmeier, F.; Gossens, C.; Lindemann, M. Gait characteristics harvested during a smartphone-based self-administered 2-minute walk test in people with multiple sclerosis: Test-retest reliability and minimum detectable change. Sensors 2020, 20, 5906. [Google Scholar] [CrossRef]
- Pau, M.; Leban, B.; Deidda, M.; Putzolu, F.; Porta, M.; Coghe, G.; Cocco, E. Kinematic analysis of lower limb joint asymmetry during gait in people with multiple sclerosis. Symmetry 2021, 13, 598. [Google Scholar] [CrossRef]
- Schumann, P.; Trentzsch, K.; Stölzer-Hutsch, H.; Jochim, T.; Scholz, M.; Malberg, H.; Ziemssen, T. Using machine learning algorithms to detect fear of falling in people with multiple sclerosis in standardized gait analysis. Mult. Scler. Relat. Disord. 2024, 88, 105721. [Google Scholar] [CrossRef] [PubMed]
- Marotta, N.; de Sire, A.; Marinaro, C.; Moggio, L.; Inzitari, M.T.; Russo, I.; Tasselli, A.; Paolucci, T.; Valentino, P.; Ammendolia, A. Efficacy of transcranial direct current stimulation (tdcs) on balance and gait in multiple sclerosis patients: A machine learning approach. J. Clin. Med. 2022, 11, 3505. [Google Scholar] [CrossRef]
- Seebacher, B.; Kuisma, R.; Glynn, A.; Berger, T. Exploring cued and non-cued motor imagery interventions in people with multiple sclerosis: A randomised feasibility trial and reliability study. Arch. Physiother. 2018, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Camerota, F.; Celletti, C.; Di Sipio, E.; De Fino, C.; Simbolotti, C.; Germanotta, M.; Mirabella, M.; Padua, L.; Nociti, V. Focal muscle vibration, an effective rehabilitative approach in severe gait impairment due to multiple sclerosis. J. Neurol. Sci. 2017, 372, 33–39. [Google Scholar] [CrossRef]
- Flachenecker, F.; Gaßner, H.; Hannik, J.; Lee, D.H.; Flachenecker, P.; Winkler, J.; Eskofier, B.; Linker, R.A.; Klucken, J. Objective sensor-based gait measures reflect motor impairment in multiple sclerosis patients: Reliability and clinical validation of a wearable sensor device. Mult. Scler. Relat. Disord. 2020, 39, 101903. [Google Scholar] [CrossRef]
- Liparoti, M.; Della Corte, M.; Rucco, R.; Sorrentino, P.; Sparaco, M.; Capuano, R.; Minino, R.; Lavorgna, L.; Agosti, V.; Sorrentino, G.; et al. Gait abnormalities in minimally disabled people with Multiple Sclerosis: A 3D-motion analysis study. Mult. Scler. Relat. Disord. 2019, 29, 100–107. [Google Scholar] [CrossRef]
- Severini, G.; Manca, M.; Ferraresi, G.; Caniatti, L.M.; Cosma, M.; Baldasso, F.; Straudi, S.; Morelli, M.; Basaglia, N. Evaluation of Clinical Gait Analysis parameters in patients affected by Multiple Sclerosis: Analysis of kinematics. Clin. Biomech. 2017, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boudarham, J.; Hameau, S.; Zory, R.; Hardy, A.; Bensmail, D.; Roche, N.; Derfuss, T. Coactivation of Lower Limb Muscles during Gait in Patients with Multiple Sclerosis. PLoS ONE 2016, 11, e0158267. [Google Scholar] [CrossRef]
- Pau, M.; Coghe, G.; Corona, F.; Marrosu, M.G.; Cocco, E. Effect of spasticity on kinematics of gait and muscular activation in people with Multiple Sclerosis. J. Neurol. Sci. 2015, 358, 339–344. [Google Scholar] [CrossRef]
- Pau, M.; Corona, F.; Pilloni, G.; Porta, M.; Coghe, G.; Cocco, E. Do gait patterns differ in men and women with multiple sclerosis? Mult. Scler. Relat. Disord. 2017, 18, 202–208. [Google Scholar] [CrossRef]
- Drebinger, D.; Rasche, L.; Kroneberg, D.; Althoff, P.; Bellmann-Strobl, J.; Weygandt, M.; Paul, F.; Brandt, A.U.; Schmitz, H. Association Between Fatigue and Motor Exertion in Patients with Multiple Sclerosis-a Prospective Study. Front. Neurol. 2020, 11, 208. [Google Scholar] [CrossRef]
- Filli, L.; Sutter, T.; Easthope, C.S.; Killeen, T.; Meyer, C.; Reuter, K.; Lörincz, L.; Bolliger, M.; Weller, M.; Curt, A.; et al. Profiling walking dysfunction in multiple sclerosis: Characterisation, classification and progression over time. Sci. Rep. 2018, 8, 4984. [Google Scholar] [CrossRef] [PubMed]
- Elsworth-Edelsten, C.; Bonnefoy-Mazure, A.; Laidet, M.; Armand, S.; Assal, F.; Lalive, P.; Allali, G. Upper limb movement analysis during gait in multiple sclerosis patients. Hum. Mov. Sci. 2017, 54, 248–252. [Google Scholar] [CrossRef]
- Kempen, J.C.E.; Doorenbosch, C.A.; Knol, D.L.; de Groot, V.; Beckerman, H. Newly Identified Gait Patterns in Patients With Multiple Sclerosis May Be Related to Push-off Quality. Phys. Ther. 2016, 96, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, A.S.; Huisinga, J.M.; Peterson, D.S. The application of principal component analysis to characterize gait and its association with falls in multiple sclerosis. Sci. Rep. 2021, 11, 12811. [Google Scholar] [CrossRef]
- Arpan, I.; Shah, V.V.; McNames, J.; Harker, G.; Carlson-Kuhta, P.; Spain, R.; El-Gohary, M.; Mancini, M.; Horak, F.B. Fall Prediction Based on Instrumented Measures of Gait and Turning in Daily Life in People with Multiple Sclerosis. Sensors 2022, 22, 5940. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.G.; Neri, S.G.R.; Motl, R.W.; Tauil, C.B.; Glehn, F.V.; Corrêa, É.C.; de David, A.C. Effect of hippotherapy on walking performance and gait parameters in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 43, 102203. [Google Scholar] [CrossRef]
- Weed, L.; Little, C.; Kasser, S.L.; McGinnis, R.S. A Preliminary Investigation of the Effects of Obstacle Negotiation and Turning on Gait Variability in Adults with Multiple Sclerosis. Sensors 2021, 21, 5806. [Google Scholar] [CrossRef] [PubMed]
- Roeing, K.L.; Moon, Y.; Sosnoff, J.J. Unplanned gait termination in individuals with multiple sclerosis. Gait Posture 2017, 53, 168–172. [Google Scholar] [CrossRef]
- Jeng, B.; Šilić, P.; Huynh, T.L.T.; Motl, R.W. Sedentary Behavior and Lower-Extremity Physical Function across the Lifespan of Adults with Multiple Sclerosis. Int. J. Environ. Res. Public Health 2022, 19, 12466. [Google Scholar] [CrossRef]
- Banks, S.A.; Howe, C.L.; Mandrekar, J.; Jahanian, O.; Pittock, S.J.; Ali, F.; Sagen, J.A.; Spence, R.; Gossman, K.A.; Baker, M.R.; et al. Assessing fall risk in multiple sclerosis using patient-reported outcomes and wearable gait metrics. Mult. Scler. J. Exp. Transl. Clin. 2025, 11, 20552173251329825. [Google Scholar] [CrossRef]
- Little, C.; Moore, C.; Bean, E.; Peters, D.M.; McGinnis, R.S.; Kasser, S.L. Acute effects of axial loading on postural control during walking and turning in people with multiple sclerosis: A pilot study. Gait Posture 2022, 94, 102–106. [Google Scholar] [CrossRef]
- Kushner, T.; Mosquera-Lopez, C.; Hildebrand, A.; Cameron, M.H.; Jacobs, P.G. Risky movement: Assessing fall risk in people with multiple sclerosis with wearable sensors and beacon-based smart-home monitoring. Mult. Scler. Relat. Disord. 2023, 79, 105019. [Google Scholar] [CrossRef]
- VanNostrand, M.V.; Bae, M.; Ramsdell, J.C.; Kasser, S.L. Information processing speed and disease severity predict real-world ambulation in persons with multiple sclerosis. Gait Posture 2024, 111, 99–104. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Motl, R.W.; Foley, F.W.; Izzetoglu, M.; Wagshul, M.; Holtzer, R. Within-session dual-task walking practice improves gait variability in older adults with multiple sclerosis. Gait Posture 2025, 119, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.W.; Richmond, S.B.; Sharp, B.E.; Fling, B.W. Middle-age people with multiple sclerosis demonstrate similar mobility characteristics to neurotypical older adults. Mult. Scler. Relat. Disord. 2021, 51, 102924. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.M.; Daugherty, A.M.; Nitta, M.; Atalla, M.; Fritz, N.E. Backward walking sensitively detects fallers in persons with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 45, 102390. [Google Scholar] [CrossRef]
- Kline, P.W.; Christiansen, C.L.; Hager, E.R.; Alvarez, E.; Mañago, M.M. Movement compensations during a step ascent task are associated with stair climbing performance in people with multiple sclerosis. Gait Posture 2021, 87, 27–32. [Google Scholar] [CrossRef]
- Hagen, A.C.; Patrick, C.M.; Bast, I.E.; Fling, B.W. Propulsive force modulation drives Split-Belt Treadmill Adaptation in people with multiple sclerosis. Sensors 2024, 24, 1067. [Google Scholar] [CrossRef] [PubMed]
- Richmond, S.B.; Swanson, C.W.; Peterson, D.S.; Fling, B.W. A temporal analysis of bilateral gait coordination in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 45, 102445. [Google Scholar] [CrossRef]
- Hu, W.; Combden, O.; Jiang, X.; Buragadda, S.; Newell, C.J.; Williams, M.C.; Critch, A.L.; Ploughman, M. Machine learning classification of multiple sclerosis patients based on raw data from an instrumented walkway. Biomed. Eng. Online 2022, 21, 21. [Google Scholar] [CrossRef]
- Hu, W.; Combden, O.; Jiang, X.; Buragadda, S.; Newell, C.J.; Williams, M.C.; Critch, A.L.; Ploughman, M. Machine learning corroborates subjective ratings of walking and balance difficulty in multiple sclerosis. Front. Artif. Intell. 2022, 5, 952312. [Google Scholar] [CrossRef]
- Craig, J.J.; Bruetsch, A.P.; Lynch, S.G.; Huisinga, J.M. Trunk and foot acceleration variability during walking relates to fall history and clinical disability in persons with multiple sclerosis. Clin. Biomech. 2020, 80, 105100. [Google Scholar] [CrossRef]
- Mañago, M.; Kline, P.; Alvarez, E.; Christiansen, C. Trunk and pelvis movement compensation in people with multiple sclerosis: Relationships to muscle function and gait performance outcomes. Gait Posture 2020, 78, 48–53. [Google Scholar] [CrossRef]
- Kaur, R.; Motl, R.W.; Sowers, R.; Hernandez, M.E. A Vision-Based Framework for Predicting Multiple Sclerosis and Parkinson’s Disease Gait Dysfunctions—A Deep Learning Approach. IEEE J. Biomed. Health Inform. 2023, 27, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Kirkland, M.C.; Wadden, K.P.; Wallack, E.M.; Ploughman, M. Reliability of gait and dual-task measures in multiple sclerosis. Gait Posture 2020, 78, 19–25. [Google Scholar] [CrossRef]
- de Aratanha, M.A.; Balardin, J.B.; Amaral, C.C.D.; Lacerda, S.S.; Sowmy, T.A.S.; Huppert, T.J.; Thomaz, R.B.; Speciali, D.S.; Machado, B.; Kozasa, E.H. The use of functional near infrared spectroscopy and gait analysis to characterize cognitive and motor processing in early-stage patients with multiple sclerosis. Front. Neurol. 2022, 13, 937231. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Kosa, P.; Bielekova, B. Smartphone tests quantify lower extremities dysfunction in multiple sclerosis. Front. Neurol. 2024, 15, 1408224. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.L.; Hoffman, R.M.; Healey, K.; Zabad, R.; Kurz, M.J. Errors in the ankle plantarflexor force production are related to the gait deficits of individuals with multiple sclerosis. Hum. Mov. Sci. 2017, 51, 91–98. [Google Scholar] [CrossRef]
- Adusumilli, G.; Lancia, S.; Levasseur, V.A.; Amblee, V.; Orchard, M.; Wagner, J.M.; Naismith, R.T.; Bayer, A. Turning is an important marker of balance confidence and walking limitation in persons with multiple sclerosis. PLoS ONE 2018, 13, e0198178. [Google Scholar] [CrossRef]
- Chen, Q.; Hattori, T.; Tomisato, H.; Ohara, M.; Hirata, K.; Yokota, T.; Initiative, F.T.A.D.N. Turning and multitask gait unmask gait disturbance in mild-to-moderate multiple sclerosis: Underlying specific cortical thinning and connecting fibers damage. Hum. Brain Mapp. 2023, 44, 1193–1208. [Google Scholar] [CrossRef]
- Shahraki, M.; Sohrabi, M.; Torbati, H.T.; Nikkhah, K.; NaeimiKia, M. Effect of rhythmic auditory stimulation on gait kinematic parameters of patients with multiple sclerosis. J. Med. Life 2017, 10, 33–37. [Google Scholar]
- Kalron, A.; Aloni, R.; Allali, G. The relationship between depression, anxiety and cognition and its paradoxical impact on falls in multiple sclerosis patients. Mult. Scler. Relat. Disord. 2018, 25, 167–172. [Google Scholar] [CrossRef]
- Kalron, A.; Frid, L. The “butterfly diagram”: A gait marker for neurological and cerebellar impairment in people with multiple sclerosis. J. Neurol. Sci. 2015, 358, 92–100. [Google Scholar] [CrossRef]
- Mofateh, R.; Salehi, R.; Negahban, H.; Mehravar, M.; Tajali, S. Effects of cognitive versus motor dual-task on spatiotemporal gait parameters in healthy controls and multiple sclerosis patients with and without fall history. Mult. Scler. Relat. Disord. 2017, 18, 8–14. [Google Scholar] [CrossRef]
- Kalron, A. The correlation between symptomatic fatigue to definite measures of gait in people with multiple sclerosis. Gait Posture 2016, 44, 178–183. [Google Scholar] [CrossRef]
- Salehi, R.; Mofateh, R.; Mehravar, M.; Negahban, H.; Tajali, S.; Monjezi, S. Comparison of the lower limb inter-segmental coordination during walking between healthy controls and people with multiple sclerosis with and without fall history. Mult. Scler. Relat. Disord. 2020, 41, 102053. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A. Symmetry in vertical ground reaction force is not related to walking and balance difficulties in people with multiple sclerosis. Gait Posture 2016, 47, 48–50. [Google Scholar] [CrossRef]
- Kalron, A.; Frid, L.; Menascu, S.; Givon, U. The association between gait variability with the energy cost of walking depends on the fall status in people with multiple sclerosis without mobility aids. Gait Posture 2019, 74, 231–235. [Google Scholar] [CrossRef] [PubMed]
- AteŞ, Y.; ÜnlÜer, N.Ö. An investigation of knee position sense, balance, and dual task performance in different phases of menstrual cycle in females with multiple sclerosis: A pilot study. Mult. Scler. Relat. Disord. 2020, 44, 102235. [Google Scholar] [CrossRef]
- Dreyer-Alster, S.; Menascu, S.; Dolev, M.; Givon, U.; Magalashvili, D.; Achiron, A.; Kalron, A. Longitudinal relationships between disability and gait characteristics in people with MS. Sci. Rep. 2022, 12, 3653. [Google Scholar] [CrossRef] [PubMed]
- Tajali, S.; Mehravar, M.; Negahban, H.; van Dieën, J.H.; Shaterzadeh-Yazdi, M.-J.; Mofateh, R. Impaired local dynamic stability during treadmill walking predicts future falls in patients with multiple sclerosis: A prospective cohort study. Clin. Biomech. 2019, 67, 197–201. [Google Scholar] [CrossRef]
- Ayvat, F.; Doğan, M.; Ayvat, E.; Kılınç, M. Reduced physical activity in multiple sclerosis: The role of gait parameters, gait variability parameters and self-reported measure of gait. Mult. Scler. Relat. Disord. 2025, 98, 106452. [Google Scholar] [CrossRef]
- Plotnik, M.; Wagner, J.M.; Adusumilli, G.; Gottlieb, A.; Naismith, R.T. Gait asymmetry, and bilateral coordination of gait during a six-minute walk test in persons with multiple sclerosis. Sci. Rep. 2020, 10, 12382. [Google Scholar] [CrossRef]
- Güner, S.; Hagharı, S.; Inanıcı, F.; Alsancak, S.; Aytekın, G. Knee muscle strength in multiple sclerosis: Relationship with gait characteristics. J. Phys. Ther. Sci. 2015, 27, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Eldemir, K.; Yildirim, M.S.; Cobanoglu, G.; Eldemir, S.; Guzel, N.A.; Irkec, C.; Guclu-Gunduz, A. Relationship between sensation and balance and gait in multiple sclerosis patients with mild disability. Mult. Scler. Relat. Disord. 2024, 87, 105690. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Soylu, E.; Terzi, M.; Turkoglu, M.; Koca, K.A. Making the Discrimination in the Walking Parameters of Individuals with Multiple Sclerosis and Parkinson’s Disease with Machine Learning/Multipl Skleroz ve Parkinson Hastaligina Sahip Bireylerin Yurume Parametrelerindeki Ayrimin Makine Ogrenimi ile Yapilmasi. Turk. J. Neurol. 2023, 29, 277–282. [Google Scholar]
- Berg, M.E.v.D.; Barr, C.J.; McLoughlin, J.V.; Crotty, M. Effect of walking on sand on gait kinematics in individuals with multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 16, 15–21. [Google Scholar] [CrossRef]
- Psarakis, M.; Greene, D.; Moresi, M.; Baker, M.; Stubbs, P.; Brodie, M.; Lord, S.; Hoang, P. Impaired heel to toe progression during gait is related to reduced ankle range of motion in people with Multiple Sclerosis. Clin. Biomech. 2017, 49, 96–100. [Google Scholar] [CrossRef]
- Hatton, A.L.; Williams, K.; Chatfield, M.D.; Hurn, S.; Maharaj, J.N.; Gane, E.M.; Cattagni, T.; Dixon, J.; Rome, K.; Kerr, G.; et al. Effects of wearing textured versus smooth shoe insoles for 12 weeks on gait, foot sensation and patient-reported outcomes, in people with multiple sclerosis: A randomised controlled trial. Brain Impair. 2023, 24, 148–167. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Verghese, J.; Coleman, S.; Burn, D.; Rochester, L. Independent domains of gait in older adults and associated motor and nonmotor attributes: Validation of a factor analysis approach. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 820–827. [Google Scholar] [CrossRef]
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait Analysis Methods: An Overview of Wearable and Non-Wearable Systems, Highlighting Clinical Applications. Sensors 2025, 14, 3362. [Google Scholar] [CrossRef]
- Leone, C.; Kalron, A.; Smedal, T.; Normann, B.; Wens, I.; Eijnde, B.O.; Feys, P. Effects of Rehabilitation on Gait Pattern at Usual and Fast Speeds Depend on Walking Impairment Level in Multiple Sclerosis. Int. J. MS Care 2018, 20, 199–209. [Google Scholar] [CrossRef]
- Hogan, N. An organizing principle for a class of voluntary movements. J. Neurosci. Off. J. Soc. Neurosci. 1984, 4, 2745–2754. [Google Scholar] [CrossRef]
- Doskas, T.; Kormas, C.; Dekavallas, L.; Kokkotis, C.; Tsiakiri, A.; Christidi, F.; Vavougios, G.; Tsiptsios, D.; Spiliopoulos, K.; Serdari, A. The mediating role of processing speed on the relationship between executive functions and social cognition in relapsing-remitting multiple sclerosis patients. Neurol. Sci. 2025, 46, 1009–1011. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Christidi, F.; Tsiptsios, D.; Vlotinou, P.; Kitmeridou, S.; Bebeletsi, P.; Kokkotis, C.; Serdari, A.; Tsamakis, K.; Aggelousis, N. Processing speed and attentional shift/mental flexibility in patients with stroke: A comprehensive review on the trail making test in stroke studies. Neurol. Int. 2024, 16, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Doskas, T.K.; Foteini, C.; Kanellos, C.S.; Dimitrios, T.; George, D.V.; Anna, T.; Theofanis, V.; Christos, K.; Ioannis, I.; Nikolaos, A.; et al. Social Cognition Impairments in Association to Clinical, Cognitive, Mood, and Fatigue Features in Multiple Sclerosis: A Study Protocol. Neurol. Int. 2023, 15, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Vlotinou, P.; Tsiakiri, A.; Frantzidis, C.A.; Katsouri, I.-G.; Aggelousis, N. The Effect of an Interventional Movement Program on the Mechanical Gait Characteristics of a Patient with Dementia. Eng. Proc. 2023, 50, 4. [Google Scholar] [CrossRef]
- Giarmatzis, G.; Giannakou, E.; Karagiannakidou, I.; Makri, E.; Tsiakiri, A.; Christidi, F.; Malliou, P.; Vadikolias, K.; Aggelousis, N. Effects of a 12-Week Moderate-to-High Intensity Strength Training Program on the Gait Parameters and Their Variability of Stroke Survivors. Brain Sci. 2025, 15, 354. [Google Scholar] [CrossRef]
- Pamboris, G.M.; Plakias, S.; Tsiakiri, A.; Karakitsiou, G.; Bebeletsi, P.; Vadikolias, K.; Aggelousis, N.; Tsiptsios, D.; Christidi, F. Physical Therapy in Neurorehabilitation with an Emphasis on Sports: A Bibliometric Analysis and Narrative Review. Sports 2024, 12, 276. [Google Scholar] [CrossRef] [PubMed]
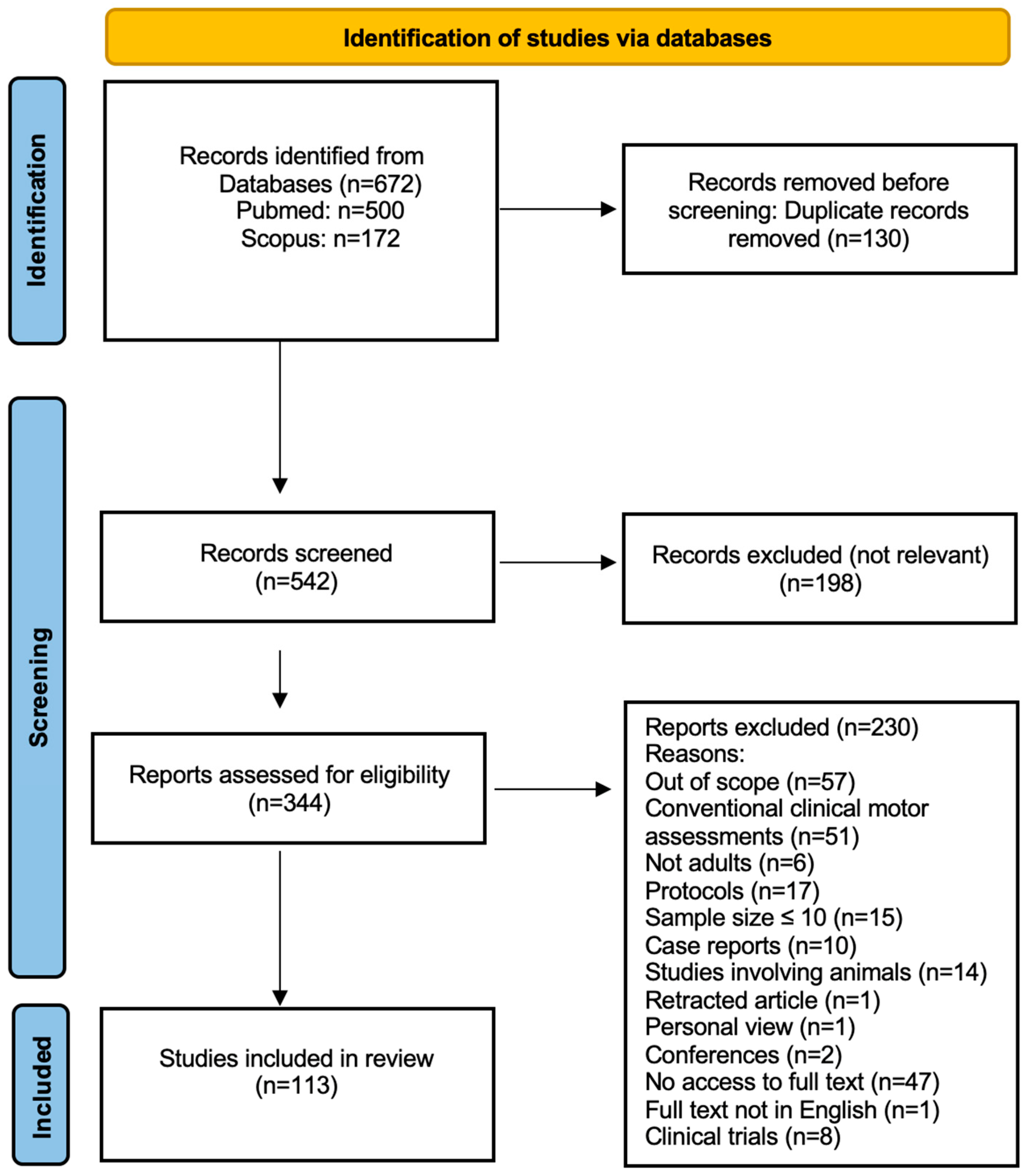
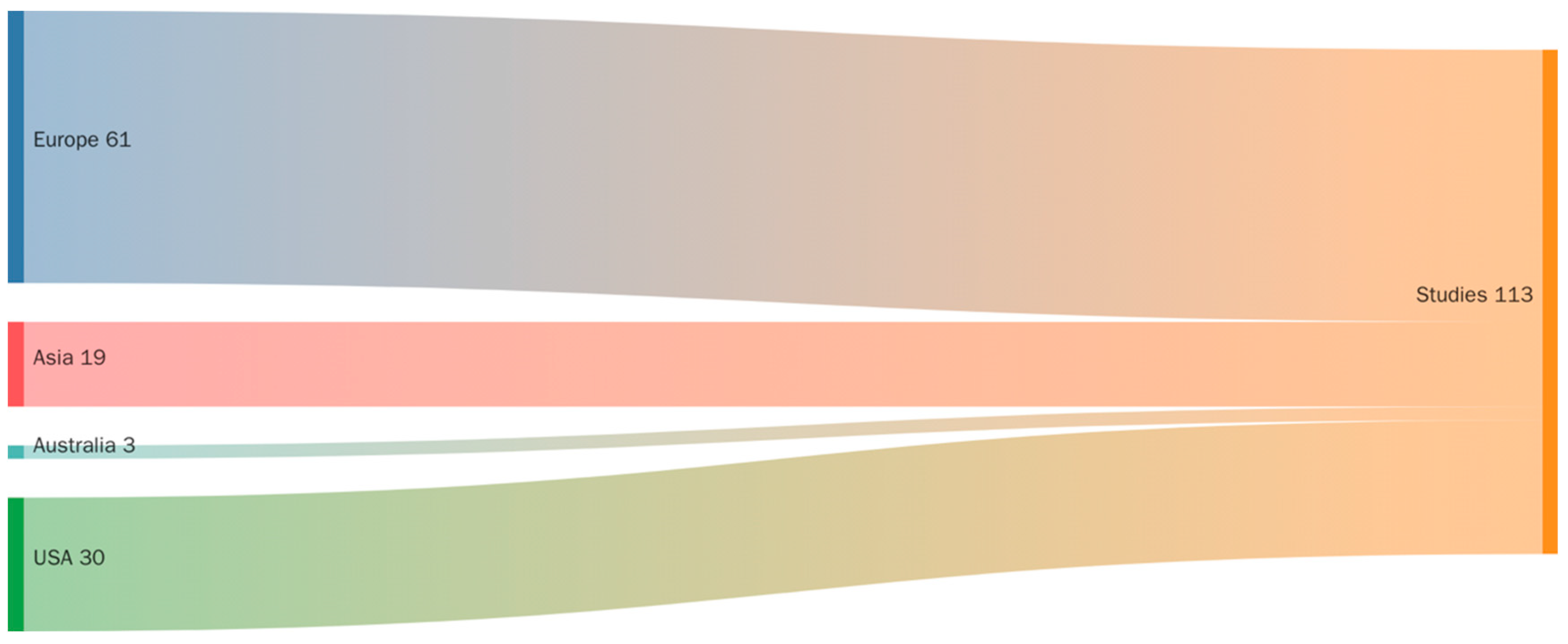

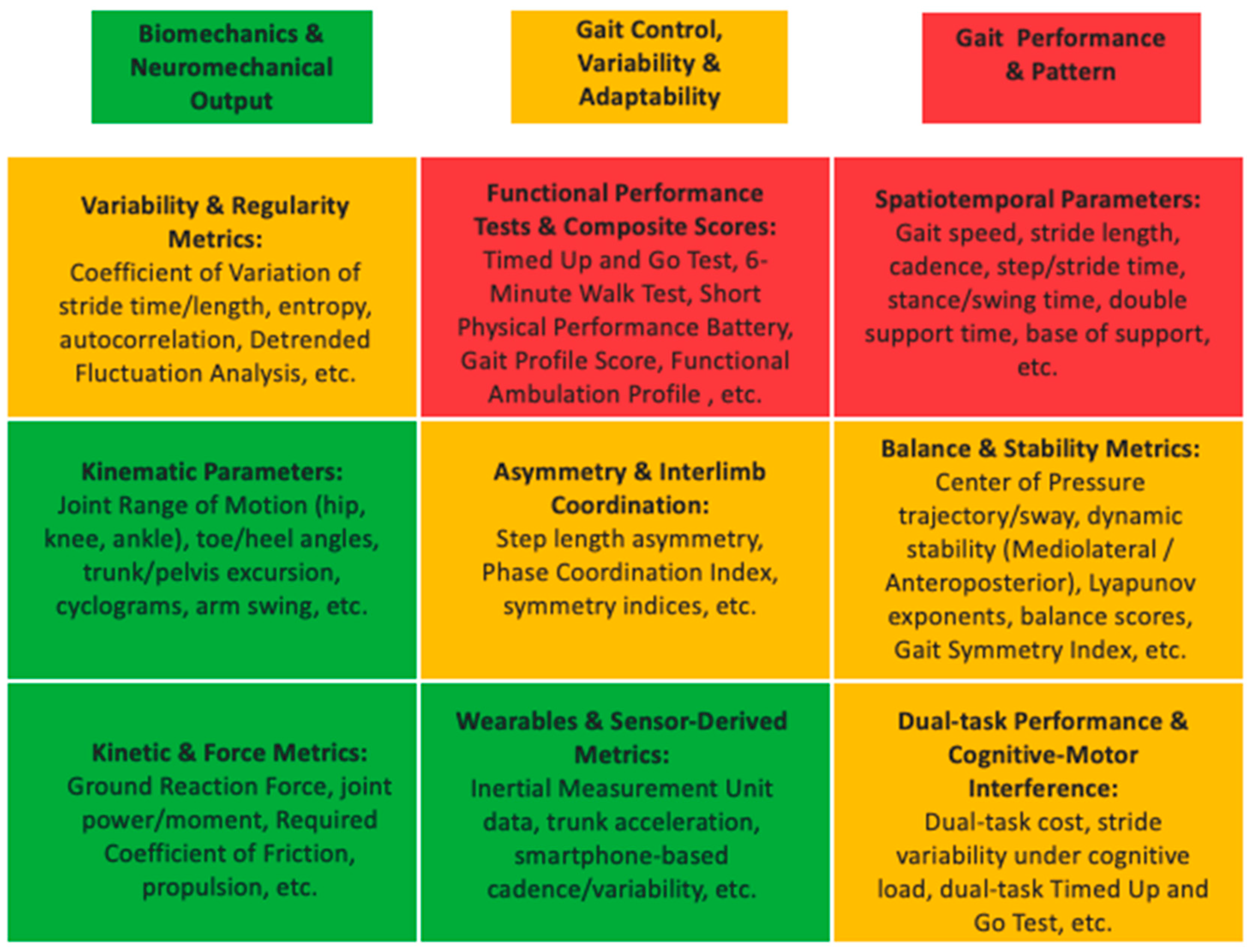
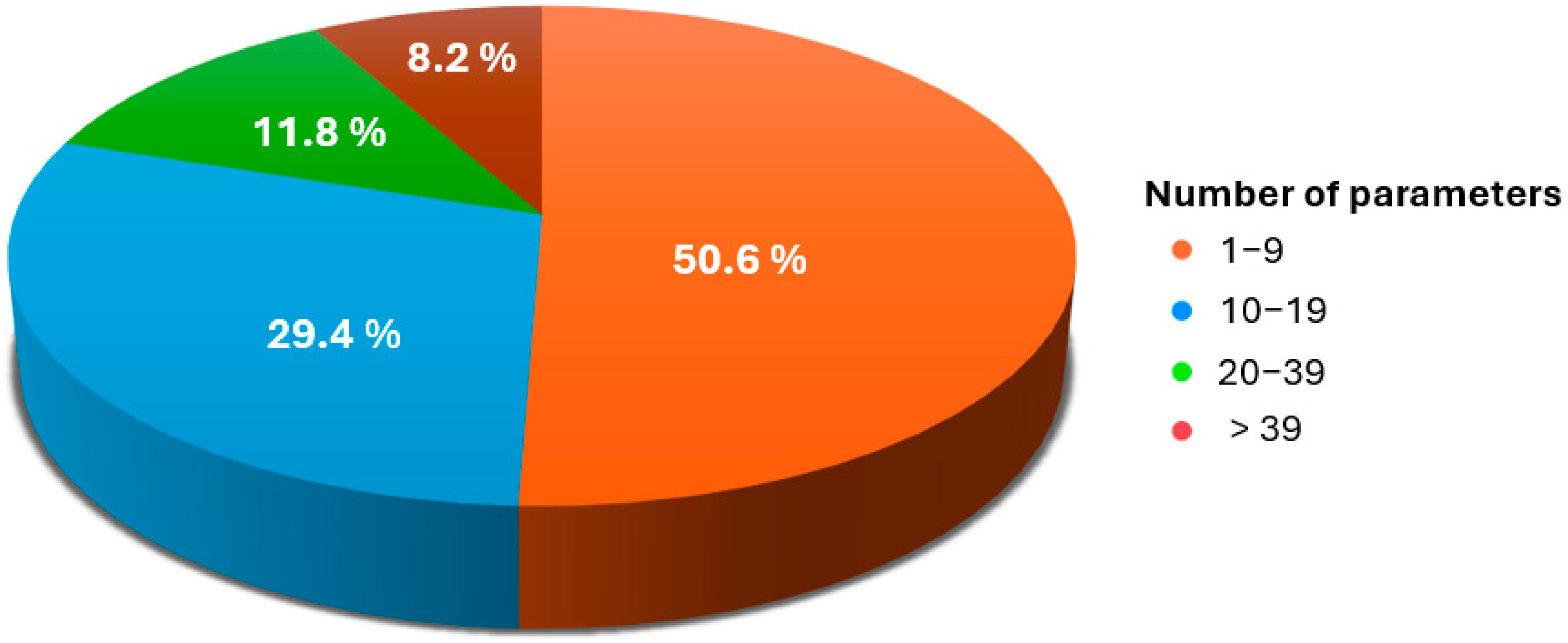
| Database | Search Date | Search Terms | Boolean Operators | Filters Applied | Timeframe |
|---|---|---|---|---|---|
| PubMed | 8 May 2025 | (gait analysis) AND (multiple sclerosis) | AND | Title/Abstract; English; Humans | Last 10 years |
| Scopus | 8 May 2025 | TITLE-ABS-KEY (“gait analysis” AND “multiple sclerosis”) | AND | Language: English; Publication Stage: Final; Document Type: Article | 2015–2025 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies published in the last decade | Studies not involving adults |
| Peer-reviewed original articles | Protocols, case reports, or conference abstracts |
| Articles written in English | Articles not available in full text |
| Human studies involving individuals with MS | Studies involving animals |
| Studies focused on gait analysis | Articles addressing only conventional clinical motor assessments |
| Sample size > 10 participants | Studies with <10 participants |
| Final publication stage | Retracted articles, personal views, or opinion pieces |
| Available in PubMed or Scopus databases | Clinical trials or studies not within scope of review |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiakiri, A.; Plakias, S.; Giarmatzis, G.; Tsakni, G.; Christidi, F.; Papadopoulou, M.; Bakalidou, D.; Vadikolias, K.; Aggelousis, N.; Vlotinou, P. Gait Analysis in Multiple Sclerosis: A Scoping Review of Advanced Technologies for Adaptive Rehabilitation and Health Promotion. Biomechanics 2025, 5, 65. https://doi.org/10.3390/biomechanics5030065
Tsiakiri A, Plakias S, Giarmatzis G, Tsakni G, Christidi F, Papadopoulou M, Bakalidou D, Vadikolias K, Aggelousis N, Vlotinou P. Gait Analysis in Multiple Sclerosis: A Scoping Review of Advanced Technologies for Adaptive Rehabilitation and Health Promotion. Biomechanics. 2025; 5(3):65. https://doi.org/10.3390/biomechanics5030065
Chicago/Turabian StyleTsiakiri, Anna, Spyridon Plakias, Georgios Giarmatzis, Georgia Tsakni, Foteini Christidi, Marianna Papadopoulou, Daphne Bakalidou, Konstantinos Vadikolias, Nikolaos Aggelousis, and Pinelopi Vlotinou. 2025. "Gait Analysis in Multiple Sclerosis: A Scoping Review of Advanced Technologies for Adaptive Rehabilitation and Health Promotion" Biomechanics 5, no. 3: 65. https://doi.org/10.3390/biomechanics5030065
APA StyleTsiakiri, A., Plakias, S., Giarmatzis, G., Tsakni, G., Christidi, F., Papadopoulou, M., Bakalidou, D., Vadikolias, K., Aggelousis, N., & Vlotinou, P. (2025). Gait Analysis in Multiple Sclerosis: A Scoping Review of Advanced Technologies for Adaptive Rehabilitation and Health Promotion. Biomechanics, 5(3), 65. https://doi.org/10.3390/biomechanics5030065










