The Impact of Fatigue on Performance and Biomechanical Variables—A Narrative Review with Prospective Methodology
Abstract
1. Introduction
2. Fatigue Overview
3. Impact of Fatigue on Performance and Biomechanical Variables
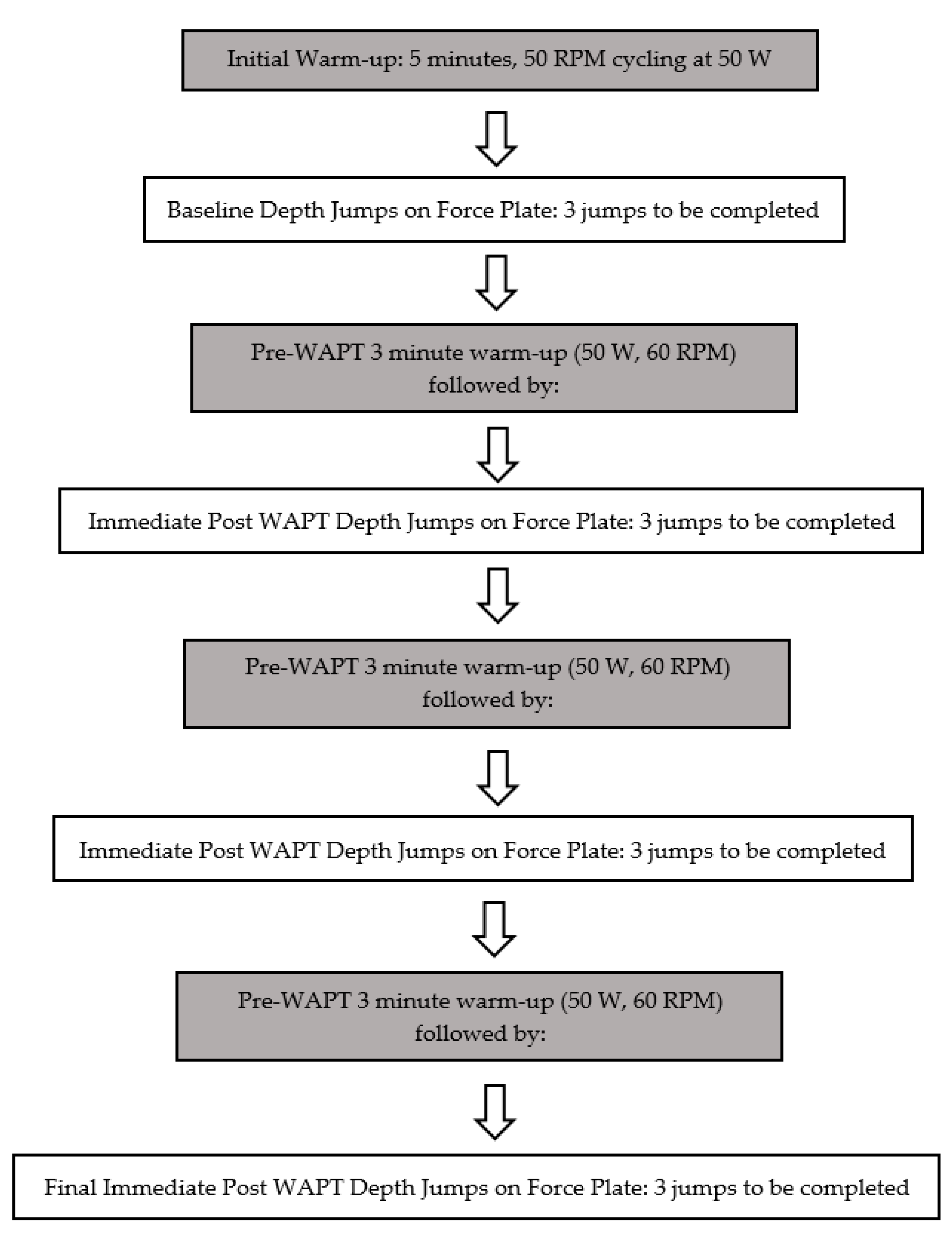
4. Proposed Protocol
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gage, B.E.; Mcilvain, N.M.; Collins, C.L.; Fields, S.K.; Comstock, R.D. Epidemiology of 6.6 Million Knee Injuries Presenting to United States Emergency Departments from 1999 through 2008. Acad. Emerg. Med. 2012, 19, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Vutescu, E.S.; Orman, S.; Garcia-Lopez, E.; Lau, J.; Gage, A.; Cruz, A.I. Psychological and Social Components of Recovery Following Anterior Cruciate Ligament Reconstruction in Young Athletes: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 9267. [Google Scholar] [CrossRef] [PubMed]
- Larwa, J.; Stoy, C.; Chafetz, R.S.; Boniello, M.; Franklin, C. Stiff landings, core stability, and dynamic knee valgus: A systematic review on documented anterior cruciate ligament ruptures in male and female athletes. Int. J. Environ. Res. Public Health 2021, 18, 3826. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, A.M.; Schneider, D.K.; Webster, K.E.; Yut, L.; Galloway, M.T.; Heidt, R.S., Jr.; Myer, G.D. Anterior cruciate ligament injury risk in sport: A systematic review and meta-analysis of injury incidence by sex and sport classification. J. Athl. Train. 2019, 54, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Bram, J.T.; Magee, L.C.; Mehta, N.N.; Patel, N.M.; Ganley, T.J. Anterior Cruciate Ligament Injury Incidence in Adolescent Athletes: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2021, 49, 1962–1972. [Google Scholar] [CrossRef]
- Agel, J.; Rockwood, T.; Klossner, D. Collegiate ACL Injury Rates Across 15 Sports: National Collegiate Athletic Association Injury Surveillance System Data Update (2004–2005 Through 2012–2013). Clin. J. Sport Med. 2016, 26, 518–523. [Google Scholar] [CrossRef]
- Pfeifer, C.E.; Beattie, P.F.; Sacko, R.S.; Hand, A. Risk Factors Associated with Non-Contact Anterior Cruciate Ligament Injury: A Systematic Review. Int. J. Sports Phys. Ther. 2018, 13, 575–587. [Google Scholar] [CrossRef]
- Wetters, N.; Weber, A.E.; Wuerz, T.H.; Schub, D.L.; Mandelbaum, B.R. Mechanism of injury and risk factors for anterior cruciate ligament injury. Oper. Tech. Sports Med. 2016, 24, 2–6. [Google Scholar] [CrossRef]
- Bisciotti, G.N.; Chamari, K.; Cena, E.; Bisciotti, A.; Bisciotti, A.; Corsini, A.; Volpi, P. Anterior cruciate ligament injury risk factors in football. J. Sports Med. Phys. Fit. 2019, 59, 1724. [Google Scholar] [CrossRef]
- Della Villa, F.; Buckthorpe, M.; Grassi, A.; Nabiuzzi, A.; Tosarelli, F.; Zaffagnini, S.; Della Villa, S. Systematic video analysis of ACL injuries in professional male football (soccer): Injury mechanisms 1738, situational patterns and biomechanics study on 134 consecutive cases. Br. J. Sports Med. 2020, 54, 1423. [Google Scholar] [CrossRef]
- Grassi, A.; Smiley, S.P.; Roberti di Sarsina, T.; Signorelli, C.; Marcheggiani Muccioli, G.M.; Bondi, A.; Zaffagnini, S. Mechanisms and situations of anterior cruciate ligament injuries in professional male soccer players: A YouTube-based video analysis. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.M.; Marshall, S.W.; Lund, J.L.; Pate, V.; Mack, C.D.; Spang, J.T. Trends in incidence of ACL reconstruction and concomitant procedures among commercially insured individuals in the United States, 2002–2014. Sports Health 2018, 10, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, M.; Razeghi, M.; Tabatabaee, H.; Piroozi, S.; Shirazi, Z.R.; Rafiee, A. A new approach to differentiate those with anterior cruciate ligament deficient knees into copers and non-copers. Physiother. Pract. Res. 2016, 37, 73–78. [Google Scholar] [CrossRef]
- Rodriguez, K.; Soni, M.; Joshi, P.K.; Patel, S.C.; Shreya, D.; Zamora, D.I.; Patel, G.S.; Grossmann, I.; Sange, I. Anterior Cruciate Ligament Injury: Conservative Versus Surgical Treatment. Cureus 2021, 13, e2. [Google Scholar] [CrossRef]
- King, E.; Richter, C.; Daniels, K.; Franklyn-Miller, A.; Falvey, E.; Myer, G.D.; Jackson, M.; Moran, R.; Strike, S. Biomechanical but Not Strength or Performance Measures Differentiate Male Athletes Who Experience ACL Reinjury on Return to Level 1 Sports. Am. J. Sports Med. 2021, 49, 918–927. [Google Scholar] [CrossRef]
- Barber-Westin, S.; Noyes, F.R. One in 5 Athletes Sustain Reinjury upon Return to High-Risk Sports after ACL Reconstruction: A Systematic Review in 1239 Athletes Younger Than 20 Years. Sports Health 2020, 12, 587–597. [Google Scholar] [CrossRef]
- Johnston, P.T.; McClelland, J.A.; Feller, J.A.; Webster, K.E. Knee muscle strength after quadriceps tendon autograft anterior cruciate ligament reconstruction: Systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 2918. [Google Scholar] [CrossRef]
- Barfod, K.W.; Feller, J.A.; Hartwig, T.; Devitt, B.M.; Webster, K.E. Knee extensor strength and hop test performance following anterior cruciate ligament reconstruction. Knee 2019, 26, 149–154. [Google Scholar] [CrossRef]
- Aune, A.K.; Holm, I.; Risberg, M.A.; Jensen, H.K.; Steen, H. Four-strand hamstring tendon autograft compared with patellar tendon-bone autograft for anterior cruciate ligament reconstruction: A randomized study with two-year follow-up. Am. J. Sports Med. 2001, 29, 722–728. [Google Scholar] [CrossRef]
- Xergia, S.A.; McClelland, J.A.; Kvist, J.; Vasiliadis, H.S.; Georgoulis, A.D. The influence of graft choice on isokinetic muscle strength 4–24 months after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 768–780. [Google Scholar] [CrossRef]
- Bizzini, M.; Gorelick, M.; Munzinger, U.; Drobny, T. Joint laxity and isokinetic thigh muscle strength characteristics after anterior cruciate ligament reconstruction: Bone patellar tendon bone versus quadrupled hamstring autografts. Clin. J. Sport Med. 2006, 16, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Mahajan, S.; Zanazzo, M.; Tuy, B. Patellar tendon versus quadrupled bone-semitendinosus anterior cruciate ligament reconstruction: A prospective clinical investigation in athletes. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 592–601. [Google Scholar] [CrossRef]
- Mattacola, C.G.; Perrin, D.H.; Gansneder, B.M.; Gieck, J.H.; Saliba, E.N.; McCue III, F.C. Strength, functional outcome, and postural stability after anterior cruciate ligament reconstruction. J. Athl. Train. 2002, 37, 262. [Google Scholar] [PubMed]
- Jansson, K.A.; Linko, E.; Sandelin, J.; Harilainen, A. A prospective randomized study of patellar versus hamstring tendon autografts for anterior cruciate ligament reconstruction. Am. J. Sports Med. 2003, 31, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Beynnon, B.D.; Hall, J.S.; Sturnick, D.R.; DeSarno, M.J.; Gardner-Morse, M.; Tourville, T.W.; Vacek, P.M. Increased slope of the lateral tibial plateau subchondral bone is associated with greater risk of noncontact ACL injury in females but not in males: A prospective cohort study with a nested, matched case-control analysis. Am. J. Sports Med. 2014, 42, 1039–1048. [Google Scholar] [CrossRef]
- Bisson, L.J.; Gurske-DePerio, J. Axial and sagittal knee geometry as a risk factor for noncontact anterior cruciate ligament tear: A case-control study. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 901–906. [Google Scholar] [CrossRef]
- Dare, D.M.; Fabricant, P.D.; McCarthy, M.M.; Rebolledo, B.J.; Green, D.W.; Cordasco, F.A.; Jones, K.J. Increased lateral tibial slope is a risk factor for pediatric anterior cruciate ligament injury: An MRI-based case-control study of 152 patients. Am. J. Sports Med. 2015, 43, 1632–1639. [Google Scholar] [CrossRef]
- de Sire, A.; Demeco, A.; Marotta, N.; Spano, R.; Curci, C.; Fari, G.; Ammendolia, A. Neuromuscular impairment of knee stabilizers muscles in a COVID-19 cluster of female volleyball players: Which role for rehabilitation in the post-COVID-19 return-to-play? Appl. Sci. 2022, 12, 557. [Google Scholar] [CrossRef]
- de Sire, A.; Marotta, A.; Demeco, A.; Moggio, L.; Paola, P.; Marotta, N.; Ammendolia, A. Electromyographic assessment of anterior cruciate ligament injury risk in male tennis players: Which role for visual input? A proof-of-concept study. Diagnostics 2021, 11, 997. [Google Scholar] [CrossRef]
- de Sire, A.; Demeco, A.; Marrota, N.; Moggio, L.; Palumbo, A.; Iona, T.; Ammendolia, A. Anterior cruciate ligament injury prevention exercises: Could a neuromuscular warm-up improve muscle pre-activation before a soccer game? A proof-of-principle study on professional football players. Appl. Sci. 2021, 11, 4958. [Google Scholar] [CrossRef]
- Leppänen, M.; Pasanen, K.; Kujala, U.M.; Vasankari, T.; Kannus, P.; Äyrämö, S.; Parkkari, J. Stiff landings are associated with increased ACL injury risk in young female basketball and floorball players. Am. J. Sports Med. 2017, 45, 386–393. [Google Scholar] [CrossRef]
- Leppänen, M.; Pasanen, K.; Krosshaug, T.; Kannus, P.; Vasankari, T.; Kujala, U.M.; Parkkari, J. Sagittal plane hip, knee, and ankle biomechanics and the risk of anterior cruciate ligament injury: A prospective study. Orthop. J. Sports Med. 2017, 5, 2325967117745487. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Garrett, W.E. Mechanisms of non-contact ACL injuries. Br. J. Sports Med. 2007, 41 (Suppl. 1), I47–I51. [Google Scholar] [CrossRef]
- Romero-Franco, N.; Ortego-Mate, M.; Molina-Mula, J. Knee Kinematics During Landing: Is It Really a Predictor of Acute Noncontact Knee Injuries in Athletes? A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2020, 8, 232596712096. [Google Scholar] [CrossRef] [PubMed]
- Nilstad, A.; Petushek, E.; Mok, K.M.; Bahr, R.; Krosshaug, T. Kiss goodbye to the ‘kissing knees’: No association between frontal plane inward knee motion and risk of future non-contact ACL injury in elite female athletes. Sports Biomech. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, N.F.; Meeuwisse, W.H.; Mendonça, L.D.; Nettel-Aguirre, A.; Ocarino, J.M.; Fonseca, S.T. Complex systems approach for sports injuries: Moving from risk factor identification to injury pattern recognition—narrative review and new concept. Br. J. Sports Med. 2016, 50, 1309–1314. [Google Scholar] [CrossRef]
- Ament, W.; Verkerke, G.J. Exercise and Fatigue. Sports Med. 2009, 39, 382–422. [Google Scholar] [CrossRef]
- Potvin, J.R.; Fuglevand, A.J. A motor unit-based model of muscle fatigue. PLoS Comput. Biol. 2017, 13, e1005581. [Google Scholar] [CrossRef]
- Kennedy, R.A.; Drake, D. The effect of acute fatigue on countermovement jump performance in rugby union players during preseason. J. Sports Med. Phys. Fit. 2017, 57, 1261. [Google Scholar] [CrossRef]
- Bedo, B.L.; Pereira, D.R.; Moraes, R.; Kalva-Filho, C.A.; Will-De-Lemos, T.; Santiago, P.R. The rapid recovery of vertical force propulsion production and postural sway after a specific fatigue protocol in female handball athletes. Gait Posture 2020, 77, 52–58. [Google Scholar] [CrossRef]
- Dotan, R.; Woods, S.; Contessa, P. On the reliability and validity of central fatigue determination. Eur. J. Appl. Physiol. 2021, 121, 2393. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Clark, C.C. Brain function during central fatigue induced by intermittent high-intensity cycling. Neurol. Sci. 2021, 2411, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.M.S.; Rabelo, P.C.R.; Moraes, M.M.; Teixeira-Coelho, F.; Coimbra, C.C.; Wanner, S.P.; Soares, D.D. Physical exercise-induced fatigue: The role of serotonergic and dopaminergic systems. Braz. J. Med. Biol. Res. 2017, 50, e6432. [Google Scholar] [CrossRef]
- Jayalath, J.L.R.; de Noronha, M.; Weerakkody, N.; Bini, R. Effects of fatigue on ankle biomechanics during jumps: A systematic review. J. Electromyogr. Kinesiol. 2018, 42, 81–91. [Google Scholar] [CrossRef]
- Wan, J.; Qin, Z.; Wang, P.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef] [PubMed]
- Debold, E.P. Recent Insights into Muscle Fatigue at the Cross-Bridge Level. Front. Physiol. 2012, 3, 151. [Google Scholar] [CrossRef]
- Scott, W.; Stevens, J.; Binder–Macleod, S.A. Human Skeletal Muscle Fiber Type Classifications. Phys. Ther. 2001, 81, 1810. [Google Scholar] [CrossRef]
- Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Murray, T.F.; Hostler, D.P.; Crill, M.T.; Toma, K. Fiber Type Composition of the Vastus Lateralis Muscle of Young Men and Women. J. Histochem. Cytochem. 2000, 48, 623–629. [Google Scholar] [CrossRef]
- Cooper, C.N.; Dabbs, N.C.; Davis, J.; Sauls, N.M. Effects of Lower-Body Muscular Fatigue on Vertical Jump and Balance Performance. J. Strength Cond. Res. 2020, 34, 2903. [Google Scholar] [CrossRef]
- Harato, K.; Morishige, Y.; Niki, Y.; Kobayashi, S.; Nagura, T. Fatigue and recovery have different effects on knee biomechanics of drop vertical jump between female collegiate and recreational athletes. J. Orthop. Surg. Res. 2021, 16, 1–7. [Google Scholar]
- Bobbert, M.F.; Van Der Krogt, M.M.; Van Doorn, H.; De Ruiter, C.J. Effects of fatigue of plantarflexors on control and performance in vertical jumping. Med. Sci. Sports Exerc. 2011, 43, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Coutts, A.J.; Merlini, M.; Deprez, D.; Lenoir, M.; Marcora, S.M. Mental fatigue impairs soccer-specific physical and technical performance. Med. Sci. Sports Exerc. 2016, 48, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Demeco, A.; de Sire, A.; Marotta, N.; Spano, R.; Lippi, L.; Palumbo, A.; Ammendolia, A. Match analysis, physical training, risk of injury, and rehabilitation in padel: Overview of the literature. Int. J. Environ. Res. Public Health 2022, 19, 4153. [Google Scholar] [CrossRef] [PubMed]
- Twomey, R.; Aboodarda, S.J.; Kruger, R.; Culos-Reed, S.N.; Temesi, J.; Millet, G.Y. Neuromuscular fatigue during exercise: Methodological considerations, etiology and potential role in chronic fatigue. Neurophysiol. Clin. 2017, 47, 95–110. [Google Scholar] [CrossRef]
- Quammen, D.; Cortes, N.; Van Lunen, B.L.; Lucci, S.; Ringleb, S.I.; Onate, J. Two Different Fatigue Protocols and Lower Extremity Motion. J. Athl. Train. 2012, 47, 32–41. [Google Scholar] [CrossRef]
- Cortes, N.; Greska, E.; Ambegaonkar, J.P.; Kollock, R.O.; Caswell, S.V.; Onate, J.A. Knee kinematics is altered post-fatigue while performing a crossover task. Knee Surg. Sports Traumatol. Arthrosc. 2013, 22, 2202. [Google Scholar] [CrossRef]
- Lucci, S.; Cortes, N.; Lunen, B.V.; Ringleb, S.; Onate, J. Knee and hip sagittal and transverse plane changes after two fatigue protocols. J. Sci. Med. Sport 2011, 14, 453–459. [Google Scholar] [CrossRef]
- Barber-Westin, S.D.; Noyes, F.R. Effect of Fatigue Protocols on Lower Limb Neuromuscular Function and Implications for Anterior Cruciate Ligament Injury Prevention Training: A Systematic Review. Am. J. Sports Med. 2017, 45, 3388. [Google Scholar] [CrossRef]
- Watanabe, S.; Aizawa, J.; Shimoda, M.; Enomoto, M.; Nakamura, T.; Okawa, A.; Yagishita, K. Effect of short-term fatigue 3396, induced by high-intensity exercise, on the profile of the ground reaction force during single-leg anterior drop-jumps. J. Phys. Ther. Sci. 2016, 28, 3371. [Google Scholar] [CrossRef]
- Kellis, E.; Kouvelioti, V. Agonist versus antagonist muscle fatigue effects on thigh muscle activity and vertical ground reaction during drop landing. J. Electromyogr. Kinesiol. 2009, 19, 55–64. [Google Scholar] [CrossRef]
- Pappas, E.; Hagins, M.; Sheikhzadeh, A.; Nordin, M.; Rose, D. Biomechanical Differences between Unilateral and Bilateral Landings from a Jump: Gender Differences. Clinical J. Sport Med. 2007, 17, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Brazen, D.M.; Todd, M.K.; Ambegaonkar, J.P.; Wunderlich, R.; Peterson, C. The Effect of Fatigue on Landing Biomechanics in Single-Leg Drop Landings. Clin. J. Sport Med. 2010, 20, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.L.; Huang, C.F.; Chen, P.C. Effects of Lower Extremity Muscle Fatigue on Knee Loading During a Forward Drop Jump to a Vertical Jump in Female Athletes. J. Hum. Kinet. 2020, 72, 5–13. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, R.; Dai, B.; Sun, X.; Fu, W. Effects of Exercise-Induced Fatigue on Lower Extremity Joint Mechanics, Stiffness, and Energy Absorption during Landings. J. Sports Sci. Med. 2018, 17, 640–649. [Google Scholar]
- Ueno, R.; Navacchia, A.; DiCesare, C.A.; Ford, K.R.; Myer, G.D.; Ishida, T.; Hewett, T.E. Knee abduction moment is predicted by lower gluteus medius force and larger vertical and lateral ground reaction forces during drop vertical jump in female athletes. J. Biomech. 2020, 103, 109669. [Google Scholar] [CrossRef]
- Evans-Pickett, A.; Davis-Wilson, H.C.; Luc-Harkey, B.A.; Blackburn, J.T.; Franz, J.R.; Padua, D.A.; Pietrosimone, B. Biomechanical effects of manipulating peak vertical ground reaction force throughout gait in individuals 6–12 months after anterior cruciate ligament reconstruction. Clin. Biomech. 2020, 76, 105014. [Google Scholar] [CrossRef]
- Heebner, N.R.; Rafferty, D.M.; Wohleber, M.F.; Simonson, A.J.; Lovalekar, M.; Reinert, A.; Sell, T.C. Landing kinematics and kinetics at the knee during different landing tasks. J. Athl. Train. 2017, 52, 1101–1108. [Google Scholar] [CrossRef]
- Benjaminse, A.; Webster, K.E.; Kimp, A.; Meijer, M.; Gokeler, A. Revised approach to the role of fatigue in anterior cruciate ligament injury prevention: A systematic review with meta-analyses. Sports Med. 2019, 49, 565–586. [Google Scholar] [CrossRef]
- Lunn, W.R.; Axtell, R.S. Validity and reliability of the lode excalibur sport cycle ergometer for the wingate anaerobic test. J. Strength Cond. Res. 2021, 35, 2894–2901. [Google Scholar]
- Borotikar, B.S.; Newcomer, R.; Koppes, R.; McLean, S.G. Combined effects of fatigue and decision making on female lower limb landing postures: Central and peripheral contributions to ACL injury risk. Clin Biomech. 2008, 23, 81–92. [Google Scholar] [CrossRef]
- Degens, H.; Stasiulis, A.; Skurvydas, A.; Statkeviciene, B.; Venckunas, T. Physiological comparison between non-athletes, endurance, power and team athletes. Eur. J. Appl. Physiol. 2019, 119, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Beneke, R.; Pollmann, C.; Bleif, I.; Leithäuser, R.M.; Hütler, M. How anaerobic is the Wingate Anaerobic Test for humans? Eur. J. Appl. Physiol. 2002, 87, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A.; Nikooyan, A.A. The effects of lower-extremity muscle fatigue on the vertical ground reaction force: A meta-analysis. Proceedings of the Institution of Mechanical Engineers Part H. J. Eng. Med. 2012, 226, 579–588. [Google Scholar] [PubMed]
- Ben Abdelkrim, N.; Castagna, C.; Jabri, I.; Battikh, T.; El Fazaa, S.; El Ati, J. Activity profile and physiological requirements of junior elite basketball players in relation to aerobic-anaerobic fitness. J. Strength Cond. Res. 2010, 24, 2330. [Google Scholar] [CrossRef] [PubMed]
- Vigne, G.; Gaudino, C.; Rogowski, I.; Alloatti, G.; Hautier, C. Activity profile in elite Italian soccer team. Int. J. Sports Med. 2010, 31, 304–310. [Google Scholar] [CrossRef]
- Sunderland, C.D.; Edwards, P.L. Activity Profile and Between-Match Variation in Elite Male Field Hockey. J. Strength Cond. Res. 2017, 31, 758–764. [Google Scholar] [CrossRef]
- Vigh-Larsen, J.F.; Haverinen, M.T.; Panduro, J.; Ermidis, G.; Andersen, T.B.; Overgaard, K.; Krustrup, P.; Parkkari, J.; Avela, J.; Kyröläinen, H.; et al. On-Ice and Off-Ice Fitness Profiles of Elite and U20 Male Ice Hockey Players of Two Different National Standards. J. Strength Cond. Res. 2020, 34, 3369. [Google Scholar] [CrossRef]
- Gharbi, Z.; Dardouri, W.; Haj-Sassi, R.; Chamari, K.; Souissi, N. Aerobic and anaerobic determinants of repeated sprint ability in team sports athletes. Biol Sport. 2015, 32, 207–212. [Google Scholar] [CrossRef]
- Yamagishi, T.; Babraj, J. Influence of recovery intensity on oxygen demand and repeated sprint performance. J. Sports Med. Phys. Fit. 2016, 56, 1103–1112. [Google Scholar]
- Orishimo, K.F.; Kremenic, I.J.; Pappas, E.; Hagins, M.; Liederbach, M. Comparison of landing biomechanics between male and female professional dancers. Am. J. Sports Med. 2009, 37, 2187. [Google Scholar] [CrossRef]
- Liederbach, M.; Kremenic, I.J.; Orishimo, K.F.; Pappas, E.; Hagins, M. Comparison of landing biomechanics between male and female dancers and athletes, part 2: Influence of fatigue and implications for anterior cruciate ligament injury. Am. J. Sports Med. 2014, 42, 1089. [Google Scholar] [CrossRef] [PubMed]
- Jurasz, M.; Boraczyński, M.; Wójcik, Z.; Gronek, P. Neuromuscular Fatigue Responses of Endurance- and Strength-Trained Athletes during Incremental Cycling Exercise. Int. J. Environ. Res. Public Health 2022, 1095, 19. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Nutrition for endurance sports: Marathon 8839, triathlon, and road cycling. J. Sports Sci. 2011, 29 (Suppl. 1), S91–S99. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Caldwell, G.E. Muscular activity patterns in 1-legged vs. 2-legged pedaling. J. Sport Health Sci. 2021, 10, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Clos, P.; Lepers, R. Leg Muscle Activity and Perception of Effort before and after Four Short Sessions of Submaximal Eccentric Cycling. Int. J. Environ. Res. Public Health 2020, 17, 7702. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, M.; Petrizzo, J.; Otto, R.M.; Wygand, J. The Impact of Fatigue on Performance and Biomechanical Variables—A Narrative Review with Prospective Methodology. Biomechanics 2022, 2, 513-524. https://doi.org/10.3390/biomechanics2040040
Aquino M, Petrizzo J, Otto RM, Wygand J. The Impact of Fatigue on Performance and Biomechanical Variables—A Narrative Review with Prospective Methodology. Biomechanics. 2022; 2(4):513-524. https://doi.org/10.3390/biomechanics2040040
Chicago/Turabian StyleAquino, Michele, John Petrizzo, Robert M. Otto, and John Wygand. 2022. "The Impact of Fatigue on Performance and Biomechanical Variables—A Narrative Review with Prospective Methodology" Biomechanics 2, no. 4: 513-524. https://doi.org/10.3390/biomechanics2040040
APA StyleAquino, M., Petrizzo, J., Otto, R. M., & Wygand, J. (2022). The Impact of Fatigue on Performance and Biomechanical Variables—A Narrative Review with Prospective Methodology. Biomechanics, 2(4), 513-524. https://doi.org/10.3390/biomechanics2040040







