Margin of Stability May Be Larger and Less Variable during Treadmill Walking Versus Overground
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
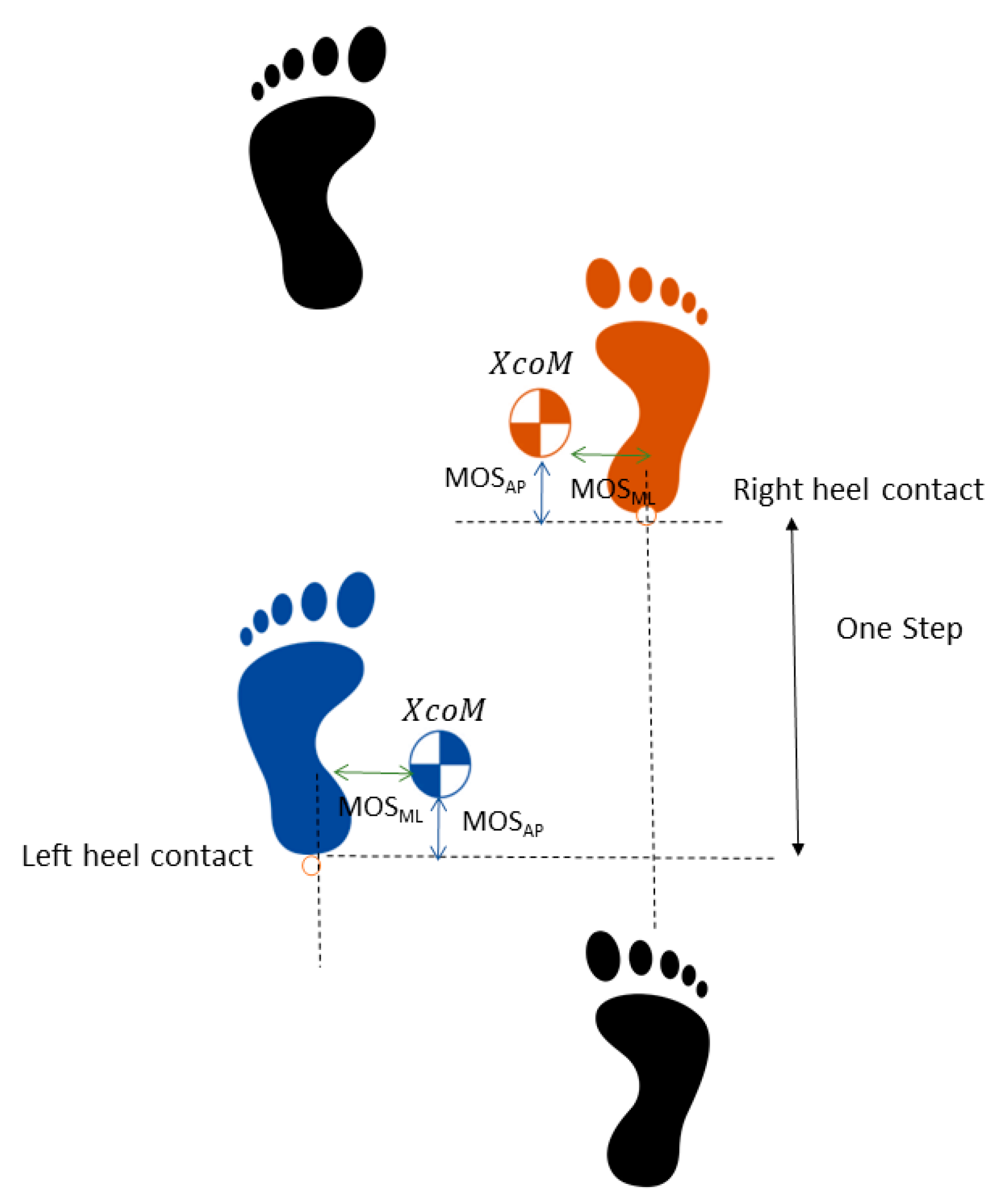
2.3. Data Analysis
2.4. Statistics
3. Results
4. Discussion
Limitations and Conclusions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caderby, T.; Yiou, E.; Peyrot, N.; Begon, M.; Dalleau, G. Influence of gait speed on the control of mediolateral dynamic stability during gait initiation. J. Biomech. 2014, 47, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Hof, A.L.; Gazendam, M.G.; Sinke, W.E. The condition for dynamic stability. J. Biomech. 2005, 38, 1–8. [Google Scholar] [CrossRef]
- Hof, A.L. The ‘extrapolated center of mass’ concept suggests a simple control of balance in walking. Hum. Mov. Sci. 2008, 27, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Peebles, A.T.; Bruetsch, A.P.; Lynch, S.G.; Huisinga, J.M. Dynamic balance in persons with multiple sclerosis who have a falls history is altered compared to non-fallers and to healthy controls. J. Biomech. 2017, 63, 158–163. [Google Scholar] [CrossRef]
- Vlutters, M.; van Asseldonk, E.H.F.; van der Kooij, H. Center of mass velocity-based predictions in balance recovery following pelvis perturbations during human walking. J. Exp. Biol. 2016, 219, 1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAndrew Young, P.M.; Wilken, J.M.; Dingwell, J.B. Dynamic margins of stability during human walking in destabilizing environments. J. Biomech. 2012, 45, 1053–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peebles, A.T.; Reinholdt, A.; Bruetsch, A.P.; Lynch, S.G.; Huisinga, J.M. Dynamic margin of stability during gait is altered in persons with multiple sclerosis. J. Biomech. 2016, 49, 3949–3955. [Google Scholar] [CrossRef] [Green Version]
- Sivakumaran, S.; Schinkel-Ivy, A.; Masani, K.; Mansfield, A. Relationship between margin of stability and deviations in spatiotemporal gait features in healthy young adults. Hum. Mov. Sci. 2018, 57, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Madehkhaksar, F.; Klenk, J.; Sczuka, K.; Gordt, K.; Melzer, I.; Schwenk, M. The effects of unexpected mechanical perturbations during treadmill walking on spatiotemporal gait parameters, and the dynamic stability measures by which to quantify postural response. PLOS ONE 2018, 13, e0195902. [Google Scholar] [CrossRef] [PubMed]
- Arampatzis, A.; Karamanidis, K.; Mademli, L. Deficits in the way to achieve balance related to mechanisms of dynamic stability control in the elderly. J. Biomech. 2008, 41, 1754–1761. [Google Scholar] [CrossRef]
- Winter, D.A. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Suptitz, F.; Karamanidis, K.; Moreno Catala, M.; Bruggemann, G.P. Symmetry and reproducibility of the components of dynamic stability in young adults at different walking velocities on the treadmill. J. Electromyogr. Kinesiol. 2012, 22, 301–307. [Google Scholar] [CrossRef]
- McCrum, C.; Willems, P.; Karamanidis, K.; Meijer, K. Stability-normalised walking speed: A new approach for human gait perturbation research. J. Biomech. 2019, 87, 48–53. [Google Scholar] [CrossRef]
- Fallahtafti, F.; Curtze, C.; Samson, K.; Yentes, J.M. Chronic obstructive pulmonary disease patients increase medio-lateral stability and limit changes in antero-posterior stability to curb energy expenditure. Gait Posture 2020, 75, 142–148. [Google Scholar] [CrossRef] [PubMed]
- McCrum, C.; Gerards, M.H.G.; Karamanidis, K.; Zijlstra, W.; Meijer, K. A systematic review of gait perturbation paradigms for improving reactive stepping responses and falls risk among healthy older adults. Eur. Rev. Aging Phys. Act. 2017, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, A.M.; van Dieën, J.H.; Daffertshofer, A.; Bruijn, S.M. Active foot placement control ensures stable gait: Effect of constraints on foot placement and ankle moments. PLoS ONE 2020, 15, e0242215. [Google Scholar] [CrossRef] [PubMed]
- Alton, F.; Baldey, L.; Caplan, S.; Morrissey, M.C. A kinematic comparison of overground and treadmill walking. Clin. Biomech. 1998, 13, 434–440. [Google Scholar] [CrossRef]
- Murray, M.P.; Spurr, G.B.; Sepic, S.B.; Gardner, G.M.; Mollinger, L.A. Treadmill vs. floor walking: Kinematics, electromyogram, and heart rate. J. Appl. Physiol. 1985, 59, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, N.J.; Grabiner, M.D. Measures of frontal plane stability during treadmill and overground walking. Gait Posture 2010, 31, 380–384. [Google Scholar] [CrossRef]
- Kao, P.C.; Dingwell, J.B.; Higginson, J.S.; Binder-Macleod, S. Dynamic instability during post-stroke hemiparetic walking. Gait Posture 2014, 40, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Havens, K.L.; Mukherjee, T.; Finley, J.M. Analysis of biases in dynamic margins of stability introduced by the use of simplified center of mass estimates during walking and turning. Gait Posture 2018, 59, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; King, G.A. Dynamic gait stability of treadmill versus overground walking in young adults. J. Electromyogr. Kinesiol. 2016, 31, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Yentes, J.M.; Rennard, S.I.; Schmid, K.K.; Blanke, D.; Stergiou, N. Patients with Chronic Obstructive Pulmonary Disease Walk with Altered Step Time and Step Width Variability as Compared with Healthy Control Subjects. Ann. Am. Thorac. Soc. 2017, 14, 858–866. [Google Scholar] [CrossRef]
- Stenum, J.; Bruijn, S.M.; Jensen, B.R. The effect of walking speed on local dynamic stability is sensitive to calculation methods. J. Biomech. 2014, 47, 3776–3779. [Google Scholar] [CrossRef] [PubMed]
- Bates, B.T.; Dufek, J.S.; Davis, H.P. The effect of trial size on statistical power. Med. Sci. Sports Exerc. 1992, 24, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Dufek, J.S.; Bates, B.T.; Davis, H.P. The effect of trial size and variability on statistical power. Med. Sci. Sports Exerc. 1995, 27, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zeni, J.A., Jr.; Richards, J.G.; Higginson, J.S. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 2008, 27, 710–714. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.; Berry, J.J. The efficiency of simulation-based multiple comparisons. Biometrics 1987, 43, 913–928. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.M.; Kuo, A.D. Direction-dependent control of balance during walking and standing. J. Neurophysiol. 2009, 102, 1411–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gates, D.H.; Scott, S.J.; Wilken, J.M.; Dingwell, J.B. Frontal plane dynamic margins of stability in individuals with and without transtibial amputation walking on a loose rock surface. Gait Posture 2013, 38, 570–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hak, L.; Houdijk, H.; Steenbrink, F.; Mert, A.; van der Wurff, P.; Beek, P.J.; van Dieën, J.H. Speeding up or slowing down?: Gait adaptations to preserve gait stability in response to balance perturbations. Gait Posture 2012, 36, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Hak, L.; van Dieën, J.H.; van der Wurff, P.; Prins, M.R.; Mert, A.; Beek, P.J.; Houdijk, H. Walking in an Unstable Environment: Strategies Used by Transtibial Amputees to Prevent Falling During Gait. Arch. Phys. Med. Rehabil. 2013, 94, 2186–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buurke, T.J.W.; Lamoth, C.J.C.; van der Woude, L.H.V.; Hof, A.L.; den Otter, R. Bilateral temporal control determines mediolateral margins of stability in symmetric and asymmetric human walking. Sci. Rep. 2019, 9, 12494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblatt, N.J.; Hurt, C.P.; Grabiner, M.D. Sensitivity of dynamic stability to changes in step width during treadmill walking by young adults. J. Appl. Biomech. 2012, 28, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Challis, J.H.; Newell, K.M. Walking speed influences on gait cycle variability. Gait Posture 2007, 26, 128–134. [Google Scholar] [CrossRef]
- Brisswalter, J.; Mottet, D. Energy Cost and Stride Duration Variability at Preferred Transition Gait Speed Between Walking and Running. Can. J. Appl. Physiol. 1996, 21, 471–480. [Google Scholar] [CrossRef] [PubMed]
- König Ignasiak, N.; Ravi, D.K.; Orter, S.; Hosseini Nasab, S.H.; Taylor, W.R.; Singh, N.B. Does variability of footfall kinematics correlate with dynamic stability of the centre of mass during walking? PLoS ONE 2019, 14, e0217460. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.H.; Kuo, A.D. Two independent contributions to step variability during over-ground human walking. PLoS ONE 2013, 8, e73597. [Google Scholar] [CrossRef] [Green Version]
- Donelan, J.M.; Kram, R.; Kuo, A.D. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J. Exp. Biol. 2002, 205, 3717. [Google Scholar] [CrossRef]
- Elftman, H. Biomechanics of Muscle with particular application to studies of gait. JBJS 1966, 48, 363–377. [Google Scholar] [CrossRef]
- McAndrew Young, P.M.; Dingwell, J.B. Voluntary changes in step width and step length during human walking affect dynamic margins of stability. Gait Posture 2012, 36, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Zelik, K.E.; Kuo, A.D. Human walking isn’t all hard work: Evidence of soft tissue contributions to energy dissipation and return. J. Exp. Biol. 2010, 213, 4257. [Google Scholar] [CrossRef] [Green Version]
- Nymark, J.R.; Balmer, S.J.; Melis, E.H.; Lemaire, E.D.; Millar, S. Electromyographic and kinematic nondisabled gait differences at extremely slow overground and treadmill walking speeds. J. Rehabil. Res. Dev. 2005, 42, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Kirtley, C.; Whittle, M.W.; Jefferson, R.J. Influence of walking speed on gait parameters. J. Biomed. Eng. 1985, 7, 282–288. [Google Scholar] [CrossRef]
- Hof, A.L.; Curtze, C. A stricter condition for standing balance after unexpected perturbations. J. Biomech. 2016, 49, 580–585. [Google Scholar] [CrossRef] [PubMed]

| Age (Years) | 23 (2.39) | ||||
| Body Mass (kg) | 72.42 (12.90) | ||||
| Height (cm) | 175.75 (5.84) | ||||
| Preferred Speed (m/s) | 1.3 (0.09) | ||||
| Treadmill | Overground | p-Value | 95% Confidence Interval of the Paired Differences | ||
| Step width (mm) | Lower | Upper | |||
| Slow | 145.78 (30.79) | 137.81 (37.24) | 0.28 | −8.25 | 24.18 |
| Preferred | 132.89 (36.39) | 130.22 (33.68) | 0.70 | −12.96 | 18.30 |
| Fast | 136.38 (39.32) | 140.41 (33.64) | 0.45 | −16.02 | 7.96 |
| Step time (s) | |||||
| Slow | 0.69 (0.03) | 0.69 (0.03) | 0.80 | −0.03 | 0.03 |
| Preferred | 0.53 (0.03) | 0.54 (0.03) | 0.16 | −0.015 | 0.01 |
| Fast | 0.46 (0.03) | 0.47 (0.03) | 0.53 | −0.01 | 0.01 |
| Step length (mm) | |||||
| Slow | 559.52 (38.94) | 538.87 (33.31) | 0.05 | 0.20 | 41.11 |
| Preferred | 716.62 (50.79) | 708.58 (49.15) | 0.14 | −3.43 | 19.51 |
| Fast | 869.79(64.96) | 857.56 (65.00) | 0.12 | −3.94 | 28.40 |
| Step speed (mm/s) | |||||
| Slow | 811.18 (57.50) | 778.84 (54.81) | 0.001 | 17.64 | 47.04 |
| Preferred | 1339.81 (101.43) | 1312.28 (107.67) | 0.01 | 8.72 | 46.36 |
| Fast | 1866.74 (136.02) | 1830.67 (150.91) | 0.004 | 15.41 | 56.74 |
| Step width variability (mm) | |||||
| Slow | 16.56 (4.06) | 20.23 (7.76) | 0.24 | −10.46 | 3.12 |
| Preferred | 17.23 (2.87) | 24.72 (4.18) | 0.01 | −12.65 | −2.33 |
| Fast | 18.98 (3.00) | 28.41 (12.41) | 0.06 | −19.19 | 0.33 |
| Step time variability (ms) | |||||
| Slow | 23.25 (6.28) | 25.92 (5.51) | 0.48 | −0.01 | 0.01 |
| Preferred | 9.84 (2.61) | 13.56 (2.83) | 0.03 | −0.01 | −0.001 |
| Fast | 8.79 (0.99) | 13.09 (3.06) | 0.004 | −0.01 | −0.001 |
| Step length variability (mm) | |||||
| Slow | 24.78 (6.91) | 25.57 (5.47) | 0.81 | −8.26 | 6.68 |
| Preferred | 15.55 (3.75) | 21.01 (5.85) | 0.03 | −10.33 | −0.58 |
| Fast | 18.27 (1.58) | 24.18 (5.43) | 0.01 | −10.21 | −1.62 |
| Step speed variability (mm/s) | |||||
| Slow | 20.26 (5.99) | 45.46 (8.17) | <0.0001 | −33.52 | −16.88 |
| Preferred | 19.32 (5.19) | 54.38 (17.22) | <0.0001 | −48.20 | −21.92 |
| Fast | 22.71 (3.4) | 64.38 (15.71) | <0.0001 | −54.34 | −29.02 |
| Pace and Condition Subgroups | Model Estimated Mean | Standard Error | 95% Confidence Interval for Estimated Mean | Significant Post Hocs | p-Value for Interaction | Group Comparison for Effect Size | Effect Size | ||
|---|---|---|---|---|---|---|---|---|---|
| MOS ML Mean | 0.78 | ||||||||
| Pace: | <0.0001 †† | ||||||||
| Fast | 38.32 | 2.98 | 31.92 | 44.72 | F vs. P | −1.96 | |||
| Preferred | 42.36 | 1.68 | 38.75 | 45.97 | P vs. S | −6.05 | |||
| Slow | 55.73 | 2.54 | 50.29 | 61.17 | F vs. S | −4.54 | |||
| Condition: | 0.01 | ||||||||
| Overground | 42.16 | 2.16 | 37.04 | 47.27 | O vs. T | −3.89 | |||
| Treadmill | 48.78 | 1.89 | 44.31 | 53.26 | |||||
| MOS AP Mean | 0.65 | ||||||||
| Pace: | <0.0001 † | ||||||||
| Fast | −317.02 | 10.76 | −340.10 | −293.93 | F vs. P | −17.94 | |||
| Preferred | −167.88 | 6.61 | −182.07 | −153.69 | P vs. S | −17.55 | |||
| Slow | −33.58 | 7.89 | −50.49 | −16.66 | F vs. S | −20.52 | |||
| Condition: | 0.89 | ||||||||
| Overground | −172.23 | 7.56 | −190.11 | −154.34 | O vs. T | 0.14 | |||
| Treadmill | −173.42 | 7.56 | −191.31 | −155.54 | |||||
| Pace and Condition Subgroups | Model Estimated Mean | Standard Error | 95% Confidence Interval for Estimated Mean | Significant Post Hocs | p-Value for Interaction | Group Comparison for Effect Size | Effect Size | ||
|---|---|---|---|---|---|---|---|---|---|
| MOS ML Variability | <0.03 | ||||||||
| Overground: | |||||||||
| Fast | 14.68 | 1.41 | 11.65 | 17.71 | * | F vs. P | 3.56 | ||
| Preferred | 12.10 | 1.27 | 9.37 | 14.82 | P vs. S | −1.35 | |||
| Slow | 13.48 | 1.32 | 10.65 | 16.32 | F vs. S | 0.94 | |||
| Treadmill: | |||||||||
| Fast | 11.17 | 0.83 | 9.38 | 12.96 | F vs. P | 1.84 | |||
| Preferred | 10.37 | 0.78 | 8.69 | 12.05 | P vs. S | −3.73 | |||
| Slow | 12.64 | 0.83 | 10.87 | 14.41 | * | F vs. S | −1.99 | ||
| MOS AP Variability | <0.0001 | ||||||||
| Overground: | |||||||||
| Fast | 55.59 | 3.46 | 48.17 | 63.02 | ** | F vs. P | 9.58 | ||
| Preferred | 33.02 | 1.69 | 29.40 | 36.64 | P vs. S | 3.19 | |||
| Slow | 25.52 | 2.31 | 20.57 | 30.47 | *,† | F vs. S | 9.37 | ||
| Treadmill: | |||||||||
| Fast | 20.39 | 2.18 | 15.72 | 25.05 | F vs. P | 1.15 | |||
| Preferred | 18.68 | 1.41 | 15.66 | 21.70 | P vs. S | −2.67 | |||
| Slow | 22.61 | 1.79 | 18.77 | 26.44 | F vs. S | −1.14 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallahtafti, F.; Mohammadzadeh Gonabadi, A.; Samson, K.; Yentes, J.M. Margin of Stability May Be Larger and Less Variable during Treadmill Walking Versus Overground. Biomechanics 2021, 1, 118-130. https://doi.org/10.3390/biomechanics1010009
Fallahtafti F, Mohammadzadeh Gonabadi A, Samson K, Yentes JM. Margin of Stability May Be Larger and Less Variable during Treadmill Walking Versus Overground. Biomechanics. 2021; 1(1):118-130. https://doi.org/10.3390/biomechanics1010009
Chicago/Turabian StyleFallahtafti, Farahnaz, Arash Mohammadzadeh Gonabadi, Kaeli Samson, and Jennifer M. Yentes. 2021. "Margin of Stability May Be Larger and Less Variable during Treadmill Walking Versus Overground" Biomechanics 1, no. 1: 118-130. https://doi.org/10.3390/biomechanics1010009






