Abstract
Dietary supplements often promote their antioxidant content as an indicator of quality on the packaging. This study evaluated the redox potential and total antioxidant capacity of various dietary supplements, using different analytical methods to obtain the complexity of antioxidant measurements. A green approach for detecting total antioxidant capacity in dietary products utilized modern electrochemical techniques, including differential pulse voltammetry (DPV) and cyclic voltammetry (CV). These rapid “green” methods measure the redox potential of samples, providing information about the electron-donating ability of antioxidants without the use of harmful chemicals or sample treatments, with minimal environmental impact. ABTS and FRAP measurements were expressed as vitamin C equivalents to allow comparison with CV measurements and actual vitamin C content. This approach enabled indirect comparison of activities obtained using different standard substances through conversion to standard equivalents. The results revealed that the claims made on product labels and packaging often overestimated the antioxidant content and did not match the measured total antioxidant capacities obtained in the current study. Measured vitamin C levels in 10 samples fell within the declared ranges (0–950 mg), but six products contained 4.85% to 49.18% less, and two had significantly higher levels (4.20% and 32.22%) than their declared (p < 0.05). Total antioxidant capacity varied from the labeled values. Similar trends were observed across methods, except for DPPH. FRAP values were correlated with ABTS and CV (r = 0.797 and r = 0.757, respectively). The DPV method provided a more detailed assessment of the redox activity of selected products based on distinct oxidation peaks. The study highlights the importance of mandatory testing and quantification of antioxidants, as well as the need for regulation of antioxidant properties through normative standards.
1. Introduction
Antioxidants are molecules that play a crucial role in reducing oxidative damage to cells, protecting them from the harmful effects of free radicals. This can be achieved through direct reactions, inhibition of enzyme generation, or by enhancing the activity of intracellular antioxidant enzymes [1,2].
Oxidation and reduction are fundamental processes in biological systems. Free radical oxidants, such as reactive oxygen species, are produced inside cells through various metabolic processes. The body is equipped with an antioxidant defense system that guards against oxidative damage caused by these reactive oxidants and plays a major role in protecting cells from oxidative stress and damage. Oxygen-derived radicals are generated constantly as a consequence of normal aerobic life. The imbalance between their production and the ability to neutralize them leads to oxidative stress of the organism and activates antioxidant defense mechanisms.
Nowadays, numerous dietary products are marketed as supplements to the normal diet, supposedly to help fulfill basic nutritional needs. Dietary supplements (DS) are concentrated sources of individual or a combination of physiologically and nutritionally active ingredients [3,4,5]. The antioxidants are widely accepted as a key factor in determining the quality of dietary supplements, as they enhance the biological value of the diet and have positive effects on the body [6,7,8,9,10]. The most commonly used antioxidants in these products are two types of non-enzymatic antioxidants, lipophilic (tocopherols, retinol, carotenoids) and hydrophilic (ascorbate–vitamin C, organically bound selenium, polyphenolics, low molecular weight thiols, plant extracts, animal-derived substances, such as bee products or bovine colostrum, enzymatic cofactors, coenzyme Q10). Their biological availability and effectiveness depend on concentration, chemical form, and interactions between different elements. The sum of antioxidant activities related to these molecules was earlier defined as total antioxidant capacity [2,11,12].
Despite the growing market for these products, there is a lack of standardized testing protocols, which leads to inconsistencies in labeling and confusion among consumers. Although some supplements claim to possess high antioxidant capacities, these claims often do not align with actual measurements [13,14,15,16,17]. The current protocols for measuring antioxidant capacity are complex, time-consuming, and lack standardization, leading to discrepancies in reported results. Therefore, a more reliable and standardized method is needed to ensure that label values accurately reflect the true antioxidant potential of the products [18,19]. The most common methods are spectrophotometric assays, including: 2,2-diphenyl-picrylhydrazyl (DPPH) radical scavenging assay, ferric reducing antioxidant power (FRAP) assay, and 2,2-azino-bis(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) radical cation decolorization assay. These methods have limitations in applicability, selectivity, and sensitivity, making direct comparison difficult and often relying on reagents that can be hazardous [20,21]. A new approach for detecting total antioxidant capacity in dietary products utilizes modern electrochemical techniques, including differential pulse voltammetry (DPV) and cyclic voltammetry (CV). These methods measure the redox potential of samples, providing information about the electron-donating ability of antioxidants without the use of harmful chemicals and sample treatment. They are considered “green techniques” because they have minimal environmental impact, as they do not use organic solvents, allow for rapid analysis, and are reliable and cost-effective [22,23]. These methods have found wide application in the rapid, reliable, and “green” analysis of the redox and antioxidant potential of food products. In addition to the fact that various methods can be developed for the selective and highly sensitive analysis of individual components based on chemically modified electrodes, the application of electrochemical methods for monitoring antioxidant capacity without complex sample preparation and with minimal consumption of additional chemicals has been demonstrated. This approach, using a standard glassy carbon electrode, enables a very high integration of these methods into modern trends in analytical and environmental chemistry [24,25,26,27]. Compared to amperometry, which is a dominantly quantitate method, cyclic voltammetry can be applied for quantitative and qualitative analysis of redox processes, providing information on oxidation and reduction potentials and the reversibility of reactions. Oxidation and reduction peaks in the voltammogram are used for the detection of active components, electron transfer mechanisms, and diffusion or kinetic properties. Chronoamperometry, on the other hand, measures current as a function of time after a potential step and is mainly used to study kinetics. Compared to previous methods, differential pulse voltammetry shows higher sensitivity and lower detection limits compared to CV and amperometry, as it applies a pulsed signal that minimizes background current. It effectively separates closely spaced oxidation peaks, allowing the detection of multiple antioxidant components in complex matrices and highly accurate quantitative analysis. It can detect redox potential within a mixture, which is especially important for complex samples such as dietary supplements or herbal extracts [28,29].
Analysis of such samples is a necessity in the current supplement market, as their consumption has increased dramatically. Most manufacturers declare the content of antioxidant components without specifying the method of determination. The existence of a standardized method as well as rapid and commercially available tests for this type of analysis is of great importance for consumers. Based on previous studies demonstrating that the electrochemical methods can be successfully applied in complex matrices such as wine, honey, milk, or infant formula [24,25,26,27,28], the main hypothesis was to try to apply and validate green electrochemical methods as potential standard approaches for detecting antioxidant potential of dietary supplements, a field highly relevant to health care, yet lacking standardized analytical criteria. This study aims to compare different methods for determining the total antioxidant capacity of dietary supplements, in order to obtain reliable values that can be used to assess product value based on antioxidant content. By evaluating both traditional spectrophotometric assays and modern electrochemical techniques, this study aims to identify the most accurate methods for determining total antioxidant capacity and verify the accuracy of claims related to antioxidant capacity. This will support more accurate labeling practices and help prevent consumer misconceptions about the effectiveness of dietary supplements.
2. Materials and Methods
2.1. Sample Preparation
Eighteen dietary supplements with label claims that they contained antioxidants were selected from various manufacturers on the market, including 10 domestic (D) and eight imported (I) products. Selection focused on widely available, commonly used, and reputable supplements, with diverse ingredients, a clear and complete list of ingredients, precise dosages, and an emphasis on quality and safety. The selected dietary supplements were available on the market in various pharmaceutical forms: capsules (39%), powders (28%), tablets/pastilles (28%), and solutions (5%). Products were based on different natural plant products like polyphenols, carotenoids, astaxanthin, bioflavonoids, lutein, resveratrol, and lycopene, mushroom extracts, or animal-derived products, propolis, royal jelly, with added vitamins (C, D, E, and A), and minerals, Zn, and Se. The list of biologically active ingredients declared on the labels of the selected products, the test dose (this is the recommended daily use dose), and the mass of the test dose are shown in Table 1. Dietary supplements were dissolved at the recommended dose in 250 mL (sometimes specified as a “standard glass of drinking water”) of deaerated, demineralized water, to avoid different drinking water compositions (Figure 1). L-ascorbic acid (vitamin C, 500 mg) was used as a reference substance. All chemicals and reagents used in this study were of analytical reagent grade.

Table 1.
Biologically active ingredients, pharmaceutical form, and dose of dietary supplements.
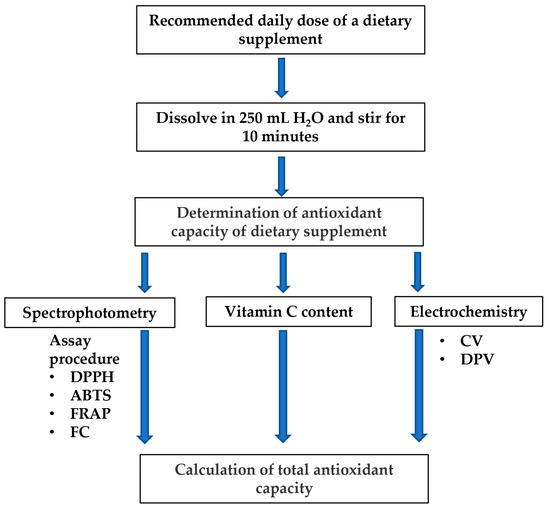
Figure 1.
Scheme of the experimental protocol.
2.2. Cyclic Voltammetry and Differential Pulse Voltammetry
Green electrochemical methods were employed for detecting the redox capacity in dietary supplements, utilizing cyclic voltammetry (CV) and differential pulse voltammetry (DPV). The results of the electrochemical methods were expressed as equivalent of vitamin C (CE). Voltammograms for cyclic and differential pulse voltammetry were recorded on the CH1760B instrument (CHInstruments, Austin, TX, USA). All measurements were performed in a three-electrode cell/quartz cuvette, where a glassy carbon (GC) electrode was used as the working electrode (Model CHI 104), and an additional large surface platinum electrode (Model CHI 221) and Ag/AgCl electrode (Model CHI 111) were used as the auxiliary and reference electrodes. The electrochemical cells had a volume of 5 mL. To ensure identical conditions throughout the experiment, each sample was diluted with 0.1 M KCl solution in a 1:1 ratio. Measurements were performed in an anaerobic atmosphere with N2 introduced into the cell. The working electrode was polished with alumina powder (1 and 0.5 µm, Buehler, IL, USA), rinsed with ethanol and deionized water, prior to each measurement. The voltammograms were recorded in the range of 0 to 1000 mV, with a scan rate 100 mV/s for CV, and 1 mV/s for DPV method. All measurements were performed at room temperature (20 °C). To produce a calibration curve for vitamin C, voltammetric cells contained 5000 µL of the supporting electrolyte (0.1M aqueous KCl solution) plus appropriate volumes of a 0.01M vitamin C solution. Measurements were performed using an Ag/AgCl reference electrode, a GC working electrode, and a platinum wire auxiliary electrode. CV and DVP voltammograms of vitamin C were taken from two peaks, approximately at 350 mV and 650 mV. Calibration curves based on peak area (Ah) were from CV and DPV measurements (at 350 mV) and provided in Figure 2A,B, respectively. In the inset Figure 2A,B, corresponding calibration equations for DPV and CV methods were provided: I (A) = 0.0015 × c(M) + 5.4234 × 10−7 (R2 = 0.9725); I (A) = 3.57 × 10−7 + 0.013 × c(M) (R2 = 0.9813), respectively. All results were recorded in triplicate, and the relative standard deviation was below 4.7%, indicating a significantly high reproducibility of the method. The detection limit (calculated as 3σ/slope) and quantification limits (calculated as 10σ/slope) for both methods were as follows: LODCV = 0.9 µM and LOQDPV = 2.9 µM; LODDP = 0.05 µM and LOQDP = 0.19 µM.
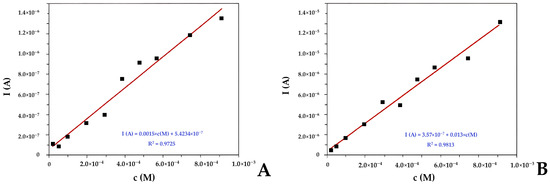
Figure 2.
Calibration curves for different concentrations of vitamin C obtained using (A) differential pulse voltammetry, and (B) cyclic voltammetry.
2.3. Methods for the Detection of Antioxidant Properties
The antioxidant properties of dietary supplements were determined by spectrophotometric methods: DPPH, Folin–Ciocalteu’s assay for phenolic substances and flavonoids, the FRAP assay for ferric reducing antioxidant power, and ABTS assay for the ability of antioxidants to scavenge the ABTS radical cation. Vitamin C content of selected products was detected using a Reflectometer RQflex® 20 (Supelco) system (Merck, Darmstadt, Germany), according to the manufacturer’s protocol, and results were expressed as mg of vitamin C per 250 mL of solution. pH values were detected using a pH meter (WTW, Weilheim, Germany).
The radical scavenging activity of supplements was detected at 517 nm (UV/VIS Spectrometer UV1280, Shimadzu), after incubation with DPPH solution (0.1 mmol/L) for 30 min at room temperature in the dark and expressed as percent of scavenging activity per 250 mL of solution [30].
The phenolic content of product samples was obtained after the addition of Folin–Ciocalteu reagent, left for 6 min before the addition of Na2CO3. After incubation for 2h at room temperature, the absorbance was measured at 765 nm. The total phenolic content was expressed as gallic acid equivalent (GAE) per 250 mL of solution [31].
The ABTS antioxidant capacity of the products was determined by a colorimetric assay using the reduction in ABTS•+ radical cation, generated overnight with potassium persulfate. The results were detected spectrophotometrically at 734 nm and expressed as mg of Vitamin C equivalent (CE) per 250 mL of solution [32].
The FRAP was determined under acidic conditions following the reduction in the ferric 2,4,6-tripyridyl-s-triazine complex. Antioxidant activity was measured spectrophotometrically at 593 nm and expressed as mg of Vitamin C equivalent (CE) per 250 mL of solution [33].
2.4. Statistical Analysis
All chemical analyses were conducted independently in triplicate, and the results were expressed as mean ± standard deviation (SD). Statistical analyses were carried out using SPSS ver. 25 software (IBM, Armonk, New York, NY, USA). A repeated measures ANOVA was applied to assess differences among the analytical methods for determining antioxidant capacity. Wilk’s test was used to test the overall model significance. When significant differences were observed (p < 0.05), pairwise comparisons were performed to identify specific differences between methods.
3. Results
A total of 18 dietary supplements labeled as containing antioxidants, from various manufacturers available on the Serbian market, were analyzed. The composition of the products differed, with 12 containing natural extracts from plants, fungi, propolis, royal jelly, and bovine colostrum. The remaining six products were formulated based on vitamins and minerals. The mass of daily dosage also differed, ranging from 130 mg to 15 g for solid forms, and up to 30 mL for liquid solutions (Table 1). These findings highlight the diversity of antioxidant sources and dosage forms in widely consumed supplements and provide insight into their potential nutritional and health contributions.
The measured vitamin C content for 10 samples fell within the declared ranges (Figure 3). However, samples 8D, 9D, 10D, 1I, 3I, 7I, and 8I exhibited lower vitamin C levels (24.22%, 4.85%, 13.90%, 39.34%, 15.91%, 31.11%, and 49.18%, respectively), while samples 2D (4.20%) and 6D (32.21%) exhibited statistically significant higher levels than their declared vitamin C contents (p < 0.05). Samples 1D, 4I, and 6I did not have any declared vitamin C contents. In sample 4I, based on mushroom extract, royal jelly, resveratrol, and lycopene (4I), a low level of vitamin C was detected (16 mg), while vitamin C was not detected in colostrum-based sample. Both samples had pH values (7.07 ± 0.01 and 6.86 ± 0.01, respectively). Product based on extract of Aronia melanocarpa (1D) contained 86 ± 6 mg vitamin C, although it did not have a declared vitamin C content. In addition to this sample, two other samples showed high measured values of vitamin C compared to their declared contents. Sample 2D exhibited a 4.20% higher vitamin C level than declared, while the vitamin C in sample 6D was 32.22% higher than the label declaration (Appendix A).
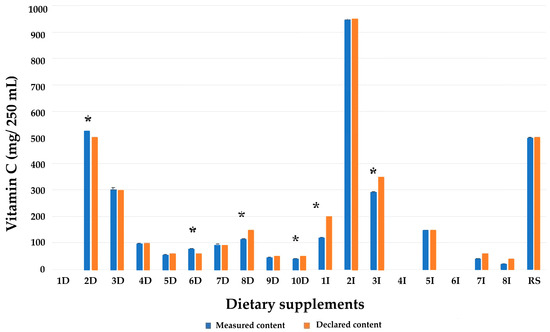
Figure 3.
Comparison of measured and declared vitamin C content in the examined dietary supplements. D indicates domestic products, I indicates imported products. * indicates a statistically significant difference between measured and declared content (p < 0.05).
The antioxidant and redox potentials of selected products were compared by four spectrophotometric methods and one electrochemical method, specifically cyclic voltammetry (Figure 4). When the total antioxidant capacity was assessed using the FRAP assay, the products 2I, 3I, and 2D displayed very high activity, while 1D and 3D showed notable levels. In contrast, 4D-7D, 1I, and 4I exhibited lower total antioxidant capacity. The Folin test indicated there was polyphenol activity in all products, expressed as GAE. The highest activity was observed in 2I, followed by 2D, 6D, 1D, 5D, and 3I, which also exhibited high activity. The total antioxidant capacities obtained by CV agreed well with those from the ABTS assay. With the CV method, the highest reductant properties were detected in 2D and 2I, indicating strong antioxidant capacity compared to the other products with lower antioxidant concentrations.
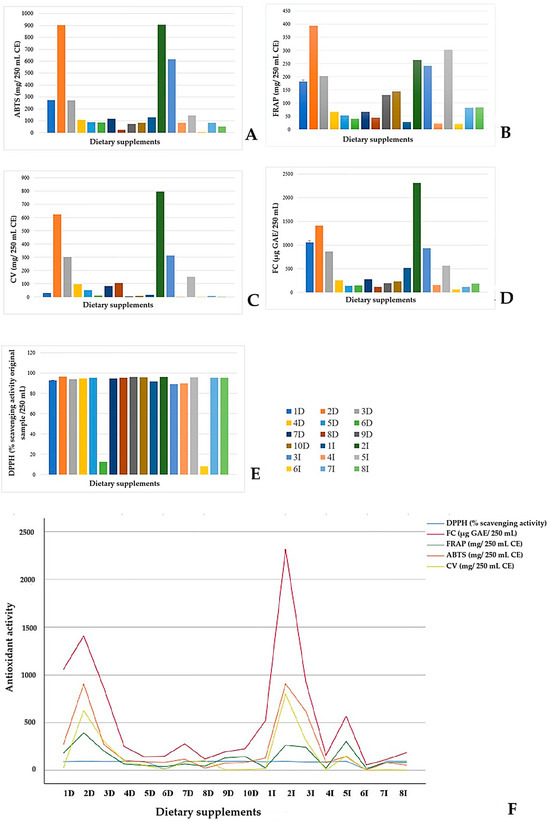
Figure 4.
The total antioxidant capacity of selected products determined by four spectrophotometric methods and one electrochemical method: (A) ABTS radical scavenging activity, (B) Ferric Reducing Antioxidant Potential (FRAP), (C) cyclic voltammetry, (D) Folin–Ciocalteu assay, and (E) DPPH radical scavenging activity. Comparison of the distribution of total antioxidant capacity in dietary supplements determined by the selected methods (F). D indicates domestic products, I indicates imported products.
The highest redox activities were detected in a propolis-based product containing vitamin C, E, D, B, and β-carotene, and in a plant flavonoid-based product containing lutein, lycopene, and B vitamins (2D and 2I, respectively). In contrast, the animal-derived colostrum dietary product, 6I, showed very low total antioxidant capacity, as measured by both spectrophotometric and electrochemical methods. The DPPH method produced exceptionally high scavenging activity for all products, ranging from 89% to 97%, while the activity for 500 mg of vitamin C per 250 mL of water was 98%. The colostrum product, 6I, and the product with Zn and vitamin C (6D) exhibited extremely low total antioxidant capacity (9% and 13%, respectively), making the DPPH method the least reliable of the methods for determining the total antioxidant capacity of the products.
However, except for DPPH method, a similar trend was observed for total antioxidant capacity across the analytical methods, for both highly- and less-active dietary supplements (Figure 4). The ABTS assay exhibited the highest total antioxidant capacities compared to other methods and vitamin C contents (1D, 2D, 5D, 7D, 2I, 3I, 4I), whereas the lowest total antioxidant capacities and vitamin C contents were measured in products 8D and 6I. In some products, the vitamin C levels corresponded to the total antioxidant capacity measured using the CV method (4D, 5D, 7D, 8D, 3I, 5I, and for the vitamin C standard), while the CV method resulted in considerably lower total antioxidant capacities for products that exhibited vitamin C levels below 60 mg (1D, 6D, 9D, 10D, 1I, 4I, 6i, 7I, and 8I). Figure 4F presents the distribution of total antioxidant capacity in the analyzed supplements, as determined by different antioxidant methods. Statistical analysis of method comparison revealed differences in variability, i.e., sensitivity, among the tested methods. An inspection of the graph shows that the FC method followed the trend observed in all other methods; however, due to differences in the measurement scales, it could not be included in the ANOVA. Furthermore, the DPPH method exhibited an almost linear response that did not align with the trends of the other methods, and therefore, this method was excluded from the ANOVA. Comparison of three methods, ABTS, FRAP, and CV, based on repeated measures ANOVA, Pairwise Comparisons showed that there was no statistically significant difference between FRAP and ABTS or FRAP and CV (p > 0.05). CV values were correlated with FRAP (r = 0.757), and ABTS values were correlated with FRAP (r = 0.797). Still, Wilk’s lambda analysis showed the ABTS method statistically differed from the CV method (p < 0.05).
Figure 5 shows the summarized redox potential, expressed as total antioxidant capacity, of the tested dietary products when measured by the CV method. This method showed that products 2D and 2I had high redox potentials compared to the other tested products. When the CV method was used, only product 2D had a total antioxidant capacity higher than the declared vitamin C level. The total antioxidant capacity measured using the CV method did not show differences among samples 3D, 4D, 5D, 7D, 8D, 3I, 5I, and 6I with regard to their declared vitamin C content. Two samples, 2D and 2I, exhibited total antioxidant capacity values higher than the declared or measured vitamin C contents. In contrast, very low vitamin C levels were detected in declared and measured samples 1D, 6D, 9D, 10D, 1I, 4I, 6I, 7I, and 8I. However, this method has shown that the content of active compounds varies among most samples. According to the data on the samples, the dominant content of antioxidant compounds in the samples is different and is based on vitamin C, propolis, plant extracts and polyphenols. The electrochemical profile obtained from this study corresponds to that distribution and electrochemical behavior of the mentioned compounds [34,35,36]. This means that the dominant carriers of antioxidative activities are ascorbate and phenolic compounds, while other components are probably present to a lesser extent but contribute to the overall value. Similar considerations have been made in the works of other authors in the literature [34,35,36,37].
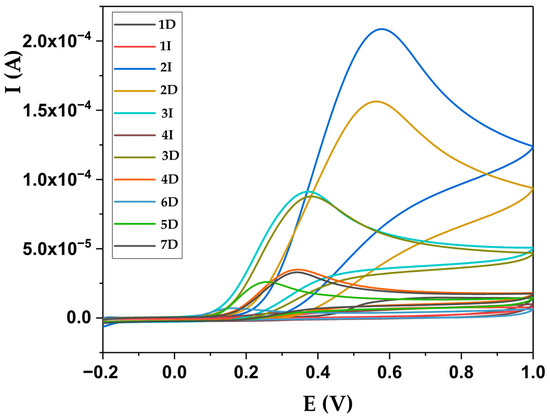
Figure 5.
Redox potential of selected dietary products measured using cyclic voltammetry. D indicates domestic products, I indicates imported products.
Figure 6 illustrates two selected products, 2D and 2I, which exhibited the highest redox potential, along with a domestic product characterized by low redox potential, based on CV measurements (1D). Further analysis used a more sensitive DPV method enabling the identification of active components in the products, as demonstrated by product 2I, with two distinct oxidation peaks (EOX1 and EOX2). The DPV analysis revealed that 2I contained various active components active at potential 0.385V and 0.425V, influencing the overall redox potential of the product. For the 2D sample, the existence of two distinct peaks (a broad peak representing the superposition of two peaks, (EOX1 and EOX2)) is also evident, which are the dominant carriers of the antioxidant activity of the sample, but which cannot be clearly separated due to the close value of the maximum potential.
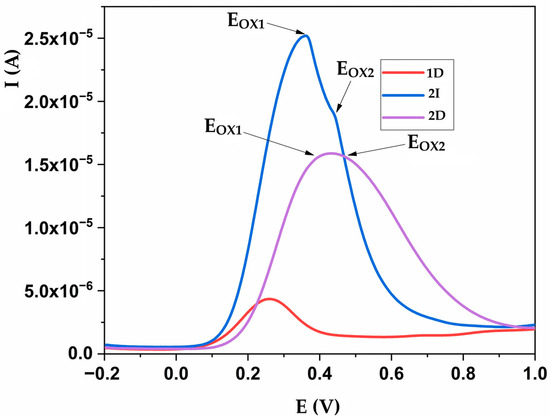
Figure 6.
Redox potential of selected dietary products measured by differential pulse voltammetry. EOX1 and EOX2 represent distinct oxidation peaks of a broad peak. D indicates domestic products, I indicates imported products.
The CV method worked over a wide range of concentrations of substances that are responsible for the total antioxidant capacity of the dietary supplements, but, as previously known, it did not show satisfactory sensitivity when the products contained low levels of the substances. In addition, the CV method did not differentiate oxidation peaks and did not produce a “fingerprint” for each product. In contrast, the DPV method showed better sensitivity, as shown in Figure 6. This method produced a significant and measurable response for products where the CV method showed shortcomings. In addition, the appearance of the signal obtained with the DPV method was such that, in addition to quantification, this method produced some additional information about the product, i.e., on the existence of different components that contribute to the total antioxidant capacity, and the mutual concentration ratio.
The DPV method provided a more detailed assessment of the redox activity of the products, enabling the determination of redox potential based on distinct oxidation peaks. A limitation of the DPV method is that high concentrations of redox-active compounds cannot be measured directly without prior sample dilution, unlike the CV method. However, samples 1D, 6D, 9D, 10D, 1I, 4I, 6I, 7I, and 8I had declared vitamin contents below 60 mg/250 mL, which fell below the detection limit of the CV method and were, thus, considered out of range.
4. Discussion
The results indicate that the statements in the materials accompanying the products or in the inscriptions and the graphic representations on the packaging regarding the antioxidant content were, to put it mildly, more optimistic than the actual measurements obtained in this study. Thus, for some classes of compounds exhibiting redox properties, such as antioxidants, the reported values were lower by several fold in some of the analyzed dietary supplements than was claimed. The mass and active substance content differed among the different products. Although the study was limited by a small number of samples, it covered a variety of antioxidant sources and dosage forms in widely consumed supplements, including both locally produced and imported products available in Serbia. These findings provide insight into their potential nutritional and health contributions. Therefore, any claim about the total antioxidant capacity of a product should be defined based on its specific activity and appropriately labeled with a standardized value.
The determination of total antioxidant capacity is primarily based on the reaction kinetics or equilibrium states of chemical assays, as assessed through spectrophotometric measurements. Antioxidant activity expressed as one standard equivalent can be converted to another using a conversion formula, allowing comparison between results based on different standards [38]. These measurements rely on detecting color changes or discoloration at defined wavelengths. Such assays have been widely utilized for antioxidant analysis and for evaluating the antioxidant capacity of complex biological and chemical samples. According to the results of the current studies, there is the same distribution of total antioxidant capacity between the studied methods. However, different methods have advantages and disadvantages for directly measuring antioxidant potential in undiluted, original dietary supplements. The DPPH method produced very high vitamin C levels for the products in which vitamin C was declared (results were 89–97% of declared levels). The FRAP method showed lower vitamin C levels compared to the ABTS and CV methods. The FC method provided insight into the activity of phenolic compounds in the tested dietary supplements, and the distribution of activity was consistent with the other methods. Previous research suggested that the DPPH method has several inconsistencies in the results it produces, making it unreliable for detecting antioxidant potential [27,39,40].
Electrochemical voltammetric methods are very sensitive for the detection of low concentrations of antioxidants, rapid measurement without sample preparation, calibration, and validation. Cloudy or complex samples with antioxidants can be analyzed to distinguish between different redox-active species, providing comprehensive insights into antioxidant mechanisms. Electrochemical methods are a valuable tool for both qualitative and quantitative antioxidant detection. CV measures current intensity, reflecting the redox activity of antioxidants. The DPV method requires prior sample dilution, making it unsuitable for determining the redox potential of highly active samples. Furthermore, the low pH of these products suggested the predominant presence of ascorbic acid, which would contribute to their high antioxidant potential. A limitation of the DPV method is that high concentrations of redox components cannot be measured directly without sample dilution, unlike the CV method. Disadvantages of CV and DPV are complex calibration and results interpretation, making them less practical for routine analyses.
ABTS and FRAP measurements were expressed as vitamin C equivalents to allow comparison with CV measurements and actual vitamin C content. The converted total antioxidant capacity of different products was consistent with the capacity measured by each individual method. This approach enabled indirect comparison of activities obtained using different standard substances through conversion to standard equivalents. The DPV method was not included in the comparison, and its results were not reported, as it is highly sensitive to low concentrations, while concentrations higher than 60 mg/250 mL require prior dilution, making standardization difficult. The CV method is suitable for antioxidant concentrations exceeding 60 mg/250 mL (daily dose), whereas the DPV method is more appropriate for detecting lower concentrations without prior treatment. In addition, the appearance of the signal obtained with the DPV method was such that, in addition to quantification, this method produced some additional information about the product, i.e., on the existence of different components that contribute to the total antioxidant capacity, and the mutual concentration ratio, which would allow individual samples to be grouped. Therefore, this method may be useful for recognizing the type of dietary supplement or the compounds that have significant antioxidant activity in the supplement, such as dietary products with bovine colostrum compared to high active human colostrum and breast milk [27].
While special medical conditions, deficiencies, or increased need require supplementation, whole foods are superior for overall health due to their complex nutrient composition, bioavailability, and natural synergy. The comparative efficacy of dietary products and fresh groceries with regard to antioxidant potential remains an open question, although current data suggest that fresh vegetables and fruits could offer superior antioxidant potential. Vitamin C, a common ingredient of dietary supplements with antioxidant properties and with enormous potential to prevent the development of oxidative reactions of macromolecules, is by definition a reductant. The emphasis on the significance of vitamin C content is somewhat relevant [6,9,41]. However, this compound in supplements does not necessarily contribute more antioxidant value than natural sources like fresh red peppers. This vegetable contains not only the antiscorbutic vitamin, but it also possesses the reducing agents that neutralize free radicals and oxidative stress.
Dietary supplements’ antioxidant effectiveness, in comparison with fresh whole foods, such as vegetables, is often overstated and should not be understood by consumers as replacements for a whole food diet that includes suitable quantities of vegetables and fruits. The discrepancy between declared and measured antioxidant capacities in dietary supplements may lead to significant underexposure or overexposure to certain redox-active compounds. Inaccurate labeling can mislead consumers regarding the actual intake of antioxidants, potentially resulting in insufficient efficacy or, conversely, unintended excessive intake. Given that some products exceeded or fell below the recommended daily allowance, such deviations may pose a risk to vulnerable populations, including pregnant women, elderly individuals, and those with chronic conditions. These findings underscore the need for mandatory quantification and accurate labeling of antioxidant content to minimize consumer health risk and ensure product efficacy.
5. Conclusions
This study compared standard spectrophotometric methods with eco-friendly electrochemical methods for determining the concentration of antioxidants in dietary supplements and evaluated their agreement with the manufacturers’ declared values. Given the current absence of a standardized method for the detection of total antioxidant capacity, the use of the electrochemical “green” cyclic voltammetry and differential pulse voltammetry methods showed potential use as faster, simpler, and more sustainable analytical approaches that require minimal use of additional chemicals and enable direct measurements. The correlation between differential pulse voltammetry and cyclic voltammetry should be considered in future studies, and further work is needed to develop the most reliable approach. Also, this study showed the shortcomings in the proper definition of the antioxidant content of various supplements, as well as the existence of a clearly defined method for determining their content in these samples. Each of the methods showed significant deviations from the declared values but excellent mutual agreement, which leads to the conclusion that this field is still very undefined. In some cases, labeling is not based on the concentration of bioactive compounds or the true characteristics of the product, which may mislead consumers. Therefore, this study highlights the need for normative regulation of antioxidant properties through the introduction of mandatory testing and quantification of total antioxidant capacity.
Author Contributions
Conceptualization, N.L.; methodology, D.S. and N.L.; formal analysis: N.L. and D.S.; investigation, D.S. and N.L.; data curation: D.S.; writing—original draft preparation, N.L.; writing—review and editing, D.S.; supervision, N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Contract No: 451-03-136/2025-03/200026 and 451-03-136/2025-03/200168), according to Agenda 2030—United Nations Sustainable Development Goals: 2—Zero hunger and 3—Good Health and well-being.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABTS | 2,2-azino-bis(3-ethyl-benzothiazoline-6-sulfonic acid |
| CE | Vitamin C equivalent |
| CV | Cyclic voltammetry |
| D | Domestic product |
| DPPH | 2,2-diphenyl-picrylhydrazyl |
| DPV | Differential pulse voltammetry |
| DS | Dietary supplements |
| FC | Folin-Ciocalteu |
| FRAP | Ferric reducing antioxidant power |
| GAE | Gallic acid equivalent |
| GC | Glassy carbon |
| I | Imported product |
Appendix A

Table A1.
Comparison of results from antioxidant assay methods, vitamin C equivalents, and pH values.
Table A1.
Comparison of results from antioxidant assay methods, vitamin C equivalents, and pH values.
| Dietary Supplement | DPPH (% Scavenging Activity Original Sample/250 mL) | FC (µg GAE/250 mL) | FRAP (mg/250 mL CE) | ABTS (mg/250 mL CE) | CV (mg/250 mL CE) | Vitamin C (mg/250 mL Daily Dose) | Vitamin C (Declared, mg) | pH |
|---|---|---|---|---|---|---|---|---|
| 1D | 92.90 ± 0.10 | 1058.67 ± 35.92 | 181.33 ± 6.11 | 273.33 ± 5.86 | 29.60 ± 0.54 | 86.00 ± 6.00 | / | 3.35 ± 0.01 |
| 2D | 96.50 ± 0.50 | 1409.67 ± 58.51 | 393.67 ± 7.23 | 904.00 ± 41.07 | 625.50 ± 25.43 | 521.33 ± 3.51 | 500 | 3.61 ± 0.01 |
| 3D | 93.97 ± 0.15 | 861.33 ± 33.56 | 202.33 ± 6.81 | 270.17 ± 12.75 | 303.67 ± 21.78 | 304.67 ± 4.51 | 300 | 4.29 ± 0.02 |
| 4D | 94.93 ± 0.06 | 250.33 ± 41.96 | 66.33 ± 1.53 | 106.83 ± 13.04 | 97.00 ± 1.00 | 97.01 ± 2.02 | 100 | 3.86 ± 0.01 |
| 5D | 95.67 ± 0.29 | 140.67 ± 25.66 | 52.33 ± 1.53 | 90.17 ± 2.25 | 52.08 ± 0.88 | 54.67 ± 2.51 | 60 | 3.31 ± 0.01 |
| 6D | 12.67 ± 0.15 | 147.67 ± 13.05 | 40.33 ± 0.58 | 85.33 ± 7.51 | 11.67 ± 0.58 | 79.33 ± 1.15 | 60 | 4.62 ± 0.02 |
| 7D | 94.67 ± 0.42 | 278.33 ± 16.20 | 66.33 ± 3.21 | 117.33 ± 7.51 | 83.25 ± 5.06 | 92.67 ± 3.78 | 90 | 3.41 ± 0.02 |
| 8D | 95.57 ± 0.67 | 118.33 ± 8.39 | 44.67 ± 0.58 | 24.833 ± 2.02 | 104.83 ± 1.26 | 113.67 ± 1.53 | 150 | 4.17 ± 0.02 |
| 9D | 96.30 ± 0.26 | 193.67 ± 3.21 | 130.33 ± 2.08 | 74.83 ± 7.29 | 5.93 ± 0.38 | 45.67 ± 0.58 | 48 | 3.05 ± 0.02 |
| 10D | 96.03 ± 0.55 | 227.33 ± 8.74 | 144.67 ± 3.79 | 84.33 ± 5.13 | 6.97 ± 0.42 | 41.33 ± 1.15 | 48 | 3.15 ± 0.01 |
| 1I | 91.67 ± 0.58 | 519.33 ± 17.50 | 28.33 ± 0.58 | 129.33 ± 11.02 | 16.00 ± 2.14 | 121.33 ± 1.15 | 200 | 5.86 ± 0.02 |
| 2I | 96.33 ± 0.58 | 2316.33 ± 48.85 | 263.33 ± 3.79 | 906.33 ± 35.13 | 797.67 ± 32.47 | 947.00 ± 3.01 | 950 | 3.35 ± 0.01 |
| 3I | 89.07 ± 0.06 | 931.33 ± 38.85 | 241.33 ± 4.93 | 618.33 ± 29.28 | 314.42 ± 19.99 | 294.33 ± 0.58 | 350 | 6.20 ± 0.0 |
| 4I | 89.93 ± 0.21 | 157.33 ± 18.93 | 22.67 ± 0.58 | 83.33 ± 5.20 | 3.11 ± 0.16 | 15.67 ± 0.58 | / | 7.07 ± 0.01 |
| 5I | 95.77 ± 0.32 | 566.33 ± 16.50 | 302.33 ± 3.21 | 144.33 ± 9.02 | 151.50 ± 6.26 | 148.33 ± 1.53 | 150 | 4.97 ± 0.01 |
| 6I | 8.16 ± 0.97 | 57.67 ± 15.04 | 20.67 ± 2.51 | 3.42 ± 0.72 | 0.27 ± 0.03 | 0 | / | 6.86 ± 0.01 |
| 7I | 95.57 ± 0.81 | 114.67 ± 7.02 | 82.33 ± 3.06 | 81.67 ± 12.58 | 7.01 ± 0.01 | 41.33 ± 1.15 | 60 | 3.98 ± 0.01 |
| 8I | 95.57 ± 0.60 | 186.67 ± 14.50 | 83.67 ± 3.21 | 53.67 ± 6.03 | 3.01 ±0.01 | 20.33 ± 0.58 | 40 | 3.71 ± 0.01 |
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; et al. Guidance on safety evaluation of sources of nutrients and bioavailability of nutrient from the sources (Revision 1). EFSA J. 2021, 19, e06552. [Google Scholar] [CrossRef]
- EFSA NDA Panel; Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health (Revision 1). EFSA J. 2018, 16, e05136. [Google Scholar] [CrossRef]
- Kalogerakou, T.; Antoniadou, M. The Role of Dietary Antioxidants, Food Supplements and Functional Foods for Energy Enhancement in Healthcare Professionals. Antioxidants 2024, 13, 1508. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Brzezińska-Rojek, J.; Sagatovych, S.; Malinowska, P.; Gadaj, K.; Prokopowicz, M.; Grembecka, M. Antioxidant Capacity, Nitrite and Nitrate Content in Beetroot-Based Dietary Supplements. Foods 2023, 12, 1017. [Google Scholar] [CrossRef]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Lam, M.; Khoshkhat, P.; Chamani, M.; Shahsavari, S.; Dorkoosh, F.A.; Rajabi, A.; Maniruzzaman, M.; Nokhodchi, A. In-depth multidisciplinary review of the usage, manufacturing, regulations & market of dietary supplements. J. Drug Deliv. Sci. Technol. 2022, 67, 102985. [Google Scholar] [CrossRef]
- Bartosz, G. Total antioxidant capacity. Adv. Clin. Chem. 2003, 37, 219–292. [Google Scholar] [CrossRef]
- Meščić Macan, A.; Gazivoda Kraljević, T.; Raić-Malić, S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef]
- Cecchini, M.; Warin, L. Impact of food labelling systems on food choices and eating behaviours: A systematic review and meta-analysis of randomized studies. Obes. Rev. 2016, 17, 201–210. [Google Scholar] [CrossRef]
- Djaoudene, O.; Romano, A.; Bradai, Y.D.; Zebiri, F.; Ouchene, A.; Yousfi, Y.; Amrane-Abider, M.; Sahraoui-Remini, Y.; Madani, K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients 2023, 15, 3320. [Google Scholar] [CrossRef]
- Bensa, M.; Vovk, I.; Glavnik, V. Resveratrol Food Supplement Products and the Challenges of Accurate Label Information to Ensure Food Safety for Consumers. Nutrients 2023, 15, 474. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Pohanka, M. Assays of antioxidant capacity: Optics and voltammetry. Int. J. Electrochem. Sci. 2023, 18, 100276. [Google Scholar] [CrossRef]
- Bisby, R.H.; Brooke, R.; Navaratnam, S. Effect of antioxidant oxidation potential in the oxygen radical absorption capacity (ORAC) assay. Food Chem. 2008, 108, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.D.; Bains, A.; Sridhar, K.; Sharma, M.; Dhull, S.B.; Goksen, G.; Chawla, P.; Inbaraj, B.S. Recent advances in the analytical methods for quantitative determination of antioxidants in food matrices. Food Chem. 2025, 463, 141348. [Google Scholar] [CrossRef]
- Tuna, B.H.; Gürbüz, M.; Uğur, H.; Çatak, J.; Yaman, M. Vitamin C bioaccessibility of commercially available dietary supplements: Quantity vs. efficiency, does it matter? J. Food Compos. Anal. 2023, 123, 105558. [Google Scholar] [CrossRef]
- Huang, L.; Waseem Shah, M.; Wang, Y.; Nam, Y.; Sun, G. Exploring the association between dietary patterns and the types of dietary supplements used. J. Funct. Foods 2024, 113, 106030. [Google Scholar] [CrossRef]
- Vasić, V.; Gašić, U.; Stanković, D.; Lušić, D.; Vukić-Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Towards Better Quality Criteria of European Honeydew Honey: Phenolic Profile and Antioxidant Capacity. Food Chem. 2019, 274, 629–641. [Google Scholar] [CrossRef]
- Osorio-Valencia, A.I.; de Jesús Franco-Mejía, J.; Hoyos-Arbeláez, J.A.; Blandón-Naranjo, L.; Vega-Castro, O.A.; Contreras-Calderón, J. Evaluation of antioxidant capacity in different food matrices through differential pulse voltammetry and its correlation with spectrophotometric methods. J. Appl. Electrochem. 2023, 53, 2495–2505. [Google Scholar] [CrossRef]
- Lugonja, N.M.; Stanković, D.M.; Spasić, S.D.; Veličković, D.; Manojlović, D.D. Comparative Electrochemical Determination of Total Antioxidant Activity in Infant Formula with Breast Milk. Food Anal. Methods 2014, 7, 337–344. [Google Scholar] [CrossRef]
- Lugonja, N.M.; Stanković, D.M.; Miličić, B.; Spasić, S.D.; Marinković, V.; Vrvić, M.M. Electrochemical Monitoring of the Breast Milk Quality. Food Chem. 2018, 240, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Ristivojević, P.; Trifković, J.; Stanković, D.M.; Radoičić, A.; Manojlović, D.; Milojković-Opsenica, D. Cyclic voltammetry and UV/Vis spectroscopy in combination with multivariate data analysis for the assessment of authenticity of poplar type propolis. J. Apic. Res. 2017, 56, 559–568. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Begines, E.; Heredia, F.J.; Escudero-Gilete, M.L.; Hernanz, D. Effectiveness of Cyclic Voltammetry in Evaluation of the Synergistic Effect of Phenolic and Amino Acids Compounds on Antioxidant Activity: Optimization of Electrochemical Parameters. Foods 2024, 13, 906. [Google Scholar] [CrossRef]
- Silva, T.M.S.; Camara, C.A.; Lins, A.C.S.; Barbosa-Filho, J.M.; Silva, E.M.S.; Freitas, B.M.; Santos, F.A.R. Chemical composition and free radical scavenging activity of pollen loads from stingless bee Melipona subnitida Ducke. J. Food Compos. Anal. 2006, 19, 507–511. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Mannino, G.; Serio, G.; Gaglio, R.; Busetta, G.; La Rosa, L.; Lauria, A.; Settanni, L.; Gentile, C. Phytochemical Profile and Antioxidant, Antiproliferative, and Antimicrobial Properties of Rubus idaeus Seed Powder. Foods 2022, 1, 2605. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of Antioxidant Power: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Suliborska, K.; Baranowska, M.; Bartoszek, A.; Chrzanowski, W.; Namieśnik, J. Determination of Antioxidant Activity of Vitamin C by Voltammetric Methods. Proceedings 2019, 11, 23. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Arteaga, J.F.; Ruiz-Montoya, M.; Palma, A.; Alonso-Garrido, G.; Pintado, S.; Rodríguez-Mellado, J.M. Comparison of the Simple Cyclic Voltammetry (CV) and DPPH Assays for the Determination of Antioxidant Capacity of Active Principles. Molecules 2012, 17, 5126–5138. [Google Scholar] [CrossRef]
- Zielińska, D.; Wiczkowski, W.; Piskuła, M.K. Determination of the relative contribution of quercetin and its glucosides to the antioxidant capacity of onion by cyclic voltammetry and spectrophotometric methods. J. Food Sci. 2008, 73, C97–C102. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Pruteanu, L.L.; Bailey, D.S.; Grădinaru, A.C.; Jäntschi, L. The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae. Antioxidants 2023, 12, 860. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).