1. Introduction
Lower limb amputations significantly impact individuals and healthcare systems globally. In 2017, approximately 57.7 million people worldwide were living with limb amputations due to traumatic causes, with lower limb amputations accounting for a substantial portion of these cases. The leading causes of these amputations include falls (36.2%), road injuries (15.7%), other transportation injuries (11.2%), and mechanical forces (10.4%) [
1]. In the United States (U.S.), lower limb amputations constitute about 83% of all amputations, with vascular diseases such as diabetes and peripheral arterial disease being primary contributors [
2]. Also, over 150,000 individuals undergo lower limb amputations each year, with more than 90% of these cases linked to vascular conditions such as diabetes and peripheral arterial disease [
3]. Projections indicate that the number of individuals living with limb loss in the U.S. could more than double by 2050, reaching 3.6 million [
4].
A population-based study conducted in Sao Paulo, Brazil, found 180,595 lower-limb amputations performed between 2009 and 2020 across public hospitals, with major amputations accounting for 33% of cases and peripheral arterial disease and diabetes as the leading causes [
5]. Togo, West Africa, between 2016 and 2021, the incidence rate for non-traumatic lower-limb amputations was reported at 8.5 per million per year, with diabetic and vascular complications being the primary causes [
6]. Meanwhile, in Kolkata, India, lower-limb amputations made up 94.8% of all amputations in a local hospital-based study, with trauma (70.3%) and peripheral vascular disease (27.7%) being the most common reasons [
7]. Between 1990 and 2019, the global incidence of traumatic amputations increased from 11.37 million to 13.23 million, while the number of prevalent cases increased from approximately 370 million to over 552 million. Years lived with disability (YLDs) due to amputations also increased by 39.2%, from 5.28 million to 7.35 million during this period [
8]. These numbers highlight the need for accessible rehabilitation services and improved prosthetic socket technologies that can adapt to diverse clinical, social, and geographic settings.
For individuals who have undergone limb amputation, regaining mobility and function hinges on a well-designed and well-fitting prosthetic socket. The socket is the primary interface between the residual limb and the prosthesis, playing a main role in comfort, stability, and the success of prosthetic rehabilitation. Socket fabrication has a long and evolving history that reflects advances in material science, patient-centered care, and biomechanical understanding.
Historically, early socket designs were often rudimentary, using materials such as wood and leather. Leather, with its flexibility and ability to conform to the limb, became popular in the early 20th century, although its durability and hygiene were limited [
9]. These early designs prioritized simplicity over customization, with craftsmen manually shaping the socket to approximate the limb’s shape. With limited materials and techniques, achieving a precise fit was challenging, often leading to pressure sores and discomfort for the wearer.
The mid-20th century introduced major shifts as war demands and returning veterans accelerated the need for durable and effective prosthetics. In the 1950s and 1960s, thermosetting plastics and fiberglass became popular, marking the beginning of more stable and customizable sockets. These materials allowed for a more secure fit and reduced socket weight, enhancing both comfort and mobility [
10]. The 1980s brought further innovation with thermoplastics, which could be molded more precisely and reheated to adjust fit, allowing for better alignment and adaptation to the residual limb’s changes over time [
11].
In recent decades, advancements in computer-aided design (CAD) and computer-aided manufacturing (CAM) have revolutionized socket fabrication. CAD/CAM technologies, introduced in the 1990s, enabled the digital mapping of the shape of the limb, leading to more consistent and individualized socket designs. This technology made it possible to produce sockets with high precision, significantly improving comfort and reducing pressure points [
12]. Alongside CAD/CAM, 3D printing emerged as a disruptive innovation in the 21st century, promising faster production times and increased customization potential. By printing sockets layer by layer, clinicians could make highly detailed adjustments adapted to each patient’s unique limb shape and preferences [
13].
Despite these advancements, challenges remain in achieving an optimal fit, minimizing skin irritation, and ensuring long-term usability. A recent study by Armitage in 2023 provides insights into current transtibial prosthetic socket fitting practices in Australia, highlighting the variability in clinical approaches [
14]. Similarly, a systematic review by Safari in 2015 consolidates the qualitative outcomes of existing socket designs, emphasizing the need for further innovation in materials and fitting methodologies [
15].
Prosthetic sockets exert force on the surface of the residual limb, influencing mobility, functionality, and user satisfaction. The magnitude, location, and management of these forces play an important role in the effectiveness of the prosthesis. Distributing pressure over a larger surface area enhances comfort during usage. While most parts of the residual limb can handle pressure, certain sensitive regions cannot tolerate any pressure. Therefore, socket designs should aim to evenly distribute forces across pressure-tolerant areas to minimize the risk of skin issues. Temporary redness might occur in pressure-tolerant zones, while pressure-sensitive areas can lead to more severe skin problems. Identifying these areas helps therapists address issues promptly, often through collaboration with prosthetists [
16,
17].
Transtibial socket designs can be categorized into two primary types: patellar tendon bearing (PTB) and total surface bearing. Within the PTB category, we have the Socket Patellar Tendon Bearing (PTB) design, which involves weight-bearing beneath the patella, supported by a thigh belt. However, this suspension method may restrict circulation and potentially lead to muscle atrophy with prolonged use [
16,
18]. In contrast, the Socket PTB SC (Patellar Tendon Bearing Supracondylar) design distributes weight-bearing below the patella, with suspension at the medial and lateral femoral condyles. This design avoids circulation and atrophy issues associated with the belt suspension. Lastly, the Socket PTB SC SP (Patellar Tendon Bearing Supracondylar Suprapatellar) design supports weight under the patella and employs suspension at the femoral condyles and the suprapatellar area, making it suitable for short stumps and addressing antero-posterior knee instability. The PTB SC design is commonly used for medium and long stumps [
16,
19].
In contrast, the Silicon Suction Socket (SSS) design evenly distributes weight-bearing over the entire stump surface. Suspension is achieved through firm adhesion and friction between the stump and a silicone liner, incorporating a pin at its distal end. This pin can be secured using a built-in blocking mechanism within the prosthetic components or using a suction mechanism for reliable suspension. The SSS design is versatile and suitable for various stump configurations [
16,
20,
21,
22].
A novel strain-gauged load cell system was developed to measure pressure at the stump/socket interface. This system was employed to study pressure distribution in different socket types among trans-femoral amputees during standing and walking. Results indicated maximum pressures of 34 kPa and 95 kPa, respectively. Comparisons revealed higher pressures at the proximal brim of the quadrilateral socket, while the ischial containment socket exhibited a more uniform pressure distribution. Similar pressure patterns were observed on the medial and lateral walls, with notable differences on the anterior and posterior walls. These findings offer valuable insights into the biomechanics of these socket types, aligning with existing literature [
23].
Despite these advancements, traditional casting and lamination techniques remain widely used in many settings due to their practicality and cost-effectiveness. While casting requires skill and experience, it allows for a high degree of customization without the need for advanced technology. However, comparing these established techniques with newer methods like 3D printing and CAD reveals critical differences in adaptability, efficiency, and clinical outcomes. Emerging technologies also open the door to increased patient involvement, as direct feedback can be integrated into the socket’s design and fit, better aligning with the user’s specific needs and enhancing long-term comfort and function [
24,
25,
26].
This review explores the current landscape of prosthetic socket design and fabrication, focusing on how a historical foundation has guided the way for modern advancements. By critically analyzing established methods alongside emerging technologies, we aim to provide healthcare professionals and amputees with a comprehensive understanding of the factors shaping socket fabrication. Finally, this review seeks to foster discussion and innovation, furthering the evolution of socket fabrication toward enhanced mobility, comfort, and rehabilitation success.
2. Methodology
A search strategy was developed to identify studies focusing on transtibial prosthetic socket design, validation, and computational modeling. The search was conducted across three electronic databases: PubMed, Scopus, and Google Scholar, covering articles published from 1999 to May 2025. Boolean operators (AND, OR) and truncation were applied to combine and expand search terms, which included keywords such as “Prosthetic Socket”, “Transtibial Socket”, “Below-Knee Prosthesis”, “Design”, “Validation”, “Computational Models”, and “Biomechanics”. These terms were chosen because they represent the main concepts related to the new perspectives in transtibial socket design and fabrication. This systematic approach ensured the identification of relevant studies meeting the predefined criteria for inclusion.
The search terms included combinations of the following keywords:
Prosthetic Socket OR Transtibial Socket OR Below-Knee Prosthesis
Design OR Shape OR Fabrication OR Construction
Validation OR Performance OR Evaluation OR Fitting
Computational Models OR Finite Element Models OR Biomechanics OR Simulation
Boolean operators (AND, OR) were used to combine search terms, and truncation was applied where appropriate to capture various word endings (e.g., “model*” for model, models, modeling).
Studies were included if they met the following criteria:
2.1. Inclusion Criteria
1. Peer-reviewed journal articles or conference papers published in English.
2. Focused on transtibial prosthetic sockets in terms of design, biomechanical validation, or computational models.
3. Provided quantitative data or computational simulations related to socket performance, pressure distribution, or mechanical properties.
4. Included human subjects or cadaveric models for physical validation or simulations for computational validation.
5. Articles published between 1999–2025.
2.2. Exclusion Criteria
1. Studies focusing on upper-limb prosthetics.
2. Reviews, editorials, commentaries, and case reports without original data.
3. Studies that did not involve validation or computational modeling aspects.
2.3. Study Selection and Data Extraction
After performing the database search, all retrieved records were imported into EndNote reference management software. Duplicates were removed before the initial screening. Two independent reviewers screened titles and abstracts for relevance based on the predefined inclusion and exclusion criteria. Studies that passed this stage were subjected to full-text screening.
As shown in
Figure 1, in the studies selected, we prioritized those that addressed key methodological features aligned with the goals of this review. Specifically, each included article was evaluated based on (1) the nature of the socket design—whether conventional, anatomical, or customized; (2) the validation methodology employed such as pressure mapping, mechanical testing, or clinical assessment of fit and comfort; (3) the inclusion of computational modeling approaches, including finite element analysis or simulation of biomechanical behavior; and (4) the type of subject data used—ranging from human participants and cadaveric models to synthetic test benches. Additionally, there was an emphasis on studies that provided quantitative outcomes related to interface pressures, material performance, or functional evaluation. This structured selection process ensured a comprehensive synthesis of the most relevant and scientifically rigorous evidence on lower limb socket design and validation.
3. Socket Design
Socket design for prosthetic limbs involves careful consideration of pressure distribution to ensure patient comfort. Pressure areas are categorized into pressure-tolerant and non-tolerant zones, with bone prominences and post-surgical scar areas being particularly sensitive and requiring pressure relief [
16,
27,
28,
29]. These distributions can be achieved through socket geometry modifications or suspension systems. Geometry adjustments target these sensitive areas [
30]. To assess pressure distribution, various strategies employing sensors and imaging have been developed. These tools help analyze high-pressure areas when the patient uses the prosthesis.
Various designs, such as the patellar tendon-bearing (PTB) socket and total surface-bearing (TSB) socket, have been developed to distribute forces effectively across the residual limb. Comprehensive reviews by Safari and Gholizadeh discuss the evolution of these socket designs, along with their interface materials and suspension mechanisms [
15,
31]. Additionally, recent research explores how different socket structures impact residual limb health, demonstrating the importance of considering pressure distribution and soft tissue response when designing prosthetic interfaces [
32,
33]. Moreover, recent studies highlight the biomechanical factors influencing socket performance, further underscoring the necessity for a personalized approach to socket design [
26,
34].
To compare the clinical features of commonly used prosthetic socket configurations,
Table 1 summarizes their suspension mechanisms, core benefits, and known drawbacks as reported in the literature.
Recent advancements in socket design hold promises for improving clinical practices and biomechanics research. These methods aim to streamline socket fitting, reducing time and iterations. However, it’s crucial to note the study’s limitations. Proposed socket design guidelines should be validated through controlled clinical studies comparing templates with customized sockets [
38]. The authors stress the importance of skilled prosthetists integrating templates for optimal outcomes.
Another innovative approach is the use of sensors to optimize design criteria. One study focused on developing and validating a sensor for measuring shear forces between the residual limb and socket in lower-extremity amputees. The sensor exhibited excellent linearity and sensitivity, and testing amputees consistently revealed shear forces. These findings suggest that such sensors could significantly enhance understanding of prosthetic fit, leading to improved patient comfort and overall performance [
39].
In developing countries, the process is often manual due to cost constraints. A study explored a cost-effective and low-skilled pressure casting technique (PCAST) for creating and fitting transtibial prosthetic sockets. Results showed high satisfaction levels and improved physical function among participants fitted with PCAST sockets. This approach has the potential to benefit underserved areas by reducing costs and increasing access to prosthetic fitting [
40,
41,
42].
These methods typically involve creating molds from wrapping casts or digital models reconstructed from scanned data. However, they are time-consuming, exhibit low accuracy, and may require extensive manual labor. To address these issues, there’s a need for a more data-driven approach, incorporating high-resolution residuum models that include internal tissue structures.
Medical imaging techniques like ultrasound, CT scans, and MRI have been used to reconstruct residuum models, but they often don’t capture natural, unloaded shapes and lack dynamic skin surface deformations. To overcome these limitations, researchers at MIT developed a multi-camera imaging system coupled with a 3D digital image correlation (3D-DIC) toolbox. This setup allows for full-field deformation and strain measurements, including internal tissue structures. The combination of MRI, CT, or ultrasound with 3D-DIC data using registration markers and algorithms results in accurate digital models with detailed skin surface geometry and internal structures. This approach has been used to design variable-thickness custom liners, improving comfort and reducing skin irritation by adjusting thickness based on skin strain measurements during flexion. Thermal imaging confirmed the effectiveness of this variable thickness liner in reducing skin irritation and thermal output in high-strain regions [
43].
In their study, Dickinson et al. analyzed digital records from expert clinicians to understand variations in transtibial prosthetic socket designs [
44]. They found that most sockets exhibited a hybrid approach, blending elements of both Patella Tendon Bearing (PTB) and Total Surface Bearing (TSB) philosophies. Notably, while certain rectifications, such as paratibial carves and gross volume reduction, showed significant correlations, the depth of the patellar tendon carve remained relatively independent, suggesting its design insensitivity. The authors suggest that leveraging these insights can enhance evidence-based socket design, potentially leading to the development of smart CAD/CAM templates that predict design features adapted to individual patient demographics and limb characteristics.
A clinical investigation conducted by van der Stelt et al. titled Evaluating the Effectiveness of Transtibial Prosthetic Socket Shape Design Using Artificial Intelligence: A Clinical Comparison with Traditional Plaster Cast Socket Designs explored the application of artificial intelligence (AI) in the design of transtibial prosthetic sockets. The study involved a comparative analysis between AI-generated socket designs and traditionally fabricated plaster cast sockets. The authors assessed both approaches in a clinical setting, focusing on patient satisfaction, functional performance, and socket fit. The AI-based workflow integrated patient-specific limb geometry with algorithmic optimization techniques to generate individualized socket shapes. Clinical trials involving transtibial amputees demonstrated that AI-designed sockets achieved comparable, and in some cases superior, outcomes in comfort and mobility compared to conventional methods. This case exemplifies how digital innovations such as AI can enhance prosthetic design and streamline the fabrication process without compromising clinical effectiveness [
45].
Table 2 compares traditional and emerging approaches—including manual modification, CAD-CAM workflows, FEM optimization, and AI-assisted customization—outlining their tools, strengths, and limitations within real-world contexts.
3.1. Segmentation of Body Parts
In patient care and prosthetic design, various techniques for digitizing patient geometry have emerged, including infrared, laser, and structured light methods [
24,
25,
49,
50,
51,
52,
53,
54]. These techniques allow for the creation of precise 3D models of patients’ bodies, enabling the design of custom sockets that reduce stress in critical areas. However, a delicate balance between accuracy and cost must be maintained, as each technique comes with its own margin of error. Additionally, 3D human body scanning, particularly the 3D human body scanner (HBS), has found applications in fields like clothing design and anthropology, and is increasingly being explored for clinical use in estimating body composition, fat distribution, and anthropometric profiling [
55]. Still, challenges persist in handling and interpreting HBS data. Recent innovations have demonstrated that low-cost, scalable scanning systems such as arrays of low-cost cameras can produce high-resolution full-body 3D reconstructions suitable for anthropometric analysis as seen in
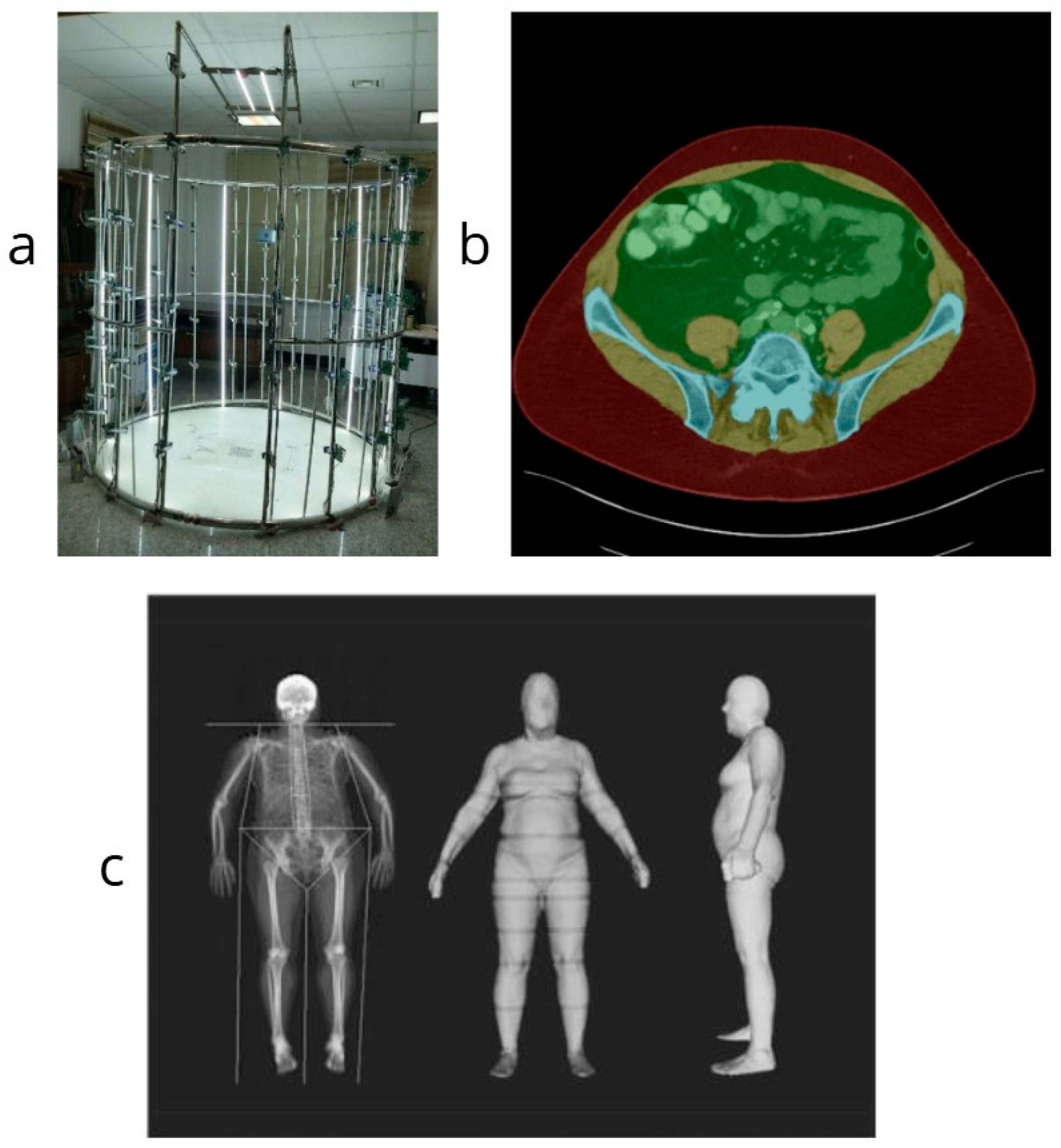
Figure 2 [
56]. These systems offer alternatives for clinical applications where traditional commercial scanners are not an option, making digital capture more accessible for prosthetic design and other healthcare applications.
In the medical field, computer-aided image analysis, driven by convolutional neural networks (CNNs), has become important for clinical diagnoses and treatment planning. Notably, threedimensional (3D) imaging systems have rapidly advanced, enabling the processing of extensive medical data in 3D. This progress has brought interest in 3D deep learning methods adapted for medical image segmentation—a crucial step in understanding the spatial distribution of structures within the body. A comprehensive review is dedicated to these emerging 3D deep learning methodologies, exploring their applicability, advantages, and potential limitations [
57,
58].
Beyond the current state of medical image analysis, the review identifies gaps in 3D medical image segmentation research, highlighting areas for further exploration and innovation [
59]. It serves as a valuable guide, directing researchers and practitioners toward uncharted territories in this dynamic field. The review encapsulates the synergy between cutting-edge deep learning techniques, the evolving landscape of 3D medical imaging, and the urgent needs of medical diagnosis and treatment planning. By offering insights into the present and a roadmap for the future, it ensures that technological advancements continue to enhance healthcare outcomes [
60,
61]. In recent advancements, convolutional neural network-based approaches have been used for the segmentation of key body compartments directly from volumetric CT data [
62]. These models facilitate automated, repeatable extraction of anatomical structures such as muscle, bone, and adipose tissue. These applications are increasingly valuable for diagnostic support, surgical planning, and outcome monitoring in medical imaging.
To summarize the various scanning technologies explored in clinical and research settings, the
Table 3 provides a comparative overview. This comparison highlights the key benefits and limitations of each approach, considering factors such as cost, accuracy, portability, and suitability for clinical environments.
Figure 2.
Collage illustrating segmentation and analysis of body parts through various 3D scanning and imaging techniques. (
a) Developed low-cost 3D scanning system using Raspberry Pi cameras [
56]; (
b) Cropped abdominal CT segmentation showing key anatomical regions [
62]; (
c) Cropped 3D optical scan and DXA comparison for anthropometric assessment [
54]. Images adapted under CC BY 4.0 where applicable.
Figure 2.
Collage illustrating segmentation and analysis of body parts through various 3D scanning and imaging techniques. (
a) Developed low-cost 3D scanning system using Raspberry Pi cameras [
56]; (
b) Cropped abdominal CT segmentation showing key anatomical regions [
62]; (
c) Cropped 3D optical scan and DXA comparison for anthropometric assessment [
54]. Images adapted under CC BY 4.0 where applicable.
3.2. Current Strategies for Socket Design
Some proposed solutions take advantage of new technologies related to materials, design, and geometry segmentation. For example, Quatro Socket focuses on a modular design that simplifies fitting and enhances patient comfort. The socket is adjustable, allowing for modifications in alignment and volume changes without needing complete re-fabrication. This adjustability offers a custom-like fit while reducing the need for multiple prosthetic sockets over time, making it ideal for patients experiencing volume fluctuation in their residual limbs [
63].
Martin Bionic has introduced innovative socket designs with an emphasis on biomechanics and individualized customization. Their flagship socket technology integrates advanced pressure distribution and energy management systems, making it particularly suited for high-activity users who demand superior dynamic performance and comfort. The technology behind Martin Bionic’s sockets aims to reduce skin irritation and pressure points [
64].
Ottobock MyFit socket technology is a key component in their line of advanced prosthetic solutions, focusing on personalization and enhanced fit. MyFit sockets utilize innovative liner and suspension systems that create a vacuum seal for superior attachment and comfort. With a focus on maintaining limb health, technology allows for precise volume management and excellent suspension without sacrificing comfort [
65].
Vytruve focuses on leveraging digital tools and scanning technology to create highly personalized prosthetic sockets. Their approach streamlines the fitting process through 3D scanning and printing, offering a high level of precision and customization. Vytruve sockets are known for their lightweight design, which enhances mobility and reduces user fatigue. The emphasis is on providing a fast, digital-first solution that delivers accuracy and comfort [
66].
The innovations in prosthetic socket technologies by companies like Quattro Socket, Martin Bionic, Ottobock MyFit, and Vytruve demonstrate significant advancements in patient comfort, mobility, and adaptability. Whether through modular design, biomechanical optimization, or digital customization, each company offers unique solutions adapted to specific user needs, from high performance athletes to everyday users. As technology continues to evolve, prosthetic sockets will likely become even more sophisticated, offering greater flexibility, function, and ease of use for individuals with limb loss.
Recent technological advancements have introduced new possibilities for socket customization and adjustability. Digital tools, such as smartphone applications and 3D printing, are revolutionizing prosthetic socket fabrication by allowing for real-time adjustments and improved user-specific customization. A feasibility study by Kaile in 2021 explores the potential of mobile applications to enable users to fine-tune socket fit, thereby enhancing comfort and usability [
67]. Additionally, Kim S (2022) discusses the role of additive manufacturing in socket design, illustrating how 3D printing technologies can facilitate the production of highly customized prosthetic components [
68]. These innovations represent a significant shift toward more adaptable and user-friendly prosthetic solutions.
3.3. Advancements in Adaptive Prosthetic Socket Technologies
In their mixed methods research study, Carroll [
69] evaluated the impact of the Smart Adaptive Socket System (SASS) on transtibial prosthesis users. The study combined quantitative assessments of socket fit and user activity levels with qualitative interviews to gather user experiences. The findings indicated that the SASS improved socket fit, reduced the need for manual adjustments, and enhanced overall user satisfaction. Participants reported increased comfort and a greater sense of security during ambulation, attributing these benefits to the system’s automatic adjustments accommodating residual limb volume fluctuations. This research underscores the potential of adaptive technologies in prosthetic socket design to enhance user comfort and mobility.
Building upon these findings, Sanders et al. [
26] conducted a study on a motor-driven adaptive socket that automatically adjusted its size during walking. The socket utilized inductive sensors to measure the distance between the elastomeric liner and the socket’s inner surface, allowing for real time adjustments. Another approach is presented by Lee et al. [
70], who developed a pneumatically controlled socket system for transfemoral amputees. Their design combines pressure sensors and a closed-loop control mechanism that regulates air bladders to maintain uniform contact pressure between the socket and residual limb. This technology offers a lightweight, responsive method of fit regulation that can adapt to limb volume changes without requiring rigid motorized adjustments. Back to Sanders study, participants who used the adaptive socket reported greater activity levels, less time spent removing the prosthesis, and fewer manual adjustments compared to using a locked non-adjustable socket or a motor-driven socket adjusted via smartphone. This study suggests that an adaptive prosthetic socket could provide a practical solution to the challenge of maintaining proper socket fit, enhancing daily comfort and functionality for people with transtibial amputation [
26].
Furthermore, a systematic review by Baldock examined adjustable prosthetic socket designs and their potential to improve prosthetic fit and comfort. The review highlighted various sensing and actuation technologies that have been proposed for improving socket performance, including pressure sensors and mechanical actuators. The authors concluded that through the continued development and integration of these technologies, it is possible to realize smart socket prostheses that not only function as a socket but also sense parameters that cause amputee discomfort and self-adjust to optimize fit, function, and performance [
71]. These innovations, including both electromechanical and pneumatic systems [
70] demonstrate an increasing number of technical solutions being explored to address socket fit and comfort challenges.
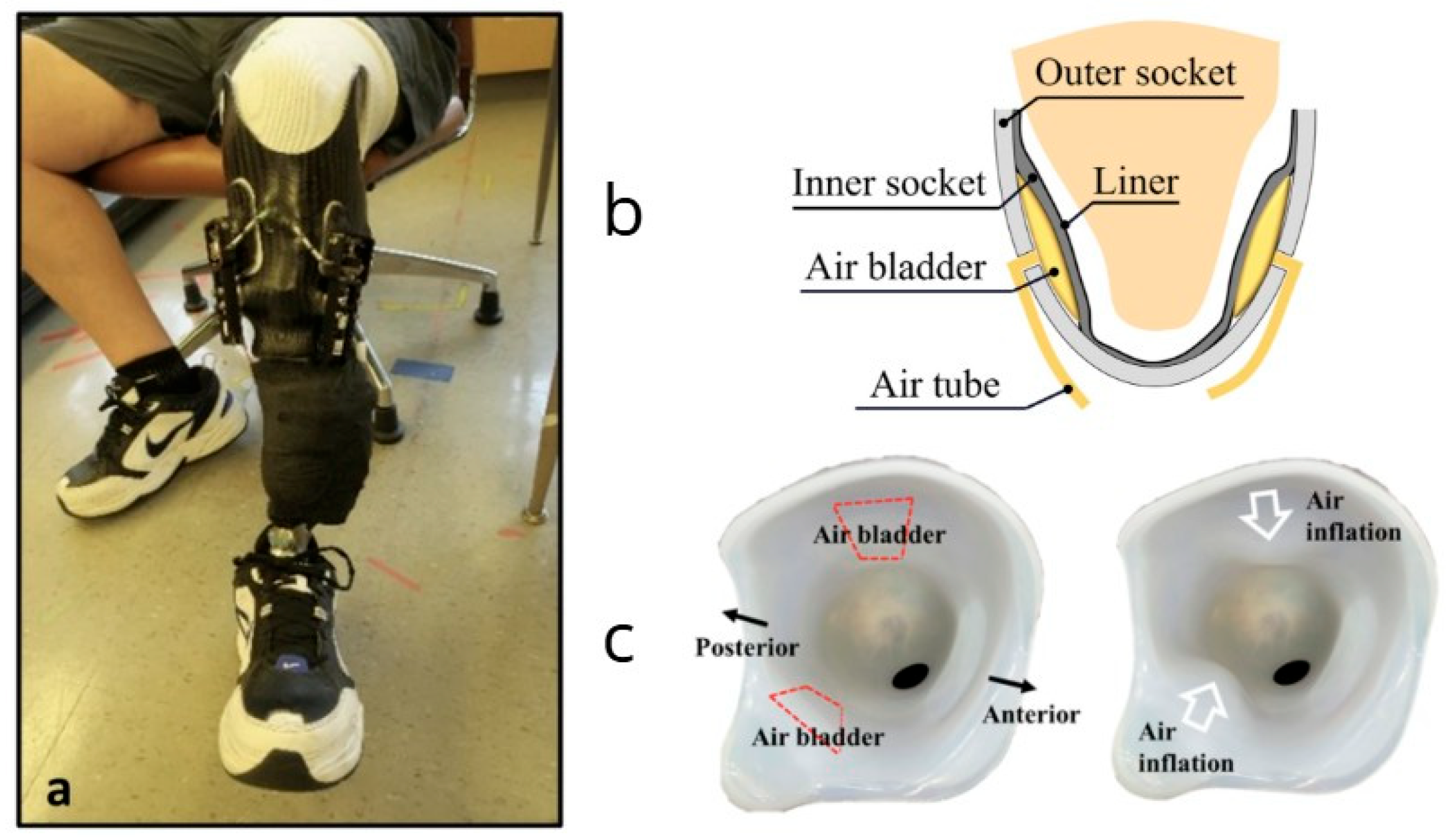
These studies highlight the advancements in adaptive prosthetic socket technologies and their positive impact on user experience. The integration of sensing and actuation mechanisms in socket design represents a promising avenue for enhancing comfort, fit, and overall satisfaction among transtibial prosthesis users. These solutions are shown in
Figure 3, which highlights different adaptive socket designs: (a) a motor-driven socket with inductive sensors [
26], and (b,c) a pneumatically controlled transfemoral socket using pressure-sensing bladders [
70].
4. Materials
The selection of materials for lower limb sockets, prosthetic, and orthotic interfaces is a process driven by the careful alignment of clinical objectives and functional prerequisites. Whether designing orthoses to address multifaceted clinical goals, including pain alleviation, deformity control, or joint motion management, or crafting prosthetic devices for the purpose of restoring lost limb functionality, the critical factor is the specific mechanical properties these materials must possess to effectively fulfill their intended functions.
In this context, the materials used must exhibit resilience to the stresses from the dynamics of human movement and the weight-bearing demands of the human body. Tensile strength is important in contexts where suspension and overall stability are critical, while material rigidity must harmonize with the soft tissue dynamics of the limb. Moreover, these materials must possess the flexibility to adapt to changes in limb morphology and accommodate the dynamic forces encountered during the act of locomotion [
48].
Predominantly, the materials selected for these applications include thermostable or thermoplastic polymers. Among these, high-density polyethylene (PE) stands as a favored choice, known for its flexibility, whereas polypropylene (PP) is recognized for its rigidity and mechanical strength as seen in
Table 4. Furthermore, composite materials, composed of matrices such as polyester resins, epoxy, and vinyl ester, combined with reinforcing agents like fibrous materials, serve to elevate mechanical attributes. For instance, the inclusion of carbon fibers heightened stiffness and tensile strength, demanding precision in shaping [
71,
72]. Recent studies have explored bio-based alternatives such as natural fiber-reinforced composites, which offer lower environmental impact, adequate strength, and cost-effectiveness for socket fabrication [
73]. These sustainable materials, often incorporating flax, kenaf, or jute fiber, show promising mechanical performance and adaptability in lower limb prosthetic applications.
Within the sphere of soft interfaces, the absorbing foams often serve as the cushioning medium. These foams, typically crafted from materials such as urethane, latex, polyurethane, or polyethylene, are engineered to provide the necessary cushioning effects. In prosthetic socket liners, silicone gels and thermoplastic elastomers (TPE) emerge as materials of choice, as stated in
Table 5’s benefits. Silicone gels offer the advantages of adaptability to limb contours and effective cushioning. In contrast, elastomers provide structural stability. However, due to the diversity of liner materials and a lack of standardized testing protocols, comparisons of mechanical properties across manufacturers remain inconsistent. Recent efforts have proposed unified methodologies to assess shear resistance, compressive response, and thermal properties in clinical conditions [
75]. These approaches aim to guide material selection based on quantifiable performance rather than anecdotal or manufacturer-provided data. Silicone gel liners are particularly prized for their attributes of cushioning and adhesion, while silicone elastomer liners can retain shape and offer mechanical robustness. Clinical investigations comparing different silicone liner types, such as Dermo and Seal-In X5, have demonstrated their varying effects on interface pressure and patient satisfaction during ambulation [
76]. Also, empirical research findings highlight the potential of silicone interfaces to not only enhance prosthetic control but also mitigate issues such as abrasion and cutaneous irritation [
73]. In the other hand, recent gait analysis studies reinforce that the type of liner material significantly influences biomechanical gait patterns, with silicone-based liners generally offering more consistent motion and pressure distribution across the gait cycle [
77].
Proper fit is crucial when using prostheses and orthoses to prevent potential complications, including trauma and skin-related problems. Skin concerns often arise due to factors such as pressure, friction, shear, and tension. Friction can lead to damage to superficial tissues, while shear injuries may affect deeper skin layers. Mechanical stress has the potential to harm the dermis and induce lesions. Furthermore, the adherence of prosthetic liners can result in pistoning, causing uncomfortable pulling on the limb during motion. Excessive pressure, particularly in bony areas, can also result in skin issues. Additionally, the heat generated by these devices can lead to perspiration, increasing the risk of injuries and infections. Skin problems can be further exacerbated by factors such as bacterial or fungal infections, hypersensitivity reactions, and insufficient hygiene practices [
32,
78]. Innovations such as perforated prosthetic liners have shown promise in improving thermal comfort and reducing sweating-related complications, thereby enhancing user experience and liner wearability [
79].
In an effort to enhance comfort and suspension, the Icelandic Roll on Silicone Socket (ICEROSS) was developed. Nevertheless, studies have shown that skin problems persist despite such innovations. Research has illuminated a spectrum of skin-related issues associated with the use of prosthetic and orthotic devices, spanning from mild irritation to more severe discomfort and pain. The frequency and nature of these issues can vary across different studies and devices, highlighting the critical significance of meticulous design considerations adapted to individual needs [
32].
5. Computational Modelling
Computational modeling in prosthetic design and validation has become a fundamental tool for understanding the biomechanical interactions between the residual limb and prosthetic socket. This section explores computational approaches used to analyze and optimize the fit, comfort, and function of prosthetic devices, focusing on mechanical validation, finite element method (FEM) analyses, and the impact of dynamic loading conditions. Through simulations and experiments, these models provide valuable insights into how design and material properties influence pressure distribution, stress behavior, and overall user experience in prosthetic applications. The outcomes contribute to advancing prosthetic socket design, enhancing user comfort, and improving functional performance, ultimately driving innovations in patient-centered prosthetic care.
5.1. Sensor Validation
Ensuring an optimal fit of transtibial prosthetic sockets is crucial for the comfort, functionality, and health of amputees. Recent advancements have focused on integrating sensor technologies to assess and enhance socket fit objectively. These innovations aim to monitor real-time interactions between the residual limb and the prosthetic socket, facilitating timely adjustments and improving overall user experience.
Adaptive prosthetic sockets are designed to automatically detect and adjust to changes in socket fit, often before the user becomes aware of any issues. A notable development in this area involves sockets equipped with pressure sensors and mechanical actuators that modify socket size in response to detected pressure variations. In a study by Sanders in 2024, an investigational prosthesis featured motor-driven adjustable panels controlled by a microcontroller [
26]. This system utilizes inductive sensors embedded in the socket to measure limb motion and calculate a socket fit metric, enabling real-time adjustments during ambulation. Participants reported increased daily step counts and reduced time spent adjusting the socket manually, indicating enhanced comfort and activity levels with the adaptive system.
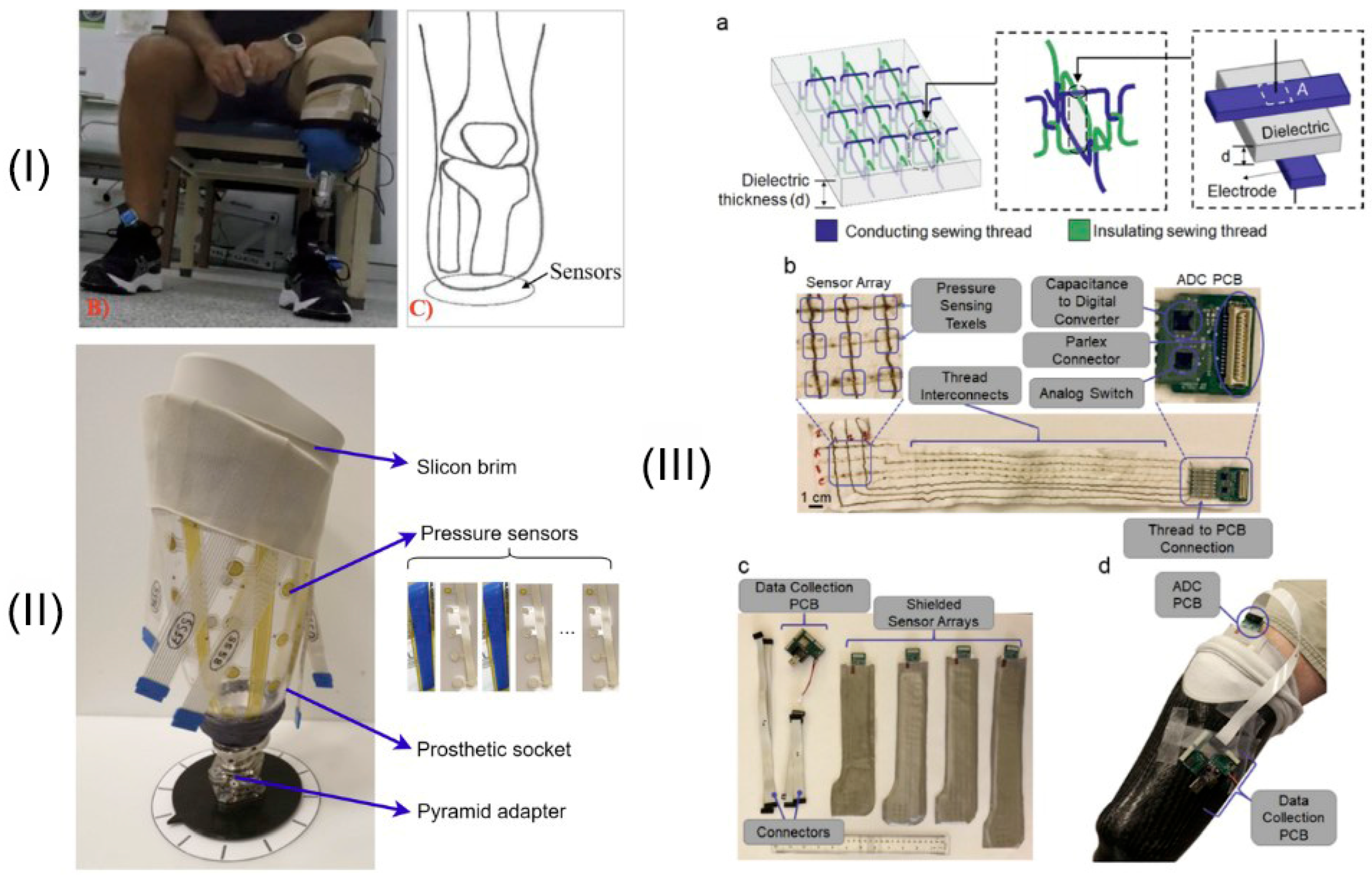
Accurate evaluation of interface pressure is critical for ensuring optimal fit during the fitting of transtibial prosthetic sockets. In 2023, Armitage developed and validated a custom-built wireless interface pressure measurement system designed to assist in improving the fitting of prosthetic check sockets. Their study demonstrated that the custom system produced reliable pressure measurements that were comparable to those obtained using commercial pressure sensors across static and dynamic loading conditions. This innovation presents a promising approach for enhancing the objectivity of socket fitting, offering clinicians an accessible tool to refine socket adjustments and potentially improve patient outcomes during the early stages of prosthetic rehabilitation [
80]. The system’s design, shown in
Figure 4I, emphasizes portability and real-time pressure capture, offering a solution for clinical environments.
Recent advances in prosthetic socket design have underscored the importance of understanding how minor changes in fit influence biomechanical forces at the limb-socket interface. In a study by Devin et al. [
83], the effects of socket fit alterations on pressure and shear distributions at a transtibial residuum/socket interface were systematically evaluated. Using experimental measurements, the researchers demonstrated that even slight deviations in socket volume can lead to localized increases in pressure and shear forces, particularly in vulnerable anatomical regions. These mechanical changes were found to have direct implications for user comfort and tissue health, highlighting the need for continuous monitoring and adaptive fitting strategies to optimize prosthetic performance.
When positioning the sensor between the stump and the liner in prosthetic systems, it was noted that significantly higher and even extreme pressure values were recorded compared to when the sensor was situated between the liner and the socket itself. This observation implies that the liner plays a crucial role in the even distribution of pressure within the socket and serves as a cushioning element, absorbing potential impacts during the use of the prosthetic device. These findings underscore the importance of liner design and placement in optimizing pressure distribution and user comfort in prosthetic applications [
84,
85,
86].
The field has seen significant progress in the development of measurement and sensing techniques aimed at evaluating prosthetic socket interfaces. A comprehensive review by Guerra [
87] highlights various technologies, including pressure sensors, shear sensors, and distance sensing devices, that have been explored to monitor socket fit and residual limb health. To reduce hardware complexity while preserving measurement quality, Zhu [
81] proposed a redundancy reduction method for sensor deployment, illustrated in
Figure 4II. Additionally, Tabor and Agcayazi [
82] developed a flexible textile-based pressure sensor array that integrates into the socket lining, enabling continuous and comfortable pressure monitoring, as shown in
Figure 4III. These technologies provide critical data that can inform the design of adaptive sockets and personalized fitting strategies, ultimately enhancing the comfort and functionality of prosthetic devices.
5.2. Mechanical Strength and Structural Integrity of Prosthetic Sockets
The mechanical strength and structural integrity of prosthetic sockets are critical factors in ensuring the safety and functionality of lower-limb prostheses. Various studies have evaluated different materials and fabrication methods to determine their impact on socket performance.
Gerschutz conducted a comprehensive evaluation comparing prosthetic check sockets, copolymer sockets, and definitive laminated sockets [
88]. Their findings indicated that check socket strengths were influenced by thickness, material choice, and fabrication method, while copolymer socket strengths depended on thickness and fabrication methods. Notably, a majority of the check sockets and all of the copolymer sockets failed to meet the ISO 10328 [
89] ductile loading criteria, whereas the strengths of definitive laminated sockets were more affected by construction material and technique. This study highlighted significant variability in socket performance across different facilities, underscoring the need for standardized fabrication practices.
In a systematic review, Gariboldi examined structural testing methods for lower-limb prosthetic sockets [
90]. The review revealed that while ISO 10328 is commonly adapted for socket testing, there is a lack of standardized guidelines specific to sockets. The authors emphasized the necessity for clear definitions of anatomical landmarks and socket axes to implement representative and repeatable test methods.
Further exploration studies have investigated factors affecting the static strength of lower-limb prosthetic sockets, including stratigraphy, distal adapter, and lamination resin [
91]. These studies aim to identify optimal combinations that meet strength requirements while minimizing weight. For instance, certain laminated sockets have been found to overcome ISO 10328 P6 loading levels and weigh less than 600 grams, suggesting potential pathways for optimizing socket design. Similarly, Nagarajan [
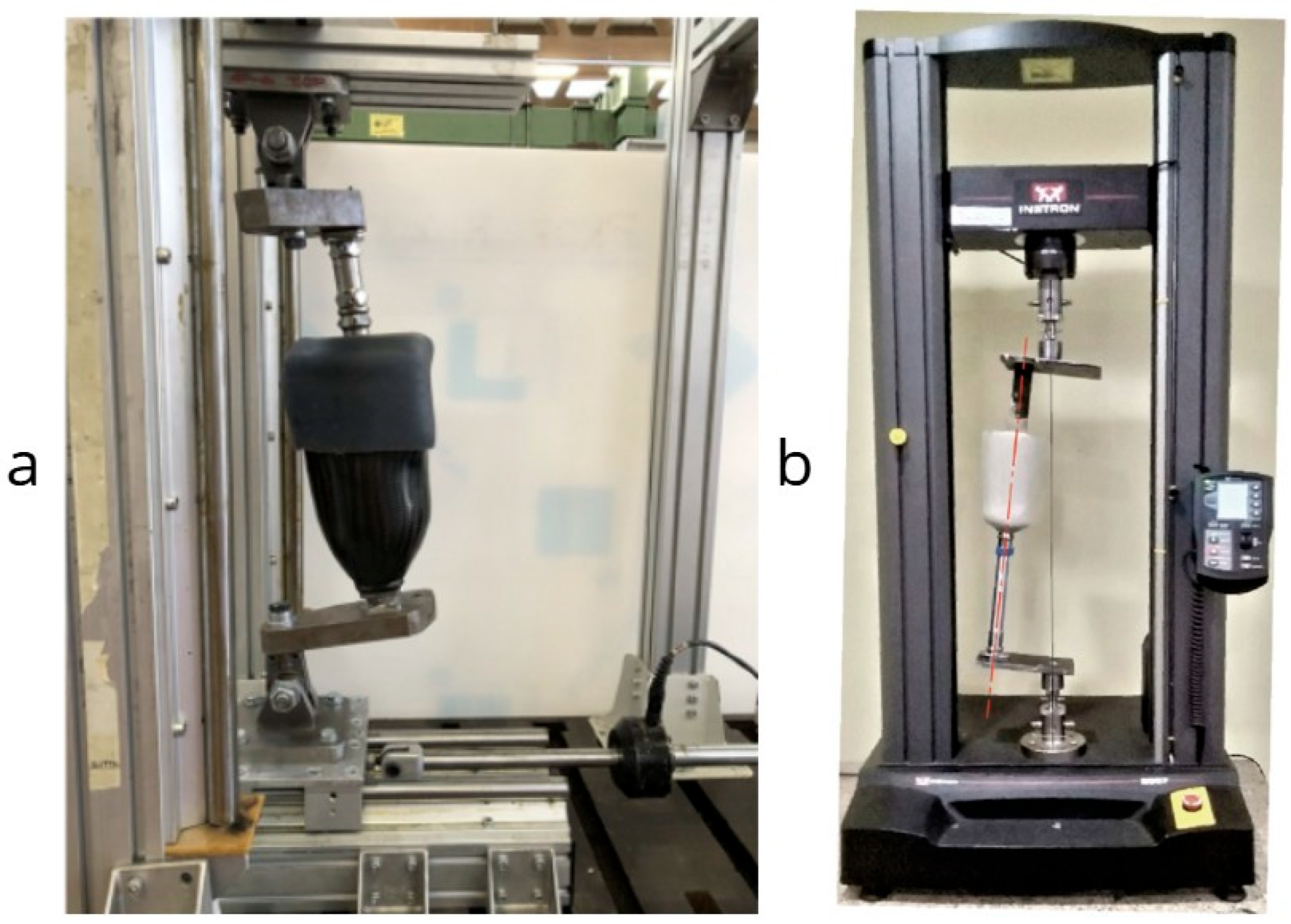
92] evaluated the compressive strength of transtibial sockets made from polyethylene terephthalate (PET) composites. Their study employed a universal testing machine setup to assess load-bearing capacity under ISO 10328-like conditions. The results demonstrated that PET composites offer an affordable yet structurally viable alternative for socket fabrication, withstanding loads comparable to traditional materials. Their methodology, alongside other structural testing rigs such as that used by Gariboldi [
91], is illustrated in
Figure 5.
The advent of 3D printing technology has introduced new possibilities in prosthetic socket fabrication. The authors of [
93] explored the strength testing of definitive transtibial prosthetic sockets made using 3D-printing technology. Through an iterative design process with integral structural testing, the study demonstrated that it is possible to produce strong, durable prosthetic sockets using 3D-printing methods. However, the authors noted that further research is needed to ensure these sockets can withstand the mechanical demands of daily use.
Collectively, these studies underscore the importance of rigorous mechanical testing and standardized protocols in the design and fabrication of prosthetic sockets. As new materials and technologies emerge, ongoing research and development are essential to ensure that prosthetic sockets provide both safety and comfort for users.
5.3. FEM-Based Design
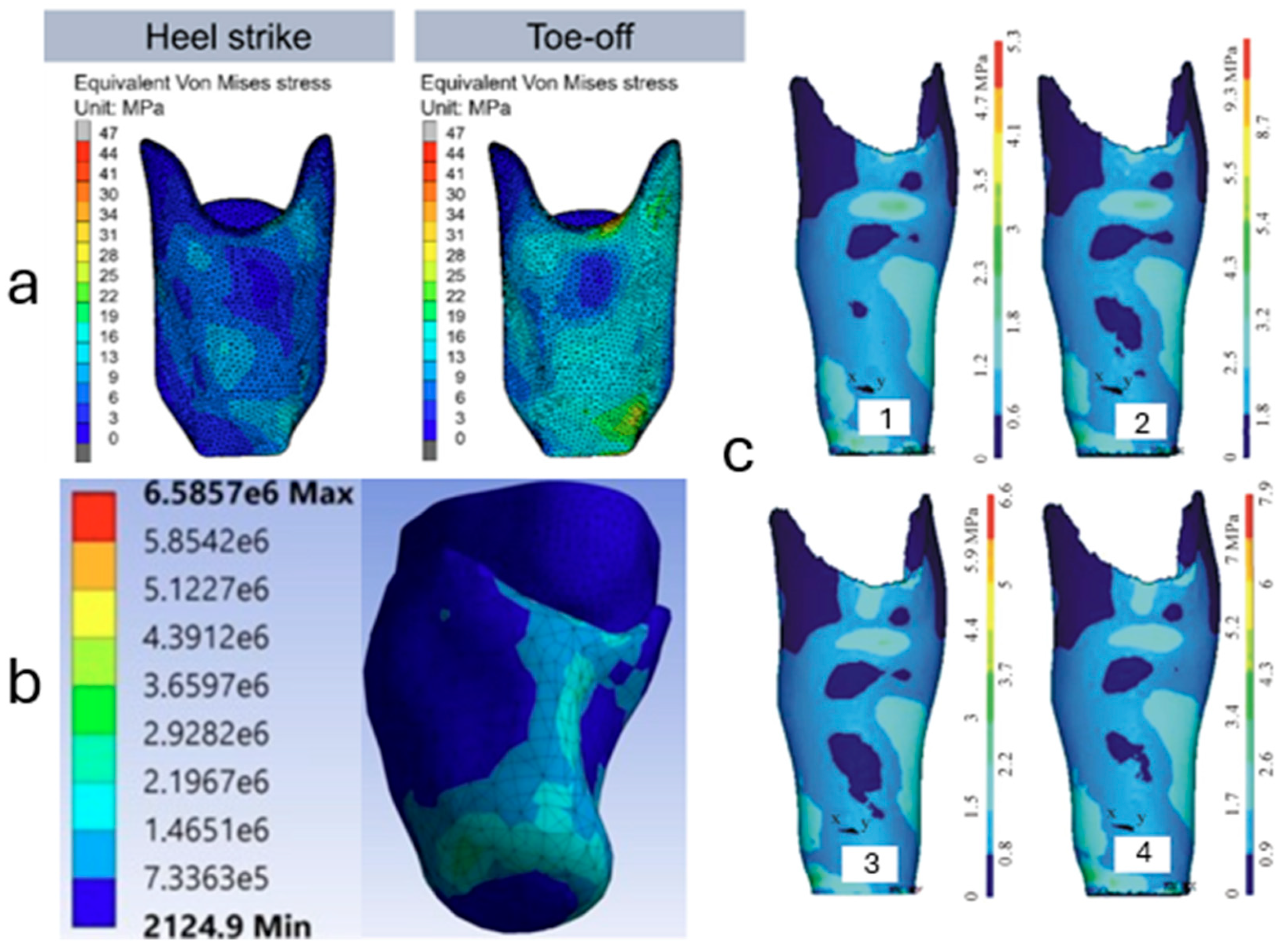
Finite Element Modeling (FEM) has become an indispensable tool in the design and optimization of transtibial prosthetic sockets, providing detailed insights into stress distributions and mechanical behavior at the limb-socket interface. Recent advancements in FEM applications have focused on creating more accurate and patient-specific models, incorporating complex geometries, material properties, and loading conditions to enhance prosthetic fit and comfort.
Traditional FEM studies often relied on simplified geometries and linear material assumptions, which could limit the accuracy of stress predictions. To address these limitations, researchers have developed more sophisticated models that capture the intricate anatomy of the residual limb and the nonlinear behavior of soft tissues. For instance, Sengeh in 2016 utilized MRI data to construct a threedimensional, multi-material viscoelastic model of a transtibial residuum. This model incorporated detailed tissue boundaries and nonlinear elastic properties, enabling a more precise simulation of limb-socket interactions under various loading scenarios. The study demonstrated that such comprehensive modeling could predict force-time responses with a mean percentage error of 7%, highlighting the potential for improved socket designs adapted to individual patients [
49].
Accurate representation of soft tissue mechanics is crucial for reliable FEM analyses. Recent efforts have focused on in vivo characterization of residual limb tissues to inform model parameters. Ranger introduced a novel approach combining ultrasound indentation and shear wave elastography to measure local shear modulus variations across different anatomical regions of the residual limb. This method allows for the identification of constitutive parameters that reflect regional differences in tissue properties, such as scarring and atrophy, which are essential for patient-specific FEM simulations. Incorporating these detailed material properties into FEM can lead to more accurate predictions of interface pressures and internal tissue strains, ultimately guiding the design of more comfortable and effective prosthetic sockets [
94].
The integration of FEM with additive manufacturing techniques has opened new avenues for the rapid prototyping and testing of prosthetic sockets. Van der Stelt et al. conducted a study where Fused Filament Fabrication (FFF) was used to produce transtibial prosthetic sockets, which were then evaluated through both long-term clinical follow-up and FEM analysis. The FEM simulations identified high-stress regions, particularly in the patella tendon area, leading to design modifications such as increasing wall thickness in critical areas. These adjustments resulted in improved durability and user satisfaction, demonstrating the efficacy of combining FEM-driven design with additive manufacturing to create patient-specific prosthetic solutions [
95]. Additional insights into stress behavior under dynamic loading were provided by Lenka and Choudhury [
96], who used FEM simulations to analyze Von Mises stress distributions in transtibial sockets during different phases of gait. Their study highlighted stress variations across stance phases and emphasized the impact of material and wall thickness selection on load distribution and socket durability. Similarly, Gubbala and Inala [
97] developed a patient-specific socket using CT imaging and FEM analysis to simulate the stress interaction at the stump–socket interface. Their findings support the notion that personalized modeling contributes significantly to better load management and improved comfort. These modeling approaches are shown in
Figure 6, showcasing the evolving complexity and resolution of FEM applications in socket design, reinforcing their value in optimizing both material selection and anatomical fit.
Despite significant progress, several challenges persist in the application of FEM to prosthetic socket design. One major hurdle is the accurate modeling of the complex, nonlinear, and viscoelastic behavior of biological tissues under dynamic loading conditions. Additionally, the interfacial interactions between the residual limb and socket, including frictional effects and potential relative motion, require further investigation to enhance model fidelity. Future research should aim to develop more comprehensive models that incorporate patient-specific anatomical and material data, possibly through advanced imaging techniques and in vivo measurements. Such models could serve as predictive tools in the prosthetic fitting process, enabling the customization of socket designs to individual needs and improving overall prosthetic function and comfort [
98,
99].
5.4. Loading Conditions Trend
Employing Finite Element Analysis (FEA), the intricate interaction between residual limbs and prosthetic sockets was examined, particularly under the dynamic loads encountered during the gait cycle. The study involved the digital reconstruction of the residual limbs and sockets of fourteen transfemoral amputees. Elastic properties were assigned to both the socket and femur, while bulk soft tissues were modeled as hyperelastic. The models were created to simulate various phases, including donning, standing, and the gait cycle. The analysis revealed a noteworthy 23% ± 19% shift in maximum stress values and an expansion of stress-bearing regions when comparing the effects of dynamic gait loads to static standing loads. This research also explored the potential correlation between comfort and the positions of peak load at the interface between the residual limb and the socket, suggesting possible links between high-stress areas and regions that are either pressure-tolerant or pressure-sensitive [
100].
In addressing the balance and fall risks faced by patients with lower limb amputations due to the absence of proprioception, current methods employ force-sensing resistors and vibrotactile feedback systems. However, a novel research initiative explores the utilization of a liquid-filled elastomeric sensor for the acquisition of proprioceptive data during the gait cycle. The study, which includes simulations of a transtibial prosthesis, highlights a specific area at the ankle-front intersection characterized by significant deformation. Both static and dynamic analyses revealed a deformation value of 0.02 m/m. This research suggests the potential incorporation of an elastomeric strain sensor in this region as a means to enhance proprioception in future prosthetic applications [
101].
6. Discussion
Prosthetic sockets exert significant external forces on the residual limb, impacting mobility, functionality, and patient satisfaction. Achieving effective pressure distribution within the socket is critical to minimizing discomfort and preventing skin complications. This literature review underscores the importance of differentiating between pressure-tolerant and pressure-sensitive regions. While most of the residual limb can tolerate certain pressures, highly sensitive areas, such as bony prominences and post-surgical scars, require careful pressure relief to avoid complications.
The categorization of transtibial socket designs, such as PTB, PTB SC, and PTB SC SP, demonstrates the diversity of solutions available to address the varying needs of amputees. For example, while the PTB designs are suitable for medium and long stumps, the SSS (Silicon Suction Socket) design offers more even weight distribution across the stump’s surface. Its versatility in accommodating different stump configurations shows promise for increased comfort and improved performance.
Innovative sensor technologies, such as the strain-gauged load cell system used to measure pressure distribution, provide new insights into the biomechanical performance of different socket designs. These findings indicate that sockets like the ischial containment design yield a more uniform pressure distribution compared to more traditional quadrilateral sockets, which can help inform future prosthetic designs.
The study also highlights how advanced imaging techniques, such as finite element analysis (FEA), can provide valuable data for optimizing socket design. By simulating various stress scenarios on the residual limb during dynamic activities like walking, researchers can better understand how socket designs affect patient comfort and performance. Such computational approaches have the potential to improve the fitting process and enhance patient outcomes while reducing the time required for socket adjustments.
Despite these advancements, challenges remain, particularly in areas like heat retention, material limitations, and skin-related issues. The persistence of skin complications, even with advanced designs such as ICEROSS, indicates that more research is needed to address friction, shear forces, and pressure-related injuries. Moreover, limb volume fluctuations present a continuing challenge, affecting socket fit and comfort over time.
The prosthetics field includes a variety of settings, spanning military institutions, the National Health Service (NHS), and private clinics. Notably, clinicians have observed service disparities among these settings, with private and military contexts often delivering higher-quality sockets but subjecting patients to longer waiting times in comparison to the NHS. A challenge in this is the continuity of care, which can be delayed by management changes and the presence of temporary clinicians, impeding the seamless sharing of patient medical histories. To mitigate these issues, collaborative efforts between physiotherapists and prosthetists have proven to be valuable [
102].
Evaluating prosthetic socket performance from the user’s perspective remains a key component in prosthetic design improvement. Hanspal introduced the Prosthetic Socket Fit Comfort Score (SCS), highlighting the importance of systematically quantifying user comfort to better tailor socket fitting processes. Their findings emphasize that even small adjustments in socket fit can significantly affect patient satisfaction and functionality [
103]. Complementing this, Hagberg and Brånemark demonstrated that advances in prosthetic technology, particularly in socket design and interface quality, are directly correlated with improved quality of life among amputees, reinforcing the clinical value of individualized approaches [
104]. Furthermore, Young reviewed emerging measurement techniques for the prosthetic socket interface, underscoring the need for dynamic, real-time assessment tools to capture the complex interactions between the residual limb and socket during daily activities [
105]. Together, these studies suggest that integrating quantitative comfort assessments with technological advancements in socket design could substantially enhance patient-reported outcomes and long-term prosthesis use.
A qualitative survey conducted among 12 Malaysian below-knee amputees reported that durability and comfort were rated as the most important features by 83% of participants, while the esthetic appearance of the socket material was a key factor for 67% of users. Overall satisfaction (satisfied or somewhat satisfied) was reported by 67% of participants, underlining the importance of design quality in supporting user mobility and adherence to prosthesis use [
106]. A UK-based survey including 94 amputees and clinicians revealed that 48% of amputees and 66% of clinicians identified socket fit as the largest challenge during rehabilitation. Amputees emphasized impacts on daily activities and quality of life, while clinicians highlighted limitations in socket adjustability and gait training. The differing perceptions suggest a need for patient-centered fit criteria, as “socket fit does not mean the same for all patients” [
107].
Common challenges encountered in prosthetic care involve the impact of limb shape changes on socket fit, leading to discomfort. Skin-related problems are mainly heating retention and moisture buildup due to non-breathable socket materials, while adherent scars and tissue stiffness can cause pain and skin breakdown. The success of rehabilitation also depends on psychological factors and patient motivation.
Miss-adjusted sockets carry physical health implications, including discomfort, skin breakdown, and pressure sores. Prolonged use of such sockets can even negatively influence the body’s biomechanics and musculoskeletal health. Prosthetic-related irritations are also complex to resolve, primarily due to constraints imposed by socket design, as the rigid socket is unable to accommodate limb size fluctuations adequately. Liners, though essential, can worsen issues, particularly concerning heat and sweat buildup. Establishing a strong clinician-patient rapport, crucial for effective care, often faces time limitations within clinical settings.
Furthermore, the practice of prosthetic care faces limitations such as increased demands on clinician-patient interaction time, a lack of standardized approaches for problem-solving, and limited follow-up in the NHS after initial goals are met, which can pose challenges when adapting to evolving goals and lifestyle changes [
100].
Collaboration between prosthetists, engineers, and clinicians will be essential to overcome these limitations. Integrating patient feedback into the design process can also lead to more personalized solutions that address individual biomechanical needs. Furthermore, increasing access to affordable, customizable prosthetic solutions, especially in low-resource settings, should be a priority in future research and development efforts.
7. Conclusions
Innovations in socket design for prosthetic limbs have the potential to transform clinical practices, biomechanics research, and patient outcomes. By prioritizing pressure relief, materials selection, and computational modeling, designers and clinicians can optimize socket comfort, functionality, and accessibility. Collaborative efforts between healthcare professionals and ongoing research initiatives are essential for addressing existing challenges and advancing prosthetic care, ultimately enhancing the quality of life for individuals with limb loss.
Prosthetic socket design is at the intersection of technology, biomechanics, and patient care. Recent advancements hold the promise of improved comfort, accessibility, and overall quality of life for amputees. However, these innovations must be tempered with a recognition of their limitations and the indispensable role of skilled prosthetists. As we continue to push the boundaries of prosthetic socket design, our goal remains clear: to enhance the lives of those who rely on these life-changing devices. The text underscores the complexity and importance of prosthetic socket design, where considerations of pressure distribution, sensitivity, and individual patient needs are paramount. It also highlights the significance of research and technology in improving the design and functionality of prosthetic sockets to enhance the lives of amputees.
Despite significant progress in prosthetic socket design, several challenges persist, including residual limb volume fluctuations, skin irritation, and long-term durability concerns. Future research must focus on integrating advanced materials, pressure-sensing technologies, and AI-driven customization to enhance socket performance. By leveraging interdisciplinary approaches and incorporating user feedback, the field can continue to evolve toward more adaptive, comfortable, and efficient prosthetic solutions.
8. Future Directions
The advancements in prosthetic socket design significantly impact rehabilitation outcomes for individuals experiencing limb loss. Effective rehabilitation is closely linked to the comfort, functionality, and overall fit of prosthetic sockets, emphasizing that socket design is not only about mechanical stability but also influences users’ daily experiences and long term health.
One critical factor is identifying which parts of the residual limb can comfortably bear pressure and which are sensitive and prone to skin issues. Poor pressure management can cause skin breakdown, discomfort, ulcers, and chronic pain, which frequently lead to reduced prosthesis usage or abandonment. Conversely, well-designed sockets such as ischial containment or silicon suction sockets (SSS) distribute pressure evenly, reducing complications, improving walking efficiency, and promoting regular prosthetic use. Rehabilitation professionals must thoroughly understand these design principles and incorporate them into patient education and clinical care.
Emerging technologies such as finite element analysis (FEA) and real-time pressure sensors are transforming rehabilitation practices. These tools provide detailed, actionable data, allowing clinicians to identify areas of high pressure and discomfort and proactively adjust socket fittings. This approach reduces trial and error adjustments, accelerates the fitting process, and enhances rehabilitation effectiveness.
Finally, the integration of patient feedback through structured assessments such as the Socket Comfort Score (SCS) gives useful insights. Regular use of these assessment tools can ensure clinical decisions align closely with patient comfort and individual needs.
In summary, effective prosthetic rehabilitation depends on integrating innovative technologies, clinical expertise, and direct patient input. By prioritizing comfort, psychological support, and consistent follow-up care, clinicians can greatly improve prosthetic socket performance, leading to enhanced quality of life for individuals with limb loss.