Volatile Compounds from Northern Moroccan Medicinal Plants: Phytochemical Analysis, Antioxidant and Antimicrobial Potential, and In Silico Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Essential Oil Extraction
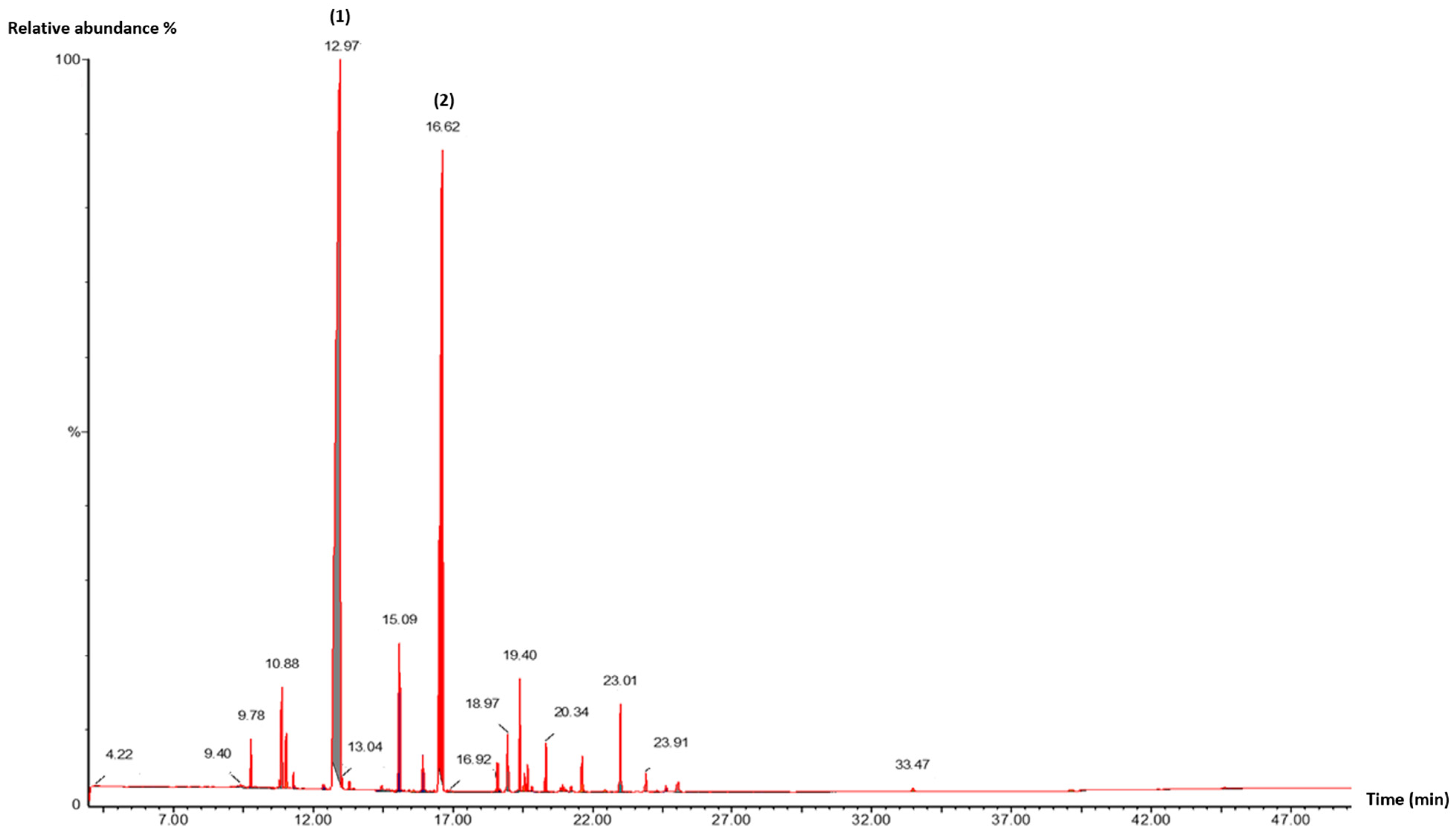
2.2. Essential Oils Characterization by Gas Chromatography/Mass Spectrometry (GC/MS)
2.3. Antimicrobial Activity
2.3.1. Microbial Strains
2.3.2. Antimicrobial Screening
2.3.3. MIC and MMC Determination
2.4. Antioxidant Activity
2.4.1. DPPH Free Radical-Scavenging Activity
2.4.2. Reducing Power Determination
2.5. ADMET Analysis
2.6. Molecular Docking Studies
2.6.1. Protein Preparation
2.6.2. Ligand Preparation
2.6.3. Molecular Docking Analysis
3. Statistical Analysis
4. Results and Discussion
4.1. Essential Oil Yield and Chemical Composition
4.2. Antimicrobial Activity
4.3. Antioxidant Capacity
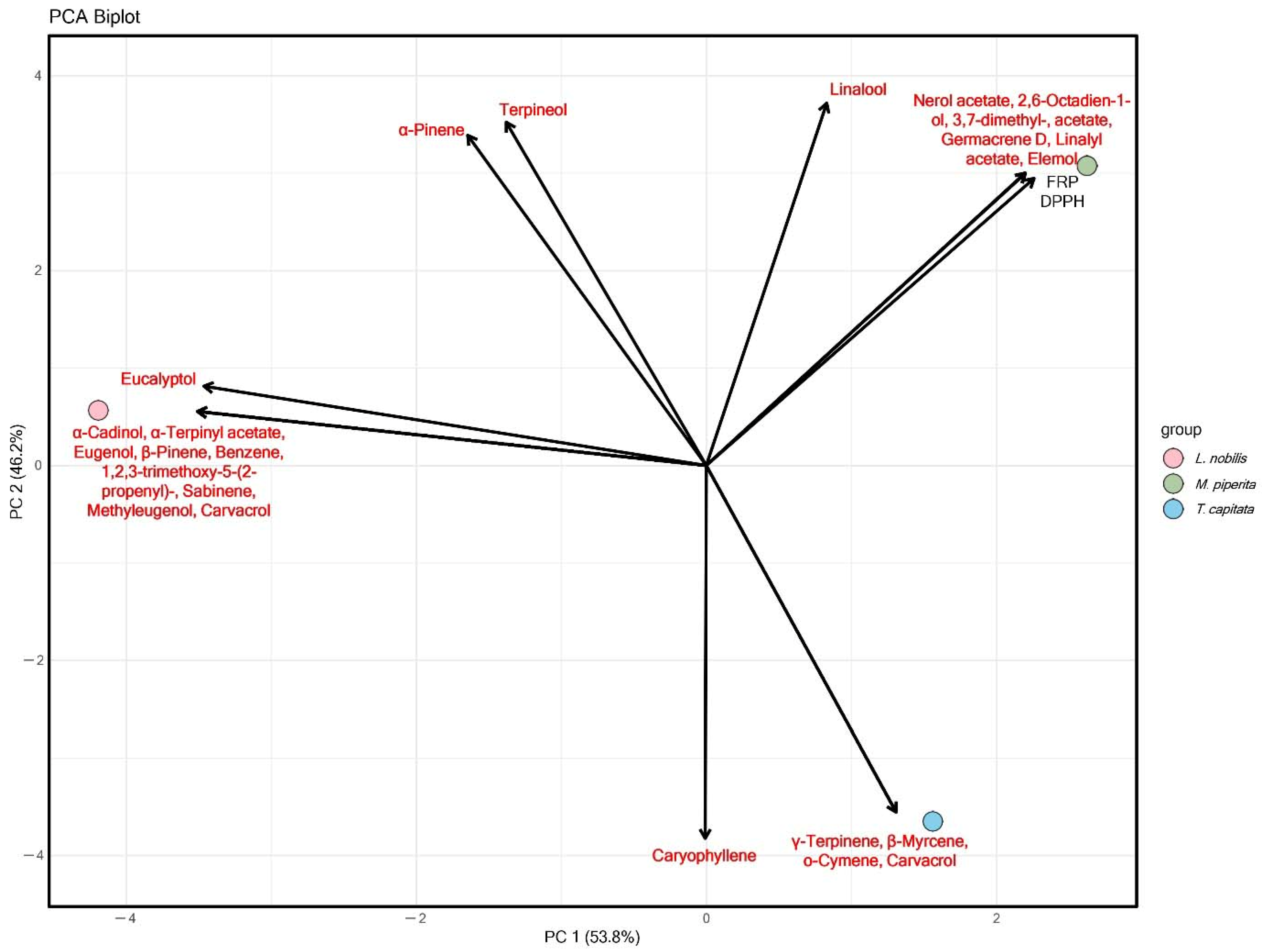
4.4. PCA
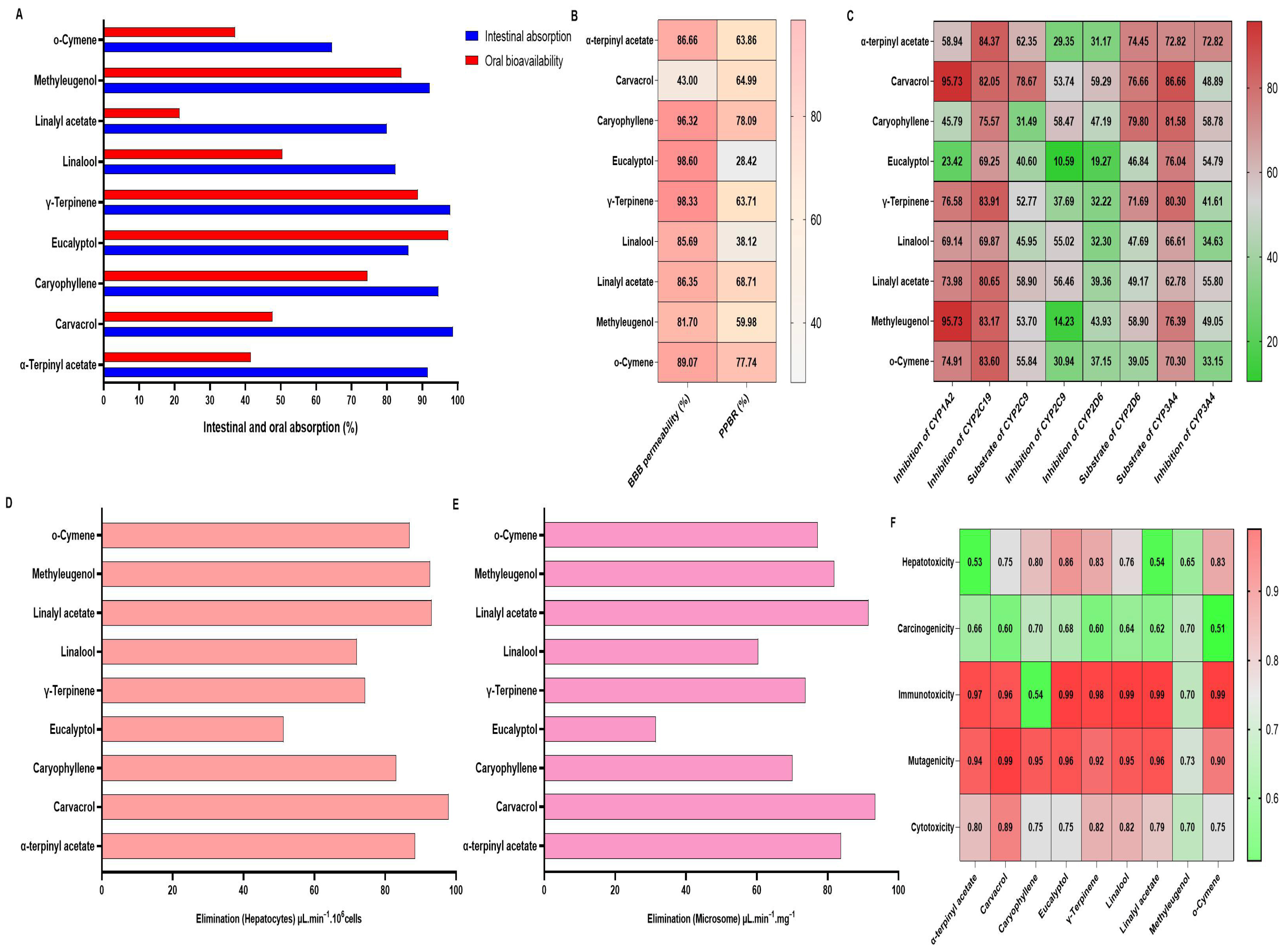
4.5. ADMET Analysis
4.6. Molecular Docking
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHT | Butylated hydroxytoluene |
| DMSO | Dimethyl Sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| E.Os | Essential oils |
| GC-MS | Gas chromatography/Mass spectrometry |
| IC50 | Inhibitory concentration 50 |
| IZ | Inhibition Zone |
| MAPs | Medicinal and Aromatic Plants |
| MHA | Mueller Hinton Agar |
| MIC | Minimum Inhibitory Concentration |
| MMC | Minimum Microbicidal Concentration |
| NCCLS | National Committee for Clinical Laboratory Standards |
| PCA | Principal component analysis |
| PDB | Protein Data Bank |
| SDA | Sabouraud Dextrose Agar |
References
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, W. The mechanism of bacterial resistance and potential bacteriostatic strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, L.; Tang, X.; Peng, L.; Li, X.; Zhao, G.; Zhong, L. Chemical Composition, Antimicrobial and Antioxidant Activities of the Flower Volatile Oils of Fagopyrum esculentum, Fagopyrum tataricum and Fagopyrum Cymosum. Molecules 2018, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Kelen, M.; Tepe, B. Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora. Bioresour. Technol. 2008, 99, 4096–4104. [Google Scholar] [CrossRef]
- Alaoui Jamali, C.; Kasrati, A.; Fadli, M.; Hassani, L.; Leach, D.; Abbad, A. Synergistic effects of three Moroccan thyme essential oils with antibiotic cefixime. Phytotherapie 2018, 16, 149–154. [Google Scholar] [CrossRef]
- Imelouane, B.; Amhamdi, H.; Wathelet, J.P.; Ankit, M.; Khedid, K.; El Bachiri, A. Chemical composition and antimicrobial activity of essential oil of thyme (Thymus vulgaris) from Eastern Morocco. Int. J. Agric. Biol. 2009, 11, 205–208. [Google Scholar]
- Benali, T.; Habbadi, K.; Bouyahya, A.; Khabbach, A.; Marmouzi, I.; Aanniz, T.; Hammani, K. Phytochemical analysis and study of antioxidant, anticandidal, and antibacterial activities of Teucrium polium subsp. polium and Micromeria graeca (Lamiaceae) essential oils from Northern Morocco. Evid.-Based Complement. Altern. Med. 2021, 2021, 6641720. [Google Scholar] [CrossRef]
- Douhri, B.; Douhri, H.; Farah, A.; Idaomar, M.; Senhaji, N.S.; Abrini, J. Phytochemical Analysis and Antibacterial Activity of Essential Oil of Lavandula multifida L. Int. J. Innov. Sci. Res. 2014, 1, 116–126. [Google Scholar]
- Laghmouchi, Y.; Belmehdi, O.; Senhaji, N.S.; Abrini, J. Chemical composition and antibacterial activity of Origanum compactum Benth. essential oils from different areas at northern Morocco. S. Afr. J. Bot. 2018, 115, 120–125. [Google Scholar] [CrossRef]
- Chellappandian, M.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Karthi, S.; Thanigaivel, A.; Ponsankar, A.; Kalaivani, K.; Hunter, W.B. Botanical essential oils and uses as mosquitocides and repellents against dengue. Environ. Int. 2018, 113, 214–230. [Google Scholar] [CrossRef]
- Latif, M.; Elkoraichi, I.; El Faqer, O.; Wahnou, H.; Mtairag, E.M.; Oudghiri, M.; Rais, S. Phytochemical analysis and immunomodulatory activities in vitro and in vivo of Aframomum melegueta K Schum seed extracts. Inflammopharmacology 2024, 32, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Mabou, F.D.; Yossa, I.B.N. TERPENES: Structural classification and biological activities. IOSR J. Pharm. Biol. Sci. 2021, 16, 25–40. [Google Scholar] [CrossRef]
- Ma, G.H.; Chen, K.X.; Zhang, L.Q.; Li, Y.M. Advance in biological activities of natural guaiane-type sesquiterpenes. Med. Chem. Res. 2019, 28, 1339–1358. [Google Scholar] [CrossRef]
- Fennane, M.; Tattou, M.I. Statistics and comments on the current inventory of the vascular flora of Morocco. Bull. Institut Sci. Sect. Sci. Vie 2012, 34, 1–9. [Google Scholar]
- Benabid, A. Les écosystèmes forestiers, préforestiers et presteppiques du Maroc: Diversité, répartition biogéographique et problèmes posés par leur aménagement. Forêt Méditerranéenne 1985, 7, 53–64. Available online: https://www.foret-mediterraneenne.org/upload/biblio/FORET_MED_1985_1_53.pdf (accessed on 4 November 2025).
- Grovel, R. La préservation des forêts du Rif centro-occidental: Un enjeu de développement de la montagne rifaine. Rev. Geogr. Alp. 1996, 84, 75–94. [Google Scholar] [CrossRef]
- Chebli, Y.; Chentouf, M.; Cabaraux, J.F.; El Otmani, S. Floristic Composition, Diversity, Palatability, and Forage Availability of Forest Rangelands in the Southern Mediterranean Region of Northern Morocco. Land 2023, 12, 215. [Google Scholar] [CrossRef]
- Ennabili, A.; Gharnit, N.; El hamdouni, E. Inventory and social interest of medicinal, aromatic and honey-plants from mokrisset region (Nw of Morocco). J. Med. Plant 2000, 19, 39–56. [Google Scholar]
- Bouyahya, A.; Abrini, J.; Et-Touys, A.; Bakri, Y.; Dakka, N. Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. Eur. J. Integr. Med. 2017, 13, 9–25. [Google Scholar] [CrossRef]
- Labiad, H.; Aljaiyash, A.; Ghanmi, M.; Satrani, B.; Ettahir, A.; Aouane, M.; Fadli, M.; Chaouch, A. Exploring the provenance effect on chemical composition and pharmacological bioactivity of the Moroccan essential oils of Laurus nobilis. Res. J. Pharm. Technol. 2020, 13, 4067–4076. [Google Scholar] [CrossRef]
- Costa, P.; Medronho, B.; Gonçalves, S.; Romano, A. Cyclodextrins enhance the antioxidant activity of essential oils from three Lamiaceae species. Ind. Crops Prod. 2015, 70, 341–346. [Google Scholar] [CrossRef]
- Khabbach, A.; Libiad, M.; Ennabili, A.; Bousta, D. Medicinal and cosmetic use of plants from the province of Taza, Northern Morocco. Bol. Latinoam. Caribe Plantas Med. Aromat. 2012, 11, 46–60. [Google Scholar]
- Benali, T.; Khabbach, A.; Ennabili, A.; Hammani, K. Ethnopharmacological prospecting of medicinal plants from the Province of Guercif (NE of Morocco). Mor. J. Biol. 2017, 14, 1–14. [Google Scholar] [CrossRef]
- Abdelaty, N.A.; Attia, E.Z.; Hamed, A.N.E.; Desoukey, S.Y. A review on various classes of secondary metabolites and biological activities of Lamiaceae (Labiatae) (2002–2018). JABPS 2021, 4, 16–31. [Google Scholar] [CrossRef]
- Approved Standard M2-A6; Performance Standards for Antimicrobial Disk Susceptibility Test. 6th ed. National Committee for Clinical Laboratory Standards (NCCLS): Wayne, PA, USA, 1997.
- Approved Standard M7-A4; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 4th ed. National Committee for Clinical Laboratory Standards (NCCLS): Wayne, PA, USA, 1997.
- Approved Standard M38-A; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. National Committee for Clinical Laboratory Standards (NCCLS): Wayne, PA, USA, 2002.
- Bendiar, S.; El Faqer, O.; Chennaoui, S.; Benjelloun, N.; Mtairag, E.M.; Oudghiri, M. Phytochemical Screening and in vivo Immunosuppressive, Antioxidant and Anti-hemolytic Activities of Zea mays Silk Aqueous Extract. Pharmacogn. J. 2020, 12, 1412–1420. [Google Scholar] [CrossRef]
- Alaoui Jamali, C.; Kasrati, A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Cultivation and the application of inorganic fertilizer modifies essential oil composition in two Moroccan species of Thymus. Ind. Crops Prod. 2014, 62, 113–118. [Google Scholar] [CrossRef]
- Latif, M.; Elkoraichi, I.; El Faqer, O.; Wahnou, H.; Elaaj, R.; Mtairag, E.M.; Oudghiri, M.; Rais, S. Aframomum Melegueta: Evaluation of Chronic Toxicity, HPLC Profiling, and In Vitro/In Vivo Antioxidant Assessment of Seeds Extracts. Chem. Biodivers. 2024, 22, e202400942. [Google Scholar] [CrossRef]
- El Faqer, O.; Rais, S.; Ouadghiri, Z.; El Faqer, A.; Benchama, Z.; El Ouaddari, A.; Dakir, M.; El Amrani, A.; Mtairag, E.M. Physicochemical properties, GC–MS profiling, and antibacterial potential of Allium sativum essential oil: In vitro and in silico approaches. Sci. Afr. 2024, 26, e02484. [Google Scholar] [CrossRef]
- El Faqer, O.; Elkoraichi, I.; Latif, M.; Debierre-Grockiego, F.; Ouadghiri, Z.; Rais, S.; Dimier-Poisson, I.; Mtairag, E.M. Pharmacological insights into Laurus nobilis: HPLC profiling and evaluation of its anti-Toxoplasma, antioxidant, and anti-hemolytic properties. Biochem. Syst. Ecol. 2024, 117, 104891. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Custódio, L.; Vitalini, S.; Iriti, M.; Hassani, L. A comparative study of the in vitro antimicrobial and synergistic effect of essential oils from Laurus nobilis L. and Prunus armeniaca L. from Morocco with antimicrobial drugs: New approach for health promoting products. Antibiotics 2020, 9, 140. [Google Scholar] [CrossRef]
- Ben Bakrim, W.; Aghraz, A.; Hriouch, F.; Larhsini, M.; Markouk, M.; Bekkouche, K.; Dugo, G. Phytochemical study and antioxidant activity of the most used medicinal and aromatic plants in Morocco. J. Essent. Oil Res. 2022, 34, 131–142. [Google Scholar] [CrossRef]
- Tagnaout, I.; Zerkani, H.; Hadi, N.; El Moumen, B.; El Makhoukhi, F.; Bouhrim, M.; Zair, T. Chemical composition, antioxidant and antibacterial activities of Thymus broussonetii Boiss and Thymus capitatus (L.) Hoffmann and Link essential oils. Plants 2022, 11, 954. [Google Scholar] [CrossRef]
- Feknous, S.; Saidi, F.; Said, R.M. Extraction, caractérisation et identification de quelques métabolites secondaires actifs de la mélisse (Melissa officinalis L.). Nat. Technol. 2014, 6, 7–13. [Google Scholar]
- Luz, T.R.S.A.; Leite, J.A.C.; de Mesquita, L.S.S.; Bezerra, S.A.; Silveira, D.P.B.; de Mesquita, J.W.C.; Coutinho, D.F. Seasonal variation in the chemical composition and biological activity of the essential oil of Mesosphaerum suaveolens (L.) Kuntze. Ind. Crops Prod. 2020, 153, 112600. [Google Scholar] [CrossRef]
- El Faqer, O.; Rais, S.; Elkoraichi, I.; El Amrani, A.; Dakir, M.; Zaid, Y.; Mtairag, E.M. Phytochemical characterization and immunomodulatory effects of aqueous and ethanolic extracts and essential oil of Moroccan Laurus nobilis L. (Lauraceae) on human neutrophils. J. Herbmed Pharmacol. 2023, 12, 92–99. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; De Feo, V. Laurus nobilis: Composition of essential oil and its biological activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical composition and antimicrobial activity of Laurus nobilis L. essential oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef]
- Ekren, S.; Yerlikaya, O.; Tokul, H.E.; Akpınar, A.; Accedil, M. Chemical composition, antimicrobial activity and antioxidant capacity of some medicinal and aromatic plant extracts. Afr. J. Microbiol. Res. 2013, 7, 383–388. [Google Scholar] [CrossRef]
- Snuossi, M.; Trabelsi, N.; Ben Taleb, S.; Dehmeni, A.; Flamini, G.; De Feo, V. Laurus nobilis, Zingiber officinale and Anethum graveolens essential oils: Composition, antioxidant and antibacterial activities against bacteria isolated from fish and shellfish. Molecules 2016, 21, 1414. [Google Scholar] [CrossRef]
- Benoutman, A.; Erbiai, E.H.; Edderdaki, F.Z.; Cherif, E.K.; Saidi, R.; Lamrani, Z.; Maouni, A. Phytochemical composition, antioxidant and antifungal activity of Thymus capitatus, a medicinal plant collected from Northern Morocco. J. Antibiot. 2022, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Moukhles, A.; Mansour, A.; Ellaghdach, A.; Abrini, J. Chemical composition and in vitro antibacterial activity of the pure essential oils and essential oils extracted from their corresponding hydrolats from different wild varieties of Moroccan thyme. J. Mater. Environ. Sci. 2018, 9, 235–244. [Google Scholar] [CrossRef]
- El Moussaoui, N.; Sanchez, G.; Idaomar, M.; Mansour, A.I.; Abrini, J.; Aznar, R. Antibacterial and antiviral activities of essential oils of Northern Moroccan plant. Br. Biotechnol. J. 2013, 3, 318. [Google Scholar] [CrossRef]
- Moukhles, A.; Mansour, I.A. The Effect of Drying Time on the Yield and the Chemical Composition of Essential Oil and Dissolved Oil in Hydrolat from Aerial Parts of Moroccan Thymbra capitata (L.) Cav. Mediterr. J. Chem. 2020, 10, 716–722. [Google Scholar] [CrossRef]
- Hamad Al-Mijalli, S.; Elsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Bouyahya, A. Determination of volatile compounds of Mentha piperita and Lavandula multifida and investigation of their antibacterial, antioxidant, and antidiabetic properties. Evid.-Based Complement. Altern. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef]
- Tsai, M.L.; Wu, C.T.; Lin, T.F.; Lin, W.C.; Huang, Y.C.; Yang, C.H. Chemical composition and biological properties of essential oils of two mint species. Trop. J. Pharm. Res. 2013, 12, 577–582. [Google Scholar] [CrossRef]
- Zahli, R.; Abrini, J.; El Baaboua, A.; Belmehdi, O.; El Maadoudi, M.; Souhail, B.; Senhaji, N.S. Synergistic action of Thymus capitatus or Syzygium aromaticum essential oils and antibiotics combinations against multi-resistant Salmonella strains. Biocatal. Agric. Biotechnol. 2023, 50, 102752. [Google Scholar] [CrossRef]
- Drioiche, A.; Radi, F.Z.; Ailli, A.; Bouzoubaa, A.; Boutakiout, A.; Mekdad, S.; Zair, T. Correlation between the chemical composition and the antimicrobial properties of seven samples of essential oils of endemic Thymes in Morocco against multi-resistant bacteria and pathogenic fungi. Saudi Pharm. J. 2022, 30, 1200–1214. [Google Scholar] [CrossRef]
- Alaoui Jamali, C.; Kasrati, A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Phenological changes to the chemical composition and biological activity of the essential oil from Moroccan endemic thyme (Thymus maroccanus Ball). Ind. Crops Prod. 2013, 49, 366–372. [Google Scholar] [CrossRef]
- Mahboubi, M.; Heidarytabar, R.; Mahdizadeh, E.; Hosseini, H. Antimicrobial activity and chemical composition of Thymus species and Zataria multiflora essential oils. Agric. Nat. Resour. 2017, 51, 395–401. [Google Scholar] [CrossRef]
- El Bouzidi, L.; Jamali, C.A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Chemical composition, antioxidant and antimicrobial activities of essential oils obtained from wild and cultivated Moroccan Thymus species. Ind. Crops Prod. 2013, 43, 450–456. [Google Scholar] [CrossRef]
- Zhang, D.; Gan, R.Y.; Ge, Y.Y.; Yang, Q.Q.; Ge, J.; Li, H.B.; Corke, H. Research progress on the antibacterial mechanisms of carvacrol: A mini-review. Bioact. Compd. Health Dis. 2018, 1, 71–81. [Google Scholar] [CrossRef]
- Menicucci, F.; Pizzo, B.; Salvadori, B.; Chelazzi, L.; Ienco, A.; Palagano, E. Antifungal activity of carvacrol-based solids and their effects on Whatman and Kraft paper. Int. Biodeterior. Biodegrad. 2024, 195, 105894. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Khribch, J.; Nassik, S.; EL Houadfi, M.; Zrira, S.; Oukessou, M. Activité antibactérienne de l’huile essentielle d’origan et du carvacrol sur des souches d’Escherichia coli d’origine aviaire. Rev. Marocaine Sci. Agron. Vétérinaires 2018, 6, 300–307. [Google Scholar]
- Gandova, V.; Lazarov, A.; Fidan, H.; Dimov, M.; Stankov, S.; Denev, P.; Bari, A. Physicochemical and biological properties of carvacrol. Open Chem. J. 2023, 21, 20220319. [Google Scholar] [CrossRef]
- Kaspute, G.; Ivaskiene, T.; Ramanavicius, A.; Ramanavicius, S.; Prentice, U. Terpenes and Essential Oils in Pharmaceutics: Applications as Therapeutic Agents and Penetration Enhancers with Advanced Delivery Systems for Improved Stability and Bioavailability. Pharmaceutics 2025, 17, 793. [Google Scholar] [CrossRef]
- Ali, T.; Majeed, S.T.; Majeed, R.; Bashir, R.; Mir, S.A.; Jan, I.; Andrabi, K.I. Recent advances in the pharmacological properties and molecular mechanisms of carvacrol. Rev. Bras. Farmacogn. 2024, 34, 35–47. [Google Scholar] [CrossRef]
- Sousa, L.G.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Cerca, N. Synergistic effects of carvacrol, α-terpinene, γ-terpinene, ρ-cymene and linalool against Gardnerella species. Sci. Rep. 2022, 12, 4417. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Martins, N. Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci. Technol. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Chen, W. Antimicrobial activity and proposed action mechanism of linalool against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef] [PubMed]
- Mączka, W.; Duda-Madej, A.; Grabarczyk, M.; Wińska, K. Natural compounds in the battle against microorganisms—Linalool. Molecules 2022, 27, 6928. [Google Scholar] [CrossRef]
- Khayyat, S. Thermal, photo-oxidation and antimicrobial studies of linalyl acetate as a major ingredient of lavender essential oil. Arab. J. Chem. 2020, 13, 1575–1581. [Google Scholar] [CrossRef]
- Şimşek, M.; Duman, R. Investigation of effect of 1, 8-cineole on antimicrobial activity of chlorhexidine gluconate. Pharmacogn. Res. 2017, 9, 234. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Górny, A.; Grabarczyk, M.; Wińska, K. Can eucalyptol replace antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.; Soković, M. Camphor and eucalyptol—Anticandidal spectrum, antivirulence effect, efflux pumps interference and cytotoxicity. Int. J. Mol. Sci. 2021, 22, 483. [Google Scholar] [CrossRef]
- Cai, Z.M.; Peng, J.Q.; Chen, Y.; Tao, L.; Zhang, Y.; Fu, L.Y.; Shen, X.C. 1, 8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef]
- Guenane, H.; Gherib, A.; Carbonell-Barrachina, Á.; Cano-Lamadrid, M.; Krika, F.; Berrabah, M.; Bakchiche, B. Minerals analysis, antioxidant and chemical composition of extracts of Laurus nobilis from southern Algeria. J. Mater. Environ. Sci. 2016, 7, 4253–4261. [Google Scholar]
- Mssillou, I.; Agour, A.; El Ghouizi, A.; Hamamouch, N.; Lyoussi, B.; Derwich, E. Chemical composition, antioxidant activity, and antifungal effects of essential oil from Laurus nobilis L. flowers growing in Morocco. J. Food Qual. 2020, 2020, 8819311. [Google Scholar] [CrossRef]
- Taroq, A.; El-Kamari, F.; Aouam, I.; El Atki, Y.; Lyoussi, B.; Abdellaoui, A. Phytochemical screening, polyphenols content and a novel source of antibacterial and antioxidant activities of essential oil of Laurus nobilis from Morocco. Int. J. Pharm. Sci. Res. 2019, 10, 3770–3776. [Google Scholar] [CrossRef]
- Zaïri, A.; Nouir, S.; Zarrouk, A.; Haddad, H.; Khélifa, A.; Achour, L.; Trabelsi, M. Chemical composition, Fatty acids profile and Biological properties of Thymus capitatus (L.) Hoffmanns, essential Oil. Sci. Rep. 2019, 9, 20134. [Google Scholar] [CrossRef] [PubMed]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Elbouzidi, A.; Bouhrim, M.; Bencheikh, N.; Bousta, D. Assessment of antidepressant-like, anxiolytic effects and impact on memory of Pimpinella anisum L. Total extract on swiss albino mice. Plants 2021, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Sabzalian, M.R. Thymol, carvacrol, and antioxidant accumulation in Thymus species in response to different light spectra emitted by light-emitting diodes. Food Chem. 2020, 307, 125521. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Islam, S. Therapeutic application of carvacrol: A comprehensive review. Int. J. Food Sci. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Mir, M.; Permana, A.D.; Ahmed, N.; Khan, G.M.; ur Rehman, A.; Donnelly, R.F. Enhancement in site-specific delivery of carvacrol for potential treatment of infected wounds using infection responsive nanoparticles loaded into dissolving microneedles: A proof of concept study. Eur. J. Pharm. Biopharm. 2020, 147, 57–68. [Google Scholar] [CrossRef]
- Hervin, V.; Roy, V.; Agrofoglio, L.A. Antibiotics and Antibiotic Resistance—Mur Ligases as an Antibacterial Target. Molecules 2023, 28, 8076. [Google Scholar] [CrossRef]
- Rambaher, M.H.; Zdovc, I.; Glavač, N.K.; Gobec, S.; Frlan, R. Mur ligase F as a new target for the flavonoids quercitrin, myricetin, and (–)-epicatechin. J. Comput. Aided Mol. Des. 2023, 37, 721–733. [Google Scholar] [CrossRef]
- Tsvetkova, N.; Harizanov, R.; Rainova, I.; Ivanova, A.; Yancheva-Petrova, N. Molecular analysis of dihydropteroate synthase gene mutations in pneumocystis jirovecii isolates among Bulgarian patients with pneumocystis pneumonia. Int. J. Mol. Sci. 2023, 24, 16927. [Google Scholar] [CrossRef]
- Cipriano, A.; Viviano, M.; Feoli, A.; Milite, C.; Sarno, G.; Castellano, S.; Sbardella, G. NADPH oxidases: From molecular mechanisms to current inhibitors. J. Med. Chem. 2023, 66, 11632–11655. [Google Scholar] [CrossRef]
- Soares-Santos, R.R.; Jaiswal, A.K.; Ferreira, R.C.M.; Azevedo, V.A.d.C.; Aburjaile, F.F.; Soto-Blanco, B. Interactions of Linalool and Linalyl Acetate with Selected Dog Cytochrome P450 (CYP) Proteins Identified by In Silico Drug Discovery Followed by Molecular Docking Analysis. Pharmaceuticals 2025, 18, 1499. [Google Scholar] [CrossRef]
- Noui Mehidi, I.; Ait Ouazzou, A.; Tachoua, W.; Hosni, K. Investigating the Antimicrobial Properties of Essential Oil Constituents and Their Mode of Action. Molecules 2024, 29, 4119. [Google Scholar] [CrossRef] [PubMed]
- Başar, Y.; Gök, M.; Erenler, R.; Demirtas, İ. Phytochemical profiling, molecular docking and ADMET prediction of essential oil of Ocimum basilicum. Int. J. Second. Metab. 2025, 12, 146–157. [Google Scholar] [CrossRef]
- Venkataraman, B.; Almarzooqi, S.; Raj, V.; Bhongade, B.A.; Patil, R.B.; Subramanian, V.S.; Subramanya, S.B. Molecular docking identifies 1, 8-Cineole (Eucalyptol) as a novel PPARγ agonist that alleviates colon inflammation. Int. J. Mol. Sci. 2023, 24, 6160. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]



| Species | Local Name | Harvesting Place | Harvesting Time | Voucher Specimen | Latitude/Longitude | Altitude (m) |
|---|---|---|---|---|---|---|
| L. nobilis | Rand | Serrour (El Haouz) | March 2022 | LAUNO052 | 35°33′36″ N/5° 46′48″ W | 300 |
| T. capitata | Zaitra | Dar Ben Karrich | April 2023 | TC036 | 35°30′36″ N/5° 25′12″ W | 166 |
| M. piperita | Naanaa lhor | Khemiss Anjra (Merzouka) | June 2023 | MENPIP059 | 35°39′36″ N/5° 30′36″ W | 73 |
| Target | PDB ID | Coordinates | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Muramyl ligase E (MurE) | 4C13 | −23.122 | 2.508 | 9.873 |
| Dihydropteroate synthase | 1AD4 | 33.106 | 8.125 | 41.463 |
| Lanosterol 14α-demethylase | 5V5Z | −40.656170 | −10.617936 | 25.111266 |
| Crystal structure of NOX | 2CDU | 10.532406 | 4.053750 | 25.952356 |
| Molecular Formula | Compounds a | Retention Parameters | Abundance (%) | |||
|---|---|---|---|---|---|---|
| RI b | RT c | L. nobilis | T. capitata | M. piperita | ||
| C10H16 | α-Pinene | 929 | 8.27 | 1.14 | - d | 1.01 |
| C10H16 | Sabinene | 974 | 9.37 | 1.76 | - | - |
| C10H16 | β-Pinene | 979 | 9.45 | 1.01 | - | - |
| C10H16 | β-Myrcene | 991 | 9.80 | - | 1.41 | - |
| C10H14 | o-Cymene | 1022 | 10.75 | - | 4.15 | - |
| C10H18O | Eucalyptol | 1032 | 11.07 | 26.09 | - | 2.00 |
| C10H16 | γ-Terpinene | 1060 | 11.68 | - | 5.47 | - |
| C10H18O | Linalool | 1099 | 12.98 | 25.64 | 2.58 | 57.37 |
| C10H18O | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)- | 1182 | 14.81 | 3.04 | - | - |
| C10H18O | Terpineol | 1188 | 15.17 | 4.07 | - | 3.97 |
| C12H20O2 | Linalyl acetate | 1275 | 16.61 | - | - | 28.56 |
| C10H14O | Carvacrol | 1299 | 18.26 | - | 80.95 | - |
| C12H20O2 | α-Terpinyl acetate | 1350 | 18.83 | 9.03 | - | - |
| C10H12O2 | Eugenol | 1357 | 19.06 | 4.33 | - | - |
| C12H20O2 | 2,6-Octadien-1-ol, 3,7-dimethyl-, acetate | 1365 | 19.40 | - | - | 2.31 |
| C11H14O2 | Methyleugenol | 1402 | 20.11 | 11.95 | - | - |
| C12H20O2 | Nerol acetate | 1406 | 20.33 | - | - | 1.01 |
| C15H24 | Caryophyllene | 1419 | 20.40 | 2.04 | 5.44 | - |
| C15H24 | Germacrene D | 1481 | 21.62 | - | - | 0.72 |
| C15H26O | Elemol | 1549 | 23.00 | - | - | 1.92 |
| C12H16O3 | Benzene, 1,2,3-trimethoxy-5-(2-propenyl)- | 1554 | 23.14 | 1.49 | - | - |
| C15H24O | Caryophyllene oxide | 1581 | 23.83 | 3.21 | - | - |
| C15H26O | α-Cadinol | 1653 | 25.16 | 1.45 | - | - |
| Total (%) | 96.25 | 100 | 98.87 | |||
| Yield (%, v/w) | 1.12 ± 0.06 | 1.2 ± 0.0 | 1.53 ± 0.28 | |||
| Monoterpene hydrocarbons | 3.91 | 11.03 | 1.01 | |||
| Terpenic compounds | Sesquiterpene hydrocarbons | 2.04 | 5.44 | 0.72 | ||
| Oxygenated monoterpenes | 85.64 | 83.53 | 95.22 | |||
| Oxygenated sesquiterpenes | 8.39 | 0 | 1.92 | |||
| Test Microorganisms | Inhibition Zone Diameter (mm) a | ||||||
|---|---|---|---|---|---|---|---|
| Essential Oils | Antibiotics | Antifungal | |||||
| L. nobilis | T. capitata | M. piperita | Ciprofloxacin | Gentamicin | Cefixime | Fluconazol | |
| Gram+ Bacteria | |||||||
| S. aureus | 12.00 ± 1.73 | 44.00 ± 1.00 | 13.00 ± 1.00 | 39.66 ± 0.57 | 27.00 ± 0.00 | NA b | NT c |
| MRSA | 30.66 ± 1.52 | 44.66 ± 0.57 | 21.66 ± 1.52 | 43.33 ± 1.52 | 43.33 ± 1.52 | 41.00 ± 1.00 | NT |
| M. luteus | 50.00 ± 0.00 | 45.00 ± 0.00 | 49.33 ± 1.15 | 50.00 ± 0.00 | 36.00 ± 1.00 | NA | NT |
| Gram− Bacteria | |||||||
| E. coli ATCC 8739 | 10.33 ± 0.57 | 44.00 ± 1.73 | 16.00 ± 1.00 | 35.33 ± 0.57 | 26.33 ± 0.57 | NA | NT |
| E. coli ATCC 25922 | 11.00 ± 1.00 | 26.00 ± 1.00 | 11.00 ± 1.00 | 30.66 ± 0.57 | 22.00 ± 1.00 | NA | NT |
| P. aeruginosa DSM 50090 | 23.33 ± 1.52 | 44.00 ± 1.73 | 28.66 ± 1.52 | 44.33 ± 1.15 | 35.33 ± 1.52 | 34.33 ± 1.52 | NT |
| P. aeruginosa ATCC 27853 | 45.66 ± 1.15 | 46.33 ± 1.15 | 45.66 ± 1.15 | 42.66 ± 2.08 | 41.00 ± 1.00 | 34.33 ± 1.52 | NT |
| Fungal strains | |||||||
| C. albicans | 34.66 ± 1.52 | 38.33 ± 1.52 | 36.00 ± 1.73 | NT | NT | NT | 26.33 ± 1.15 |
| C. glabrata | 24.00 ± 1.73 | 44.00 ± 1.73 | 29.33 ± 1.52 | NT | NT | NT | 6.66 ± 1.15 |
| C. krusei | 38.33 ± 1.52 | 30.00 ± 0.00 | 20.33 ± 1.52 | NT | NT | NT | 7.00 ± 1.00 |
| C. parapsilosis | 15.33 ± 1.52 | 26.33 ± 1.15 | 15.66 ± 1.15 | NT | NT | NT | 22.66 ± 0.57 |
| Test Microorganisms | Essential Oils (mg/mL) | Antibiotics (µg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. nobilis | T. capitata | M. piperita | Ciprofloxacin | Gentamicin | Cefixime | Fluconazol | ||||||||
| MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | |
| Gram+ bacteria | ||||||||||||||
| S. aureus | 54.24 | 54.24 | 9.47 | 9.47 | 51.90 | 51.90 | 35.00 | 35.00 | 5.60 | 5.60 | 270 | 270 | NT | NT |
| MRSA | 54.24 | 54.24 | 9.47 | 9.47 | 51.90 | 51.90 | 35.00 | 35.00 | 2.80 | 2.80 | 135 | 135 | NT | NT |
| M. luteus | 13.56 | 13.56 | 9.47 | 9.47 | 51.90 | 51.90 | 17.50 | 17.50 | 1.40 | 1.40 | 540 | 540 | NT | NT |
| Gram− bacteria | ||||||||||||||
| E. coli ATCC 8739 | 27.12 | 54.24 | 9.47 | 9.47 | 51.90 | 51.90 | 70.00 | 70.00 | 5.60 | 5.60 | 135 | 135 | NT | NT |
| E. coli ATCC 25922 | 54.24 | 54.24 | 4.73 | 9.47 | 51.90 | 51.90 | 70.00 | 70.00 | 5.60 | 5.60 | 135 | 135 | NT | NT |
| P. aeruginosa DSM 50090 | 13.56 | 13.56 | 0.073 | 0.073 | 51.90 | 51.90 | 1000 | 1000 | 22.40 | 22.40 | 540 | 540 | NT | NT |
| Gram− bacteria | ||||||||||||||
| P. aeruginosa ATCC 27853 | 54.24 | 54.24 | 9.47 | 9.47 | 51.90 | 51.90 | 1000 | 1000 | 22.40 | 22.40 | 540 | 540 | NT | NT |
| Yeasts | ||||||||||||||
| C. albicans | 54.24 | 54.24 | 9.47 | 9.47 | 17.30 | 17.30 | NT | NT | NT | NT | NT | NT | 4 | 8 |
| C. glabrata | 54.24 | 54.24 | 4.73 | 9.47 | 17.30 | 17.30 | NT | NT | NT | NT | NT | NT | 4 | 8 |
| C. krusei | 27.12 | 54.24 | 9.47 | 9.47 | 17.30 | 17.30 | NT | NT | NT | NT | NT | NT | 4 | 8 |
| C. parapsilosis | 54.24 | 54.24 | 9.47 | 9.47 | 25.95 | 25.95 | NT | NT | NT | NT | NT | NT | 4 | 8 |
| Essential Oils | ||||||
|---|---|---|---|---|---|---|
| Test Microorganisms | L. nobilis | T. capitata | M. piperita | |||
| MBC/MIC Ratio | Effect | MBC/MIC Ratio | Effect | MBC/MIC Ratio | Effect | |
| Gram+ Bacteria | ||||||
| S. aureus | 1 | Bactericidal | 1 | Bactericidal | 1 | Bactericidal |
| MRSA | 1 | Bactericidal | 1 | Bactericidal | 1 | Bactericidal |
| M. luteus | 1 | Bactericidal | 1 | Bactericidal | 1 | Bactericidal |
| Gram− Bacteria | ||||||
| E. coli ATCC 8739 | 2 | Bactericidal | 1 | Bactericidal | 1 | Bactericidal |
| E. coli ATCC 25922 | 1 | Bactericidal | 2 | Bactericidal | 1 | Bactericidal |
| P. aeruginosa DSM 50090 | 1 | Bactericidal | 1 | Bactericidal | 1 | Bactericidal |
| P. aeruginosa ATCC 27853 | 1 | Bactericidal | 1 | Bactericidal | 1 | Bactericidal |
| Fungal strains | ||||||
| C. albicans | 1 | Fungicidal | 1 | Bactericidal | 1 | Bactericidal |
| C. glabrata | 1 | Fungicidal | 2 | Bactericidal | 1 | Bactericidal |
| C. krusei | 2 | Fungicidal | 1 | Bactericidal | 1 | Bactericidal |
| C. parapsilosis | 1 | Fungicidal | 1 | Bactericidal | 1 | Bactericidal |
| Antioxidant Tests | Essential Oils (mg/mL) | Synthetic Antioxidants (mg/mL) | |||
|---|---|---|---|---|---|
| L. nobilis | T. capitata | M. piperita | Quercetin | BHT | |
| DPPH | 0.037 ± 0.001 * | 0.143 ± 0.002 * | 4.189 ± 0.008 * | 0.002 ± 0.0003 | 0.006 ± 0.0001 |
| Reducing power | 0.058 ± 0.001 ns | 0.064 ± 0.001 ns | 6.457 ± 0.006 * | 0.004 ± 0.0001 | 0.009 ± 0.0001 |
| Compounds | Predicted LD50 (mg/kg) | Predicted Toxicity Class |
|---|---|---|
| Alpha terpinyl acetate | 4800 | 5 |
| Carvacrol | 810 | 4 |
| Caryophyllene | 5300 | 5 |
| Eucalyptol | 2480 | 5 |
| Gamma terpinene | 2500 | 5 |
| Linalool | 2200 | 5 |
| Linalyl acetate | 12,000 | 5 |
| Methylisoeugenol | 2500 | 5 |
| O-cymene | 113 | 3 |
| Bacterial Enzymes | Fungal Enzyme | Oxidant Enzyme | ||||||
|---|---|---|---|---|---|---|---|---|
| 4C13 | 1AD4 | 5V5Z | 2CDU | |||||
| Compounds | ΔGb a (Kcal/mol) | Ki b (µM) | ΔGb a (Kcal/mol) | Ki b (µM) | ΔGb a (Kcal/mol) | Ki b (µM) | ΔGb a (Kcal/mol) | Ki b (µM) |
| 1 | −5.05 | 199.40 | −5.90 | 47.74 | −7.44 | 3.54 | −6.32 | 23.31 |
| 2 | −3.34 | 3.59 × 103 | −4.59 | 433.18 | −5.51 | 91.00 | −5.32 | 125.74 |
| 3 | 4.43 | 567.69 | −5.38 | 113.31 | −6.84 | 9.65 | ||
| 4 | −4.34 | 658.64 | −4.28 | 731.24 | −6.38 | 21.23 | −1.78 | 49.78 |
| 5 | −2.87 | 7.83 × 103 | −3.94 | 1.30 × 103 | −4.87 | 267.33 | −4.31 | 690.71 |
| 6 | −2.76 | 9.44 × 103 | −4.35 | 652.45 | −5.04 | 200.56 | −6.17 | 29.97 |
| 7 | −3.16 | 4.79 × 103 | −4.33 | 672.57 | −5.64 | 73.28 | −5.94 | 44.59 |
| 8 | −3.65 | 2.10 × 103 | −4.98 | 225.03 | −5.96 | 42.54 | −4.66 | 383.46 |
| 9 | −2.98 | 6.54 × 103 | −4.07 | 1.05 × 103 | −5.23 | 146.70 | −3.41 | 3.18 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Alla, K.; El Faqer, O.; Jahjah, S.; Labhar, A.; Alaoui Jamali, C.; Kasrati, A.; Souhail, B.; Legssyer, M.; Maouni, A.; Saidi, R. Volatile Compounds from Northern Moroccan Medicinal Plants: Phytochemical Analysis, Antioxidant and Antimicrobial Potential, and In Silico Investigations. Compounds 2025, 5, 49. https://doi.org/10.3390/compounds5040049
Ait Alla K, El Faqer O, Jahjah S, Labhar A, Alaoui Jamali C, Kasrati A, Souhail B, Legssyer M, Maouni A, Saidi R. Volatile Compounds from Northern Moroccan Medicinal Plants: Phytochemical Analysis, Antioxidant and Antimicrobial Potential, and In Silico Investigations. Compounds. 2025; 5(4):49. https://doi.org/10.3390/compounds5040049
Chicago/Turabian StyleAit Alla, Karima, Othman El Faqer, Sanae Jahjah, Amina Labhar, Chaima Alaoui Jamali, Ayoub Kasrati, Badredine Souhail, Mounir Legssyer, Abdelfettah Maouni, and Rabah Saidi. 2025. "Volatile Compounds from Northern Moroccan Medicinal Plants: Phytochemical Analysis, Antioxidant and Antimicrobial Potential, and In Silico Investigations" Compounds 5, no. 4: 49. https://doi.org/10.3390/compounds5040049
APA StyleAit Alla, K., El Faqer, O., Jahjah, S., Labhar, A., Alaoui Jamali, C., Kasrati, A., Souhail, B., Legssyer, M., Maouni, A., & Saidi, R. (2025). Volatile Compounds from Northern Moroccan Medicinal Plants: Phytochemical Analysis, Antioxidant and Antimicrobial Potential, and In Silico Investigations. Compounds, 5(4), 49. https://doi.org/10.3390/compounds5040049







