Effects of Artificial Achilles Tendon on Hindlimb Movement Biomechanics and Muscle Morphology in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Artificial Tendon
2.2. Animal Handling and Surgery
2.3. Data Collection
2.4. Data Analysis
3. Results
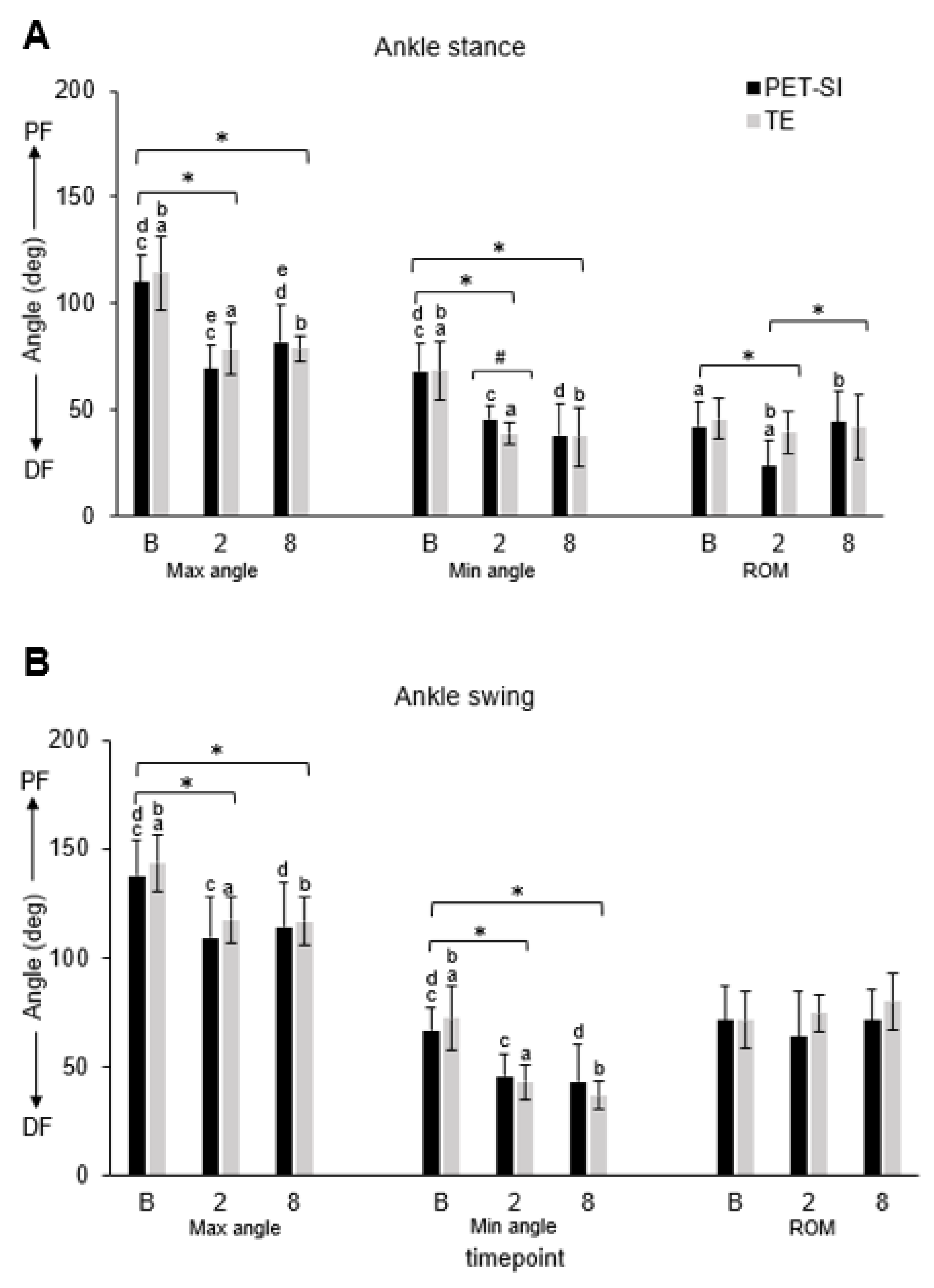
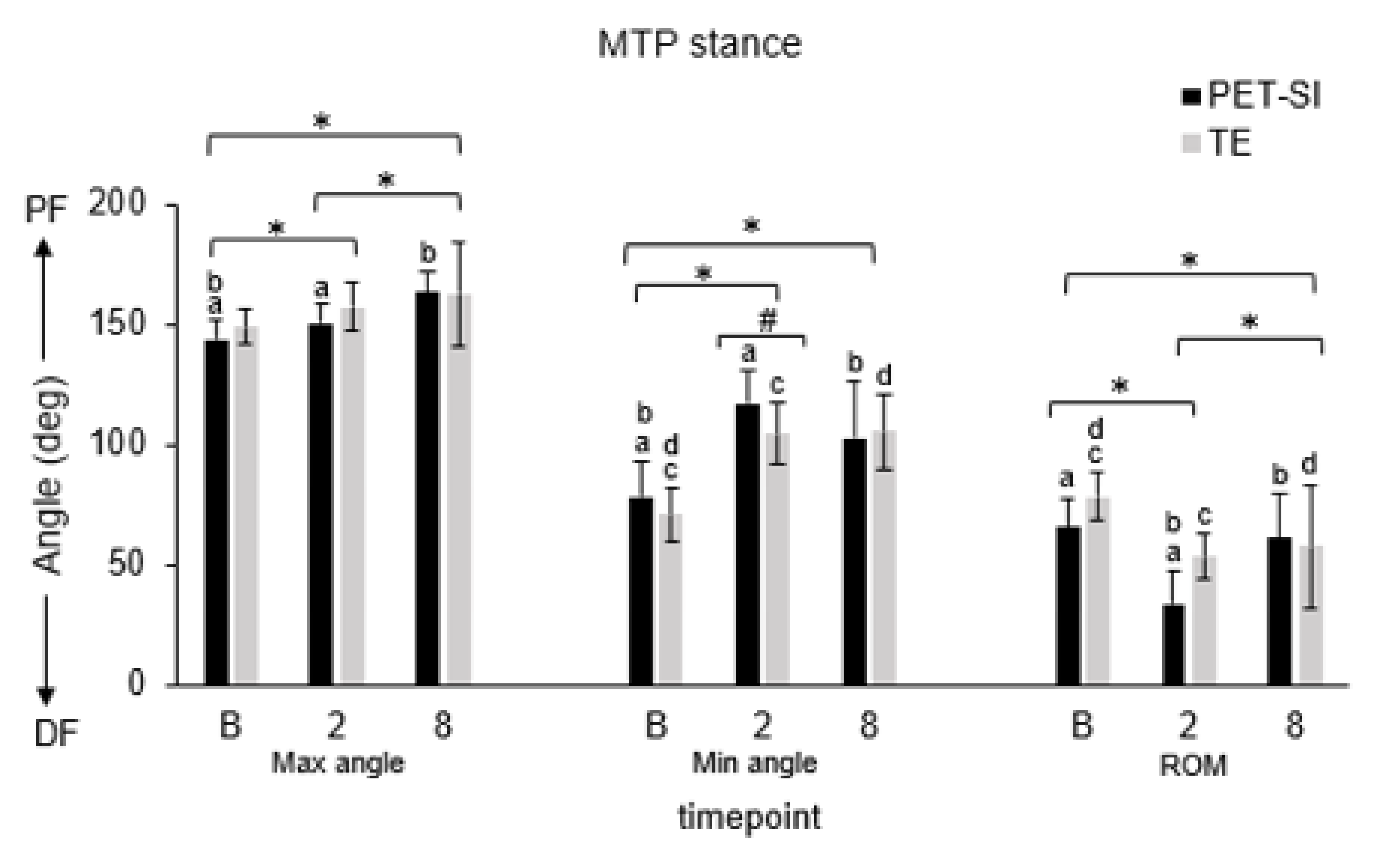
3.1. Biomechanics: Kinematics
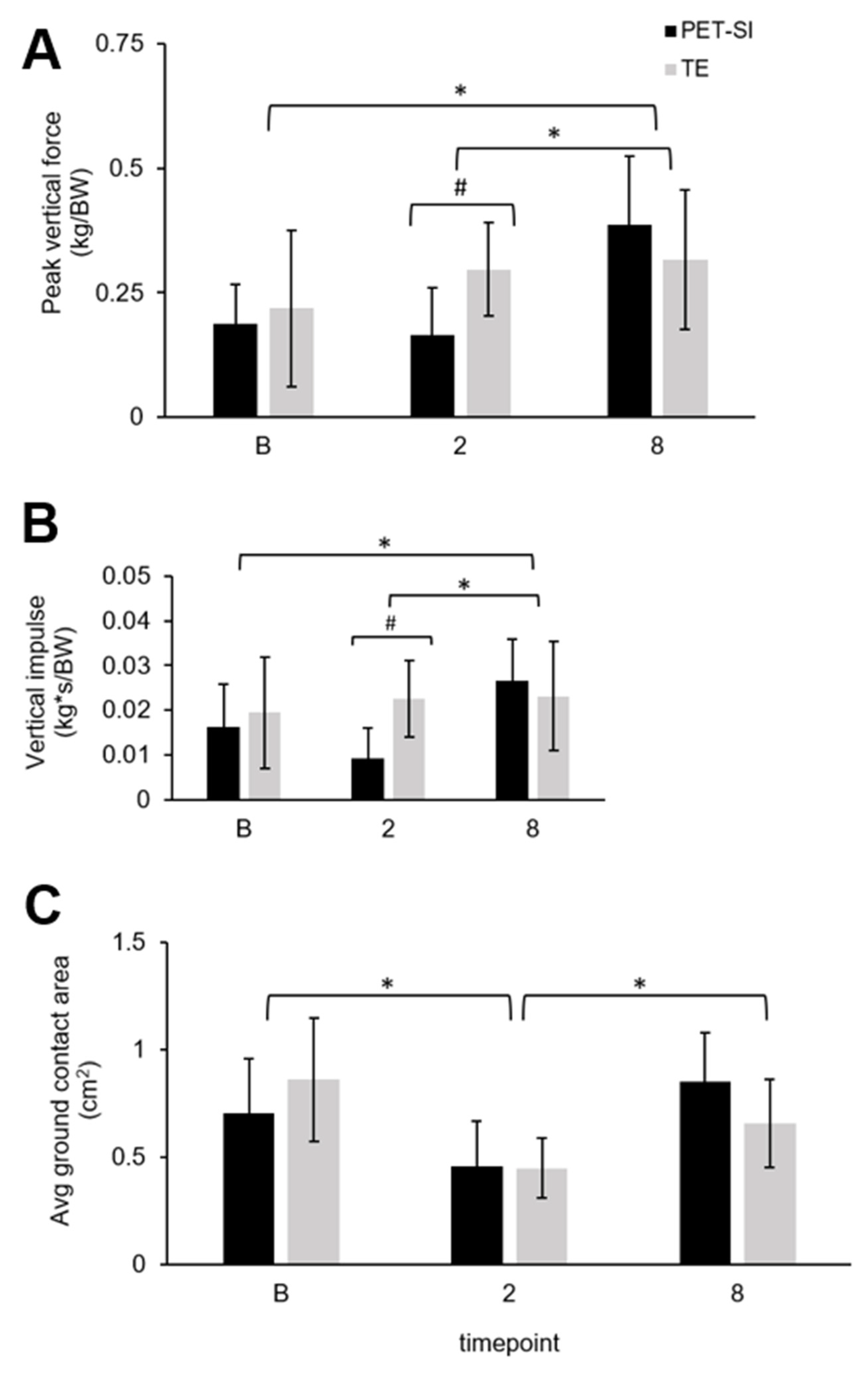
3.2. Biomechanics: Pressure Data
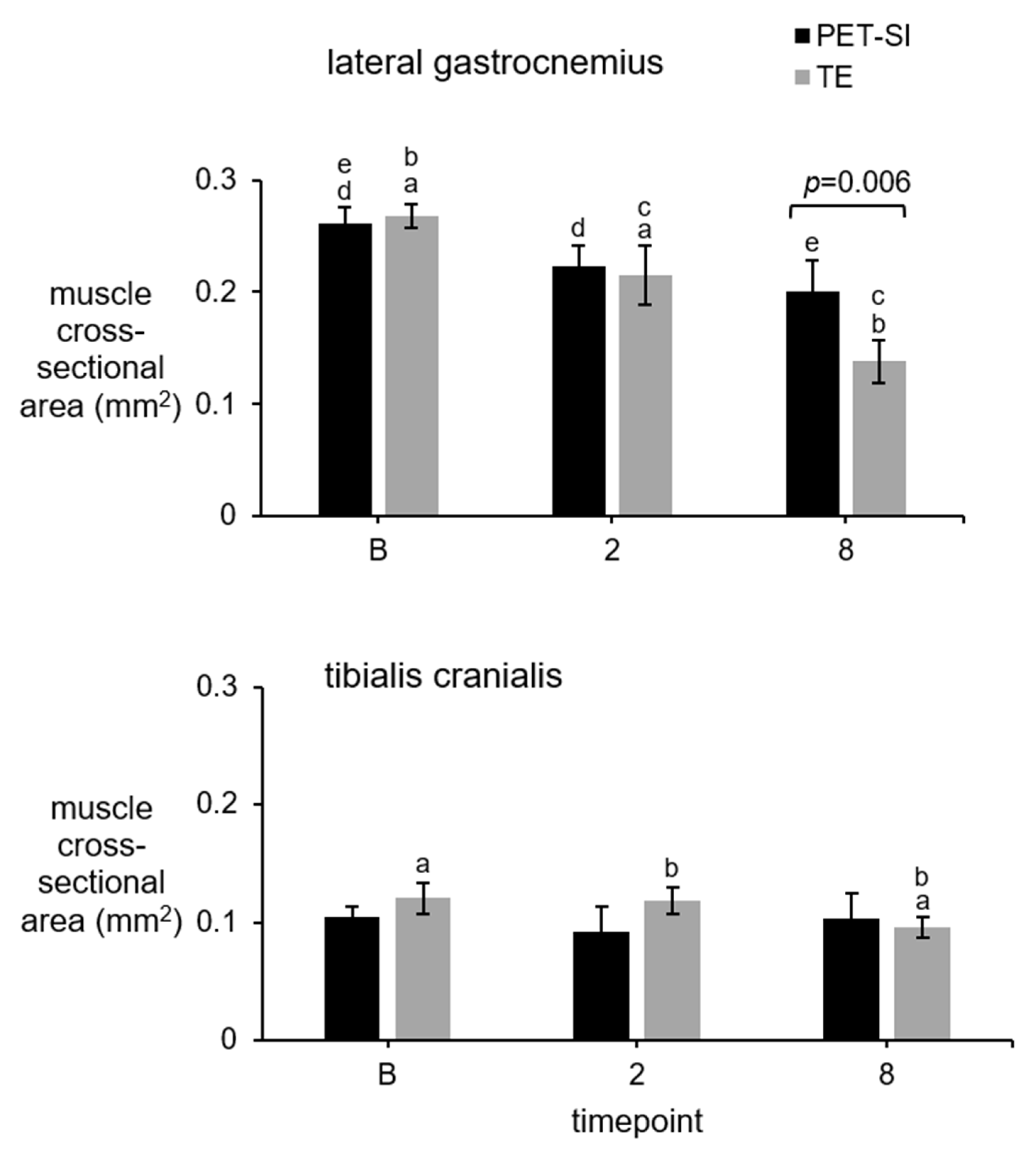
3.3. Muscle Cross-Sectional Area (CSA)
3.4. Muscle Mass and Length
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, L.; Yin, L.; Duan, X. Surgical Strategy for the Chronic Achilles Tendon Rupture. BioMed Res. Int. 2016, 2016, 14169–14171. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Jones, K.; Raikin, S.M. Treatment of Chronic Achilles Tendon Ruptures With Large Defects. Foot Ankle Spec. 2016, 9, 400–408. [Google Scholar] [CrossRef]
- Erickson, B.J.; Cvetanovich, G.L.; Nwachukwu, B.U.; Villarroel, L.D.; Lin, J.L.; Bach, B.R.; McCormick, F.M. Trends in the Management of Achilles Tendon Ruptures in the United States Medicare Population, 2005–2011. Orthop. J. Sports Med. 2014, 2, 2325967114549948. [Google Scholar] [CrossRef]
- Hardy, A.; Casabianca, L.; Andrieu, K.; Baverel, L.; Noailles, T. Complications following harvesting of patellar tendon or hamstring tendon grafts for anterior cruciate ligament reconstruction: Systematic review of literature. Orthop. Traumatol. Surg. Res. 2017, 103, S245–S248. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.R.; Mameri, E.S.; Tuthill, T.; Wessels, M.; Asif, S.; Sugrañes, J.; Batra, A.K.; McCormick, J.R.; Obioha, O.A.; Kaplan, D.J.; et al. Adverse Events and Complications After Primary ACL Reconstruction With Quadriceps Tendon Autograft: A Systematic Review. Orthop. J. Sports Med. 2023, 11, 23259671231199728. [Google Scholar] [CrossRef]
- Shearn, J.T.; Kinneberg, K.R.; Dyment, N.A.; Galloway, M.T.; Kenter, K.; Wylie, C.; Butler, D.L. Tendon tissue engineering: Progress, challenges, and translation to the clinic. J. Musculoskelet. Neuronal Interact. 2011, 11, 163–173. [Google Scholar]
- Maffulli, N.; Bartoli, A.; Sammaria, G.; Migliorini, F.; Karlsson, J.; Oliva, F. Free tendon grafts for surgical management of chronic tears of the main body of the Achilles tendon: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 4526–4538. [Google Scholar] [CrossRef]
- Melvin, A.; Litsky, A.; Mayerson, J.; Witte, D.; Melvin, D.; Juncosa-Melvin, N. An artificial tendon with durable muscle interface. J. Orthop. Res. 2010, 28, 218–224. [Google Scholar] [CrossRef]
- Melvin, A.; Litsky, A.; Mayerson, J.; Stringer, K.; Melvin, D.; Juncosa-Melvin, N. An artificial tendon to connect the quadriceps muscle to the tibia. J. Orthop. Res. 2011, 29, 1775–1782. [Google Scholar] [CrossRef]
- Easton, K.L.; Hatch, C.; Stephens, K.; Marler, D.; Fidelis, O.; Sun, X.; Bowers, K.M.; Billings, C.; Greenacre, C.B.; Anderson, D.E.; et al. Replacement of tibialis cranialis tendon with polyester, silicone-coated artificial tendon preserves biomechanical function in rabbits compared to tendon excision only. J. Orthop. Surg. Res. 2024, 19, 108. [Google Scholar] [CrossRef]
- Murray, G.A.W.; Semple, J.C. A review of work on artificial tendons. J. Biomed. Eng. 1979, 1, 177–184. [Google Scholar] [CrossRef]
- Melvin, A.J.; Litsky, A.S.; Mayerson, J.L.; Stringer, K.; Juncosa-Melvin, N. Extended healing validation of an artificial tendon to connect the quadriceps muscle to the Tibia: 180-day study. J. Orthop. Res. 2012, 30, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Melvin, D.B.; Klosterman, B.; Gramza, B.R.; Byrne, M.T.; Weisbrode, S.L.; Litsky, A.S.; Clarson, S.J. A durable load bearing muscle to prosthetic coupling. Asaio J. 2003, 49, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology—A minireview of basic concepts and recent advancements. J. Hand Ther. 2012, 25, 133–140; quiz 141. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, D.T.; Garrett, W.E. Function and biomechanics of tendons. Scand. J. Med. Sci. Sports 1997, 7, 62–66. [Google Scholar] [CrossRef]
- Jin, Z.; Dowson, D. Bio-friction. Friction 2013, 1, 100–113. [Google Scholar] [CrossRef]
- Zajac, F.E. Muscle and tendon: Properties, models, scaling, and application to biomechanics and motor control. Crit. Rev. Biomed. Eng. 1989, 17, 359–411. [Google Scholar]
- Lieber, R.L. Skeletal muscle architecture: Implications for muscle function and surgical tendon transfer. J. Hand Ther. 1993, 6, 105–113. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, H.S.; Young, K.W.; Seo, S.G. Treatment of Acute Achilles Tendon Rupture. Clin. Orthop. Surg. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Ponkilainen, V.; Kuitunen, I.; Liukkonen, R.; Vaajala, M.; Reito, A.; Uimonen, M. The incidence of musculoskeletal injuries: A systematic review and meta-analysis. Bone Jt. Res. 2022, 11, 814–825. [Google Scholar] [CrossRef]
- Wu, F.; Nerlich, M.; Docheva, D. Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2017, 2, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Rawson, S.; Cartmell, S.; Wong, J. Suture techniques for tendon repair; a comparative review. Muscles Ligaments Tendons J. 2013, 3, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Jardaly, A.; Kiel, J. Anatomy, Bony Pelvis and Lower Limb: Achilles Tendon; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Speedtsberg, M.B.; Kastoft, R.; Barfod, K.W.; Penny, J.; Bencke, J. Gait Function and Postural Control 4.5 Years After Nonoperative Dynamic Treatment of Acute Achilles Tendon Ruptures. Orthop. J. Sports Med. 2019, 7, 2325967119854324. [Google Scholar] [CrossRef]
- Lehr, N.L.; Clark, W.H.; Lewek, M.D.; Franz, J.R. The effects of triceps surae muscle stimulation on localized Achilles subtendon tissue displacements. J. Exp. Biol. 2021, 224, jeb242135. [Google Scholar] [CrossRef]
- Neptune, R.R.; Kautz, S.A.; Zajac, F.E. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J. Biomech. 2001, 34, 1387–1398. [Google Scholar] [CrossRef]
- Noskovicova, N.; Hinz, B.; Pakshir, P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 2021, 10, 1794. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.P.; Koike, Y.; Uhthoff, H.K.; Lecompte, M.; Trudel, G. Comparative anatomy of rabbit and human achilles tendons with magnetic resonance and ultrasound imaging. Comp. Med. 2006, 56, 68–74. [Google Scholar]
- Hall, P.T.; Stubbs, C.; Pedersen, A.P.; Billings, C.; Stephenson, S.M.; Greenacre, C.B.; Anderson, D.E.; Crouch, D.L. Gait biomechanics of rabbits with either Achilles or tibialis cranialis artificial tendons. bioRxiv 2022. [Google Scholar] [CrossRef]
- Fidelis, O.P.; Stubbs, C.; Easton, K.L.; Billings, C.; Pedersen, A.P.; Anderson, D.E.; Crouch, D.L. Attaching artificial Achilles and tibialis cranialis tendons to bone using suture anchors in a rabbit model: Assessment of outcomes. PeerJ 2025, 13, e18756. [Google Scholar] [CrossRef]
- Lichtwark, G.A.; Wilson, A.M. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J. Exp. Biol. 2005, 208 Pt 24, 4715–4725. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.R.; Pfister, T.; MacIntosh, B.R. Energy cost of running and achilles tendon stiffness in man and woman trained runners. Physiol. Rep. 2013, 1, e00178. [Google Scholar] [CrossRef] [PubMed]
- Lichtwark, G.A.; Wilson, A.M. Is Achilles tendon compliance optimised for maximum muscle efficiency during locomotion? J. Biomech. 2007, 40, 1768–1775. [Google Scholar] [CrossRef]
- Khair, R.A.M.; Stenroth, L.; Cronin, N.J.; Reito, A.; Paloneva, J.; Finni, T. Muscle-tendon morphomechanical properties of non-surgically treated Achilles tendon 1-year post-rupture. Clin. Biomech. 2022, 92, 105568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Garofalo, J.; Abuhantash, M.; McRae, S.; MacDonald, P.; Longstaffe, R.; Ogborn, D. Functional Performance and Tendon Morphology After Operative or Nonoperative Treatment of Achilles Tendon Ruptures. Int. J. Sports Phys. Ther. 2024, 19, 670–680. [Google Scholar] [CrossRef]
- Hoeffner, R.; Agergaard, A.-S.; Svensson, R.B.; Cullum, C.; Mikkelsen, R.K.; Konradsen, L.; Krogsgaard, M.; Boesen, M.; Kjaer, M.; Magnusson, S.P. Tendon Elongation and Function After Delayed or Standard Loading of Surgically Repaired Achilles Tendon Ruptures: A Randomized Controlled Trial. Am. J. Sports Med. 2024, 52, 1022–1031. [Google Scholar] [CrossRef]
- Koh, T.J.; Herzog, W. Increasing the moment arm of the tibialis anterior induces structural and functional adaptation: Implications for tendon transfer. J. Biomech. 1998, 31, 593–599. [Google Scholar] [CrossRef]
- Laurent, D.; Walsh, L.; Muaremi, A.; Beckmann, N.; Weber, E.; Chaperon, F.; Haber, H.; Goldhahn, J.; Klauser, A.S.; Blauth, M.; et al. Relationship between tendon structure, stiffness, gait patterns and patient reported outcomes during the early stages of recovery after an Achilles tendon rupture. Sci. Rep. 2020, 10, 20757. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Tsujii, A.; Nakamura, N.; Mitsuoka, T. Ultrasonographic Evaluation of the Early Healing Process After Achilles Tendon Repair. Orthop. J. Sports Med. 2018, 6, 2325967118789883. [Google Scholar] [CrossRef]
- Mashimo, S.; Nozaki, T.; Amaha, K.; Tanaka, K.; Kubota, J.; Sato, H.; Kitamura, N. Quantitative Assessment of Calf Muscle Volume, Strength, and Quality After Achilles Tendon Rupture Repair: A 1-Year Prospective Follow-up Study. Am. J. Sports Med. 2023, 51, 3781–3789. [Google Scholar] [CrossRef]
- Yoneno, M.; Minegishi, Y.; Takahashi, H.; Takahata, K.; Miyamoto, H.; Usami, Y.; Kokubun, T. Muscle Contraction Is Essential for Tendon Healing and Muscle Function Recovery After Achilles Tendon Rupture and Surgical Repair. J. Orthop. Res. 2025, 43, 746–755. [Google Scholar] [CrossRef] [PubMed]

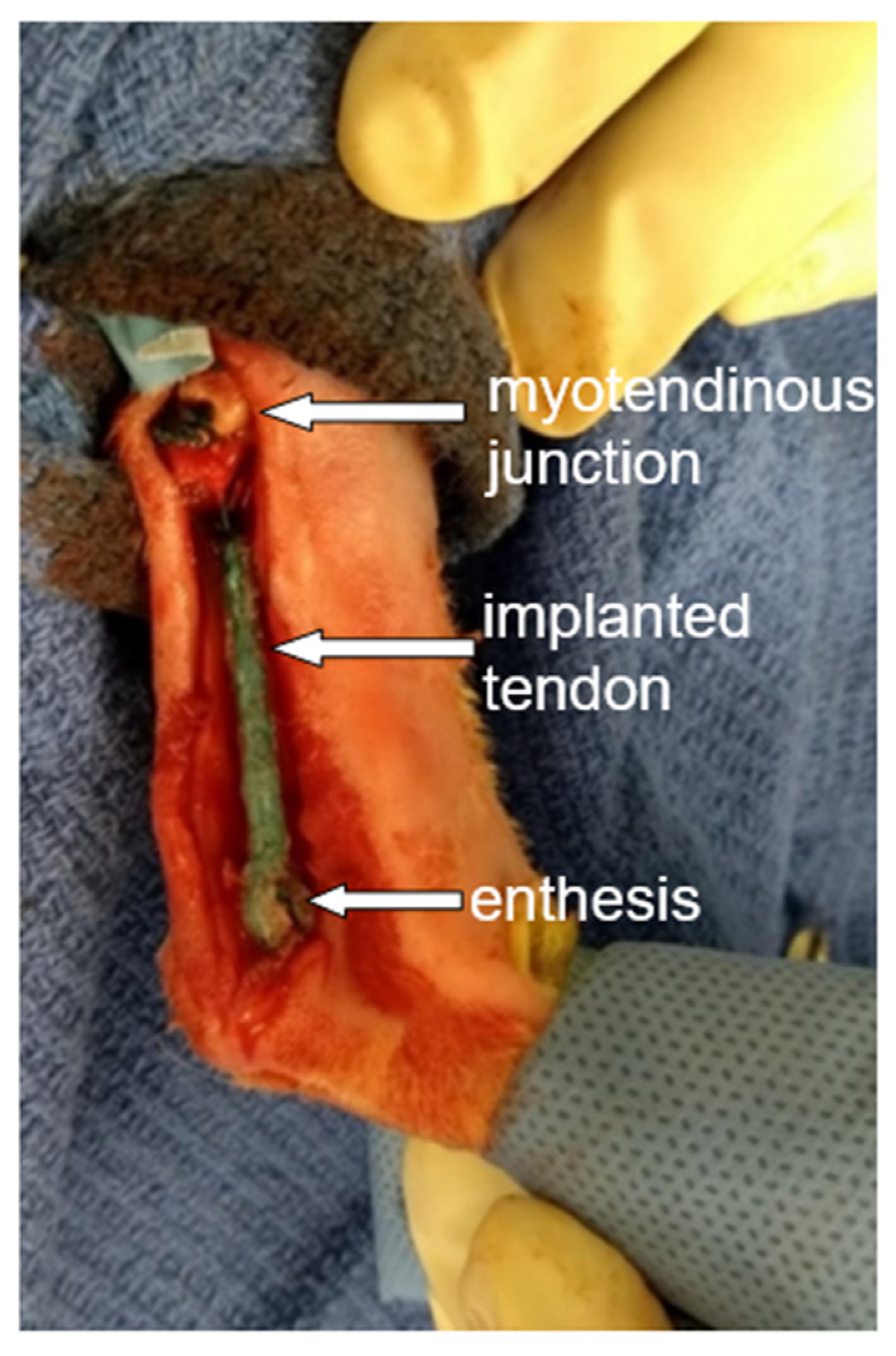

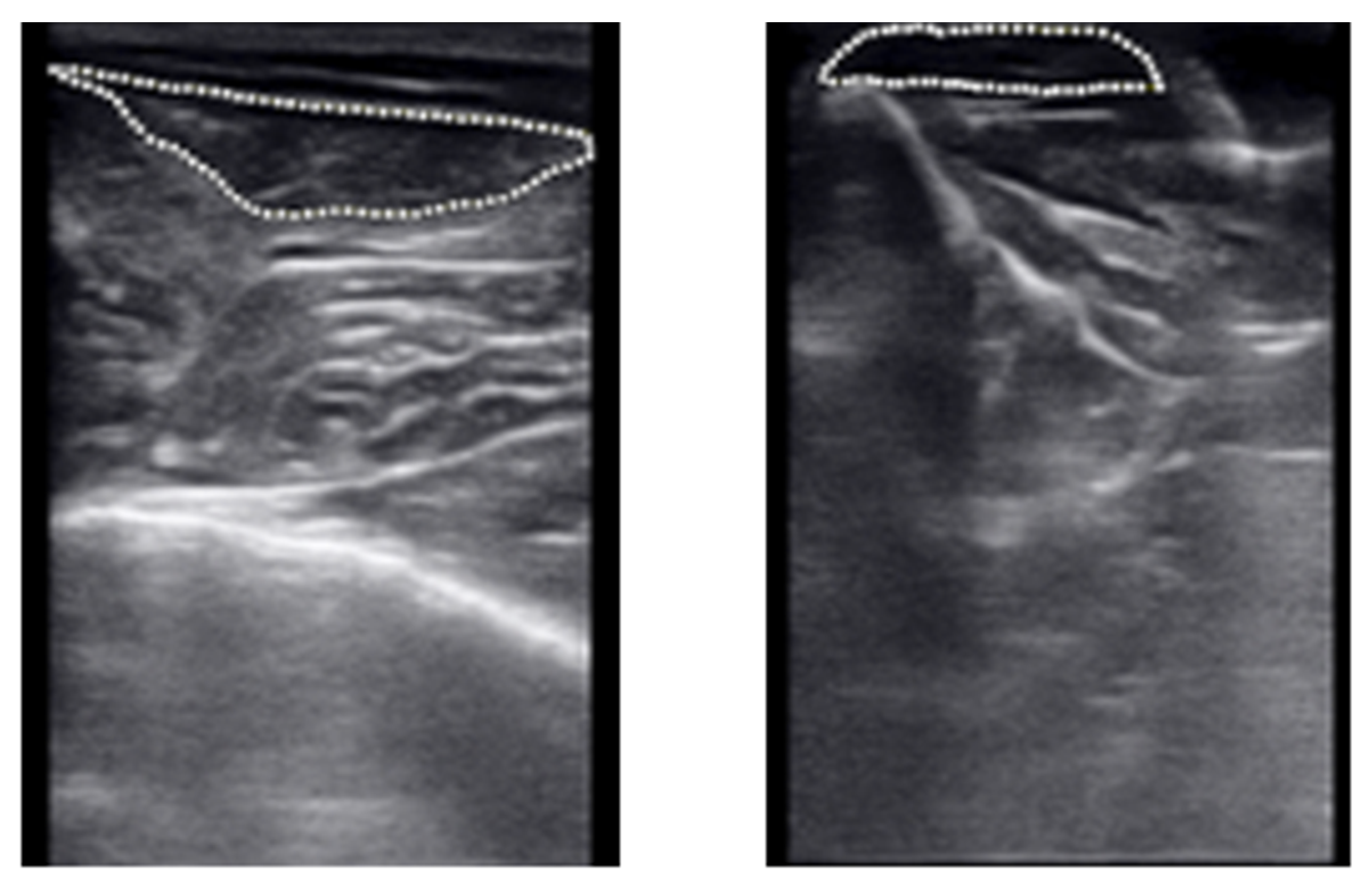



| * Housing | Age (Weeks) | Body Mass at Surgery (kg) | Body Mass at Euthanasia (kg) | Biological Tendon Length (mm) | Artificial Tendon Length (mm) | Percent of Biological Tendon (%) | |
|---|---|---|---|---|---|---|---|
| Tendon excision (TE)-only group | |||||||
| TE 1 | P | 17.3 | 2.85 | 3.15 | 40 | NA | NA |
| TE 2 | P | 17.4 | 2.84 | 3.36 | 33 | NA | NA |
| TE 3 | P | 17.4 | 2.83 | 3.45 | 37 | NA | NA |
| TE 4 | P, I | 17.4 | 3.13 | 3.71 | 44 | NA | NA |
| Mean (std) | 17.38 (0.04) | 2.91 (0.13) | 3.42 (0.2) | 38.50 (4.03) | NA | NA | |
| Tendon excision and replacement with polyester silicone-coated (PET-SI) tendon group | |||||||
| PET-SI 1 | I | 23.4 | 2.76 | 3.05 | 35 | 30 | 86% |
| PET-SI 2 | I | 18.7 | 2.78 | 3.03 | -- | 27 | -- |
| PET-SI 3 | # P, I | 17.3 | 2.97 | 3.44 | 38 | 33 | 87% |
| PET-SI 4 | P | 17.3 | 2.88 | 3.41 | 35 | 30 | 86% |
| PET-SI 5 | P, I | 17.4 | 3.00 | 3.45 | 32 | 27 | 84% |
| Mean (std) | 18.83 (2.35) | 2.87 (0.10) | 3.28 (0.2) | 35 (2.12) | 29.40 (2.24) | 85.75 (1.09) | |
| Variable | Gait phase | Group | Timepoint | GxT | B vs. 2 | B vs. 8 | 2 vs. 8 |
|---|---|---|---|---|---|---|---|
| Knee | |||||||
| Max angle | Stance | 0.588 | <0.001 | <0.001 | 0.007 | <0.001 | 0.231 |
| Swing | 0.193 | 0.017 | <0.001 | 0.289 | 0.015 | 0.380 | |
| Min angle | Stance | 0.525 | <0.001 | <0.001 | <0.001 | <0.001 | 0.989 |
| Swing | 0.035 | <0.001 | <0.001 | <0.001 | <0.001 | 0.556 | |
| Avg angle | Stance | 0.704 | <0.001 | <0.001 | <0.001 | <0.001 | 0.873 |
| Swing | 0.969 | <0.001 | <0.001 | 0.016 | <0.001 | 0.532 | |
| Ankle | |||||||
| Max angle | Stance | 0.271 | <0.001 | <0.001 | <0.001 | <0.001 | 0.177 |
| Swing | 0.147 | <0.001 | <0.001 | <0.001 | <0.001 | 0.912 | |
| Min angle | Stance | 0.403 | <0.001 | <0.001 | <0.001 | <0.001 | 0.390 |
| Swing | 0.822 | <0.001 | <0.001 | <0.001 | <0.001 | 0.603 | |
| Avg angle | Stance | 0.651 | <0.001 | <0.001 | <0.001 | <0.001 | 0.984 |
| Swing | 0.564 | <0.001 | <0.001 | <0.001 | <0.001 | 0.992 | |
| MTP | |||||||
| Max angle | Stance | 0.148 | <0.001 | <0.001 | 0.040 | <0.001 | 0.012 |
| Swing | - | - | - | - | - | - | |
| Min angle | Stance | 0.123 | <0.001 | <0.001 | <0.001 | <0.001 | 0.297 |
| Swing | - | - | - | - | - | - | |
| Avg angle | Stance | 0.550 | <0.001 | <0.001 | <0.001 | <0.001 | 0.486 |
| Swing | - | - | - | - | - | - | |
| Knee | Ankle | MTP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Variable | Gait Phase | B vs. 2 | B vs. 8 | 2 vs. 8 | Normality | B vs. 2 | B vs. 8 | 2 vs. 8 | Normality | B vs. 2 | B vs. 8 | 2 vs. 8 | Normality |
| TE | Max angle | Stance | 0.032 | 0.008 | 0.164 | 0.080 | <0.001 | <0.001 | 0.910 | 0.022 | 0.033 | 0.062 | 0.459 | 0.192 |
| Swing | 0.187 | 0.062 | 0.354 | 0.069 | <0.001 | <0.001 | 0.869 | 0.200 | - | - | - | - | ||
| Min angle | Stance | <0.001 | <0.001 | 0.145 | 0.064 | <0.001 | <0.001 | 0.755 | 0.080 | <0.001 | <0.001 | 0.850 | 0.200 | |
| Swing | 0.008 | <0.001 | 0.132 | 0.200 | <0.001 | <0.001 | 0.091 | 0.012 | - | - | - | - | ||
| Avg angle | Stance | <0.001 | <0.001 | 0.172 | 0.104 | <0.001 | <0.001 | 0.972 | 0.041 | <0.001 | <0.001 | 0.527 | 0.200 | |
| Swing | 0.082 | 0.009 | 0.163 | 0.080 | <0.001 | <0.001 | 0.763 | 0.049 | - | - | - | - | ||
| PET-SI | Max angle | Stance | 0.057 | 0.006 | 0.127 | 0.004 | <0.001 | <0.001 | 0.034 | 0.195 | 0.033 | <0.001 | <0.001 | 0.200 |
| Swing | 0.532 | 0.089 | 0.052 | 0.132 | <0.001 | <0.001 | 0.530 | 0.200 | - | - | - | - | ||
| Min angle | Stance | <0.001 | 0.005 | 0.012 | 0.002 | <0.001 | <0.001 | 0.099 | 0.200 | <0.001 | <0.001 | 0.075 | 0.077 | |
| Swing | 0.007 | 0.002 | 0.987 | 0.004 | <0.001 | <0.001 | 0.670 | 0.200 | - | - | - | - | ||
| Avg angle | Stance | 0.009 | 0.003 | 0.466 | <0.001 | <0.001 | <0.001 | 0.740 | 0.200 | <0.001 | <0.001 | 0.399 | 0.200 | |
| Swing | 0.096 | 0.036 | 0.436 | 0.061 | <0.001 | <0.001 | 0.816 | 0.025 | - | - | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidelis, O.P.; Easton, K.L.; Smith, M.; Bastos, G.; Bowers, K.; Anderson, D.E.; Crouch, D.L. Effects of Artificial Achilles Tendon on Hindlimb Movement Biomechanics and Muscle Morphology in Rabbits. Biomechanics 2025, 5, 47. https://doi.org/10.3390/biomechanics5030047
Fidelis OP, Easton KL, Smith M, Bastos G, Bowers K, Anderson DE, Crouch DL. Effects of Artificial Achilles Tendon on Hindlimb Movement Biomechanics and Muscle Morphology in Rabbits. Biomechanics. 2025; 5(3):47. https://doi.org/10.3390/biomechanics5030047
Chicago/Turabian StyleFidelis, Obinna P., Katrina L. Easton, Madison Smith, Gabriela Bastos, Kristin Bowers, David E. Anderson, and Dustin L. Crouch. 2025. "Effects of Artificial Achilles Tendon on Hindlimb Movement Biomechanics and Muscle Morphology in Rabbits" Biomechanics 5, no. 3: 47. https://doi.org/10.3390/biomechanics5030047
APA StyleFidelis, O. P., Easton, K. L., Smith, M., Bastos, G., Bowers, K., Anderson, D. E., & Crouch, D. L. (2025). Effects of Artificial Achilles Tendon on Hindlimb Movement Biomechanics and Muscle Morphology in Rabbits. Biomechanics, 5(3), 47. https://doi.org/10.3390/biomechanics5030047








