Development of a Lower Limb Finite Element Musculoskeletal Gait Simulation Framework Driven Solely by Inertial Measurement Unit Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Finite Element Musculoskeletal Model
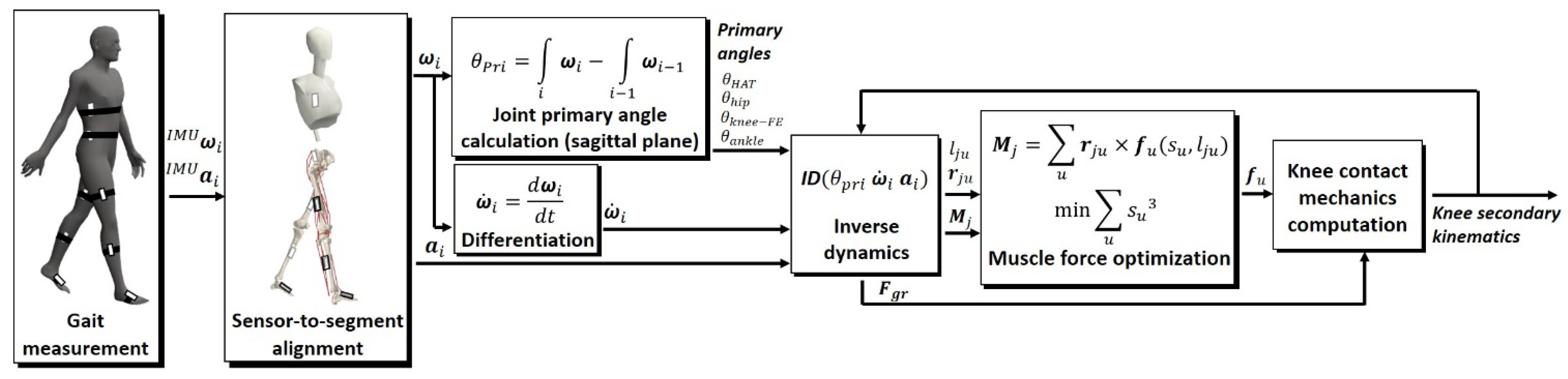
2.2. Prediction of the Knee Contact Mechanics and Muscle Forces
2.2.1. Estimation of the Ground Reaction Force and Moments
2.2.2. Muscle Force Optimization and Knee Contact Mechanics Computation
2.3. Subject Experiments
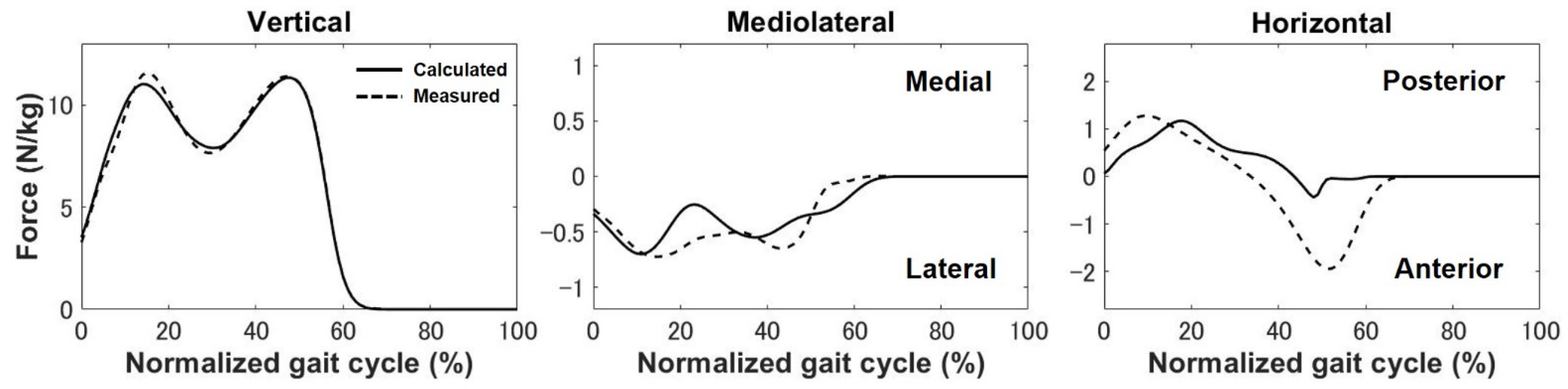
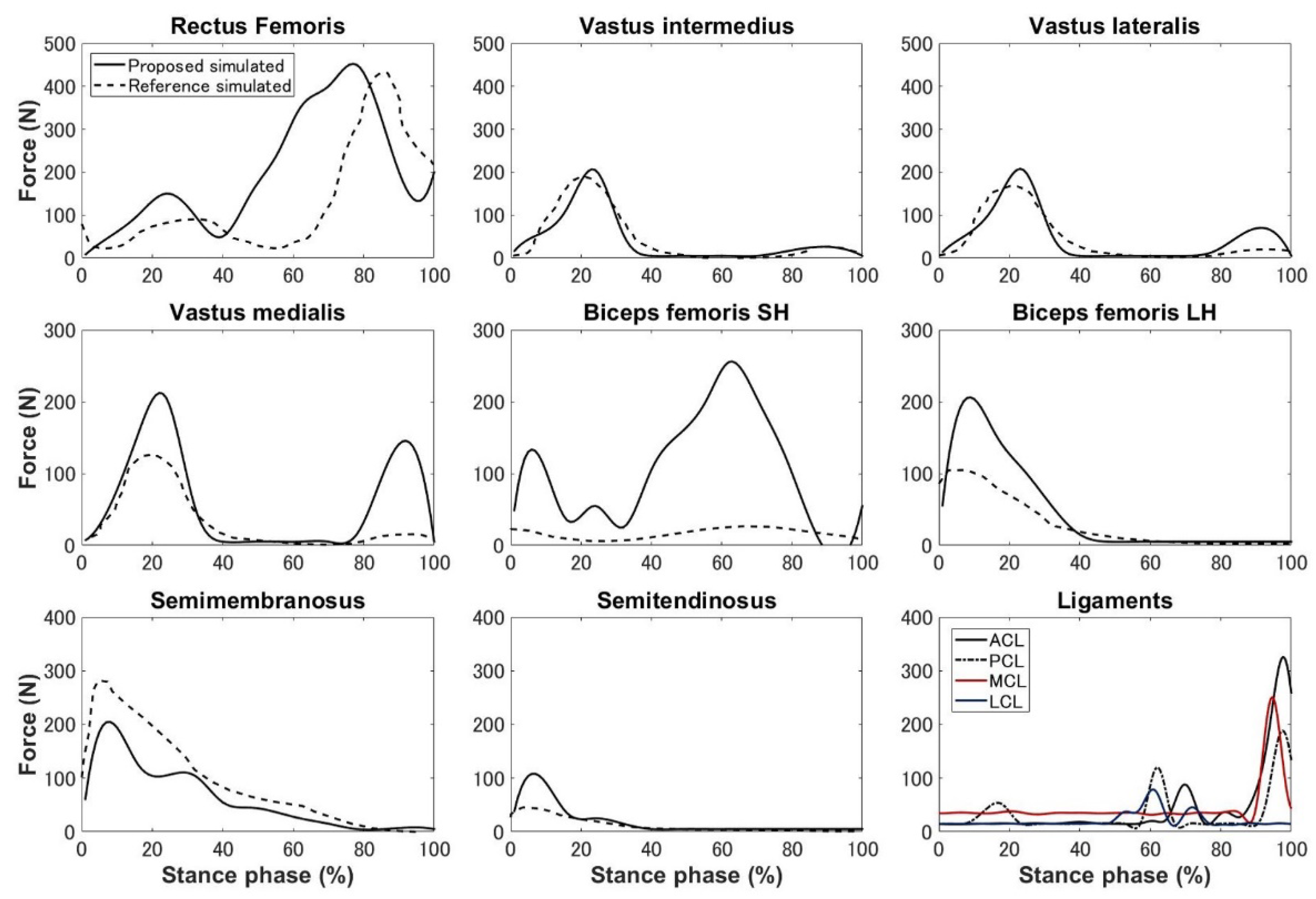
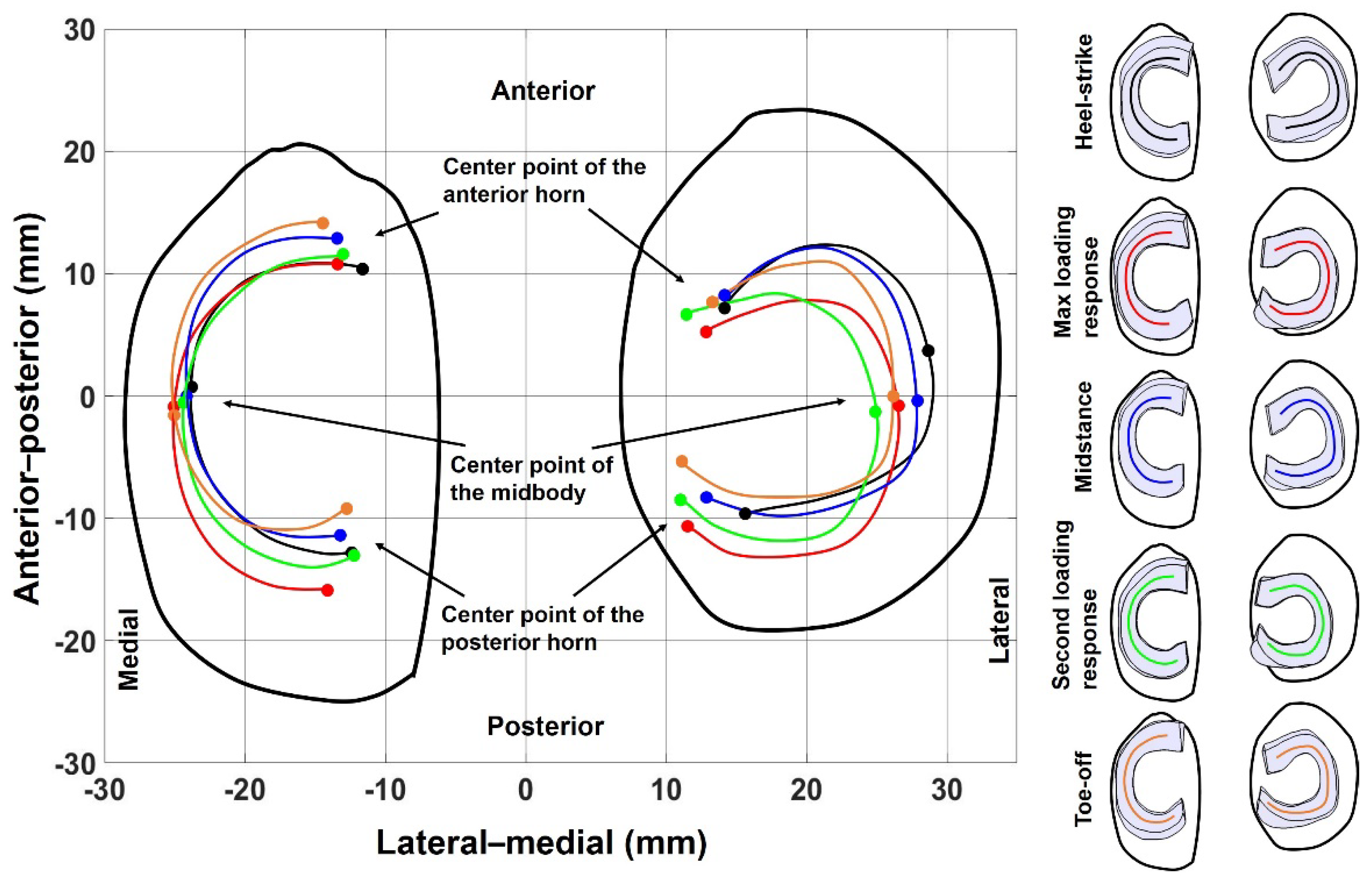
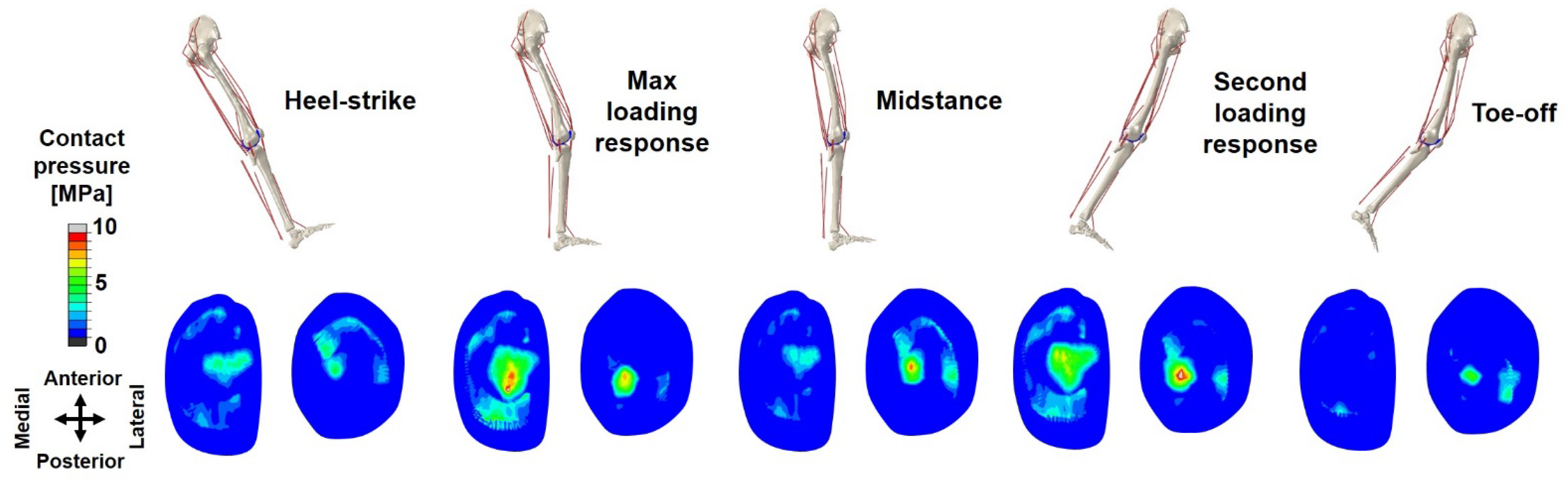
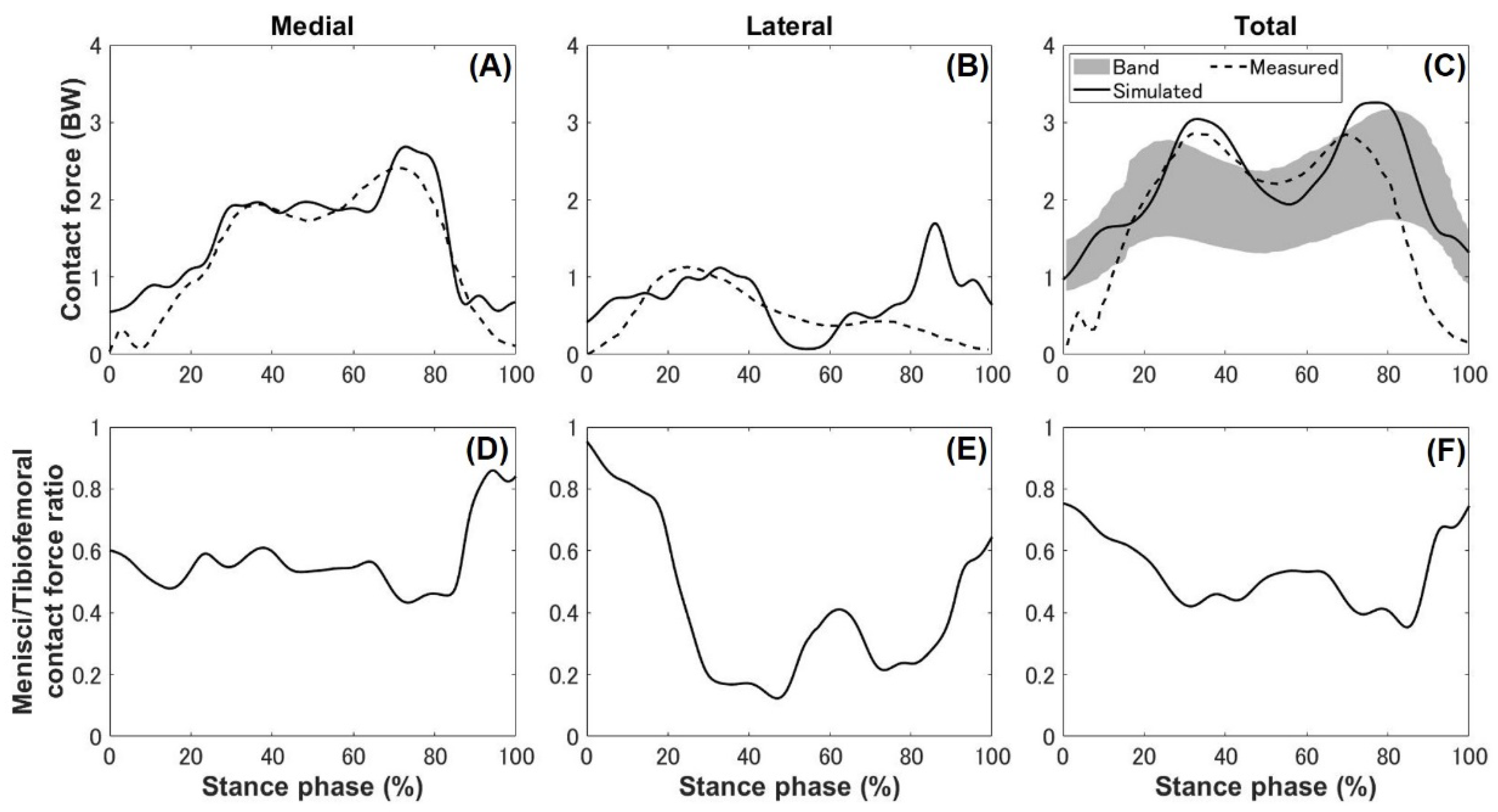
3. Results
4. Discussion
4.1. Kinematics, Kinetics, and Ligament Force
4.2. Meniscal Translocation and Deformation
4.3. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Baliunas, A.J.; Hurwitz, D.E.; Ryals, A.B.; Karrar, A.; Case, J.P.; Block, J.A.; Andriacchi, T.P. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthr. Cartil. 2002, 10, 573–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiden, T.L.; Lloyd, D.G.; Ackland, T.R. Knee joint kinematics, kinetics and muscle co-contraction in knee osteoarthritis patient gait. Clin. Biomech. 2009, 24, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Delp, S.L.; Loan, J.P.; Hoy, M.G.; Zajac, F.E.; Topp, E.L.; Rosen, J.M. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans. Biomed. Eng. 1990, 37, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Donahue, T.L.H.; Hull, M.L.; Rashid, M.M.; Jacobs, C.R. A finite element model of the human knee joint for the study of tibio-femoral contact. J. Biomech. Eng. 2002, 124, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Halonen, K.S.; Dzialo, C.M.; Mannisi, M.; Venäläinen, M.S.; Zee, M.D.; Andersen, M.S. Workflow assessing the effect of gait alterations on stresses in the medial tibial cartilage-combined musculoskeletal modelling and finite element analysis. Sci. Rep. 2017, 7, 17396. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.; Yoon, J.; Chae, S.W. Finite element analysis of knee and ankle joint during gait based on motion analysis. Med. Eng. Phys. 2019, 63, 33–41. [Google Scholar] [CrossRef]
- Shu, L.; Yamamoto, K.; Yao, J.; Saraswat, P.; Liu, Y.; Mitsuishi, M.; Sugita, N. A subject-specific finite element musculoskeletal framework for mechanics analysis of a total knee replacement. J. Biomech. 2018, 77, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.R.; Navacchia, A.; Ali, N.A.; Shelburne, K.B. The interaction of muscle moment arm, knee laxity, and torque in a multi-scale musculoskeletal model of the lower limb. J. Biomech. 2016, 76, 173–180. [Google Scholar] [CrossRef]
- Marouane, H.; Adl, A.S.; Adouni, M. 3D active-passive response of human knee joint in gait is markedly altered when simulated as a planar 2D joint. Biomech. Model. Mechanobiol. 2017, 16, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Shalhoub, S.S.; Cyr, A.J.; Fitzpatrick, C.K.; Maletsky, L.P.; Rullkoetter, P.J.; Shelburne, K.B. Validation of predicted patellofemoral mechanics in a finite element model of the healthy and cruciate-deficient knee. J. Biomech. 2016, 49, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenhart, R.L.; Kaiser, J.; Smith, C.R.; Thelen, D.G. Prediction and validation of load-dependent behavior of the tibiofemoral and patellofemoral joints during movement. Ann. Biomed. Eng. 2015, 43, 2675–2685. [Google Scholar] [CrossRef] [Green Version]
- Mononen, M.E.; Jurvelin, J.S.; Korhonen, R.K. Effects of radial tears and partial meniscectomy of lateral meniscus on the knee joint mechanics during the stance phase of the gait cycle-a 3D finite element study. J. Orthop. Res. 2012, 31, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Harato, K.; Sakurai, A.; Kudo, Y.; Nagura, T.; Masumoto, K.; Otani, T.; Niki, Y. Three-dimensional knee kinematics in patients with a discoid lateral meniscus during gait. Knee 2016, 23, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Burke, D. In-vitro of measurement of static pressure distribution in synovial joints–part I: Tibial surface of the knee. J. Biomech. Eng. 1983, 105, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xin, H.; Chen, Z.; Zhang, Q.; Peng, Y.; Jin, Z. The role of menisci in knee contact mechanics and secondary kinematics during human walking. Clin. Biomech. 2019, 61, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, F.; Li, J.; Dan, M.; Liu, T.; Behr, M. Implementation of controlling strategy in a biomechanical lower limb model with active muscles for coupling multibody dynamics and finite element analysis. J. Biomech. 2019, 91, 51–60. [Google Scholar] [CrossRef]
- Cardona, M.; Cena, C.E.G. Biomechanical analysis of the lower limb: A full-body musculoskeletal model for muscle-driven simulation. IEEE Access 2019, 7, 92709–92723. [Google Scholar] [CrossRef]
- Lathrop, R.L.; Chaudhari, A.M.W.; Siston, R.A. Comparative assessment of bone pose estimation using point cluster technique and OpenSim. J. Biomech. Eng. 2011, 133, 114503. [Google Scholar] [CrossRef] [PubMed]
- Navacchia, A.; Hume, D.R.; Rullkoetter, P.J.; Shelburne, K.B. A computationally efficient strategy to estimate muscle forces in a finite element musculoskeletal model of the lower limb. J. Biomech. 2019, 84, 94–102. [Google Scholar] [CrossRef]
- Gray, H.A.; Guan, S.; Thomeer, L.T.; Schache, A.G.; Steiger, R.D.; Pandy, M.G. Three-dimensional motion of the knee-joint complex during normal walking revealed by mobile biplane X-ray imaging. J. Orthop. Res. 2019, 37, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Jang, S.H.; Cho, J.S.; Kim, M.J.; Lee, H.D.; Lee, S.Y.; Moom, S.B. Evaluation of validity and reliability of inertial measurement unit-based gait analysis systems. Ann. Rehabil. Med. 2018, 42, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Hindle, B.R.; Keogh, J.W.; Lorimer, A.V. Inertial-based human motion capture: A technical summary of current processing methodologies for spatiotemporal and kinematic measures. Appl. Bionics Biomech. 2021, 2021, 6628320. [Google Scholar] [CrossRef] [PubMed]
- McCann, L.; Ingham, E.; Jin, Z.; Fisher, J. Influence of the meniscus on friction and degradation of cartilage in the natural knee joint. Osteoarthr. Cartil. 2009, 17, 995–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.A.; Harris, M.D.; Shalhoub, S.; Maletsky, L.P.; Rullkoetter, P.J.; Shelburne, K.B. Combined measurement and modeling of specimen-specific knee mechanics for healthy and ACL-deficient conditions. J. Biomech. 2017, 57, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar]
- Li, G.; Lopez, O.; Rubash, H. Variability of a three dimensional finite element model constructed using magnetic resonance images of a knee for joint contact stress analysis. J. Biomech. Eng. 2001, 123, 341–346. [Google Scholar] [CrossRef]
- Yao, J.; Snibbe, J.; Maloney, M.; Lerner, A.L. Stresses and strains in the medial meniscus of an ACL deficient knee under anterior loading: A finite element analysis with image-based experimental validation. J. Biomech. Eng. 2006, 128, 135–141. [Google Scholar] [CrossRef]
- Ren, L.; Jones, R.K.; Howard, D. Whole body inverse dynamics over a complete gait cycle based only on measured kinematics. J. Biomech. 2008, 41, 2750–2759. [Google Scholar] [CrossRef]
- Hase, K.; Yamazaki, N. Computer simulation study of human locomotion with a three-dimensional entire-body neuro-musculo-skeletal model (I. Acquisition of normal walking). JSME Int. J. 2002, 45, 1040–1050. [Google Scholar] [CrossRef] [Green Version]
- O’Donovan, K.J.; Kamnik, R.; O’Keeffe, D.T.; Lyons, G.M.A. An inertial and magnetic sensor based technique for joint angle measurement. J. Biomech. 2007, 40, 2604–2611. [Google Scholar] [CrossRef]
- Du, J.; Gerdtman, C.; Lindén, M. Signal quality improvement algorithms for MEMS gyroscope-based human motion analysis systems: A systematic review. Sensors 2018, 18, 1123. [Google Scholar] [CrossRef] [Green Version]
- Kozanek, M.; Hosseini, A.; Liu, F.; Van de Velde, S.K.; Gill, T.J.; Rubash, H.E.; Li, G. Tibiofemoral kinematics and condylar motion during the stance phase of gait. J. Biomech. 2009, 43, 1877–1884. [Google Scholar] [CrossRef] [Green Version]
- Clément, J.; Toliopoulos, P.; Hagemeister, N.; Desmeules, F.; Fuentes, A.; Vendittoli, P.A. Healthy 3D knee kinematics during gait: Differences between women and men, and correlation with X-ray alignment. Gait Post. 2018, 64, 198–204. [Google Scholar] [CrossRef]
- Trinler, U.; Schwameder, H.; Baker, R.; Alexander, N. Muscle force estimation in clinical gait analysis using AnyBody and OpenSim. J. Biomech. 2019, 86, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Cen, X.; Lu, Z.; Baker, J.; István, B.; Gu, Y. A Comparative Biomechanical Analysis during Planned and Unplanned Gait Termination in Individuals with Different Arch Stiffnesses. Appl. Sci. 2021, 11, 1871. [Google Scholar] [CrossRef]
- Dempster, J.; Dutheil, F.; Ugbolue, U.C. The Prevalence of Lower Extremity Injuries in Running and Associated Risk Factors: A Systematic Review. J. Phys. Act. Health 2021, 5, 133–145. [Google Scholar] [CrossRef]
- Arias, L.M.; Iwaniec, J.; Iwaniec, M. Modeling and Analysis of the Power Conditioning Circuit for an Electromagnetic Human Walking-Induced Energy Harvester. Energies 2021, 14, 3367. [Google Scholar] [CrossRef]
- Bergmann, G.; Bender, A.; Graichen, F.; Dymke, J.; Rohlmann, A.; Trepczynski, A.; Heller, M.O.; Kutzner, I. Standardized loads acting in knee implants. PLoS ONE 2014, 9, e86035. [Google Scholar] [CrossRef] [Green Version]
- Zeighami, A.; Aissaoui, R.; Dumas, R. Knee medial and lateral contact forces in musculoskeletal model with subject-specific contact point trajectories. J. Biomech. 2018, 69, 138–145. [Google Scholar] [CrossRef] [Green Version]
- D’Lima, D.D.; Fregly, B.J.; Patil, S.; Steklov, N.; Colwell, C.W.C., Jr. Knee joint force: Prediction, measurement, and significance. Proc. Inst. Mech. Eng. H 2012, 226, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, P.S.; Arno, S.; Bell, C.; Salvadore, G.; Borukhov, I.; Oh, C. Function of the medial meniscus in force transmission and stability. J. Biomech. 2015, 48, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Catalfamo, P.F.; Aguiar, G.; Curi, J.; Braidot, A. Anterior cruciate ligament injury: Compensation during gait using hamstring muscle activity. Open Biomed. Eng. J. 2010, 4, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Vedi, V.; Williams, A.; Tennant, S.J.; Spouse, E.; Hunt, D.M.; Gedroyc, W.M.W. Meniscal movement: An in-vivo study using dynamic MRI. Bone Jt. J. 1999, 81, 37–41. [Google Scholar] [CrossRef]
- Thompson, W.O.; Thaete, F.L.; Fu, F.H.; Dye, S.F. Tibial meniscal dynamics using three-dimensional reconstruction of magnetic resonance images. Am. J. Sports Med. 1991, 19, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Guess, T.M.; Razu, S. Loading of the medial meniscus in the ACL deficient knee: A multibody computational study. Med. Eng. Phys. 2017, 41, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Winter, D.A. The Biomechanics and Motor Control of Human Movement; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 189–191. [Google Scholar]


| Tissues | Element Type | Young’s Modulus [MPa] | Poisson Ratio | Density [kg/m3] |
|---|---|---|---|---|
| Bones | Triangular shell | - | - | 1,100,000 |
| Cartilages [26] | 8-node hexahedra | 10 | 0.46 | 1800 |
| Menisci [4,27] | 8-node hexahedra | Radial and axial: 20 | In-plane: 0.2 | 1800 |
| Circumferential: 140 | Out-plane: 0.3 | |||
| Ligaments, Meniscal horn | Nonlinear spring | - | - | 1500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Hase, K.; Ota, S. Development of a Lower Limb Finite Element Musculoskeletal Gait Simulation Framework Driven Solely by Inertial Measurement Unit Sensors. Biomechanics 2021, 1, 293-306. https://doi.org/10.3390/biomechanics1030025
Wang S, Hase K, Ota S. Development of a Lower Limb Finite Element Musculoskeletal Gait Simulation Framework Driven Solely by Inertial Measurement Unit Sensors. Biomechanics. 2021; 1(3):293-306. https://doi.org/10.3390/biomechanics1030025
Chicago/Turabian StyleWang, Sentong, Kazunori Hase, and Susumu Ota. 2021. "Development of a Lower Limb Finite Element Musculoskeletal Gait Simulation Framework Driven Solely by Inertial Measurement Unit Sensors" Biomechanics 1, no. 3: 293-306. https://doi.org/10.3390/biomechanics1030025
APA StyleWang, S., Hase, K., & Ota, S. (2021). Development of a Lower Limb Finite Element Musculoskeletal Gait Simulation Framework Driven Solely by Inertial Measurement Unit Sensors. Biomechanics, 1(3), 293-306. https://doi.org/10.3390/biomechanics1030025






