Synthesis and Optical Properties of Red Carbon@(NH4)3ZnCl5 Hybrid Heterostructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of the Materials
2.2.1. Preparation of Red Carbon
2.2.2. Preparation of (NH4)3ZnCl5 Perovskites and Heterostructure
2.3. Characterizations
3. Results
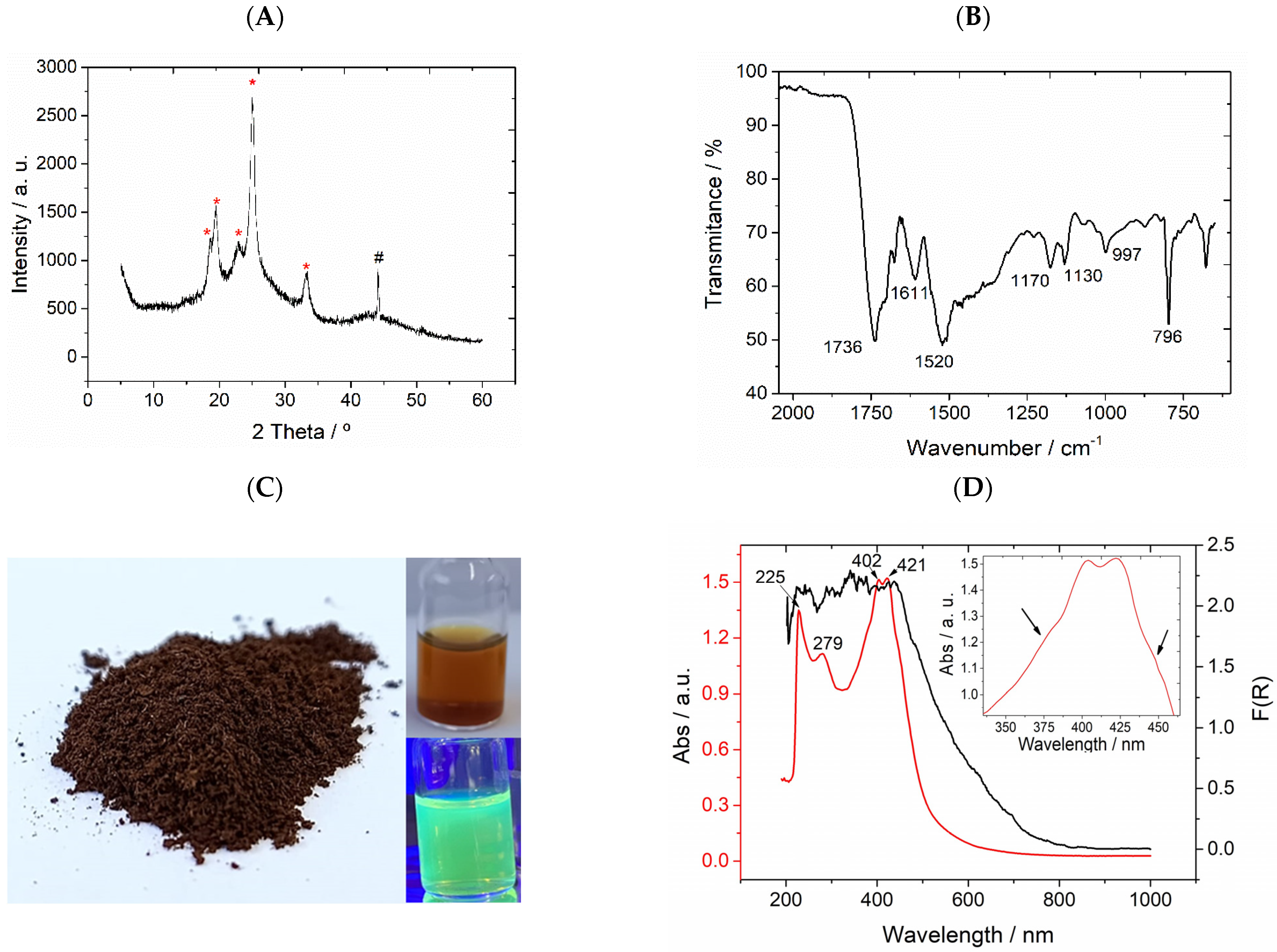
3.1. Red Carbon Characterization
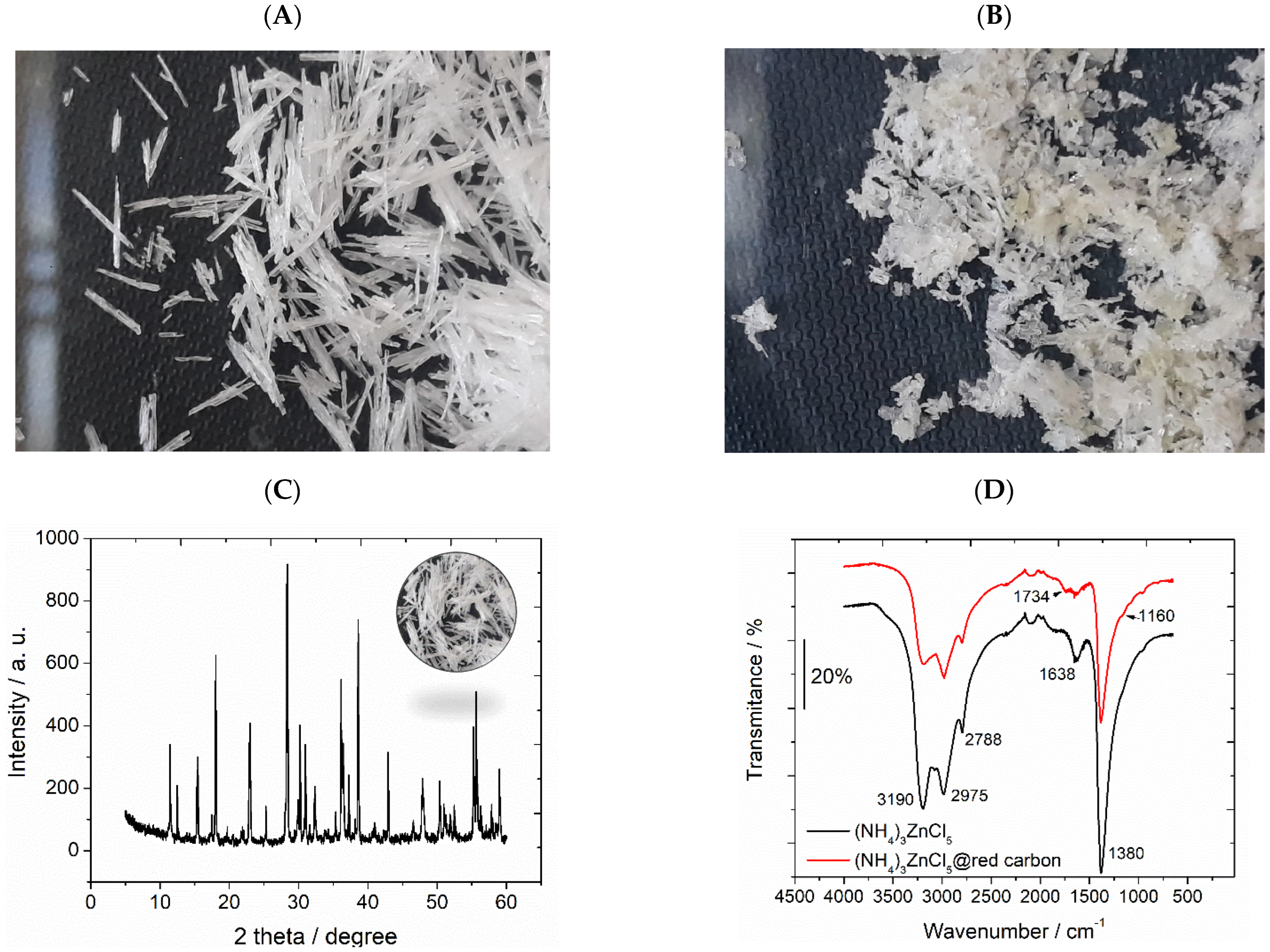
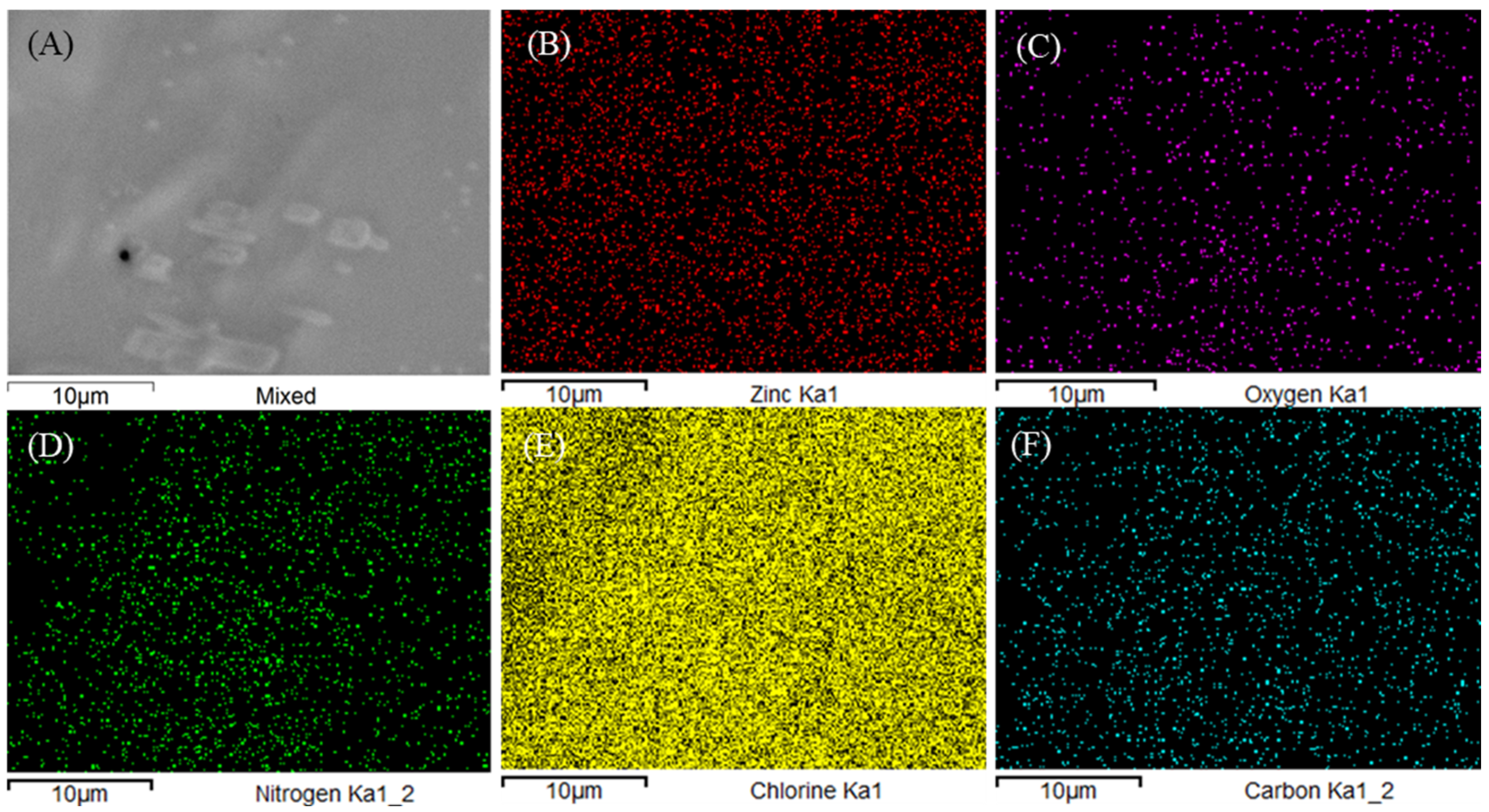
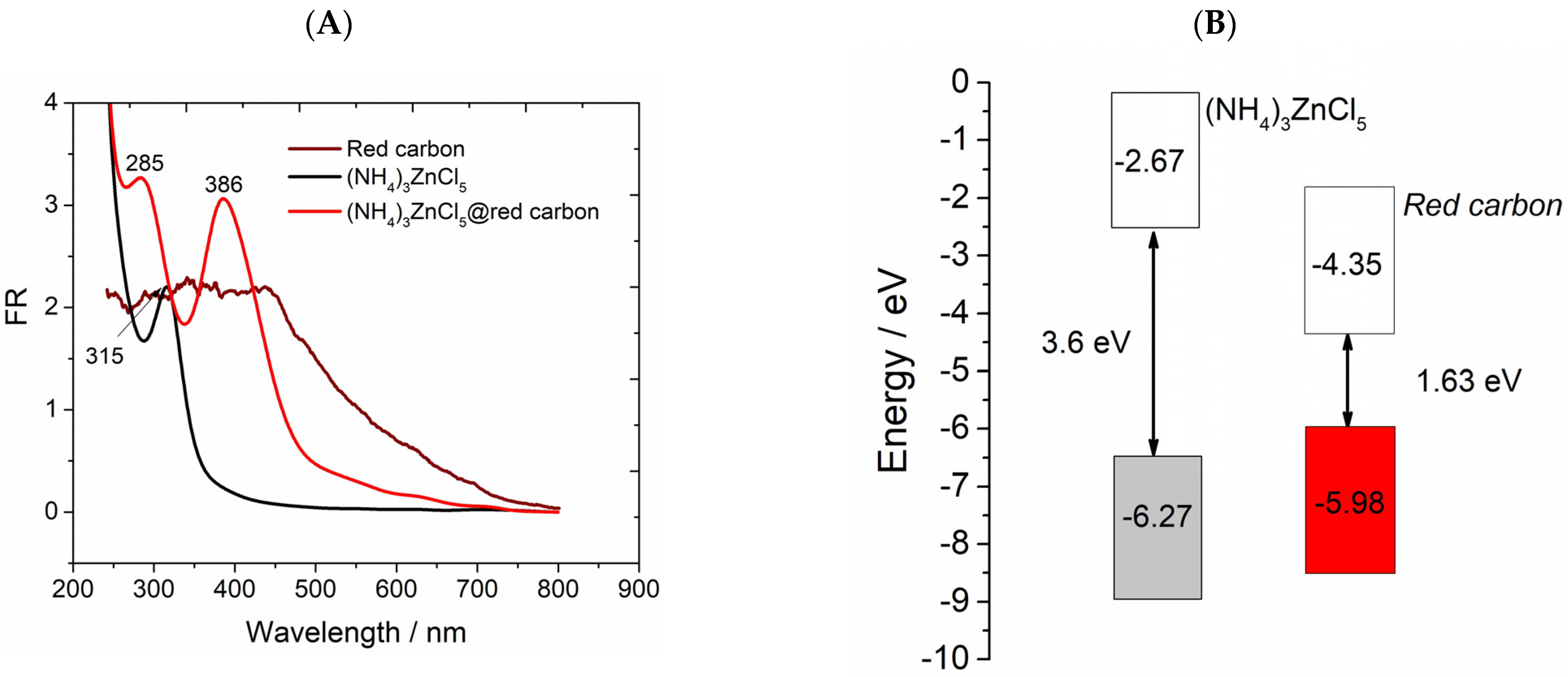
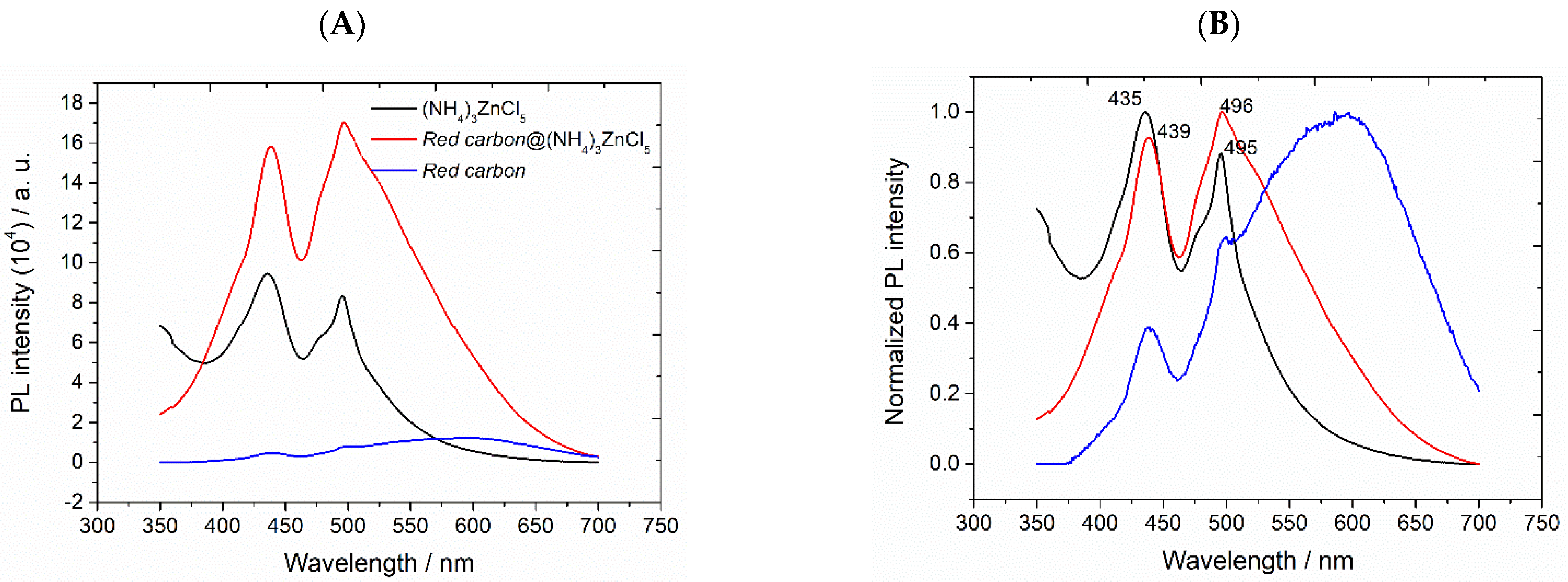
3.2. Characterization of (NH4)3ZnCl5 Perovskite@Red Carbon Heterostructures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Paul, T.; Hiralal Makani, N.; Sahoo, A.; Singh Tanwar, L.; Singh, M.; Banerjee, R. Halide perovskites for optoelectronic application. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Yuan, C.; He, Y.; Chen, R.; Sun, Y.; Li, J.; Cui, W.; Chen, P.; Sheng, J.; Dong, F. Perovskite Nanocrystals-Based Heterostructures: Synthesis Strategies, Interfacial Effects, and Photocatalytic Applications. Sol. RRL 2021, 5, 2000419. [Google Scholar] [CrossRef]
- Shi, E.; Gao, Y.; Finkenauer, B.P.; Akriti; Coffey, A.H.; Dou, L. Two-dimensional halide perovskite nanomaterials and heterostructures. Chem. Soc. Rev. 2018, 47, 6046–6072. [Google Scholar] [CrossRef]
- Bera, S.; Pradhan, N. Perovskite Nanocrystal Heterostructures: Synthesis, Optical Properties, and Applications. ACS Energy Lett. 2020, 5, 2858–2872. [Google Scholar] [CrossRef]
- Hautzinger, M.P.; Raulerson, E.K.; Harvey, S.P.; Liu, T.; Duke, D.; Qin, X.; Scheidt, R.A.; Wieliczka, B.M.; Phillips, A.J.; Graham, K.R.; et al. Metal Halide Perovskite Heterostructures: Blocking Anion Diffusion with Single-Layer Graphene. J. Am. Chem. Soc. 2023, 145, 2052–2057. [Google Scholar] [CrossRef]
- Ray, S.; Sahoo, M.R.; Mukherjee, S.; Perumal, A.; Nayak, S.K.; Bhaumik, S. Understanding the charge transfer mechanism in CsPbBr3 nanocrystals and nitrogen-doped carbon quantum dot heterostructures: Effect of nanocrystal encapsulation. RSC Adv. 2023, 13, 35551–35561. [Google Scholar] [CrossRef]
- Algadi, H.; Ren, J.; Alqarni, A. A high-performance self-powered photodetector based on solution-processed nitrogen-doped graphene quantum dots/all-inorganic perovskite heterostructures. Adv. Compos. Hybrid Mater. 2023, 6, 98. [Google Scholar] [CrossRef]
- Tyznik, C.; Lee, J.; Sorli, J.; Liu, X.; Holland, E.K.; Day, C.S.; Anthony, J.E.; Loo, Y.-L.; Vardeny, Z.V.; Jurchescu, O.D. Photocurrent in Metal-Halide Perovskite/Organic Semiconductor Heterostructures: Impact of Microstructure on Charge Generation Efficiency. ACS Appl. Mater. Interfaces 2021, 13, 10231–10238. [Google Scholar] [CrossRef]
- Liu, C.-K.; Tai, Q.; Wang, N.; Tang, G.; Hu, Z.; Yan, F. Lead-Free Perovskite/Organic Semiconductor Vertical Heterojunction for Highly Sensitive Photodetectors. ACS Appl. Mater. Interfaces 2020, 12, 18769–18776. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Liu, X.; Wang, L.; Xiong, L.; Huang, J. Optoelectronic Synapses and Photodetectors Based on Organic Semiconductor/Halide Perovskite Heterojunctions: Materials, Devices, and Applications. Adv. Funct. Mater. 2023, 33, 2305508. [Google Scholar] [CrossRef]
- Cheng, X.; Han, Y.; Cui, B.-B. Fabrication Strategies and Optoelectronic Applications of Perovskite Heterostructures. Adv. Opt. Mater. 2022, 10, 2102224. [Google Scholar] [CrossRef]
- Rathore, E.; Maji, K.; Rao, D.; Saha, B.; Biswas, K. Charge Transfer in the Heterostructure of CsPbBr3 Nanocrystals with Nitrogen-Doped Carbon Dots. J. Phys. Chem. Lett. 2020, 11, 8002–8007. [Google Scholar] [CrossRef]
- DuBose, J.T.; Kamat, P.V. Efficacy of Perovskite Photocatalysis: Challenges to Overcome. ACS Energy Lett. 2022, 7, 1994–2011. [Google Scholar] [CrossRef]
- Mahmood, A.; Shi, G.; Sun, J.; Liu, J. Investigation of electronic structure and optical properties of thallium lead halides: First principle calculations. J. Appl. Phys. 2018, 124, 093102. [Google Scholar] [CrossRef]
- Odziomek, M.; Giusto, P.; Kossmann, J.; Tarakina, N.V.; Heske, J.; Rivadeneira, S.M.; Keil, W.; Schmidt, C.; Mazzanti, S.; Savateev, O.; et al. “Red Carbon”: A Rediscovered Covalent Crystalline Semiconductor. Adv. Mater. 2022, 34, 2206405. [Google Scholar] [CrossRef]
- Brodie, B.C., IV. On the action of electricity on gases.—II. On the electric decomposition of carbonic-acid gas. Philos. Trans. R. Soc. Lond. 1997, 164, 83–103. [Google Scholar] [CrossRef]
- Ellern, A.; Drews, T.; Seppelt, K. The Structure of Carbon Suboxide, C3O2, in the Solid State. Z. Anorg. Allg. Chem. 2001, 627, 73–76. [Google Scholar] [CrossRef]
- Smith, R.N.; Young, D.A.; Smith, E.N.; Carter, C.C. The Structure and Properties of Carbon Suboxide Polymer. Inorg. Chem. 1963, 2, 829–838. [Google Scholar] [CrossRef]
- Giusto, P.; Cruz, D.; Rodriguez, Y.; Rothe, R.; Tarakina, N.V. Red Carbon Thin Film: A Carbon–Oxygen Semiconductor with Tunable Properties by Amine Vapors and Its Carbonization toward Carbon Thin Films. Adv. Mater. Interfaces 2022, 9, 2200834. [Google Scholar] [CrossRef]
- Huang, H.; Verhaeghe, D.; Weng, B.; Ghosh, B.; Zhang, H.; Hofkens, J.; Steele, J.A.; Roeffaers, M.B.J. Metal Halide Perovskite Based Heterojunction Photocatalysts. Angew. Chem. Int. Ed. 2022, 61, e202203261. [Google Scholar] [CrossRef]
- Oliveira, W.L.; de Oliveira, E.F.; do Carmo Batista, W.V.F.; Mourão, H.A.J.L.; Mendes Pires, M.J.; Coelho, R.M.; Atta Diab, G.A.; Teixeira, I.F.; Marques, G.; Mastelaro, V.R.; et al. A metal-free catalyst based on g-C3N4 functionalized with cyamelurate-like groups: Catalytic properties and mechanism of a new heterogeneous Fenton-like catalyst. Carbon 2023, 214, 118366. [Google Scholar] [CrossRef]
- Oliveira, W.L.; Ferreira, M.A.; Mourão, H.A.J.L.; Pires, M.J.M.; Ferreira, V.; Gorgulho, H.F.; Cipriano, D.F.; Freitas, J.C.C.; Mastelaro, V.R.; Nascimento, O.R.; et al. Heterogeneous Fenton-like surface properties of oxygenated graphitic carbon nitride. J. Colloid Interface Sci. 2021, 587, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Ba, J.; Dong, H.; Odziomek, M.; Lai, F.; Wang, R.; Han, Y.; Shu, J.; Antonietti, M.; Liu, T.; Yang, W.; et al. Red Carbon Mediated Formation of Cu2O Clusters Dispersed on the Oxocarbon Framework by Fehling’s Route and their Use for the Nitrate Electroreduction in Acidic Conditions. Adv. Mater. 2024, 36, 2400396. [Google Scholar] [CrossRef]
- Zheng, X.; Tian, Z.; Bouchal, R.; Antonietti, M.; López-Salas, N.; Odziomek, M. Tin (II) Chloride Salt Melts as Non-Innocent Solvents for the Synthesis of Low-Temperature Nanoporous Oxo-Carbons for Nitrate Electrochemical Hydrogenation. Adv. Mater. 2024, 36, 2311575. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Manna, L. What Defines a Halide Perovskite? ACS Energy Lett. 2020, 5, 604–610. [Google Scholar] [CrossRef]
- Araújo, T.C.; Oliveira, H.d.S.; Teles, J.J.S.; Fabris, J.D.; Oliveira, L.C.A.; de Mesquita, J.P. Hybrid heterostructures based on hematite and highly hydrophilic carbon dots with photocatalytic activity. Appl. Catal. B Environ. 2016, 182, 204–212. [Google Scholar] [CrossRef]
- Lampman, G.M.; Pavia, D.L.; Kriz, G.S.; Vyvyan, J.R. Introduçao a Espectroscopia; Cengage: Boston, MA, USA, 2014. [Google Scholar]
- Klug, H.P.; Alexander, L. The Crystal Structure of Ammonium Pentachlorozincate1. J. Am. Chem. Soc. 1944, 66, 1056–1064. [Google Scholar] [CrossRef]
- Friese, K.; Madariaga, G.; Breczewski, T. Tricaesium Tetraiodozincate(II) Iodide, Cs3ZnI5. Acta Crystallogr. Sect. C 1998, 54, 1737–1739. [Google Scholar] [CrossRef]
- Gaffar, M.A.; El-Fadl, A.A.; Anooz, S.B. Effects induced by chemical non-stoichiometry and γ-irradiation on the habit and unit cell parameters of ammonium tetrachlorozincate. Cryst. Res. Technol. 2006, 41, 379–387. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Ramakrishnan, V.; Rajendran, S. Vibrational spectra of (NH4)3ZnCI5. J. Phys. 1991, 36, 8. [Google Scholar]
- Yu, W.; Li, F.; Huang, T.; Li, W.; Wu, T. Go beyond the limit: Rationally designed mixed-dimensional perovskite/semiconductor heterostructures and their applications. Innovation 2023, 4, 100363. [Google Scholar] [CrossRef] [PubMed]
- Tawil, C.A.; Kurdi, R.E.; Patra, D. Cesium Lead Bromide Perovskites: Synthesis, Stability, and Photoluminescence Quantum Yield Enhancement by Hexadecyltrimethylammonium Bromide Doping. ACS Omega 2022, 7, 20872–20880. [Google Scholar] [CrossRef] [PubMed]
- Schötz, K.; Askar, A.M.; Peng, W.; Seeberger, D.; Gujar, T.P.; Thelakkat, M.; Köhler, A.; Huettner, S.; Bakr, O.M.; Shankar, K.; et al. Double peak emission in lead halide perovskites by self-absorption. J. Mater. Chem. C 2020, 8, 2289–2300. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Deng, J.; Chu, Z.; Jiang, Q.; Meng, J.; Wang, P.; Zhang, L.; Yin, Z.; You, J. Efficient green light-emitting diodes based on quasi-two-dimensional composition and phase engineered perovskite with surface passivation. Nat. Commun. 2018, 9, 570. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, W.V.F.d.C.; de Souza, A.P.; Cruz, T.d.S.; Pimentel, D.M.; de Avelar, D.G.S.; Oliveira, S.K.N.; de Oliveira, W.L.; Ferreira, D.R.C.; Pereira, M.C.; Ferreira, R.A.d.R.; et al. Synthesis and Optical Properties of Red Carbon@(NH4)3ZnCl5 Hybrid Heterostructures. Compounds 2025, 5, 21. https://doi.org/10.3390/compounds5020021
Batista WVFdC, de Souza AP, Cruz TdS, Pimentel DM, de Avelar DGS, Oliveira SKN, de Oliveira WL, Ferreira DRC, Pereira MC, Ferreira RAdR, et al. Synthesis and Optical Properties of Red Carbon@(NH4)3ZnCl5 Hybrid Heterostructures. Compounds. 2025; 5(2):21. https://doi.org/10.3390/compounds5020021
Chicago/Turabian StyleBatista, Walker Vinícius Ferreira do Carmo, Aniely Pereira de Souza, Tais dos Santos Cruz, Dilton Martins Pimentel, Danila Graziele Silva de Avelar, Sarah Karoline Natalino Oliveira, Wanessa Lima de Oliveira, Danilo Roberto Carvalho Ferreira, Márcio Cesar Pereira, Rondinele Alberto dos Reis Ferreira, and et al. 2025. "Synthesis and Optical Properties of Red Carbon@(NH4)3ZnCl5 Hybrid Heterostructures" Compounds 5, no. 2: 21. https://doi.org/10.3390/compounds5020021
APA StyleBatista, W. V. F. d. C., de Souza, A. P., Cruz, T. d. S., Pimentel, D. M., de Avelar, D. G. S., Oliveira, S. K. N., de Oliveira, W. L., Ferreira, D. R. C., Pereira, M. C., Ferreira, R. A. d. R., & de Mesquita, J. P. (2025). Synthesis and Optical Properties of Red Carbon@(NH4)3ZnCl5 Hybrid Heterostructures. Compounds, 5(2), 21. https://doi.org/10.3390/compounds5020021







