Abstract
Essential oils (EOs) from aromatic plants are valuable sources of bioactive compounds with potential applications as natural antimicrobials and antioxidants. This study investigated the chemical composition, antimicrobial and antioxidant activities, and in silico pharmacological properties of EOs extracted from Laurus nobilis, Thymbra capitata, and Mentha piperita, three medicinal species traditionally used in northern Morocco. Hydrodistillation yielded 1.12–1.53% oils, and GC–MS analysis revealed distinct chemotypes: L. nobilis was rich in eucalyptol, linalool, methyleugenol, and α-terpinyl acetate; T. capitata was dominated by carvacrol (80.95%), and M. piperita contained high proportions of linalool (57.37%) and linalyl acetate (28.56%). Antimicrobial assays demonstrated strong activity of all oils against Gram-positive and Gram-negative bacteria as well as Candida species, with T. capitata showing the most potent and broad-spectrum effects (MIC 0.073–9.47 mg/mL), attributed to its high carvacrol content. Antioxidant assays (DPPH and ferric reducing power) identified L. nobilis as the most active radical scavenger (IC50 = 0.037 mg/mL), followed by T. capitata, whereas M. piperita displayed weaker activity. PCA confirmed that phenolic monoterpenes drive both antimicrobial and antioxidant potency, while oxygenated terpenes and sesquiterpenes contribute more selectively. ADMET predictions indicated generally favorable absorption and low toxicity, although o-cymene and carvacrol presented potential safety concerns. Molecular docking revealed α-terpinyl acetate as the most versatile ligand, with strong binding to bacterial, fungal, and oxidative enzymes, whereas other compounds exhibited more selective affinities. Collectively, these findings highlight the potential of Moroccan EOs, particularly T. capitata and L. nobilis, as promising natural alternatives to synthetic antimicrobial and antioxidant agents.
1. Introduction
From the earliest times, humans have relied on plants to meet a variety of needs. Among these natural resources, medicinal and aromatic plants (MAPs) and their extracts have been applied in numerous fields, including food preservation, cosmetics, and medicine. Bacterial resistance to antibiotics is currently one of the most serious global health issues, especially in the medical sector. Indeed, the excessive and often inappropriate use of antibiotics in the treatment of infections has promoted the development and dissemination of multidrug-resistant bacterial strains [1]. These microorganisms are able to neutralize or evade the action of antimicrobial agents through various mechanisms, such as target modification, enzymatic inactivation of antibiotic molecules, and active efflux of antimicrobial compounds [2]. This phenomenon compromises the therapeutic efficacy of conventional treatments, leading to a significant increase in morbidity, mortality, and hospital costs [3]. In this regard, identifying and characterizing new bioactive compounds from natural sources, particularly plant extracts, is a key priority in the pursuit of innovative and environmentally sustainable antimicrobial approaches. As a type of plant extract, essential oils (EOs) have received growing attention for their potential health-promoting properties and their environmental safety [4]. Furthermore, EOs are regarded as effective and safer alternatives to many synthetic and potentially harmful substances commonly used in food processing and therapeutic medicine [5]. EOs derived from MAPs have been reported to exhibit a variety of biological properties, including antimicrobial, antioxidant, immunomodulatory, and anti-inflammatory effects [6,7,8,9,10,11,12]. These activities are primarily attributed to their high content of monoterpenes and Sesquiterpenes, volatile compounds well known for their strong biological efficacy [13,14].
Morocco, located in the Mediterranean region, possesses a rich diversity of MAPs with various biological properties [15]. Owing to its geographical position and favorable climatic conditions, the northern part of the country is particularly known for its high biological diversity and abundant flora [16,17]. This floristic richness encompasses 358 species, classified into 228 genera and 66 families [18]. Previous studies have shown that the Lamiaceae family [19,20] dominates the local pharmacopeia in certain areas of northern Morocco. Other notable species belong to the Asteraceae and Lauraceae families [18,21]. Many of these plants hold significant economic value due to their essential oil production, traditional applications, and wide range of biological activities [22,23,24,25].
According to our recent ethnopharmacological investigations, Thymbra capitata L. Cav. (T. capitata), Mentha piperita L. (M. piperita), and Laurus nobilis L. (L. nobilis), locally known as “Zaitra” “Naanaa lhor”, and “Rand,” respectively, are among the most valued species by local communities. In this context, the present study aimed to characterize the volatile constituents of the hydrodistilled EOs from these three species and to evaluate their antioxidant and antimicrobial activities against various pathogenic microorganisms responsible for multiple diseases, and in silico pharmacological properties, with the goal of identifying potential novel plant-based antimicrobial agents.
2. Materials and Methods
2.1. Plant Material and Essential Oil Extraction
The aerial parts of wild-grown L. nobilis (Lauraceae), T. capitata (Lamiaceae), and M. piperita (Lamiaceae) were harvested from various locations in northern Morocco (Table 1). The Plants were identified by one of the authors (C. Alaoui Jamali), and corresponding voucher specimens were archived at the Laboratory of Biology, Environment, and Sustainable Development at the Ecole Normale Supérieure, Tetouan, Morocco. The harvested plant material was air-dried in the shade at room temperature (≈ 25 °C) and then distilled using a Clevenger-type apparatus (manufactured by Labbox Labware, Barcelone, Spain) for 3 h until complete oil recovery. EOs extraction was performed in triplicate (3 × 100 g). The obtained oils were dehydrated with anhydrous sodium sulfate, weighed, and kept in darkness at 4 °C until further use. The essential oil yield (YEO) was determined by dividing the volume of essential oil (VEO) obtained by the mass of the dried plant material (DM):

Table 1.
Locality, harvesting site, and harvesting period of the tree Northern Moroccan aromatic plants studied.
2.2. Essential Oils Characterization by Gas Chromatography/Mass Spectrometry (GC/MS)
The chemical profile of the EOs was analyzed using gas chromatography–mass spectrometry (GC–MS) on a PerkinElmer Clarus 580 gas chromatograph (Waltham, MA, USA) coupled with a PerkinElmer SQ8S quadrupole mass spectrometer. The chromatographic separation was achieved using an Rtx-5MS capillary column (30 m × 0.25 mm × 0.25 μm) provided by Restek Corporation (Bellefonte, PA, USA). The compounds were introduced into the GC column through a split/splitless injector maintained at 250 °C, operating with a split ratio of 20%. High-purity helium (99.99%) was employed as the carrier gas at a constant flow rate of 1 mL/min. The chromatographic analysis were conducted using a temperature program starting at 40 °C for 2 min, then increasing to 200 °C at a rate of 6 °C/min and held for 2 min, followed by a ramp to 280 °C at 6 °C/min, where it was maintained for 6 min. The mass spectrometer was operated under the following settings: sample components were ionized using Electron Impact (EI) mode at 70 eV, with the transfer line maintained at 250 °C. Data were acquired in full-scan mode over a mass range of m/z 50–500 and processed using TurboMass software (PerkinElmer Inc., Waltham, MA, USA, Version 6.1, 2014). The identification of components was performed by aligning their retention times with those of standards tested under identical chromatographic conditions. The mass spectral fragmentation patterns were compared with those in the National Institute of Standards and Technology (NIST) compound database. Data collection and processing were performed using LabSolutions software (Version 2.4).
2.3. Antimicrobial Activity
2.3.1. Microbial Strains
The EOs were examined against seven bacterial reference strains obtained from “The American Type Culture Collection, Manassas, VA, USA”: Escherichia coli (E. coli) ATCC 8739, Micrococcus luteus (M. luteus) ATCC 381, Methicillin-resistant Staphylococcus aureus (MRSA) ATCC 43300, Staphylococcus aureus (S. aureus) 209 PCIP 53156, Escherichia coli (E. coli) ATCC 25922, Pseudomonas aeruginosa (P. aeruginosa) DSM 50090, and Pseudomonas aeruginosa (P. aeruginosa) ATCC 27853. The fungal strains employed in this study were obtained from the “Moroccan Coordinated Collections of Microorganisms, Rabat, Morocco”: Candida glabrata (C. glabrata) CCMM L7, Candida albicans (C. albicans) CCMM L5, Candida parapsilosis (C. parapsilosis) CCMM L18, and Candida krusei (C. krusei) CCMM L10. For cultivation, bacterial and fungal strains were grown on nutrient Agar and Sabouraud Dextrose Agar (SDA), respectively, and incubated at 37 °C for bacteria and at 25 °C for yeasts before the assays. The EOs were evaluated against seven bacterial reference strains: Bacterial and fungal strains were grown on nutrient agar and Sabouraud Dextrose Agar (SDA), respectively, and maintained at 37 °C for bacteria, whereas yeasts were incubated at 25 °C before the tests.
2.3.2. Antimicrobial Screening
Assessment of the antimicrobial activities of EOs was performed using the agar disk diffusion method according to the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) [26]. Briefly, 0.1 mL of the examined microorganism in their exponential growth phase was inoculated on Mueller-Hinton Agar (MHA) for bacteria and Sabouraud Dextrose Agar (SDA) for yeasts. On the inoculated plates, sterile disks (Whatman, 6 mm in diameter) were individually loaded with 10 µL of each EOs. To improve the diffusion of EOs, the treated Petri plates were incubated for two hours at 4 °C, followed by twenty-four hours at 37 °C for bacteria and forty-eight hours at 25 °C for yeasts. Inhibition zone diameters were measured in millimeters. Ciprofloxacin (10 µg/disk), gentamycin (10 µg/disk), cefixime (10 µg/disk), and fluconazol (40 µg/disk) were employed as positive controls. Every test was conducted in triplicate.
2.3.3. MIC and MMC Determination
A minimum inhibitory concentration (MIC) was determined using the broth microdilution method by NCCLS recommendations M7-A4 [27] for bacteria and M38-A [28] for yeasts. Dimethyl Sulfoxide (DMSO) at a concentration of 4% was used to dissolve the examined oils. The assays were conducted in Mueller-Hinton Broth (MHB) for bacterial strains and Sabouraud Dextrose Broth (SDB) for yeast strains. To prepare cell suspensions that were adjusted for bacteria to 106 CFU/mL and yeasts to 1–2 × 103 cells/mL, microorganism tests were grown overnight, and then 100 μL was added to each well of 96-well plates. Then, 100 μL of each EO dilution was added. For bacteria, plates were incubated for 24 h at 37 °C, and for yeasts, they were incubated for 48 h at 25 °C. The MIC corresponded to the lowest essential oil concentration showing no visible turbidity, indicating complete inhibition of microbial growth.
For the determination of the minimum microbicidal concentration (MMC), aliquots from each microwell were plated onto MHA and incubated at 37 °C for 24 h for bacterial strains, or onto Sabouraud dextrose agar (SDA) and incubated at 25 °C for 48 h for yeast strains. The MMC corresponds to the lowest concentration of the essential oil capable of producing a microbicidal effect. Ciprofloxacin, gentamicin, and cefixime were used as reference antibacterial agents, while fluconazol served as the antifungal control. All assays were performed in triplicate.
2.4. Antioxidant Activity
2.4.1. DPPH Free Radical-Scavenging Activity
The antioxidant potential of the essential oils was evaluated through their ability to scavenge the stable DPPH free radical (2,2-diphenyl-1-picrylhydrazyl), according to the procedure outlined by Bendiar et al. [29]. The DPPH radical exhibits a deep purple color, which shifts to yellow upon interaction with a proton-donating antioxidant, resulting in a decrease in absorbance [30]. The reaction mixture was prepared by combining 2 mL of a 60 µM DPPH solution in methanol with 50 µL of the sample, EOs, or control substances prepared in methanol at different concentrations. After incubation in the dark at room temperature for 20 min, absorbance was recorded at 517 nm. Quercetin and butylated hydroxytoluene (BHT) were used as positive controls. Experiments were conducted in triplicate, and IC50 values were expressed as mean ± SD, obtained from inhibition percentage–concentration curves.
The percentage inhibition of the DPPH radical was calculated using the following equation:
where Ab is the absorbance of the blank sample and Aa is the absorbance of the test sample.
2.4.2. Reducing Power Determination
The reductive capacity of the EOs was determined according to Latif et al. [31], by measuring the reduction of Fe3+ to Fe2+ in the presence of the test samples. In brief, 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide were mixed with different concentrations of the EOs or control samples. After incubation at 50 °C for 20 min, 2.5 mL of 10% trichloroacetic acid was added, and the mixtures were centrifuged at 3000 rpm for 10 min. The supernatant (2.5 mL) was combined with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride (FeCl3). Absorbance was recorded at 700 nm, and the EC50 was defined as the concentration yielding an absorbance of 0.5, calculated from the absorbance–concentration curve. BHT and Quercetin served as standard antioxidant controls. Experiments were conducted in triplicate, and EC50 values are expressed as mean ± SD.
2.5. ADMET Analysis
The pharmacokinetic behavior of the principal compounds identified in the EOs including their absorption, distribution, metabolism, excretion, and toxicity (ADMET) characteristics was assessed using three in silico prediction platforms. The methodology was adapted from El Faqer et al. [32]. Specifically, the online tools SwissADME (http://www.swissadme.ch, accessed on 7 August 2025), ADMET-AI (https://admet.ai.greenstonebio.com/; accessed on 7 August 2025), and ProTox-II (https://tox.charite.de/protox3/; accessed on 7 August 2025) were employed. These platforms collectively provided complementary insights into the pharmacokinetic and toxicological profiles of the investigated EOs constituents.
2.6. Molecular Docking Studies
2.6.1. Protein Preparation
To elucidate the molecular interactions underlying the antimicrobial and antioxidant potential of the EOs components, four protein targets were selected: two bacterial enzymes (Muramyl ligase E and Dihydropteroate synthase), one fungal enzyme (Lanosterol 14α-demethylase), and one oxidative stress-related enzyme (NADPH oxidase). These targets correspond to specific three-dimensional crystallographic structures retrieved from the Protein Data Bank (PDB; https://www.rcsb.org/; accessed on 7 August 2025), as listed in Table 2, along with their respective PDB identifiers.

Table 2.
Molecular docking target of bacterial and fungal target proteins, PDB ID’s, and active site coordinates.
Protein preparation was conducted using AutoDock Tools (ADT, v4.2.6) following the procedure described by El Faqer et al. [32]. In brief, non-essential atoms and water molecules were removed, and any missing residues or atoms were corrected to optimize structural integrity. Polar hydrogen atoms were subsequently added, and Kollman charges were assigned to calculate partial atomic charges. The resulting protein structures were saved in PDBQT format to enable molecular docking simulations.
2.6.2. Ligand Preparation
The ligands used for docking simulations corresponded to the principal phytoconstituents of the EOs from L. nobilis, T. capitata, and M. piperita. Specifically, L. nobilis contained eucalyptol, linalool, α-terpinyl acetate, and methyleugenol; T. capitata comprised o-cymene, γ-terpinene, carvacrol, and caryophyllene; and M. piperita included linalool and linalyl acetate. These molecules were examined for their potential as inhibitors of bacterial and fungal enzymes, as well as their interaction with oxidative stress-related targets.
The two-dimensional chemical structures of all compounds were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/; accessed on 7 August 2025) in SDF format. Structures were converted to PDBQT format using the graphical interface of Open Babel, following the procedure outlined by El Faqer et al. [33]. In addition, Gasteiger charges were applied to the ligands using ADT to ensure accurate electrostatic representation in docking simulations.
2.6.3. Molecular Docking Analysis
Molecular docking experiments were performed using AutoDock Tools (version 1.5.7). Each prepared ligand (PDBQT format) was docked into the active site of its corresponding target protein under default ADT parameters. The docking results were expressed as binding energy (ΔGb) in kcal/mol and inhibition constant (Ki) in mM, providing an estimation of binding affinity and inhibitory potential.
For visualization and further analysis of the docked complexes, BIOVIA Discovery Studio Visualizer 2021 was employed. This software enabled the identification of key hydrogen bonds, hydrophobic interactions, and van der Waals contacts between amino acid residues and ligands. The resulting three-dimensional visualizations offered detailed insights into the binding conformations and the nature of molecular interactions within each protein–ligand complex [32].
3. Statistical Analysis
Each experimentation was conducted at least three times, and the results were presented as the mean ± standard deviation. The statistical significance of the findings (p < 0.05) was determined using ANOVA, followed by Tukey’s post hoc test for multiple comparisons, performed with GraphPad Prism 8.0.2 software (GraphPad Software Inc., Boston, MA, USA). Principal Component Analysis (PCAs) was conducted using the Rstudio software for Windows, version 22.1.64970 (2020).
4. Results and Discussion
4.1. Essential Oil Yield and Chemical Composition
EOs yields of ranged from 1.12% ± 0.06 to 1.53% ± 0.28 (v/w) among the different plants studied (Table 3). The highest yield was obtained from M. piperita (1.53%), followed by T. capitata (1.20%), while L. nobilis produced the lowest yield at 1.12%. The yields obtained for all studied plants were lower than those reported by several researchers in other Moroccan regions [34,35,36]. Several factors may account for these differences, including harvesting season, drying and extraction methods, soil micronutrient composition, climate, the plant part used for extraction, as well as the plant’s age and genetic diversity [37,38].

Table 3.
Chemical composition of volatile components extracted from aerial parts of L. nobilis, T. capitata, and M. piperita from northern Morocco.
Volatile compounds from the investigated plants were analyzed by gas chromatography (GC) and characterized using mass spectrometry (MS) (Figure 1, Figure 2 and Figure 3). Between 6 and 16 constituents were detected in the studied EOs, representing 98.87% to 100% of the total oil composition (Table 3). The chemical profiles of all EOs were dominated by monoterpenoids, particularly oxygenated monoterpenes, which constituted the largest subclass. The proportion of this major subclass was 95.22%, 85.64%, and 83.53% for M. piperita, L. nobilis, and T. capitata, respectively. For each identified compound, Table 3 provides the relative percentage, elution order, retention index, and retention time.
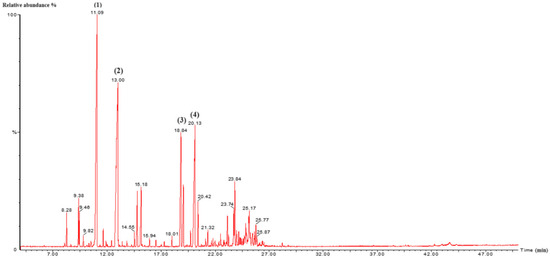
Figure 1.
GC/MS chromatogram of L. nobilis EO. (1): Eucalyptol; (2): Linalool; (3): α-Terpinyl acetate; (4): Methyl eugenol. Detector type: mass spectrometry (MS).
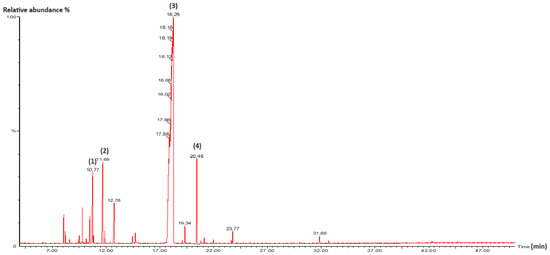
Figure 2.
GC/MS chromatogram of T. capitata EO. (1): o-Cymene; (2): γ-Terpinene; (3): Carvacrol; (4): Caryophyllene. Detector type: mass spectrometry (MS).
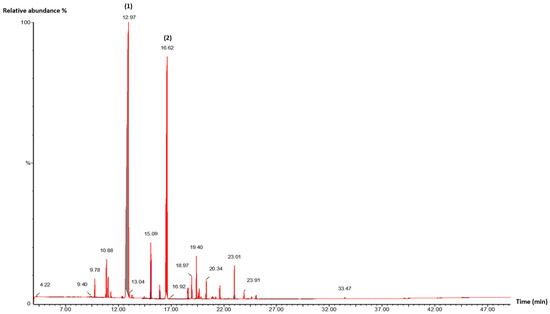
Figure 3.
GC/MS chromatogram of M. piperita EO. (1): Linalool; (2): Linalyl acetate. Detector type: mass spectrometry (MS).
In L. nobilis EO, the major constituents were eucalyptol (26.09%), linalool (25.64%), methyl eugenol (11.95%), and α-terpinyl acetate (9.03%), with other components present in lower proportions. The observed profile aligns qualitatively with findings of Nafis et al. for the same species from the High Atlas of Morocco, although some quantitative differences were noted, particularly in the contents of eucalyptol (40.85%), α-terpinyl acetate (12.64%), methyl eugenol (8.72%), and linalool (6.81%) [34]. Similarly, El Faqer et al. found that the main constituents of L. nobilis EO from Taza were eucalyptol (44.14%), α-terpinyl acetate (11.11%), and β-phellandrene (6.74%) [39]. Comparative studies on L. nobilis from Italy, Bulgaria, Turkey, and Tunisia also revealed marked variation in EO composition between regions, especially in the relative proportions of linalool, eucalyptol, methyl eugenol, sabinene, and α-terpinyl acetate [40,41,42,43].
The EO obtained from the aerial parts of T. capitata was dominated by carvacrol (80.95%), followed by minor components such as γ-terpinene (5.47%), caryophyllene (5.44%), and o-cymene (4.15%). This composition closely matches that reported by Benoutman et al. and Moukhles et al. for samples from other northern Moroccan locations [44,45]. Consistent with our findings, multiple studies from the same geographic area have identified T. capitata EO as a carvacrol chemotype, with reported carvacrol content ranging from 68.63% to 92.27% depending on the region [46,47].
In M. piperita EO, linalool (57.37%) and linalyl acetate (28.56%) were the predominant constituents. The chemical profile was qualitatively in line with that reported by Ben Bakrim et al. for samples from the Marrakech region of Morocco, though quantitative differences were observed, particularly for linalool (71.10%) and linalyl acetate (9.46%) [35]. In contrast, Hamad Al-Mijalli et al. found that M. piperita oil from the El Gharb region of Morocco was dominated by menthone (29.24%) and levomenthol (38.73%) [48]. Similarly, Tsai et al. reported that the main volatile compounds of Mentha oil from Taiwan were menthol (30.35%), menthone (21.12%), and trans-carane (10.99%) [49].
4.2. Antimicrobial Activity
Antimicrobial activity of the essential oils and reference agents were assessed against a panel of three Gram-positive, four Gram-negative bacteria, and four yeast strains. Their potencies were assessed using two methods: the disk diffusion assay and the broth microdilution technique.
According to the disk diffusion results (Table 4), all tested EOs exhibited antimicrobial activity to varying degrees against the different microorganisms. T. capitata EO demonstrated the highest antimicrobial potency across all tested strains, with inhibition zone (IZ) diameters ranging from 26.0 mm to 46.33 mm. Notably, P. aeruginosa ATCC 27853, a highly resistant Gram-negative bacteria, showed greater susceptibility to this EO than to the antibiotics tested. The EOs of L. nobilis and M. piperita displayed moderate antimicrobial effects against several strains, with IZ diameters of 10.33–50.00 mm and 11.00–49.33 mm, respectively. Interestingly, M. luteus and P. aeruginosa ATCC 27853 were more sensitive to these two EOs than to the reference antibiotics, showing inhibition zones exceeding 45 mm (Table 4).

Table 4.
Antimicrobial activity of essential oils isolated from aerial parts of L. nobilis, T. capitata, and M. piperita using disk diffusion assay.
Overall, yeast strains appeared to be the most sensitive group to all tested oils. The strongest activity was observed for T. capitata EO against all Candida species (26.33–44.00 mm), followed by M. piperita (15.66–36.00 mm) and L. nobilis (15.33–38.33 mm). These findings differ from earlier reports for the same species collected from other Moroccan regions [34,36,48].
The results of MIC and MMC determinations were consistent with those obtained from the disk diffusion assay, as shown in Table 5. For most of the tested oils, MMC values were identical to MIC values, indicating a microbicidal effect. Overall, all examined strains were sensitive to the EOs, although the degree of activity varied among species. The most sensitive microorganisms were M. luteus, P. aeruginosa DSM 50090, P. aeruginosa ATCC 27853, and C. krusei, with MIC values ranging from 9.47 to 51.90 mg/mL, 0.073 to 51.90 mg/mL, 9.47 to 54.24 mg/mL, and 9.47 to 27.12 mg/mL, respectively. As indicated in Table 5, T. capitata EO showed the highest antimicrobial activity (MIC = 0.073–9.47 mg/mL), while L. nobilis EO exhibited the lowest potency (MIC = 13.56–54.24 mg/mL). M. piperita EO demonstrated notable antifungal activity, particularly against Candida species (MIC = MMC = 17.30 mg/mL), except for C. parapsilosis, which showed moderate susceptibility (MIC = MMC = 25.95 mg/mL). The MBC/MIC ratios for the EOs of L. nobilis, T. capitata, and M. piperita were consistently low (≤2) across all tested microorganisms, indicating strong killing activity rather than mere growth inhibition (Table 6). The results indicate that all three essential oils have potent killing activity (bactericidal or fungicidal) against a wide range of Gram-positive, Gram-negative bacteria, and fungi, with little variation in efficacy between microbial groups and species.

Table 5.
Antimicrobial activity of EOs isolated from aerial parts of L. nobilis, T. capitata, and M. piperita using the minimum inhibitory concentration method.

Table 6.
MMC/MIC ratio of essential oils isolated from the aerial parts of L. nobilis, T. capitata, and M. piperita determined by the disk diffusion assay.
The data confirm the inhibitory activity of T. capitata EO against bacteria and fungi, in agreement with previous reports [44,50]. Oils extracted from various Thymus species are well known for their fungicidal and bactericidal properties [51,52,53,54]. The strong antimicrobial activity of T. capitata EO is likely attributable to its high carvacrol content (80.95%), an oxygenated phenolic monoterpene known for its higher antibacterial and antifungal potential [55,56,57,58,59]. Carvacrol has been reported to act through multiple mechanisms, including the disruption of cell membranes, depletion of intracellular ATP, the generation of reactive oxygen species, and the inhibition of efflux pumps [55,60]. This phenolic compound may affect fungi by disrupting the cell envelope, blocking ergosterol synthesis, and impairing membrane integrity. In C. albicans cells, carvacrol interferes with proper protein folding and elicits endoplasmic reticulum stress [61,62]. Additionally, minor constituents like γ-terpinene and caryophyllene also possess antimicrobial activity [63,64].
The antimicrobial activity of L. nobilis EO is presumably linked to its relatively high contents of eucalyptol (26.09%) and linalool (25.64%) [65,66], whereas the activity of M. piperita EO may be attributed to its high levels of linalool (57.37%) and linalyl acetate (28.56%), both of which have recognized antimicrobial properties [67,68]. Eucalyptol (1,8-cineole) has been reported to interact with plasma membranes and is applied therapeutically for its antitussive activity, while in aromatherapy it is used as a skin stimulant [69]. Terpenes such as eucalyptol not only demonstrate antibiotic effects but also interfere with signaling by blocking receptors responsive to different autoinducers. Furthermore, this compound is responsible for inhibiting the chemical signals between microbes, a process known as quorum sensing (QS). This inhibition plays an important biological role and contributes to eucalyptol’s antimicrobial effects [70,71]. Eucalyptol has demonstrated synergistic effects with conventional antimicrobial drugs, supporting its potential role as an adjuvant against bacterial infections [72]. Linalool, on the other hand, can disrupt cell membrane structure and function, leading to leakage of intracellular components, and may also inhibit key intracellular enzymes such as ATPases and respiratory chain dehydrogenases, ultimately causing cell death [66].
4.3. Antioxidant Capacity
Antioxidant activity of essential oils from the aerial parts of L. nobilis, T. capitata, and M. piperita was assessed using DPPH radical scavenging and ferric ion reducing assays. The IC50 results are presented in Table 7. Lower IC50 values indicate stronger antioxidant capacity.

Table 7.
IC50 values of L. nobilis, T. capitata, and M. piperita EOs, and of quercetin and BHT.
As shown in Table 7, Antioxidant activity followed the same decreasing trend in both assays: L. nobilis EO > T. capitata EO > M. piperita EO. All tested EOs were capable of reducing the purple DPPH radical to a stable yellow form, with the degree of discoloration reflecting their ability to scavenge free radicals. The oils from L. nobilis and T. capitata showed strong DPPH reduction, with IC50 values of 0.037 ± 0.001 mg/mL and 0.143 ± 0.002 mg/mL, respectively. The lowest DPPH scavenging activity was observed for M. piperita EO (IC50 = 4.189 ± 0.008 mg/mL). All oils were less potent than quercetin (IC50 = 0.002 ± 0.0003 mg/mL) and BHT (IC50 = 0.006 ± 0.0001 mg/mL). Similarly, in the ferric ion reducing power assay, L. nobilis and T. capitata EOs exhibited the highest activity, with IC50 values of 0.058 ± 0.001 mg/mL and 0.064 ± 0.001 mg/mL, respectively, whereas M. piperita EO showed a significantly lower reducing capacity (IC50 = 6.457 ± 0.006 mg/mL). As indicated by the IC50 values in Table 7, both BHT and quercetin demonstrated greater antioxidant activity than the studied EOs.
Overall, the findings highlight L. nobilis EO as a potent antioxidant. This activity is likely linked to its high eucalyptol content (26.09%), a compound reported to possess notable antioxidant properties [72]. The strong radical scavenging ability of this EO may also result from synergistic or antagonistic interactions among its constituents [73]. These results are consistent with previous reports for the same species [74,75]. Similarly, the high antioxidant activity of T. capitata EO has been confirmed in several studies [36,44,76]. Antioxidant capacity is generally attributed to the combined effect of all EO constituents’ alcohols, phenols, and terpenes acting synergistically or antagonistically. Numerous studies have also emphasized the correlation between phenolic content and antioxidant potential in plants [77,78]. Additionally, the greater anti-oxidative property of T. capitata oil may be attributed to its high carvacrol content (80.95%). This well-known compound enhance the activity of enzymatic antioxidants, including superoxide dismutase, catalase, and glutathione peroxidase, as well as the levels of nonenzymatic antioxidants such as reduced glutathione, vitamin E, and vitamin C [79,80]. Additionally, carvacrol’s radical scavenging activity against hydrogen peroxide, nitric oxide, and superoxide radicals, is mainly attributed to its hydroxyl (–OH) group [81]. The low antioxidant activity observed for M. piperita EO is consistent with previous findings for samples from the Marrakesh region [35].
4.4. PCA
The PCA analyses of both yeast and bacterial inhibition reveal a strikingly consistent separation of the three EOs according to their dominant phytochemical profiles (Figure 4). For T. capitata, clustering with carvacrol, γ-terpinene, β-myrcene, and o-cymene is observed in both the yeast and bacterial datasets (Figure 4A,B). This association underscores the central role of phenolic monoterpenes in driving strong and broad antimicrobial activity, active across Candida species as well as both Gram-positive and Gram-negative bacteria. In contrast, M. piperita is positioned with oxygenated monoterpenes such as linalool, terpineol, α-pinene, and eucalyptol, suggesting a distinct but weaker antimicrobial profile, potentially reflecting a narrower spectrum of action. L. nobilis consistently aligns with sesquiterpenes and acetates (e.g., α-terpinyl acetate, germacrene D, linalyl acetate, elemol) as well as phenylpropanoids like eugenol and methyleugenol, indicating a more selective inhibitory activity, possibly dependent on specific microbial targets rather than broad-spectrum potency. Taken together, these results demonstrate that phenolic-rich oils (T. capitata) provide the most potent and general antimicrobial effects, while peppermint and bay leaf oils show narrower, composition-driven selectivity.
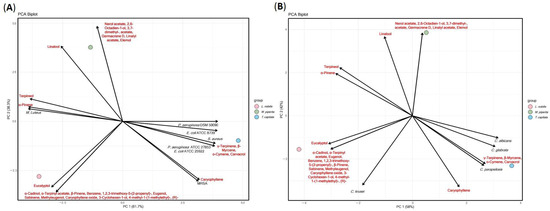
Figure 4.
Principal component analysis of chemical and antibacterial and antifungal activity of L. nobilis, T. capitata, and M. piperita EOs. (A): Bacterial strains, (B): Candida strains.
The antioxidant PCA, based on FRP and DPPH assays, mirrors the antimicrobial trends but with subtle distinctions (Figure 5). T. capitata again clusters with carvacrol, γ-terpinene, β-myrcene, and o-cymene, confirming that the same phenolic monoterpenes that drive antimicrobial potency are also responsible for strong antioxidant activity. M. piperita occupies a separate space associated with linalool, α-pinene, terpineol, and eucalyptol, which contribute moderate antioxidant effects but do not reach the strength of phenolic-rich oils. L. nobilis, linked with acetates, sesquiterpenes, and eugenol derivatives, shows weaker antioxidant positioning, reinforcing the idea that its bioactivity is more selective and limited compared to T. capitata. These results indicate that phenolic monoterpenes are multifunctional drivers of both antimicrobial and antioxidant properties, while oxygenated terpenes and sesquiterpenes contribute more modestly or in a target-specific manner.
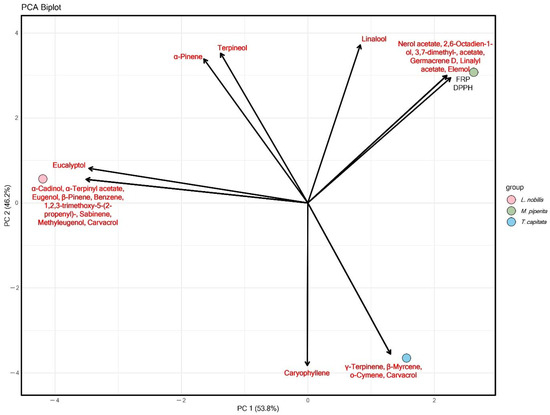
Figure 5.
Principal component analysis of chemical and antioxidant activity of L. nobilis, T. capitata, and M. piperita EOs.
Across antimicrobial and antioxidant PCAs, a unified pattern emerges: T. capitata, dominated by carvacrol and related monoterpenes, consistently demonstrates broad and potent bioactivity, spanning fungi, bacteria, and oxidative stress. M. piperita represents an intermediate profile, with oxygenated monoterpenes driving moderate but consistent effects, while L. nobilis exhibits a distinct, more selective activity spectrum linked to complex sesquiterpene and phenylpropanoid chemistry. Together, these findings highlight the dual role of chemical composition in defining both the potency and selectivity of EOs, with phenolic-rich profiles offering the most versatile and powerful bioactivity.
4.5. ADMET Analysis
ADMET profiling of the principal constituents of L. nobilis, T. capitata, and M. piperita EOs revealed distinctive pharmacokinetic and toxicological characteristics that could impact their therapeutic applicability (Figure 6).
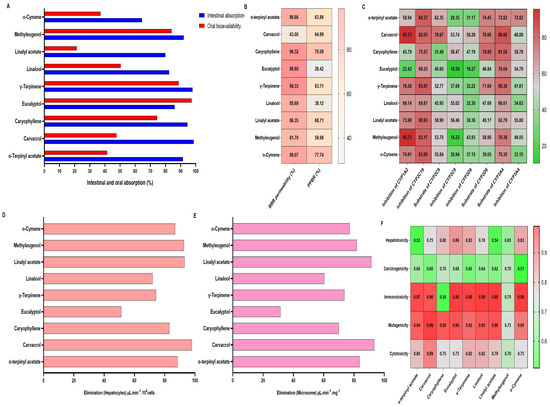
Figure 6.
Predictive results of the absorption, distribution, and Elimination of the major compounds of L. nobilis, T. capitata, and M. piperita EOs. (A): intestinal absorption (%); oral bioavailability: (B): Distribution; (C): CYP Enzyme Interactions; (D): elimination (hepatocytes); (E): elimination (microsome); (F): Toxicity Profile.
In silico ADMET profiling revealed that most tested compounds exhibited high predicted intestinal absorption (>70%), particularly α-terpinyl acetate, caryophyllene, eucalyptol, γ-terpinene, and o-cymene (Figure 6A). However, oral bioavailability values were generally lower than intestinal absorption, notably for linalool and linalyl acetate, suggesting substantial first-pass metabolism (Figure 6A). BBB permeability predictions were high (>80%) for most compounds except carvacrol (43%) and eucalyptol (23%), while plasma protein binding rates ranged from 28.4% (eucalyptol) to 78.1% (caryophyllene) (Figure 6B). CYP450 interaction analysis indicated that carvacrol, methyleugenol, and γ-terpinene strongly inhibited CYP1A2 and CYP2C19 (>80%), whereas eucalyptol showed minimal inhibition across all isoforms (Figure 6C). Hepatocyte and microsomal elimination rates were generally high, with eucalyptol showing notably lower clearance in both systems (Figure 6D,E). Toxicological prediction models identified high probabilities (>0.9) of immunotoxicity and mutagenicity for several compounds, especially methyleugenol and carvacrol, whereas eucalyptol and o-cymene exhibited comparatively lower hepatotoxicity and carcinogenicity risks (Figure 6F).
The predicted acute oral toxicity of the major constituents of L. nobilis, T. capitata, and M. piperita EOs ranged from 113 to 12,000 mg/kg, corresponding mainly to toxicity class 5 (may be harmful if ingested) (Table 8). Most compounds, including α-terpinyl acetate, caryophyllene, eucalyptol, γ-terpinene, linalool, linalyl acetate, and methylisoeugenol, displayed relatively low predicted toxicity (LD50 2000–12,000 mg/kg). In contrast, carvacrol (LD50 = 810 mg/kg, class 4) was classified as harmful if ingested, and o-cymene (LD50 = 113 mg/kg, class 3) was predicted to be toxic if ingested, representing the most concerning constituent from a safety perspective. These results suggest that while the majority of compounds present low acute oral toxicity, careful consideration should be given to formulations containing higher proportions of o-cymene to ensure safe use in therapeutic or food applications.

Table 8.
Toxicity predictions for the identified major compounds of L. nobilis, T. capitata, and M. piperita EOs.
Collectively, these results suggest that although many constituents display favorable absorption and penetration profiles, metabolic interactions and potential toxicities, particularly for methyleugenol and carvacrol, may limit their pharmacological applicability, while eucalyptol emerges as a compound with favorable metabolic and safety predictions.
4.6. Molecular Docking
Molecular docking simulations were performed to evaluate the interactions of the major constituents of L. nobilis, T. capitata, and M. piperita EOs with selected target enzymes, including the bacterial Muramyl ligase E (MurE) and Dihydropteroate synthase (DHPS), the fungal Lanosterol 14α-demethylase, and the oxidant NADPH oxidase (NOX). The estimated binding free energies (ΔGb) and inhibition constants (Ki) are summarized in Table 9.

Table 9.
Docking results of the major compounds of L. nobilis, T. capitata, and M. piperita essential oils on bacterial, fungal, and oxidant enzymes.
The in silico investigation was conceived as an exploratory yet mechanistically informative complement to the experimental assays. The selection of molecular targets was guided by their established biochemical relevance to the antibacterial, antifungal, and antioxidant properties of the EOs constituents. Specifically, MurE and DHPS were chosen as essential bacterial enzymes involved in peptidoglycan and folate biosynthesis, respectively, while Lanosterol 14α-demethylase, a cytochrome P450 enzyme required for ergosterol formation, represents a validated antifungal target [82,83,84]. In addition, NOX was selected for its central role in the generation of reactive oxygen species, thereby linking the computational analysis to the antioxidant and anti-inflammatory potential observed in vitro [85]. Collectively, these targets encompass complementary mechanisms of action and provide a coherent biochemical framework for interpreting the biological activities of the tested compounds.
For the bacterial enzyme MurE, most ligands exhibited weak to moderate binding, with docking energies generally above −5.0 kcal/mol. The best-performing compound was α-terpinyl acetate (−5.05 kcal/mol; Ki = 199.40 μM), whereas eucalyptol and methyleugenol clustered around −4.0 to −4.3 kcal/mol. Caryophyllene showed no stable binding, with a positive energy value, indicating a poor fit to the enzyme’s active site. For DHPS, the results followed a similar trend but were slightly stronger. Again, α-terpinyl acetate displayed the best affinity (−5.90 kcal/mol; Ki = 47.74 μM), while most other ligands remained within the −4.0 to −5.0 kcal/mol range. This reproducible ranking across both bacterial enzymes reinforces α-terpinyl acetate as the most competent antibacterial binder in the tested set.
In contrast, the fungal enzyme Lanosterol 14α-demethylase showed substantially higher affinities. Here, α-terpinyl acetate again ranked first (−7.44 kcal/mol; Ki = 3.54 μM), closely followed by caryophyllene (−6.84 kcal/mol; Ki = 9.65 μM) and eucalyptol (−6.38 kcal/mol; Ki = 21.2 μM). For NOX, the docking profile was intermediate between the bacterial and fungal enzymes. α-terpinyl acetate again showed the highest affinity (−6.32 kcal/mol; Ki = 23.3 μM), followed by linalool (−6.17 kcal/mol; Ki = 30.0 μM) and linalyl acetate (−5.94 kcal/mol; Ki = 44.6 μM). In contrast, eucalyptol demonstrated a very weak interaction (−1.78 kcal/mol; Ki = 50 mM), reflecting high selectivity among closely related terpenes.
The docking simulations revealed that the most active terpenoids namely α-terpinyl acetate, linalool, and eucalyptol, exhibited the lowest binding energies and most favorable interaction profiles across all selected targets. These molecules formed multiple hydrogen bonds and hydrophobic contacts with catalytically important residues, resulting in stable enzyme-ligand complexes that align with their strong in vitro activities [86,87,88,89]. In contrast, hydrocarbons such as caryophyllene and o-cymene showed weaker predicted affinities and limited polar interactions, consistent with their lower biological potency. This correlation between computational affinities and experimental results underscores a clear structure-activity relationship (SAR), in which oxygenated functional groups (hydroxyl, ester, or ether moieties) enhance molecular recognition and strengthen binding within enzyme pockets [90,91]. The molecular docking data provide a plausible mechanistic explanation for the observed differential bioactivities. Together, these findings indicate that biological potency correlates with predicted binding strength and interaction quality, thereby supporting the consistency between in silico predictions and in vitro results.
Minor discrepancies between in silico predictions and in vitro outcomes can be attributed to the simplifications inherent to docking, which models static receptor-ligand interactions and does not fully capture solvent effects, metabolic transformations, or cellular uptake. Nonetheless, the overall consistency between predicted affinities and experimental responses reinforces the biological plausibility of the computational findings. Taken together, the integrated in silico and in vitro analyses demonstrate that oxygenated terpenoids possess superior target affinity and interaction stability, providing a robust mechanistic basis for their higher antimicrobial and antioxidant efficacy. This synergy between computational and experimental approaches strengthens the pharmacological interpretation of the results and supports the rational exploration of structure based optimization in future studies.
5. Conclusions
The bioactivity of EOs obtained from the studied species L. nobilis, T. capitata, and M. piperita was elucidated by their richness in oxygenated monoterpenes, known for their great antimicrobial and antioxidant effects. The obtained results provide strong evidence that these plant species represent promising natural sources of antioxidants and antimicrobial agents, which could be incorporated as additives in food preservation to prevent oxidative deterioration and inhibit the growth of pathogenic microorganisms, including those responsible for nosocomial infections. However, despite the encouraging in vitro results, further investigations are required to assess the in vivo efficacy and safety of these essential oils in more complex biological systems. Such research should include animal model studies to elucidate their pharmacokinetic behavior, potential toxicological effects, and mechanisms of action at the molecular level.
Author Contributions
Conceptualization, K.A.A., A.L., M.L. and A.M.; Methodology, K.A.A., O.E.F. and A.M.; Formal analysis, K.A.A.; Investigation, K.A.A., C.A.J., R.S. and A.K.; Resources, K.A.A. and A.L.; Writing—original draft, K.A.A.; review & editing, O.E.F., S.J. and A.K.; Writing, C.A.J.; Data curation, C.A.J.; Visualization, C.A.J., B.S. and R.S.; Formal Analysis, B.S.; Supervision, C.A.J. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors carried out all research activities in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BHT | Butylated hydroxytoluene |
| DMSO | Dimethyl Sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| E.Os | Essential oils |
| GC-MS | Gas chromatography/Mass spectrometry |
| IC50 | Inhibitory concentration 50 |
| IZ | Inhibition Zone |
| MAPs | Medicinal and Aromatic Plants |
| MHA | Mueller Hinton Agar |
| MIC | Minimum Inhibitory Concentration |
| MMC | Minimum Microbicidal Concentration |
| NCCLS | National Committee for Clinical Laboratory Standards |
| PCA | Principal component analysis |
| PDB | Protein Data Bank |
| SDA | Sabouraud Dextrose Agar |
References
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, W. The mechanism of bacterial resistance and potential bacteriostatic strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, L.; Tang, X.; Peng, L.; Li, X.; Zhao, G.; Zhong, L. Chemical Composition, Antimicrobial and Antioxidant Activities of the Flower Volatile Oils of Fagopyrum esculentum, Fagopyrum tataricum and Fagopyrum Cymosum. Molecules 2018, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Kelen, M.; Tepe, B. Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora. Bioresour. Technol. 2008, 99, 4096–4104. [Google Scholar] [CrossRef]
- Alaoui Jamali, C.; Kasrati, A.; Fadli, M.; Hassani, L.; Leach, D.; Abbad, A. Synergistic effects of three Moroccan thyme essential oils with antibiotic cefixime. Phytotherapie 2018, 16, 149–154. [Google Scholar] [CrossRef]
- Imelouane, B.; Amhamdi, H.; Wathelet, J.P.; Ankit, M.; Khedid, K.; El Bachiri, A. Chemical composition and antimicrobial activity of essential oil of thyme (Thymus vulgaris) from Eastern Morocco. Int. J. Agric. Biol. 2009, 11, 205–208. [Google Scholar]
- Benali, T.; Habbadi, K.; Bouyahya, A.; Khabbach, A.; Marmouzi, I.; Aanniz, T.; Hammani, K. Phytochemical analysis and study of antioxidant, anticandidal, and antibacterial activities of Teucrium polium subsp. polium and Micromeria graeca (Lamiaceae) essential oils from Northern Morocco. Evid.-Based Complement. Altern. Med. 2021, 2021, 6641720. [Google Scholar] [CrossRef]
- Douhri, B.; Douhri, H.; Farah, A.; Idaomar, M.; Senhaji, N.S.; Abrini, J. Phytochemical Analysis and Antibacterial Activity of Essential Oil of Lavandula multifida L. Int. J. Innov. Sci. Res. 2014, 1, 116–126. [Google Scholar]
- Laghmouchi, Y.; Belmehdi, O.; Senhaji, N.S.; Abrini, J. Chemical composition and antibacterial activity of Origanum compactum Benth. essential oils from different areas at northern Morocco. S. Afr. J. Bot. 2018, 115, 120–125. [Google Scholar] [CrossRef]
- Chellappandian, M.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Karthi, S.; Thanigaivel, A.; Ponsankar, A.; Kalaivani, K.; Hunter, W.B. Botanical essential oils and uses as mosquitocides and repellents against dengue. Environ. Int. 2018, 113, 214–230. [Google Scholar] [CrossRef]
- Latif, M.; Elkoraichi, I.; El Faqer, O.; Wahnou, H.; Mtairag, E.M.; Oudghiri, M.; Rais, S. Phytochemical analysis and immunomodulatory activities in vitro and in vivo of Aframomum melegueta K Schum seed extracts. Inflammopharmacology 2024, 32, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Mabou, F.D.; Yossa, I.B.N. TERPENES: Structural classification and biological activities. IOSR J. Pharm. Biol. Sci. 2021, 16, 25–40. [Google Scholar] [CrossRef]
- Ma, G.H.; Chen, K.X.; Zhang, L.Q.; Li, Y.M. Advance in biological activities of natural guaiane-type sesquiterpenes. Med. Chem. Res. 2019, 28, 1339–1358. [Google Scholar] [CrossRef]
- Fennane, M.; Tattou, M.I. Statistics and comments on the current inventory of the vascular flora of Morocco. Bull. Institut Sci. Sect. Sci. Vie 2012, 34, 1–9. [Google Scholar]
- Benabid, A. Les écosystèmes forestiers, préforestiers et presteppiques du Maroc: Diversité, répartition biogéographique et problèmes posés par leur aménagement. Forêt Méditerranéenne 1985, 7, 53–64. Available online: https://www.foret-mediterraneenne.org/upload/biblio/FORET_MED_1985_1_53.pdf (accessed on 4 November 2025).
- Grovel, R. La préservation des forêts du Rif centro-occidental: Un enjeu de développement de la montagne rifaine. Rev. Geogr. Alp. 1996, 84, 75–94. [Google Scholar] [CrossRef]
- Chebli, Y.; Chentouf, M.; Cabaraux, J.F.; El Otmani, S. Floristic Composition, Diversity, Palatability, and Forage Availability of Forest Rangelands in the Southern Mediterranean Region of Northern Morocco. Land 2023, 12, 215. [Google Scholar] [CrossRef]
- Ennabili, A.; Gharnit, N.; El hamdouni, E. Inventory and social interest of medicinal, aromatic and honey-plants from mokrisset region (Nw of Morocco). J. Med. Plant 2000, 19, 39–56. [Google Scholar]
- Bouyahya, A.; Abrini, J.; Et-Touys, A.; Bakri, Y.; Dakka, N. Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. Eur. J. Integr. Med. 2017, 13, 9–25. [Google Scholar] [CrossRef]
- Labiad, H.; Aljaiyash, A.; Ghanmi, M.; Satrani, B.; Ettahir, A.; Aouane, M.; Fadli, M.; Chaouch, A. Exploring the provenance effect on chemical composition and pharmacological bioactivity of the Moroccan essential oils of Laurus nobilis. Res. J. Pharm. Technol. 2020, 13, 4067–4076. [Google Scholar] [CrossRef]
- Costa, P.; Medronho, B.; Gonçalves, S.; Romano, A. Cyclodextrins enhance the antioxidant activity of essential oils from three Lamiaceae species. Ind. Crops Prod. 2015, 70, 341–346. [Google Scholar] [CrossRef]
- Khabbach, A.; Libiad, M.; Ennabili, A.; Bousta, D. Medicinal and cosmetic use of plants from the province of Taza, Northern Morocco. Bol. Latinoam. Caribe Plantas Med. Aromat. 2012, 11, 46–60. [Google Scholar]
- Benali, T.; Khabbach, A.; Ennabili, A.; Hammani, K. Ethnopharmacological prospecting of medicinal plants from the Province of Guercif (NE of Morocco). Mor. J. Biol. 2017, 14, 1–14. [Google Scholar] [CrossRef]
- Abdelaty, N.A.; Attia, E.Z.; Hamed, A.N.E.; Desoukey, S.Y. A review on various classes of secondary metabolites and biological activities of Lamiaceae (Labiatae) (2002–2018). JABPS 2021, 4, 16–31. [Google Scholar] [CrossRef]
- Approved Standard M2-A6; Performance Standards for Antimicrobial Disk Susceptibility Test. 6th ed. National Committee for Clinical Laboratory Standards (NCCLS): Wayne, PA, USA, 1997.
- Approved Standard M7-A4; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 4th ed. National Committee for Clinical Laboratory Standards (NCCLS): Wayne, PA, USA, 1997.
- Approved Standard M38-A; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. National Committee for Clinical Laboratory Standards (NCCLS): Wayne, PA, USA, 2002.
- Bendiar, S.; El Faqer, O.; Chennaoui, S.; Benjelloun, N.; Mtairag, E.M.; Oudghiri, M. Phytochemical Screening and in vivo Immunosuppressive, Antioxidant and Anti-hemolytic Activities of Zea mays Silk Aqueous Extract. Pharmacogn. J. 2020, 12, 1412–1420. [Google Scholar] [CrossRef]
- Alaoui Jamali, C.; Kasrati, A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Cultivation and the application of inorganic fertilizer modifies essential oil composition in two Moroccan species of Thymus. Ind. Crops Prod. 2014, 62, 113–118. [Google Scholar] [CrossRef]
- Latif, M.; Elkoraichi, I.; El Faqer, O.; Wahnou, H.; Elaaj, R.; Mtairag, E.M.; Oudghiri, M.; Rais, S. Aframomum Melegueta: Evaluation of Chronic Toxicity, HPLC Profiling, and In Vitro/In Vivo Antioxidant Assessment of Seeds Extracts. Chem. Biodivers. 2024, 22, e202400942. [Google Scholar] [CrossRef]
- El Faqer, O.; Rais, S.; Ouadghiri, Z.; El Faqer, A.; Benchama, Z.; El Ouaddari, A.; Dakir, M.; El Amrani, A.; Mtairag, E.M. Physicochemical properties, GC–MS profiling, and antibacterial potential of Allium sativum essential oil: In vitro and in silico approaches. Sci. Afr. 2024, 26, e02484. [Google Scholar] [CrossRef]
- El Faqer, O.; Elkoraichi, I.; Latif, M.; Debierre-Grockiego, F.; Ouadghiri, Z.; Rais, S.; Dimier-Poisson, I.; Mtairag, E.M. Pharmacological insights into Laurus nobilis: HPLC profiling and evaluation of its anti-Toxoplasma, antioxidant, and anti-hemolytic properties. Biochem. Syst. Ecol. 2024, 117, 104891. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Custódio, L.; Vitalini, S.; Iriti, M.; Hassani, L. A comparative study of the in vitro antimicrobial and synergistic effect of essential oils from Laurus nobilis L. and Prunus armeniaca L. from Morocco with antimicrobial drugs: New approach for health promoting products. Antibiotics 2020, 9, 140. [Google Scholar] [CrossRef]
- Ben Bakrim, W.; Aghraz, A.; Hriouch, F.; Larhsini, M.; Markouk, M.; Bekkouche, K.; Dugo, G. Phytochemical study and antioxidant activity of the most used medicinal and aromatic plants in Morocco. J. Essent. Oil Res. 2022, 34, 131–142. [Google Scholar] [CrossRef]
- Tagnaout, I.; Zerkani, H.; Hadi, N.; El Moumen, B.; El Makhoukhi, F.; Bouhrim, M.; Zair, T. Chemical composition, antioxidant and antibacterial activities of Thymus broussonetii Boiss and Thymus capitatus (L.) Hoffmann and Link essential oils. Plants 2022, 11, 954. [Google Scholar] [CrossRef]
- Feknous, S.; Saidi, F.; Said, R.M. Extraction, caractérisation et identification de quelques métabolites secondaires actifs de la mélisse (Melissa officinalis L.). Nat. Technol. 2014, 6, 7–13. [Google Scholar]
- Luz, T.R.S.A.; Leite, J.A.C.; de Mesquita, L.S.S.; Bezerra, S.A.; Silveira, D.P.B.; de Mesquita, J.W.C.; Coutinho, D.F. Seasonal variation in the chemical composition and biological activity of the essential oil of Mesosphaerum suaveolens (L.) Kuntze. Ind. Crops Prod. 2020, 153, 112600. [Google Scholar] [CrossRef]
- El Faqer, O.; Rais, S.; Elkoraichi, I.; El Amrani, A.; Dakir, M.; Zaid, Y.; Mtairag, E.M. Phytochemical characterization and immunomodulatory effects of aqueous and ethanolic extracts and essential oil of Moroccan Laurus nobilis L. (Lauraceae) on human neutrophils. J. Herbmed Pharmacol. 2023, 12, 92–99. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; De Feo, V. Laurus nobilis: Composition of essential oil and its biological activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical composition and antimicrobial activity of Laurus nobilis L. essential oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef]
- Ekren, S.; Yerlikaya, O.; Tokul, H.E.; Akpınar, A.; Accedil, M. Chemical composition, antimicrobial activity and antioxidant capacity of some medicinal and aromatic plant extracts. Afr. J. Microbiol. Res. 2013, 7, 383–388. [Google Scholar] [CrossRef]
- Snuossi, M.; Trabelsi, N.; Ben Taleb, S.; Dehmeni, A.; Flamini, G.; De Feo, V. Laurus nobilis, Zingiber officinale and Anethum graveolens essential oils: Composition, antioxidant and antibacterial activities against bacteria isolated from fish and shellfish. Molecules 2016, 21, 1414. [Google Scholar] [CrossRef]
- Benoutman, A.; Erbiai, E.H.; Edderdaki, F.Z.; Cherif, E.K.; Saidi, R.; Lamrani, Z.; Maouni, A. Phytochemical composition, antioxidant and antifungal activity of Thymus capitatus, a medicinal plant collected from Northern Morocco. J. Antibiot. 2022, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Moukhles, A.; Mansour, A.; Ellaghdach, A.; Abrini, J. Chemical composition and in vitro antibacterial activity of the pure essential oils and essential oils extracted from their corresponding hydrolats from different wild varieties of Moroccan thyme. J. Mater. Environ. Sci. 2018, 9, 235–244. [Google Scholar] [CrossRef]
- El Moussaoui, N.; Sanchez, G.; Idaomar, M.; Mansour, A.I.; Abrini, J.; Aznar, R. Antibacterial and antiviral activities of essential oils of Northern Moroccan plant. Br. Biotechnol. J. 2013, 3, 318. [Google Scholar] [CrossRef]
- Moukhles, A.; Mansour, I.A. The Effect of Drying Time on the Yield and the Chemical Composition of Essential Oil and Dissolved Oil in Hydrolat from Aerial Parts of Moroccan Thymbra capitata (L.) Cav. Mediterr. J. Chem. 2020, 10, 716–722. [Google Scholar] [CrossRef]
- Hamad Al-Mijalli, S.; Elsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Bouyahya, A. Determination of volatile compounds of Mentha piperita and Lavandula multifida and investigation of their antibacterial, antioxidant, and antidiabetic properties. Evid.-Based Complement. Altern. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef]
- Tsai, M.L.; Wu, C.T.; Lin, T.F.; Lin, W.C.; Huang, Y.C.; Yang, C.H. Chemical composition and biological properties of essential oils of two mint species. Trop. J. Pharm. Res. 2013, 12, 577–582. [Google Scholar] [CrossRef]
- Zahli, R.; Abrini, J.; El Baaboua, A.; Belmehdi, O.; El Maadoudi, M.; Souhail, B.; Senhaji, N.S. Synergistic action of Thymus capitatus or Syzygium aromaticum essential oils and antibiotics combinations against multi-resistant Salmonella strains. Biocatal. Agric. Biotechnol. 2023, 50, 102752. [Google Scholar] [CrossRef]
- Drioiche, A.; Radi, F.Z.; Ailli, A.; Bouzoubaa, A.; Boutakiout, A.; Mekdad, S.; Zair, T. Correlation between the chemical composition and the antimicrobial properties of seven samples of essential oils of endemic Thymes in Morocco against multi-resistant bacteria and pathogenic fungi. Saudi Pharm. J. 2022, 30, 1200–1214. [Google Scholar] [CrossRef]
- Alaoui Jamali, C.; Kasrati, A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Phenological changes to the chemical composition and biological activity of the essential oil from Moroccan endemic thyme (Thymus maroccanus Ball). Ind. Crops Prod. 2013, 49, 366–372. [Google Scholar] [CrossRef]
- Mahboubi, M.; Heidarytabar, R.; Mahdizadeh, E.; Hosseini, H. Antimicrobial activity and chemical composition of Thymus species and Zataria multiflora essential oils. Agric. Nat. Resour. 2017, 51, 395–401. [Google Scholar] [CrossRef]
- El Bouzidi, L.; Jamali, C.A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Chemical composition, antioxidant and antimicrobial activities of essential oils obtained from wild and cultivated Moroccan Thymus species. Ind. Crops Prod. 2013, 43, 450–456. [Google Scholar] [CrossRef]
- Zhang, D.; Gan, R.Y.; Ge, Y.Y.; Yang, Q.Q.; Ge, J.; Li, H.B.; Corke, H. Research progress on the antibacterial mechanisms of carvacrol: A mini-review. Bioact. Compd. Health Dis. 2018, 1, 71–81. [Google Scholar] [CrossRef]
- Menicucci, F.; Pizzo, B.; Salvadori, B.; Chelazzi, L.; Ienco, A.; Palagano, E. Antifungal activity of carvacrol-based solids and their effects on Whatman and Kraft paper. Int. Biodeterior. Biodegrad. 2024, 195, 105894. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Khribch, J.; Nassik, S.; EL Houadfi, M.; Zrira, S.; Oukessou, M. Activité antibactérienne de l’huile essentielle d’origan et du carvacrol sur des souches d’Escherichia coli d’origine aviaire. Rev. Marocaine Sci. Agron. Vétérinaires 2018, 6, 300–307. [Google Scholar]
- Gandova, V.; Lazarov, A.; Fidan, H.; Dimov, M.; Stankov, S.; Denev, P.; Bari, A. Physicochemical and biological properties of carvacrol. Open Chem. J. 2023, 21, 20220319. [Google Scholar] [CrossRef]
- Kaspute, G.; Ivaskiene, T.; Ramanavicius, A.; Ramanavicius, S.; Prentice, U. Terpenes and Essential Oils in Pharmaceutics: Applications as Therapeutic Agents and Penetration Enhancers with Advanced Delivery Systems for Improved Stability and Bioavailability. Pharmaceutics 2025, 17, 793. [Google Scholar] [CrossRef]
- Ali, T.; Majeed, S.T.; Majeed, R.; Bashir, R.; Mir, S.A.; Jan, I.; Andrabi, K.I. Recent advances in the pharmacological properties and molecular mechanisms of carvacrol. Rev. Bras. Farmacogn. 2024, 34, 35–47. [Google Scholar] [CrossRef]
- Sousa, L.G.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Cerca, N. Synergistic effects of carvacrol, α-terpinene, γ-terpinene, ρ-cymene and linalool against Gardnerella species. Sci. Rep. 2022, 12, 4417. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Martins, N. Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci. Technol. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Chen, W. Antimicrobial activity and proposed action mechanism of linalool against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef] [PubMed]
- Mączka, W.; Duda-Madej, A.; Grabarczyk, M.; Wińska, K. Natural compounds in the battle against microorganisms—Linalool. Molecules 2022, 27, 6928. [Google Scholar] [CrossRef]
- Khayyat, S. Thermal, photo-oxidation and antimicrobial studies of linalyl acetate as a major ingredient of lavender essential oil. Arab. J. Chem. 2020, 13, 1575–1581. [Google Scholar] [CrossRef]
- Şimşek, M.; Duman, R. Investigation of effect of 1, 8-cineole on antimicrobial activity of chlorhexidine gluconate. Pharmacogn. Res. 2017, 9, 234. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Górny, A.; Grabarczyk, M.; Wińska, K. Can eucalyptol replace antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.; Soković, M. Camphor and eucalyptol—Anticandidal spectrum, antivirulence effect, efflux pumps interference and cytotoxicity. Int. J. Mol. Sci. 2021, 22, 483. [Google Scholar] [CrossRef]
- Cai, Z.M.; Peng, J.Q.; Chen, Y.; Tao, L.; Zhang, Y.; Fu, L.Y.; Shen, X.C. 1, 8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef]
- Guenane, H.; Gherib, A.; Carbonell-Barrachina, Á.; Cano-Lamadrid, M.; Krika, F.; Berrabah, M.; Bakchiche, B. Minerals analysis, antioxidant and chemical composition of extracts of Laurus nobilis from southern Algeria. J. Mater. Environ. Sci. 2016, 7, 4253–4261. [Google Scholar]
- Mssillou, I.; Agour, A.; El Ghouizi, A.; Hamamouch, N.; Lyoussi, B.; Derwich, E. Chemical composition, antioxidant activity, and antifungal effects of essential oil from Laurus nobilis L. flowers growing in Morocco. J. Food Qual. 2020, 2020, 8819311. [Google Scholar] [CrossRef]
- Taroq, A.; El-Kamari, F.; Aouam, I.; El Atki, Y.; Lyoussi, B.; Abdellaoui, A. Phytochemical screening, polyphenols content and a novel source of antibacterial and antioxidant activities of essential oil of Laurus nobilis from Morocco. Int. J. Pharm. Sci. Res. 2019, 10, 3770–3776. [Google Scholar] [CrossRef]
- Zaïri, A.; Nouir, S.; Zarrouk, A.; Haddad, H.; Khélifa, A.; Achour, L.; Trabelsi, M. Chemical composition, Fatty acids profile and Biological properties of Thymus capitatus (L.) Hoffmanns, essential Oil. Sci. Rep. 2019, 9, 20134. [Google Scholar] [CrossRef] [PubMed]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Elbouzidi, A.; Bouhrim, M.; Bencheikh, N.; Bousta, D. Assessment of antidepressant-like, anxiolytic effects and impact on memory of Pimpinella anisum L. Total extract on swiss albino mice. Plants 2021, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Sabzalian, M.R. Thymol, carvacrol, and antioxidant accumulation in Thymus species in response to different light spectra emitted by light-emitting diodes. Food Chem. 2020, 307, 125521. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Islam, S. Therapeutic application of carvacrol: A comprehensive review. Int. J. Food Sci. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Mir, M.; Permana, A.D.; Ahmed, N.; Khan, G.M.; ur Rehman, A.; Donnelly, R.F. Enhancement in site-specific delivery of carvacrol for potential treatment of infected wounds using infection responsive nanoparticles loaded into dissolving microneedles: A proof of concept study. Eur. J. Pharm. Biopharm. 2020, 147, 57–68. [Google Scholar] [CrossRef]
- Hervin, V.; Roy, V.; Agrofoglio, L.A. Antibiotics and Antibiotic Resistance—Mur Ligases as an Antibacterial Target. Molecules 2023, 28, 8076. [Google Scholar] [CrossRef]
- Rambaher, M.H.; Zdovc, I.; Glavač, N.K.; Gobec, S.; Frlan, R. Mur ligase F as a new target for the flavonoids quercitrin, myricetin, and (–)-epicatechin. J. Comput. Aided Mol. Des. 2023, 37, 721–733. [Google Scholar] [CrossRef]
- Tsvetkova, N.; Harizanov, R.; Rainova, I.; Ivanova, A.; Yancheva-Petrova, N. Molecular analysis of dihydropteroate synthase gene mutations in pneumocystis jirovecii isolates among Bulgarian patients with pneumocystis pneumonia. Int. J. Mol. Sci. 2023, 24, 16927. [Google Scholar] [CrossRef]
- Cipriano, A.; Viviano, M.; Feoli, A.; Milite, C.; Sarno, G.; Castellano, S.; Sbardella, G. NADPH oxidases: From molecular mechanisms to current inhibitors. J. Med. Chem. 2023, 66, 11632–11655. [Google Scholar] [CrossRef]
- Soares-Santos, R.R.; Jaiswal, A.K.; Ferreira, R.C.M.; Azevedo, V.A.d.C.; Aburjaile, F.F.; Soto-Blanco, B. Interactions of Linalool and Linalyl Acetate with Selected Dog Cytochrome P450 (CYP) Proteins Identified by In Silico Drug Discovery Followed by Molecular Docking Analysis. Pharmaceuticals 2025, 18, 1499. [Google Scholar] [CrossRef]
- Noui Mehidi, I.; Ait Ouazzou, A.; Tachoua, W.; Hosni, K. Investigating the Antimicrobial Properties of Essential Oil Constituents and Their Mode of Action. Molecules 2024, 29, 4119. [Google Scholar] [CrossRef] [PubMed]
- Başar, Y.; Gök, M.; Erenler, R.; Demirtas, İ. Phytochemical profiling, molecular docking and ADMET prediction of essential oil of Ocimum basilicum. Int. J. Second. Metab. 2025, 12, 146–157. [Google Scholar] [CrossRef]
- Venkataraman, B.; Almarzooqi, S.; Raj, V.; Bhongade, B.A.; Patil, R.B.; Subramanian, V.S.; Subramanya, S.B. Molecular docking identifies 1, 8-Cineole (Eucalyptol) as a novel PPARγ agonist that alleviates colon inflammation. Int. J. Mol. Sci. 2023, 24, 6160. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).