Training History-Dependent Functional Role of EMG Model-Predicted Antagonist Moments in Knee Extensor Moment Generation in Healthy Young Adults
Abstract
:1. Introduction
2. Methods
2.1. Participants and Design
2.2. Preparation for Measurements
2.3. Measurement of Quadriceps and Hamstring Maximal Moments
2.4. Data Collection and Analyses
2.5. Statistical Analyses
3. Results
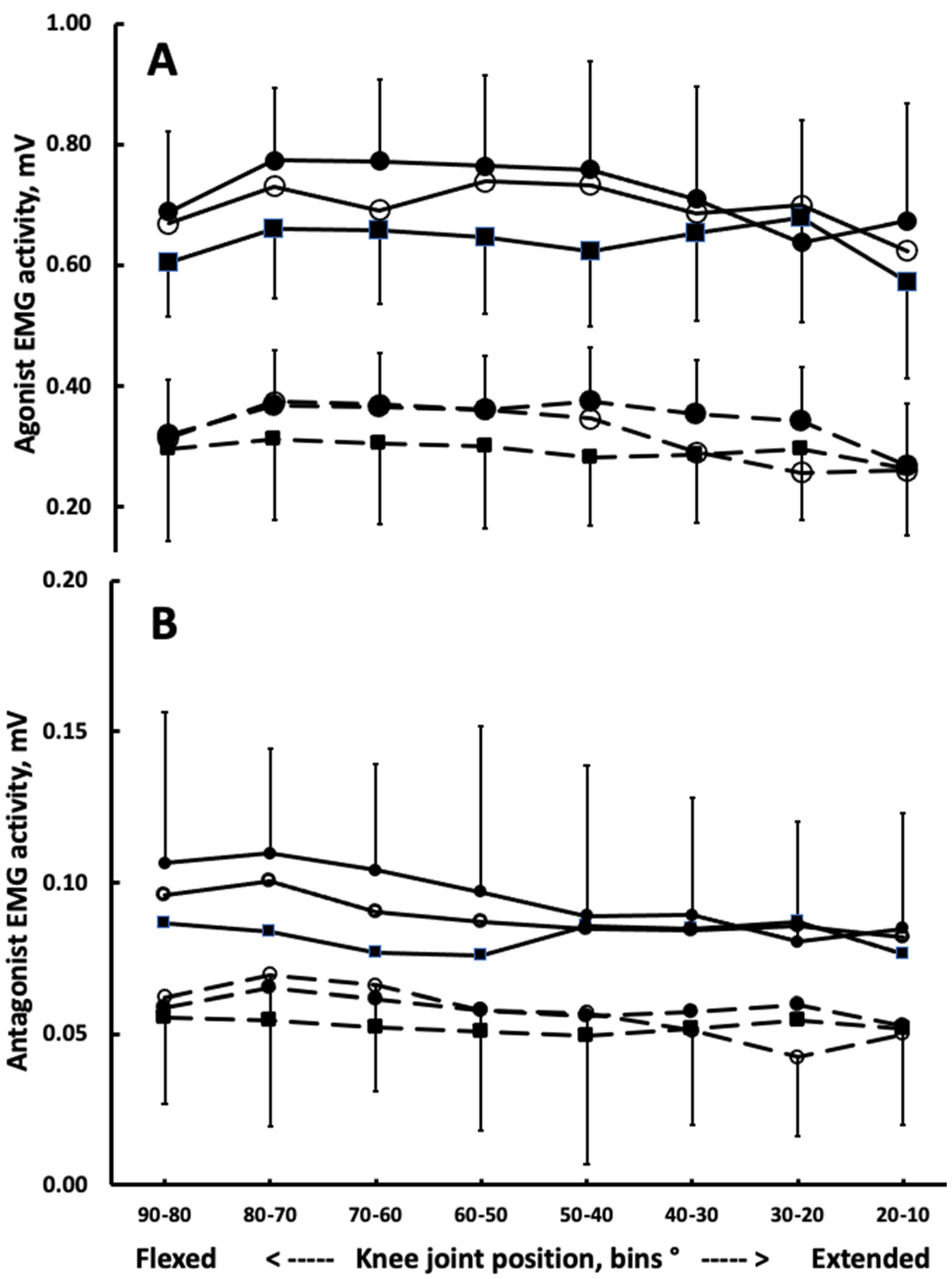
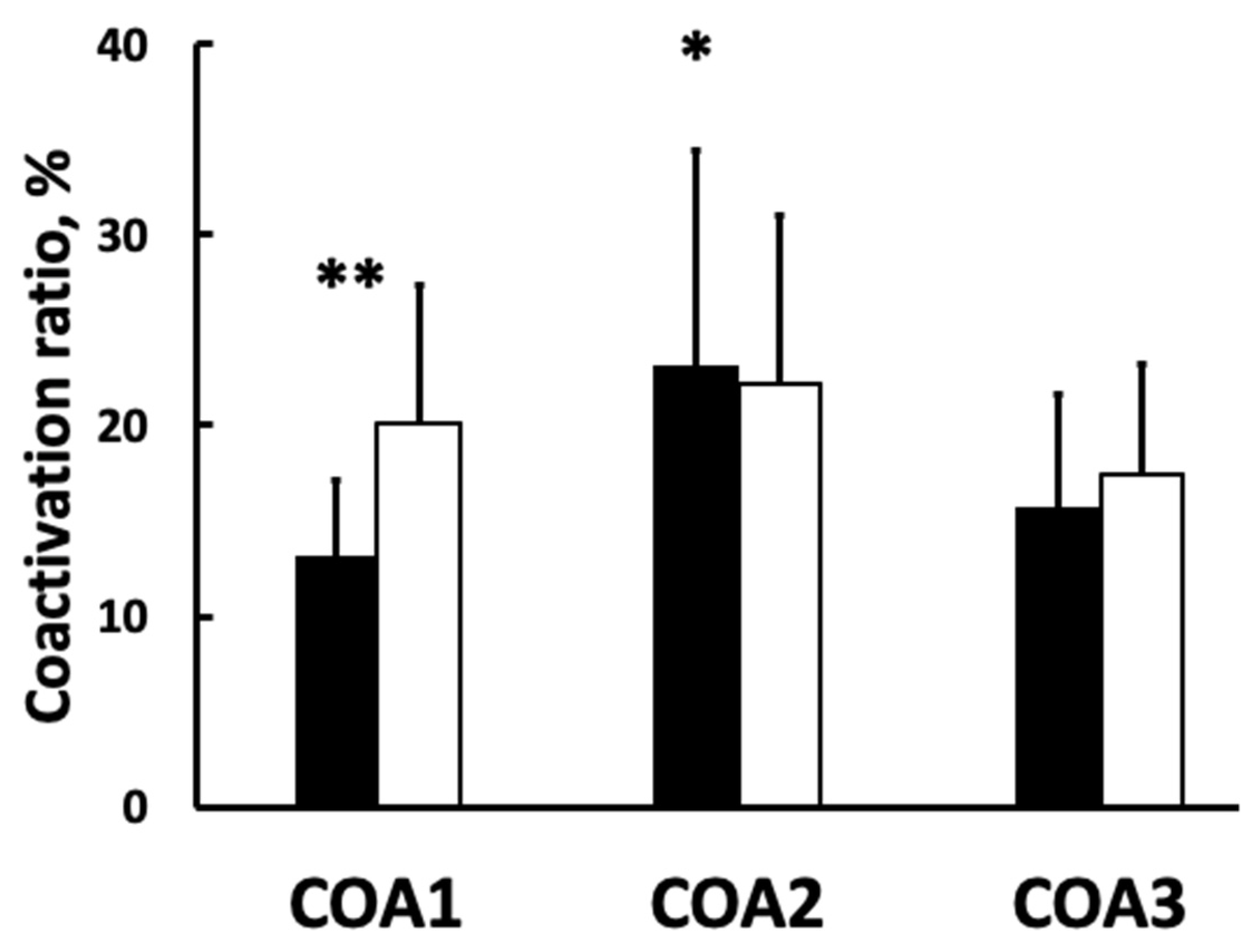
3.1. Muscle Activation and Coactivation
3.2. Moments Data
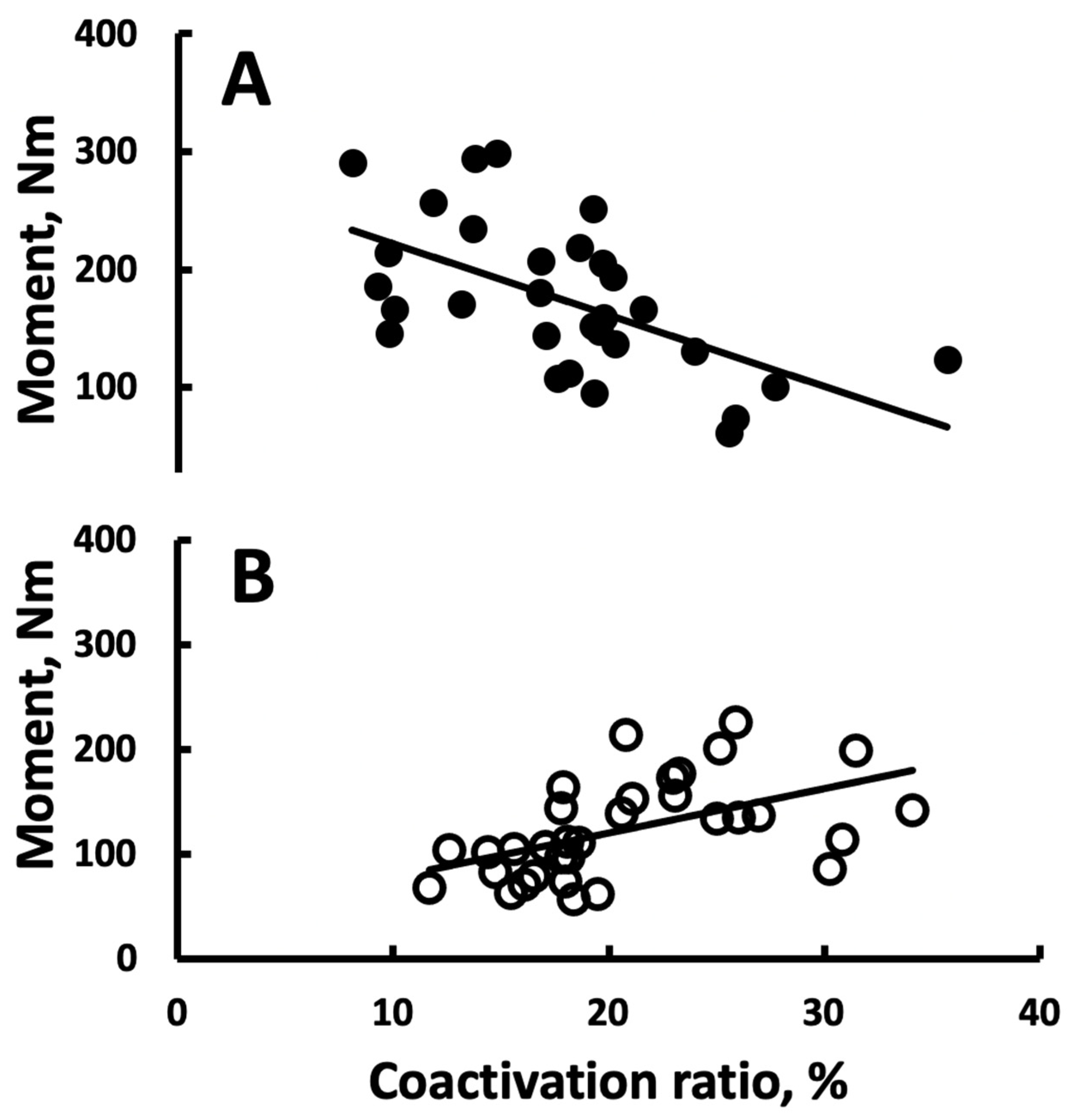
3.3. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, S.P.; Bojsen-Moller, F.; Dyhre-Poulsen, P. Antagonist muscle coactivation during isokinetic knee extension. Scand. J. Med. Sci. Sports 2000, 10, 58–67. [Google Scholar] [CrossRef]
- Latash, M.L. Muscle coactivation: Definitions, mechanisms, and functions. J. Neurophysiol. 2018, 120, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Hortobágyi, T.; Granacher, U.; Fernandez-Del-Olmo, M.; Howatson, G.; Manca, A.; Deriu, F.; Taube, W.; Gruber, M.; Marquez, G.; Lundbye-Jensen, J.; et al. Functional relevance of resistance training-induced neuroplasticity in health and disease. Neurosci. Biobehav. Rev. 2021, 122, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Maeo, S.; Balshaw, T.G.; Lanza, M.B.; Hannah, R.; Folland, J.P. Corticospinal excitability and motor representation after long-term resistance training. Eur. J. Neurosci. 2021, 53, 3416–3432. [Google Scholar] [CrossRef]
- Skarabot, J.; Brownstein, C.G.; Casolo, A.; Del Vecchio, A.; Ansdell, P. The knowns and unknowns of neural adaptations to resistance training. Eur. J. Appl. Physiol. 2021, 121, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Pearcey, G.E.P.; Alizedah, S.; Power, K.E.; Button, D.C. Chronic resistance training: Is it time to rethink the time course of neural contributions to strength gain? Eur. J. Appl. Physiol. 2021, 121, 2413–2422. [Google Scholar] [CrossRef]
- Sugawara, K.; Tanabe, S.; Higashi, T.; Suzuki, T.; Tsurumi, T.; Kasai, T. Functional plasticity of surround inhibition in the motor cortex during single finger contraction training. Neuroreport 2012, 23, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Baratta, R.; Solomonow, M.; Zhou, B.H.; Letson, D.; Chuinard, R.; D’Ambrosia, R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am. J. Sports Med. 1988, 16, 113–122. [Google Scholar] [CrossRef]
- Solomonow, M.; Baratta, R.; Zhou, B.H.; Shoji, H.; Bose, W.; Beck, C.; D’Ambrosia, R. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am. J. Sports Med. 1987, 15, 207–213. [Google Scholar] [CrossRef]
- Carolan, B.; Cafarelli, E. Adaptations in coactivation after resistance training. J. Appl. Physiol. 1992, 73, 911–917. [Google Scholar] [CrossRef]
- Häkkinen, K.; Kallinen, M.; Izquierdo, M.; Jokelainen, K.; Lassila, H.; Malkia, E.; Kraemer, W.J.; Newton, R.U.; Alen, M. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J. Appl. Physiol. 1998, 84, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, E.; Martin, A.; Van Hoecke, J. Adaptations to long-term strength training of ankle joint muscles in old age. Eur. J. Appl. Physiol. 2007, 100, 507–514. [Google Scholar] [CrossRef]
- Morse, C.I.; Thom, J.M.; Mian, O.S.; Muirhead, A.; Birch, K.M.; Narici, M.V. Muscle strength, volume and activation following 12-month resistance training in 70-year-old males. Eur. J. Appl. Physiol. 2005, 95, 197–204. [Google Scholar] [CrossRef]
- de Boer, M.D.; Morse, C.I.; Thom, J.M.; de Haan, A.; Narici, M.V. Changes in antagonist muscles’ coactivation in response to strength training in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1022–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtermann, A.; Roeleveld, K.; Vereijken, B.; Ettema, G. Changes in agonist EMG activation level during MVC cannot explain early strength improvement. Eur. J. Appl. Physiol. 2005, 94, 593–601. [Google Scholar] [CrossRef]
- Reeves, N.D.; Maganaris, C.N.; Narici, M.V. Plasticity of dynamic muscle performance with strength training in elderly humans. Muscle Nerve 2005, 31, 355–364. [Google Scholar] [CrossRef]
- Simoneau, E.; Martin, A.; Porter, M.M.; Van Hoecke, J. Strength training in old age: Adaptation of antagonist muscles at the ankle joint. Muscle Nerve 2006, 33, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Wilk, K.E.; Escamilla, R.F.; Fleisig, G.S.; Barrentine, S.W.; Andrews, J.R.; Boyd, M.L. A comparison of tibiofemoral joint forces and electromyographic activity during open and closed kinetic chain exercises. Am. J. Sports Med. 1996, 24, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Kvist, J.; Gillquist, J. Sagittal plane knee translation and electromyographic activity during closed and open kinetic chain exercises in anterior cruciate ligament-deficient patients and control subjects. Am. J. Sports Med. 2001, 29, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Messier, S.P.; Beavers, D.P.; Loeser, R.F.; Carr, J.J.; Khajanchi, S.; Legault, C.; Nicklas, B.J.; Hunter, D.J.; Devita, P. Knee joint loading in knee osteoarthritis: Influence of abdominal and thigh fat. Med. Sci. Sports Exerc. 2014, 46, 1677–1683. [Google Scholar] [CrossRef] [Green Version]
- Messier, S.P.; Pater, M.; Beavers, D.P.; Legault, C.; Loeser, R.F.; Hunter, D.J.; DeVita, P. Influences of alignment and obesity on knee joint loading in osteoarthritic gait. Osteoarthr. Cartil. 2014, 22, 912–917. [Google Scholar] [CrossRef] [Green Version]
- Hortobágyi, T. Training planning of junior high jumpers. In Planning of Training Series; Hortobágyi, T., Ed.; National Strength and Conditioning Association: Lincoln, NE, USA, 1989; pp. 1–28. [Google Scholar]
- Chavarro-Nieto, C.; Beaven, M.; Gill, N.; Hebert-Losier, K. Hamstrings injury incidence, risk factors, and prevention in Rugby Union players: A systematic review. Physician Sportsmed. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Llurda-Almuzara, L.; Labata-Lezaun, N.; Lopez-de-Celis, C.; Aiguade-Aiguade, R.; Romani-Sanchez, S.; Rodriguez-Sanz, J.; Fernandez-de-Las-Penas, C.; Perez-Bellmunt, A. Biceps Femoris Activation during Hamstring Strength Exercises: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 8733. [Google Scholar] [CrossRef] [PubMed]
- Claudino, J.G.; Cardoso Filho, C.A.; Bittencourt, N.F.N.; Goncalves, L.G.; Couto, C.R.; Quintao, R.C.; Reis, G.F.; de Oliveira Junior, O.; Amadio, A.C.; Boullosa, D.; et al. Eccentric Strength Assessment of Hamstring Muscles with New Technologies: A Systematic Review of Current Methods and Clinical Implications. Sports Med. Open 2021, 7, 10. [Google Scholar] [CrossRef]
- Kitatani, R.; Ohata, K.; Sato, S.; Watanabe, A.; Hashiguchi, Y.; Yamakami, N.; Sakuma, K.; Yamada, S. Ankle muscle coactivation and its relationship with ankle joint kinematics and kinetics during gait in hemiplegic patients after stroke. Somat. Mot. Res. 2016, 33, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cofre Lizama, L.E.; Bastani, A.; van der Walt, A.; Kilpatrick, T.; Khan, F.; Galea, M.P. Increased ankle muscle coactivation in the early stages of multiple sclerosis. Mult. Scler. J.-Exp. Transl. Clin. 2020, 6, 2055217320905870. [Google Scholar] [CrossRef] [Green Version]
- Arias, P.; Espinosa, N.; Robles-Garcia, V.; Cao, R.; Cudeiro, J. Antagonist muscle co-activation during straight walking and its relation to kinematics: Insight from young, elderly and Parkinson’s disease. Brain Res. 2012, 1455, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Hirai, H.; Miyazaki, F.; Naritomi, H.; Koba, K.; Oku, T.; Uno, K.; Uemura, M.; Nishi, T.; Kageyama, M.; Krebs, H.I. On the Origin of Muscle Synergies: Invariant Balance in the Co-activation of Agonist and Antagonist Muscle Pairs. Front. Bioeng. Biotechnol. 2015, 3, 192. [Google Scholar] [CrossRef] [Green Version]
- Unnithan, V.B.; Dowling, J.J.; Frost, G.; Bar-Or, O. Role of cocontraction in the O2 cost of walking in children with cerebral palsy. Med. Sci. Sports Exerc. 1996, 28, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Nisell, R.; Ericson, M.O.; Nemeth, G.; Ekholm, J. Tibiofemoral joint forces during isokinetic knee extension. Am. J. Sports Med. 1989, 17, 49–54. [Google Scholar] [CrossRef]
- Beynnon, B.; Howe, J.G.; Pope, M.H.; Johnson, R.J.; Fleming, B.C. The measurement of anterior cruciate ligament strain in vivo. Int. Orthop. 1992, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, S.; Solomonow, M.; Lu, Y.; Lou, Z.P.; D’Ambrosia, R. Anterior-posterior and rotational displacement of the tibia elicited by quadriceps contraction. Am. J. Sports Med. 1992, 20, 299–306. [Google Scholar] [CrossRef]
- Balshaw, T.G.; Massey, G.J.; Maden-Wilkinson, T.M.; Lanza, M.B.; Folland, J.P. Neural adaptations after 4 years vs. 12 weeks of resistance training vs. untrained. Scand. J. Med. Sci. Sports 2019, 29, 348–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Habets, B.; Staal, J.B.; Tijssen, M.; van Cingel, R. Intrarater reliability of the Humac NORM isokinetic dynamometer for strength measurements of the knee and shoulder muscles. BMC Res. Notes 2018, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Whinton, A.K.; Thompson, K.M.A.; Power, G.A.; Burr, J.F. Testing a novel isokinetic dynamometer constructed using a 1080 Quantum. PLoS ONE 2018, 13, e0201179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Araujo Ribeiro Alvares, J.B.; Rodrigues, R.; de Azevedo Franke, R.; da Silva, B.G.; Pinto, R.S.; Vaz, M.A.; Baroni, B.M. Inter-machine reliability of the Biodex and Cybex isokinetic dynamometers for knee flexor/extensor isometric, concentric and eccentric tests. Phys. Ther. Sport 2015, 16, 59–65. [Google Scholar] [CrossRef]
- Hortobagyi, T.; Zheng, D.; Weidner, M.; Lambert, N.J.; Westbrook, S.; Houmard, J.A. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, B399–B406. [Google Scholar] [CrossRef] [PubMed]
- Balshaw, T.G.; Massey, G.J.; Maden-Wilkinson, T.M.; Morales-Artacho, A.J.; McKeown, A.; Appleby, C.L.; Folland, J.P. Changes in agonist neural drive, hypertrophy and pre-training strength all contribute to the individual strength gains after resistance training. Eur. J. Appl. Physiol. 2017, 117, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarantini, D.; Bru, B. Training-related changes in the EMG-moment relationship during isometric contractions: Further evidence of improved control of muscle activation in strength-trained men? J. Electromyogr. Kinesiol. 2015, 25, 697–702. [Google Scholar] [CrossRef]
- Orssatto, L.B.R.; Wiest, M.J.; Moura, B.M.; Collins, D.F.; Diefenthaeler, F. Neuromuscular determinants of explosive torque: Differences among strength-trained and untrained young and older men. Scand. J. Med. Sci. Sports 2020, 30, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Vila-Cha, C.; Falla, D.; Farina, D. Motor unit behavior during submaximal contractions following six weeks of either endurance or strength training. J. Appl. Physiol. 2010, 109, 1455–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, R.J.; Cook, S.B. Effect of instructions on EMG during the bench press in trained and untrained males. Hum. Mov. Sci. 2017, 55, 182–188. [Google Scholar] [CrossRef]
- Besomi, M.; Hodges, P.W.; Clancy, E.A.; Van Dieen, J.; Hug, F.; Lowery, M.; Merletti, R.; Sogaard, K.; Wrigley, T.; Besier, T.; et al. Consensus for experimental design in electromyography (CEDE) project: Amplitude normalization matrix. J. Electromyogr. Kinesiol. 2020, 53, 102438. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Cerone, G.L. Tutorial. Surface EMG detection, conditioning and pre-processing: Best practices. J. Electromyogr. Kinesiol. 2020, 54, 102440. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Muceli, S. Tutorial. Surface EMG detection in space and time: Best practices. J. Electromyogr. Kinesiol. 2019, 49, 102363. [Google Scholar] [CrossRef]
- Vieira, T.M.; Botter, A. The Accurate Assessment of Muscle Excitation Requires the Detection of Multiple Surface Electromyograms. Exerc. Sport Sci. Rev. 2021, 49, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Casolo, A.; Negro, F.; Scorcelletti, M.; Bazzucchi, I.; Enoka, R.; Felici, F.; Farina, D. The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J. Physiol. 2019, 597, 1873–1887. [Google Scholar] [CrossRef] [Green Version]
- Trezise, J.; Blazevich, A.J. Anatomical and Neuromuscular Determinants of Strength Change in Previously Untrained Men Following Heavy Strength Training. Front. Physiol. 2019, 10, 1001. [Google Scholar] [CrossRef] [Green Version]
- Cadore, E.L.; Gonzalez-Izal, M.; Grazioli, R.; Setuain, I.; Pinto, R.S.; Izquierdo, M. Effects of Concentric and Eccentric Strength Training on Fatigue Induced by Concentric and Eccentric Exercise. Int. J. Sports Physiol. Perform. 2019, 14, 91–98. [Google Scholar] [CrossRef]
- Komi, P.V.; Buskirk, E.R. Effect of eccentric and concentric muscle conditioning on tension and electrical activity of human muscle. Ergonomics 1972, 15, 417–434. [Google Scholar] [CrossRef]
- Purkayastha, S.; Cramer, J.T.; Trowbridge, C.A.; Fincher, A.L.; Marek, S.M. Surface electromyographic amplitude-to-work ratios during isokinetic and isotonic muscle actions. J. Athl. Train. 2006, 41, 314–320. [Google Scholar] [PubMed]
- Rothstein, J.M.; Delitto, A.; Sinacore, D.R.; Rose, S.J. Electromyographic, peak torque, and power relationships during isokinetic movement. Phys. Ther. 1983, 63, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Bazzucchi, I.; Sbriccoli, P.; Marzattinocci, G.; Felici, F. Coactivation of the elbow antagonist muscles is not affected by the speed of movement in isokinetic exercise. Muscle Nerve 2006, 33, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Clancy, E.A.; Cairns, K.D.; Riley, P.O.; Meister, M.; Kerrigan, D.C. Effects of treadmill walking speed on lateral gastrocnemius muscle firing. Am. J. Phys. Med. Rehabil. 2004, 83, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Q.; Anderson, F.C.; Schwartz, M.H.; Delp, S.L. Muscle contributions to support and progression over a range of walking speeds. J. Biomech. 2008, 41, 3243–3252. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Trained (n = 10, 4F) | Untrained (n = 11, 5F) |
|---|---|---|
| Age, y | 22.8 ± 1.75 | 21.1 ± 2.55 |
| Height, cm | 178.1 ± 8.45 | 171.1 ± 12.10 |
| Mass, kg | 78.9 ± 13.08 | 64.6 ± 10.58 * |
| BMI, kg·m−2 | 24.7 ± 2.25 | 21.9 ± 1.59 * |
| Quadriceps MVC, Nm | 216 ± 88 | 137 ± 48 * |
| Quadriceps MVC, Nm·kg−1 | 2.80 ± 1.15 | 2.20 ± 0.97 * |
| Hamstring MVC, Nm | 202 ± 84 | 134 ± 53 * |
| Hamstring MVC, Nm·kg−1 | 2.63 ± 1.19 | 2.10 ± 0.63 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hortobágyi, T.; DeVita, P.; Brady, R.; Rider, P. Training History-Dependent Functional Role of EMG Model-Predicted Antagonist Moments in Knee Extensor Moment Generation in Healthy Young Adults. Biomechanics 2022, 2, 7-19. https://doi.org/10.3390/biomechanics2010002
Hortobágyi T, DeVita P, Brady R, Rider P. Training History-Dependent Functional Role of EMG Model-Predicted Antagonist Moments in Knee Extensor Moment Generation in Healthy Young Adults. Biomechanics. 2022; 2(1):7-19. https://doi.org/10.3390/biomechanics2010002
Chicago/Turabian StyleHortobágyi, Tibor, Paul DeVita, Robert Brady, and Patrick Rider. 2022. "Training History-Dependent Functional Role of EMG Model-Predicted Antagonist Moments in Knee Extensor Moment Generation in Healthy Young Adults" Biomechanics 2, no. 1: 7-19. https://doi.org/10.3390/biomechanics2010002
APA StyleHortobágyi, T., DeVita, P., Brady, R., & Rider, P. (2022). Training History-Dependent Functional Role of EMG Model-Predicted Antagonist Moments in Knee Extensor Moment Generation in Healthy Young Adults. Biomechanics, 2(1), 7-19. https://doi.org/10.3390/biomechanics2010002






