Recent Advances in Transition Metal-Catalyzed Ring-Opening Reaction of Aziridine
Abstract
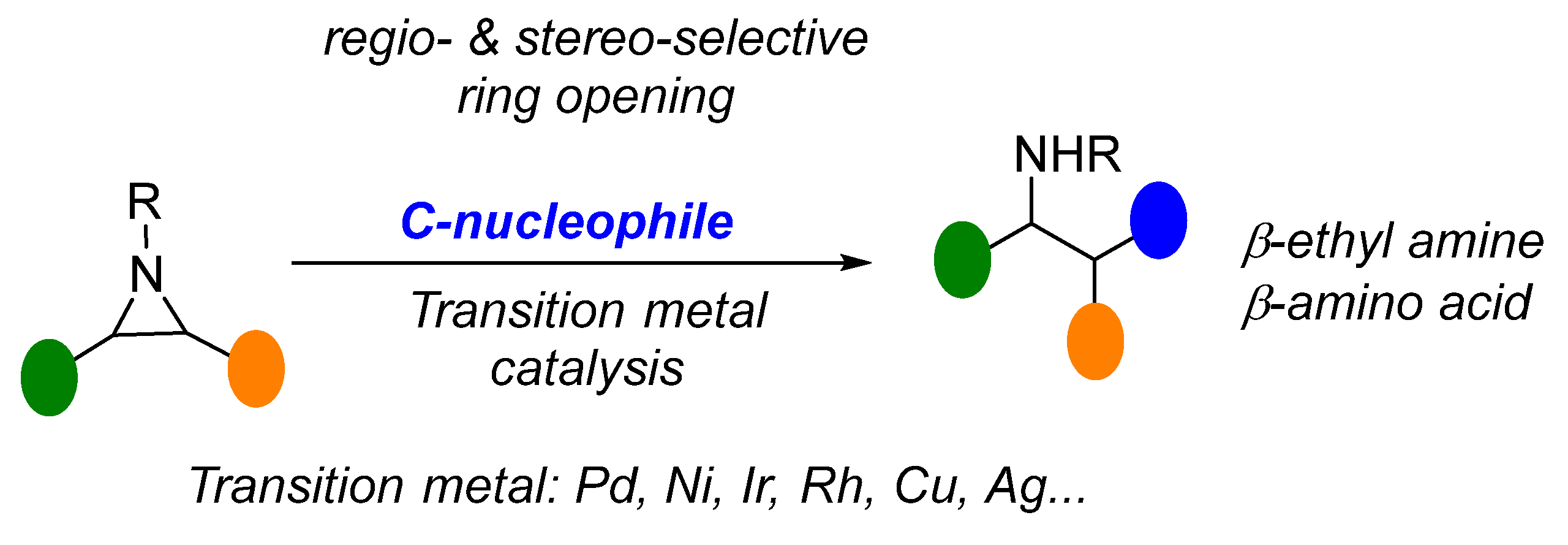
1. Introduction
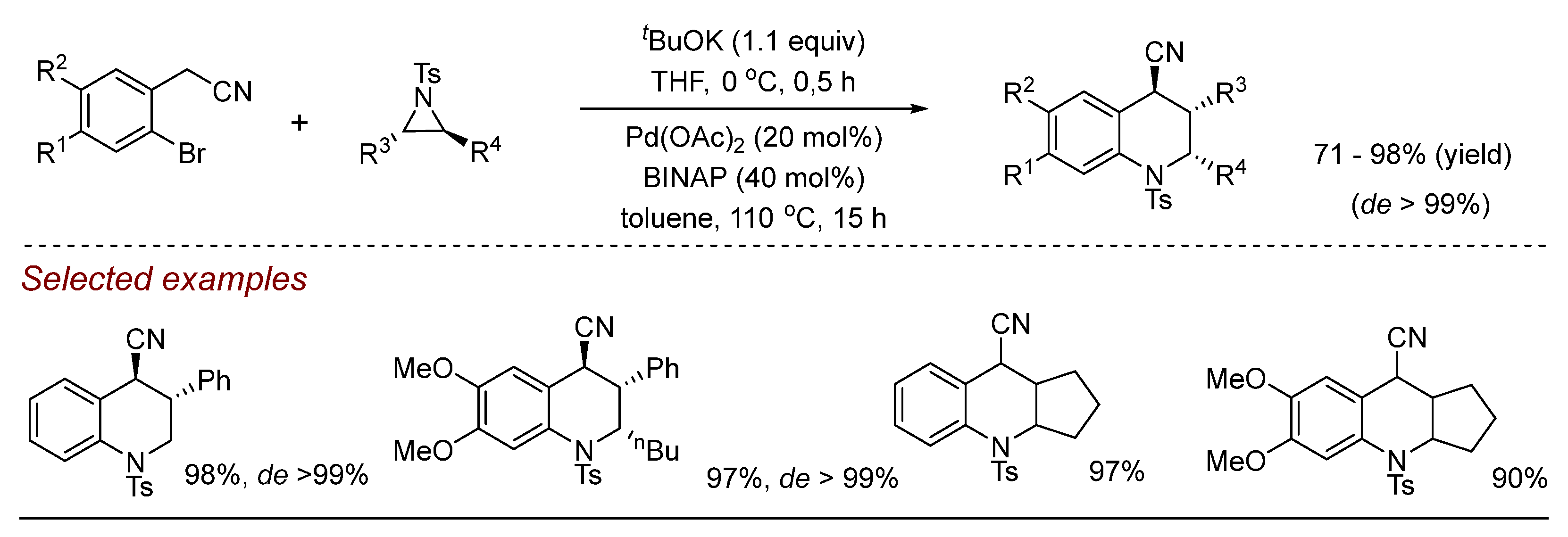
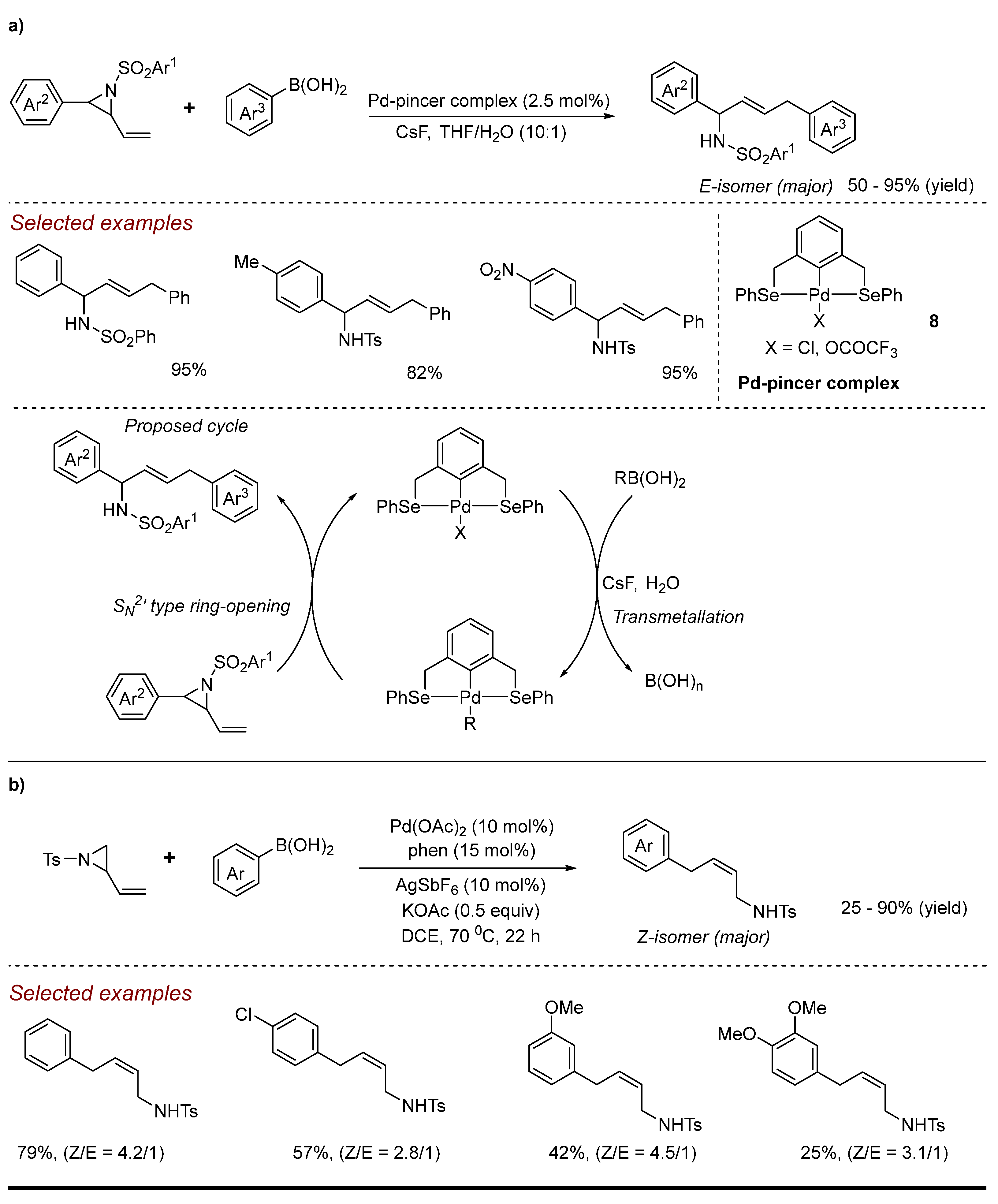
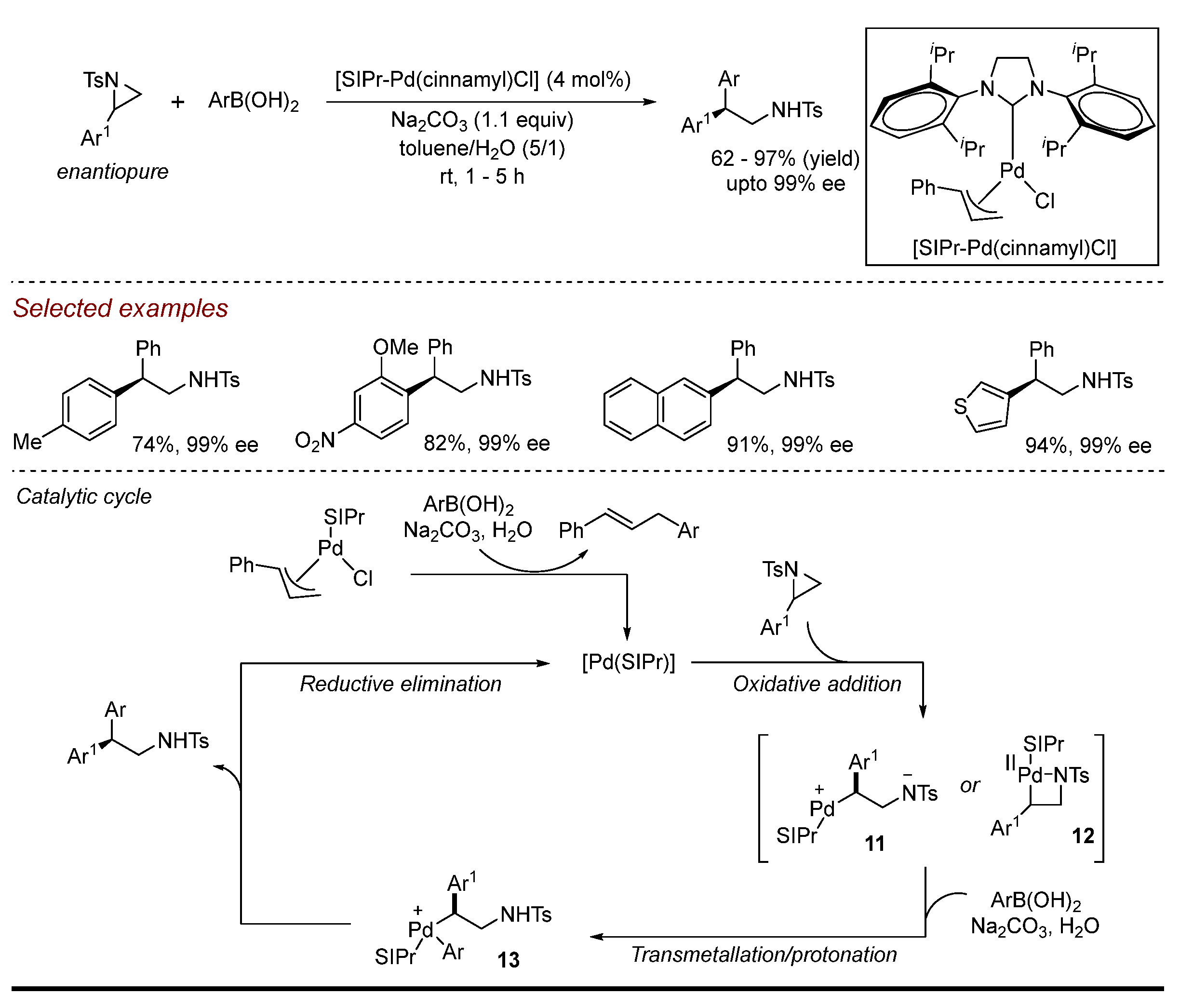
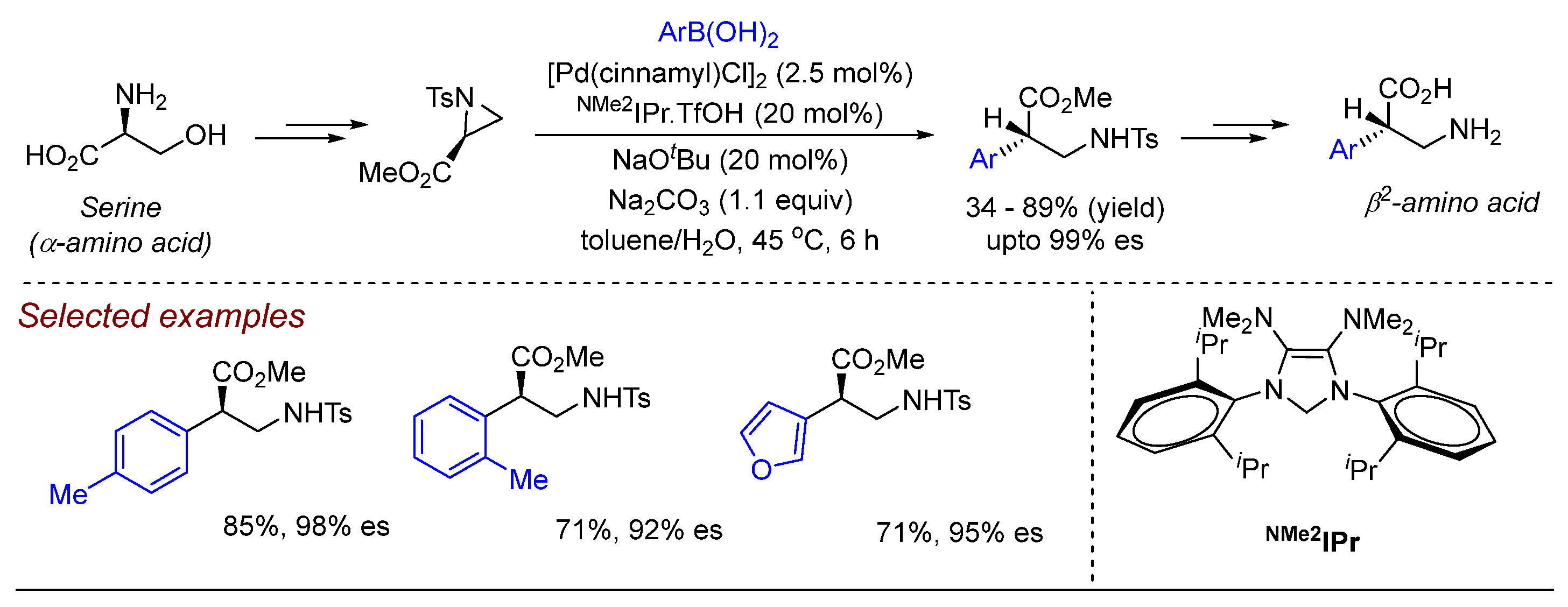
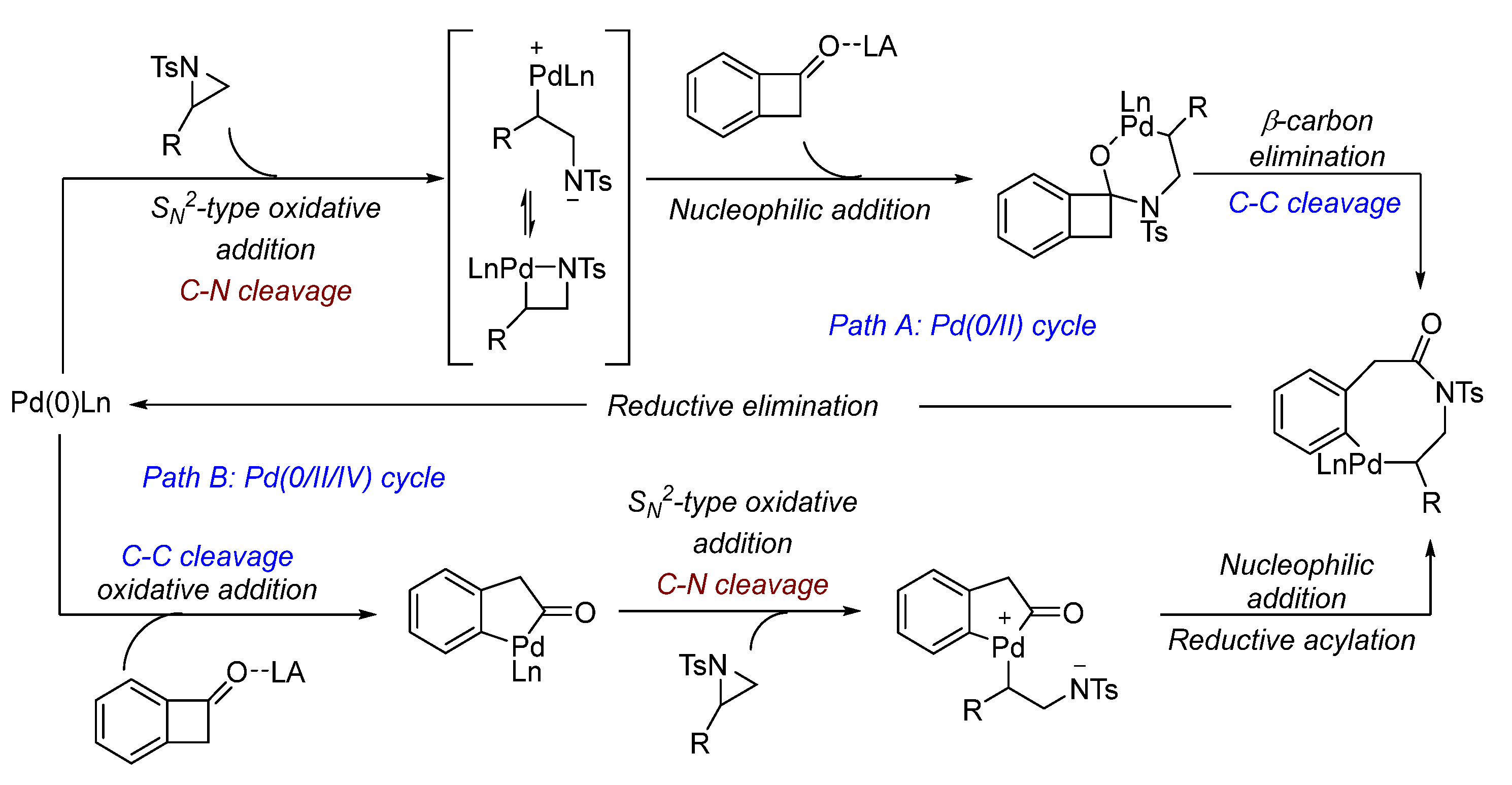
2. Palladium (Pd)-Catalyzed Aziridine Ring-Opening Reactions
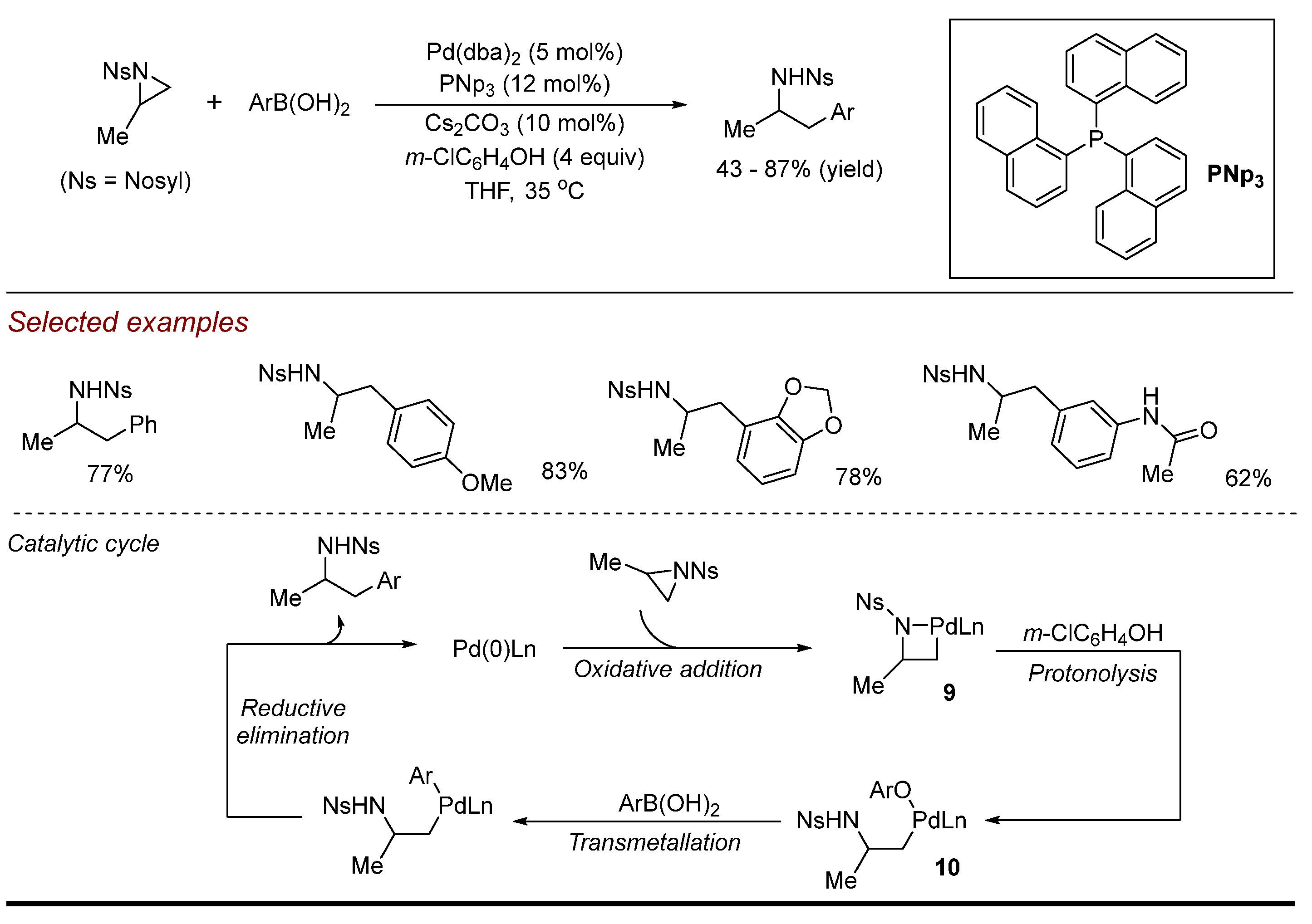
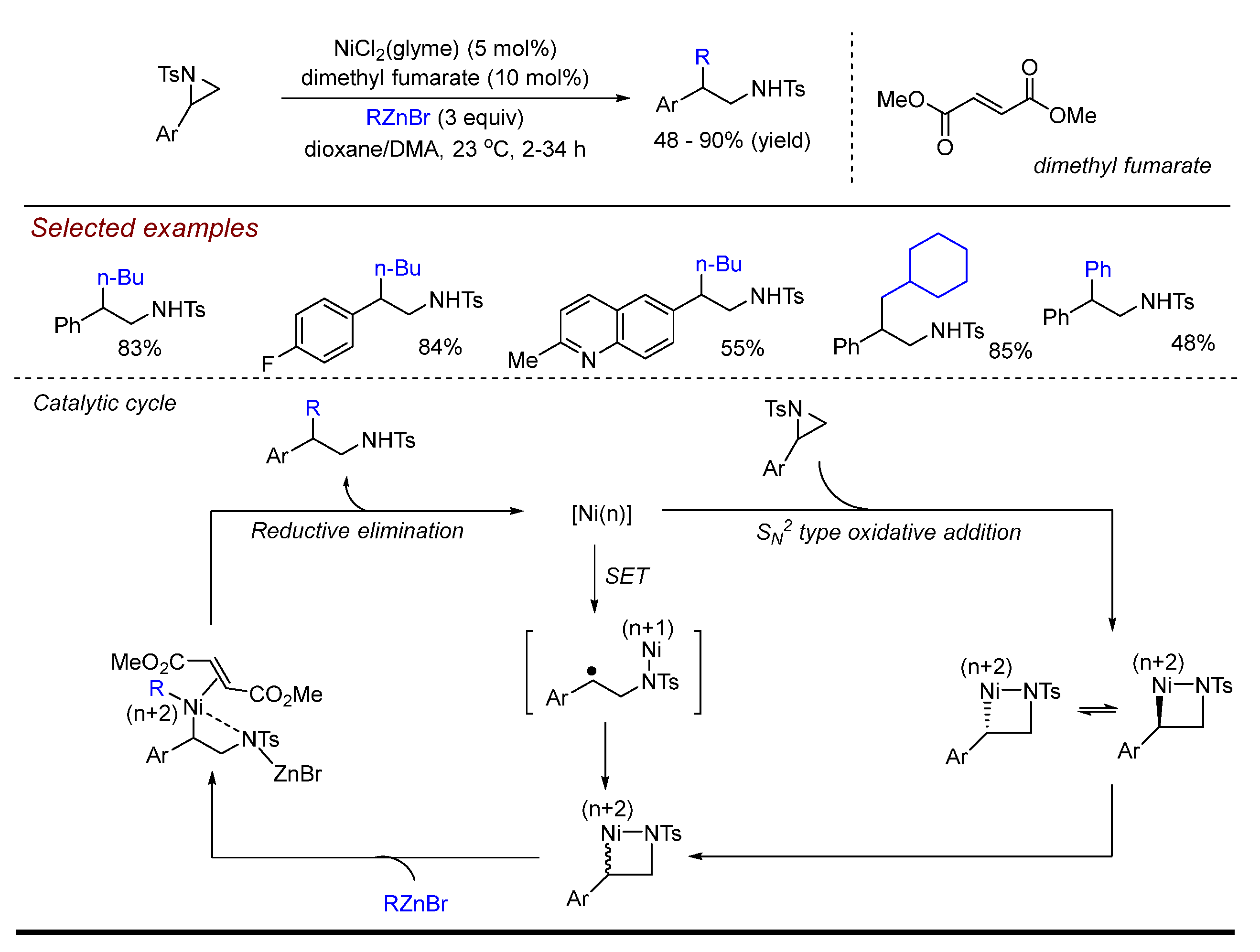
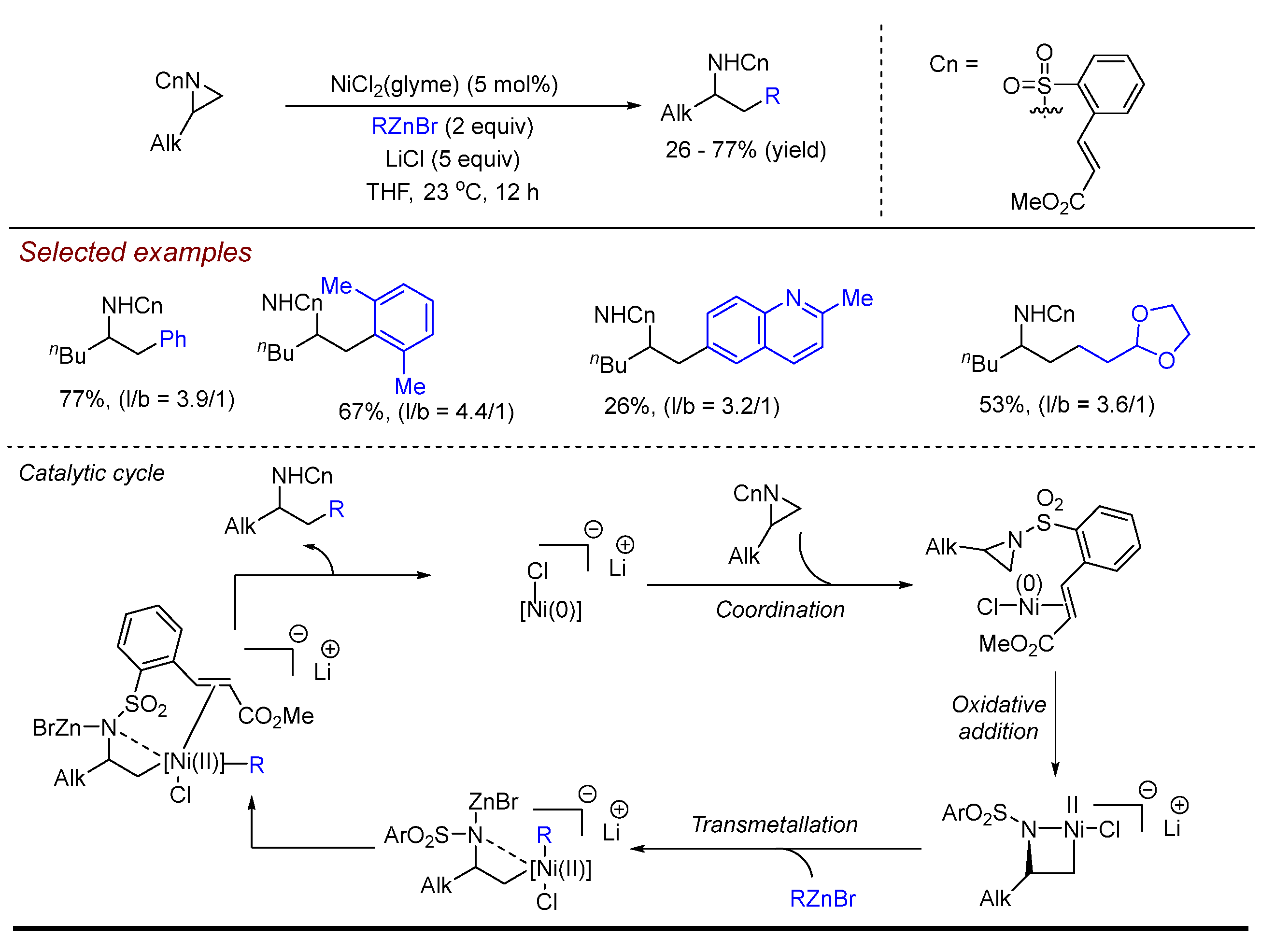
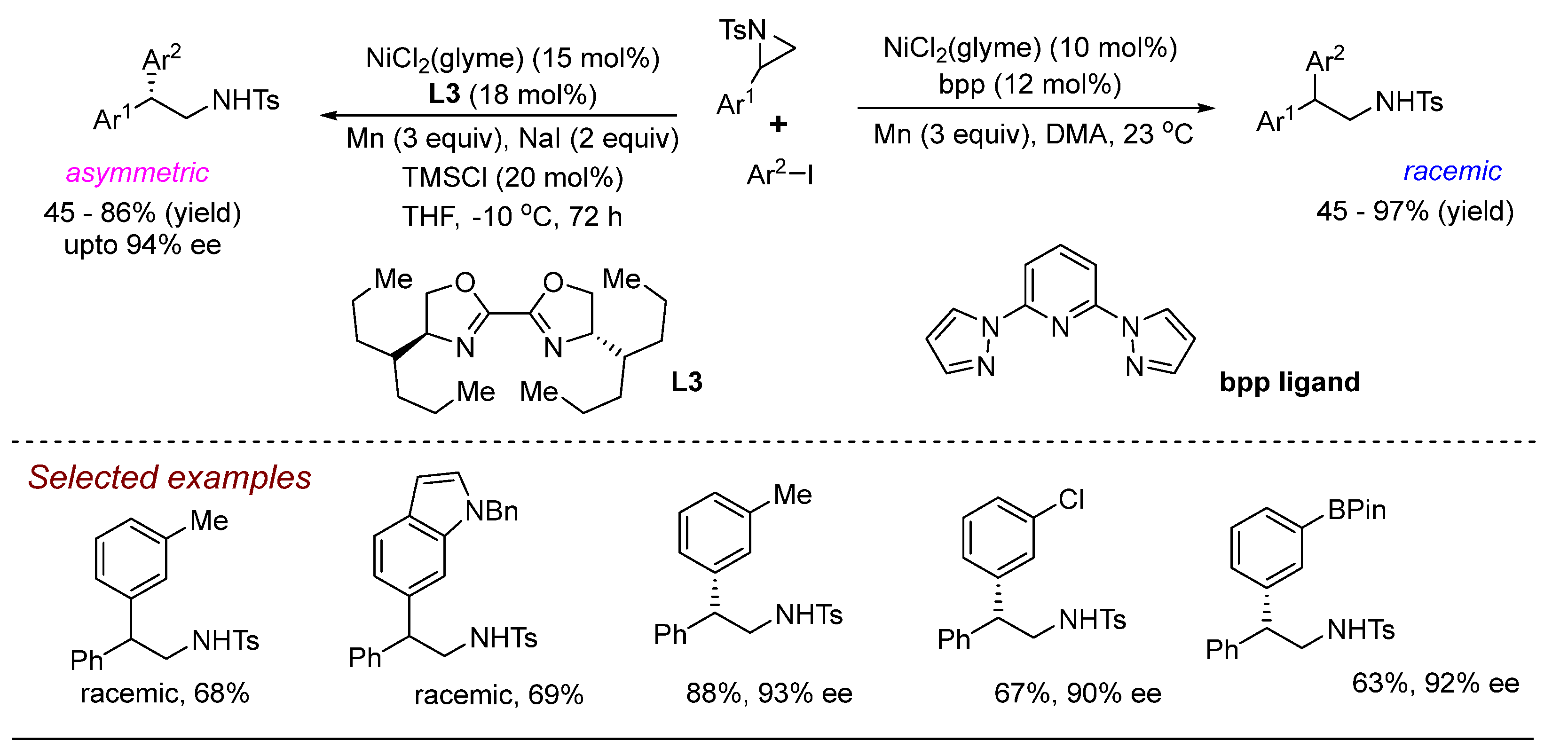
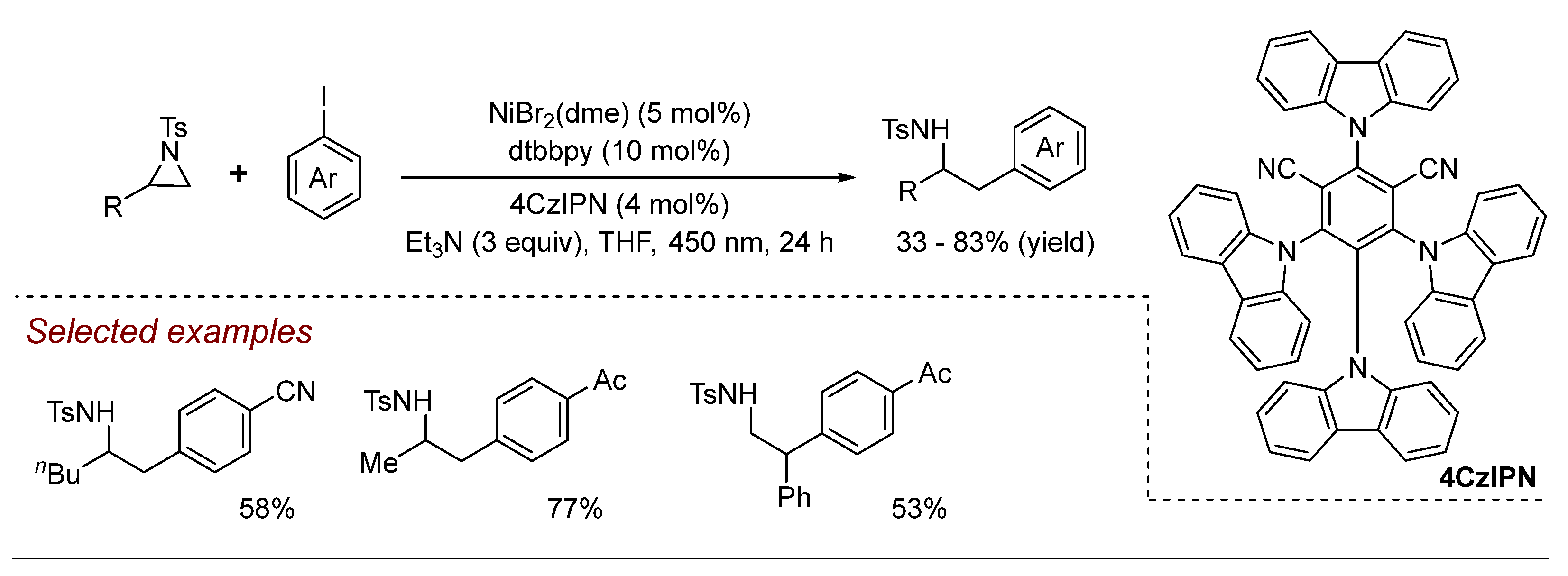
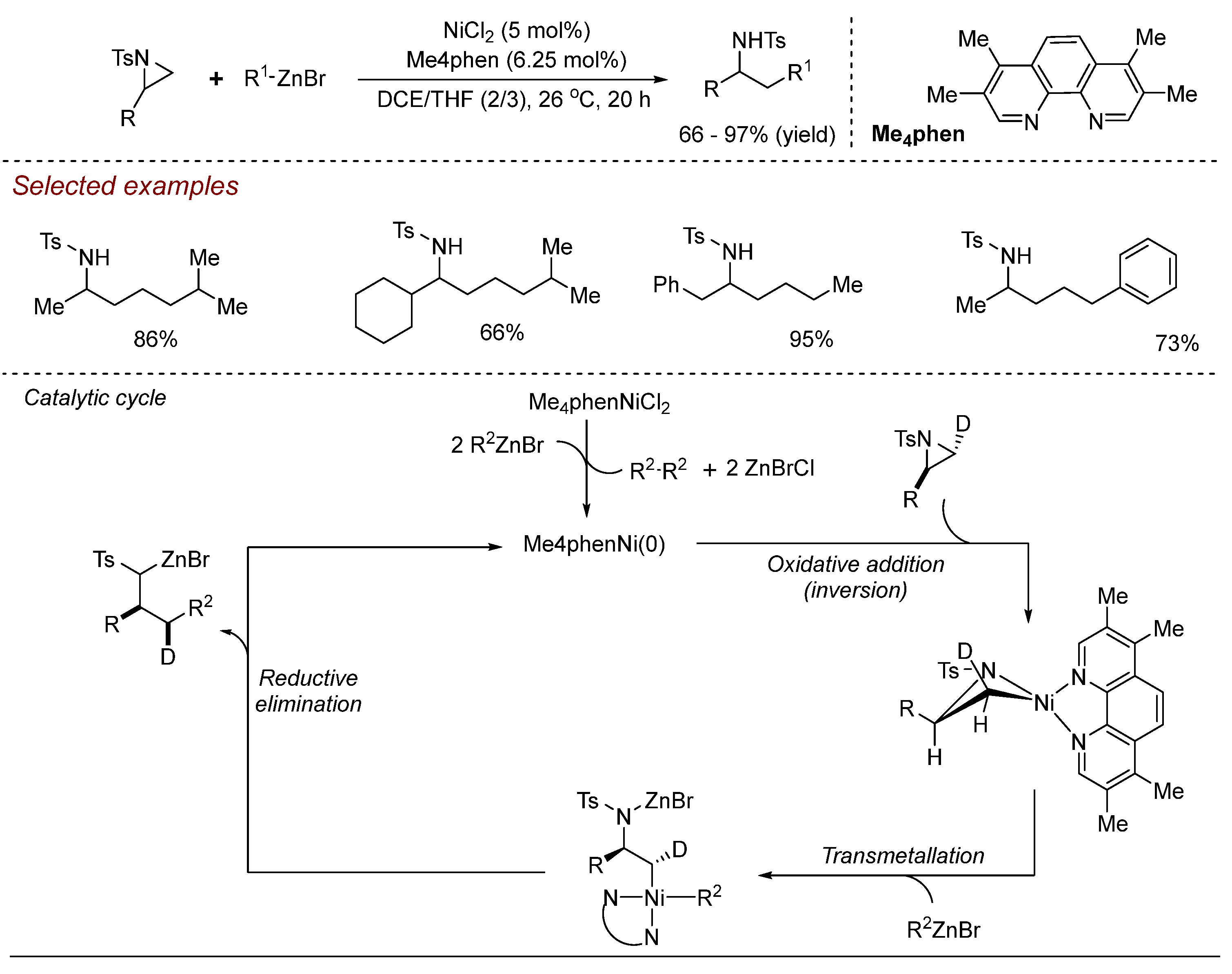
3. Nickel (Ni)-Catalyzed Aziridine Ring-Opening Reactions
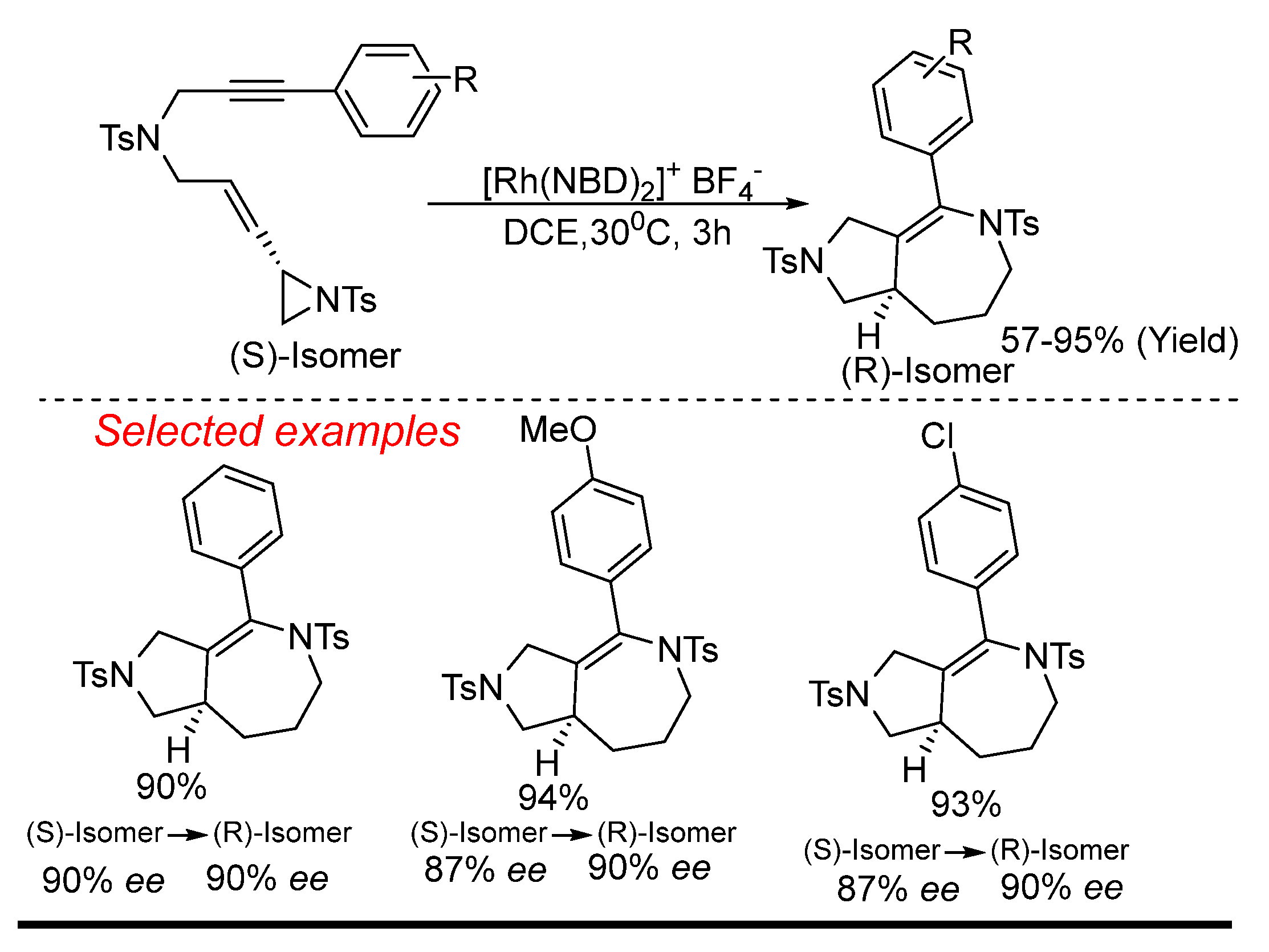
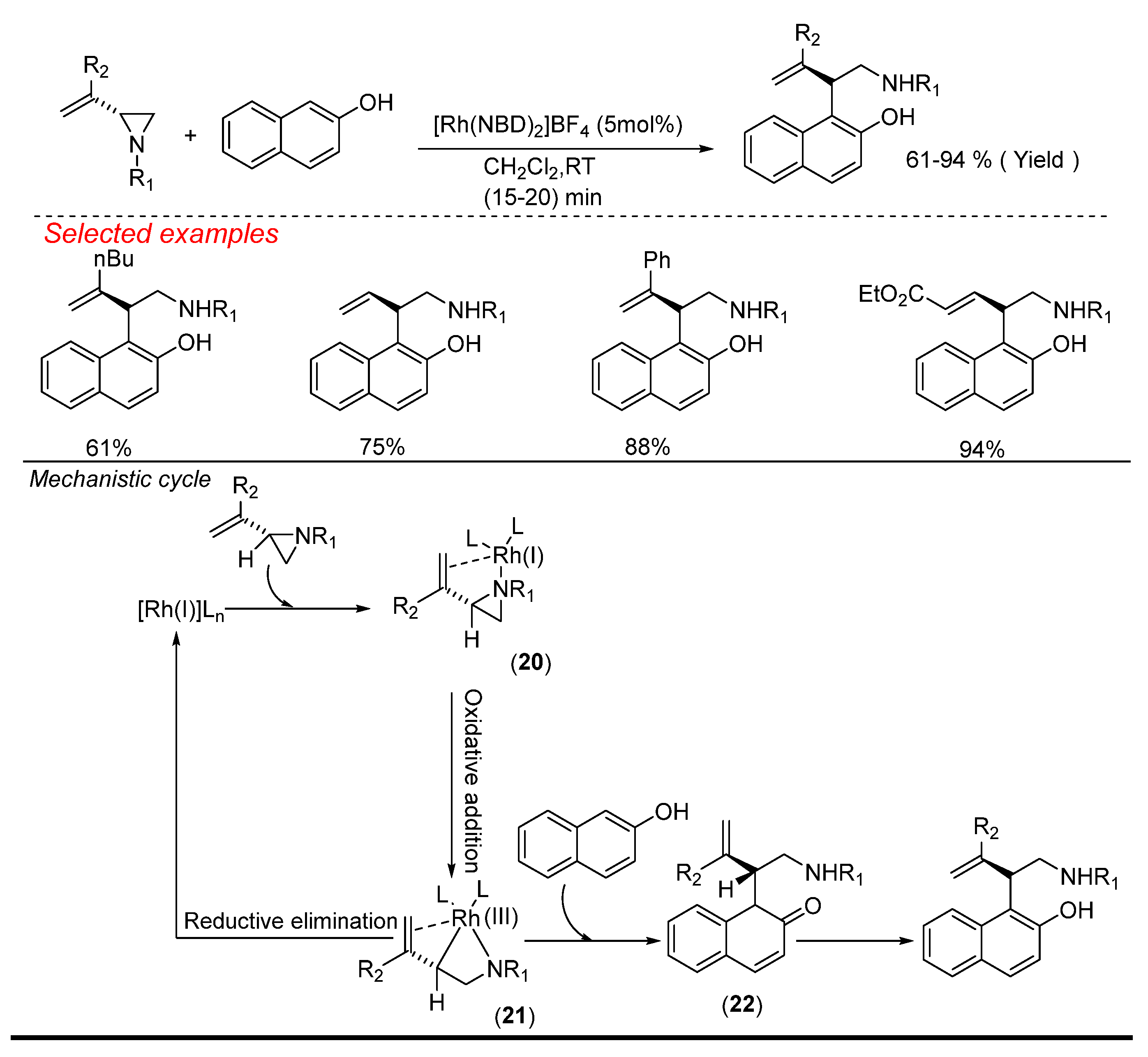
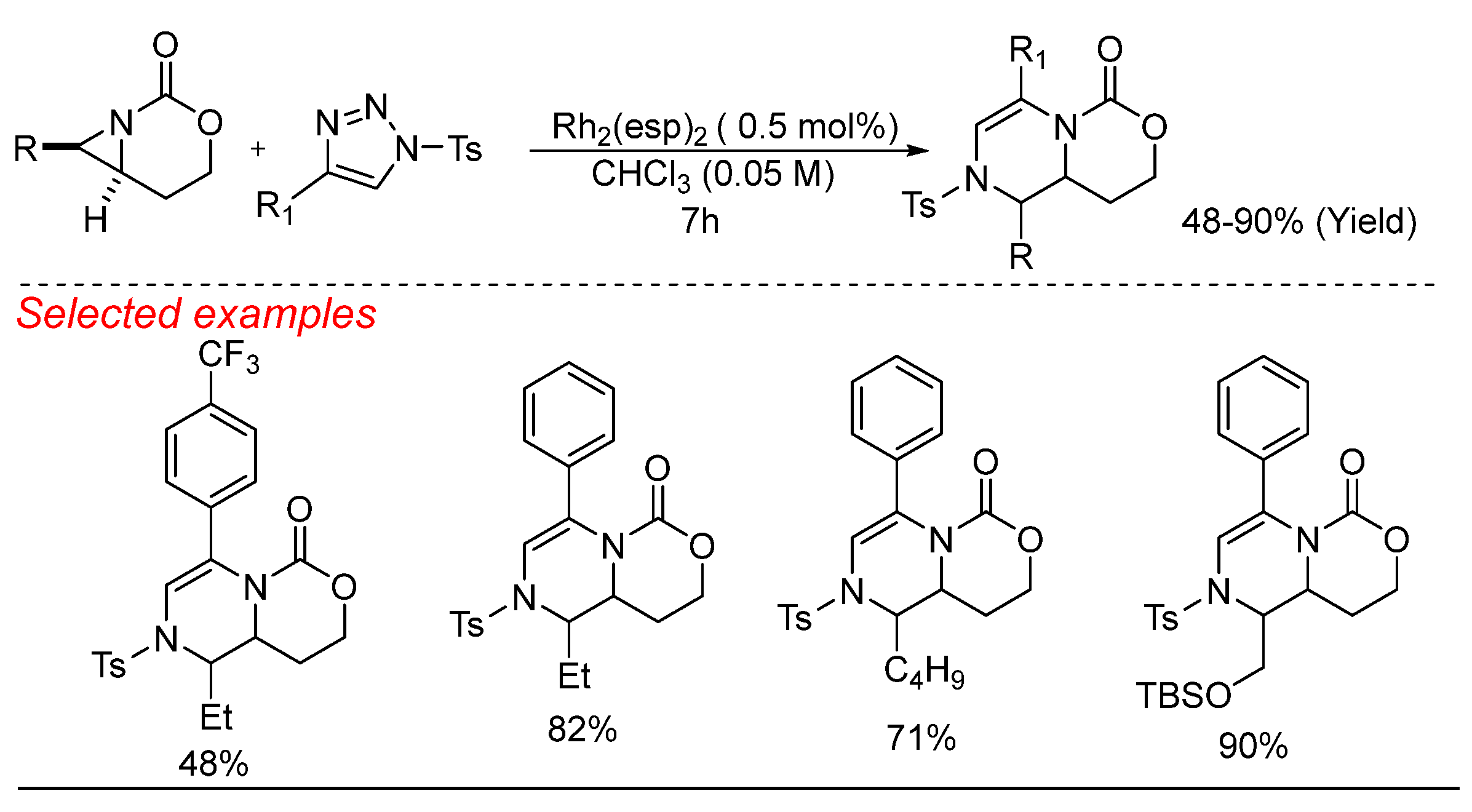
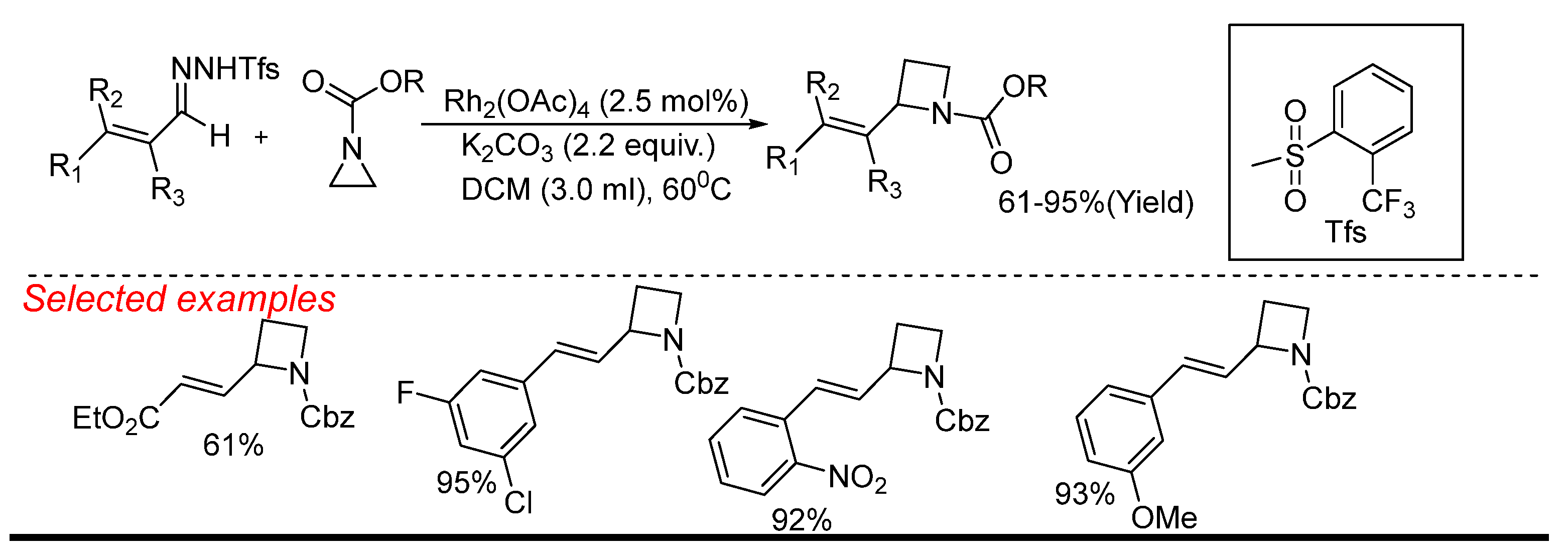
4. Rhodium (Rh)-Catalyzed Aziridine Ring-Opening Reactions
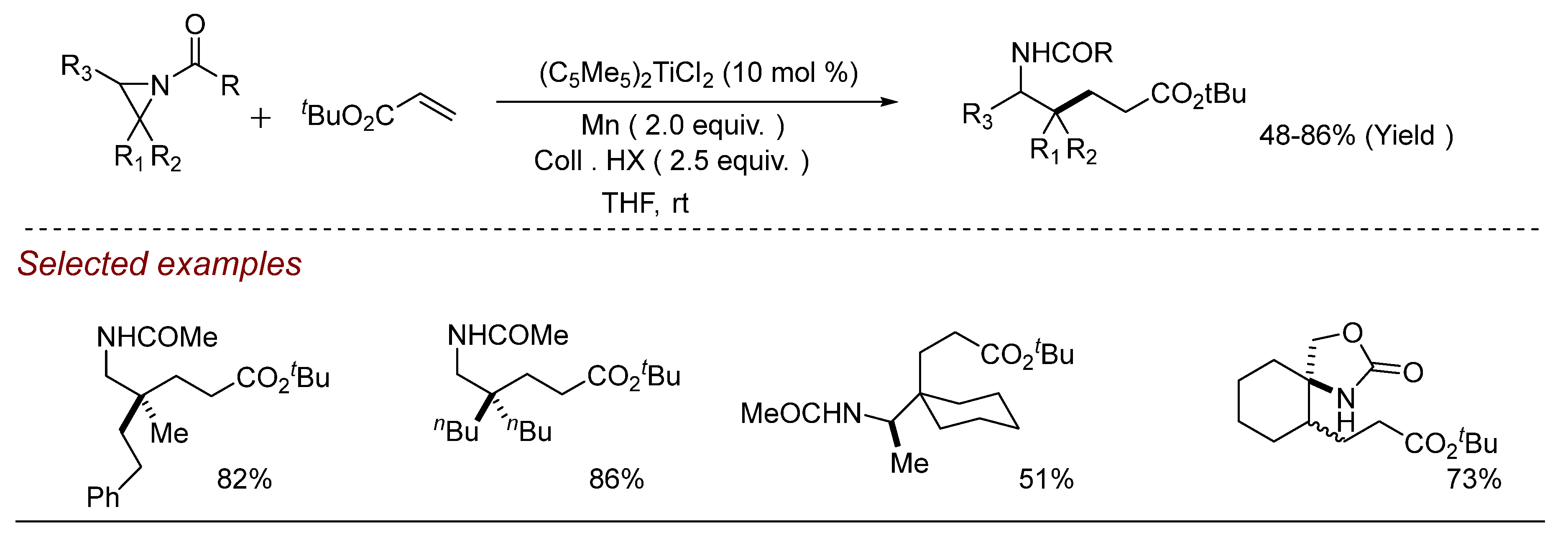
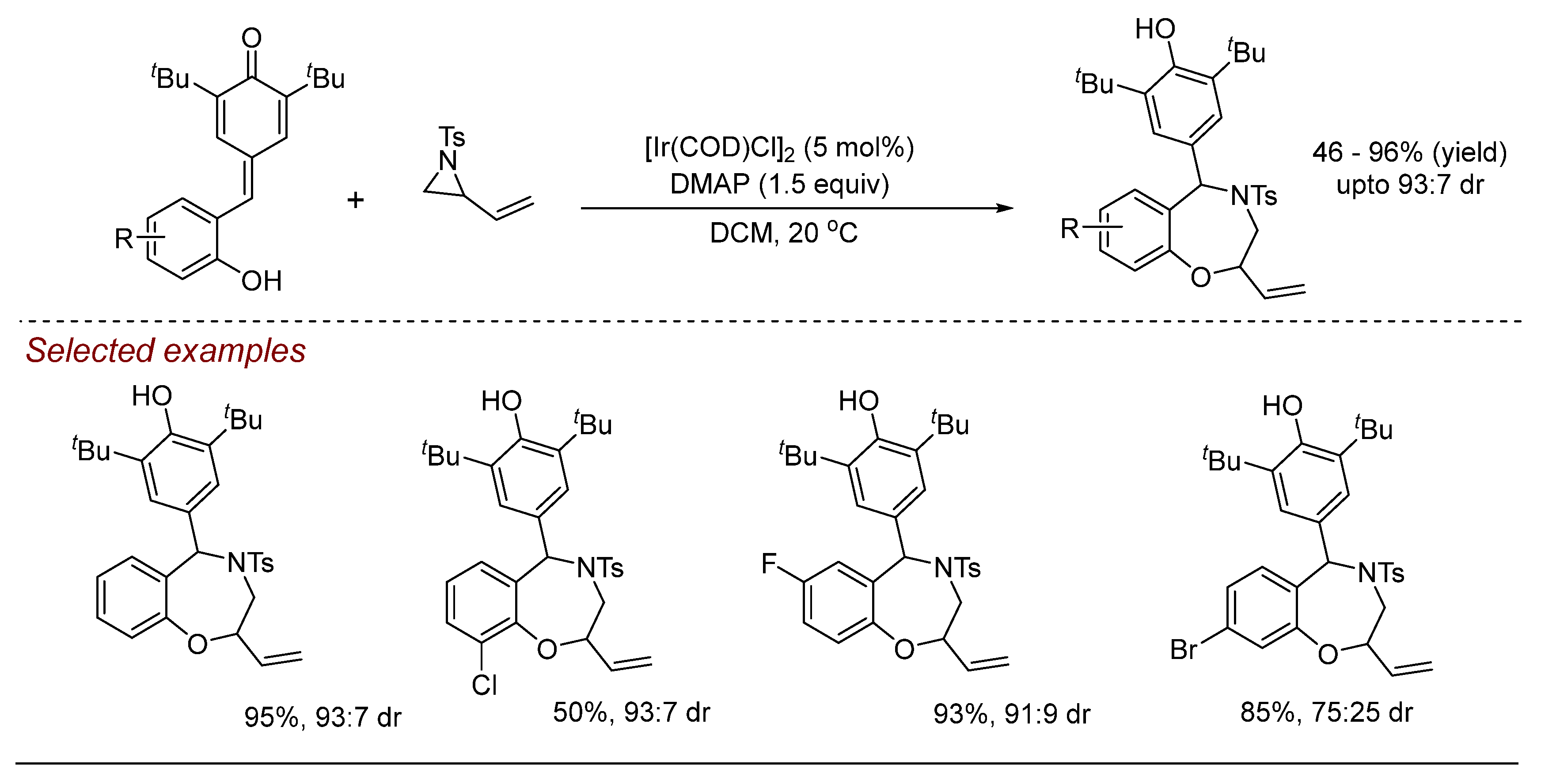
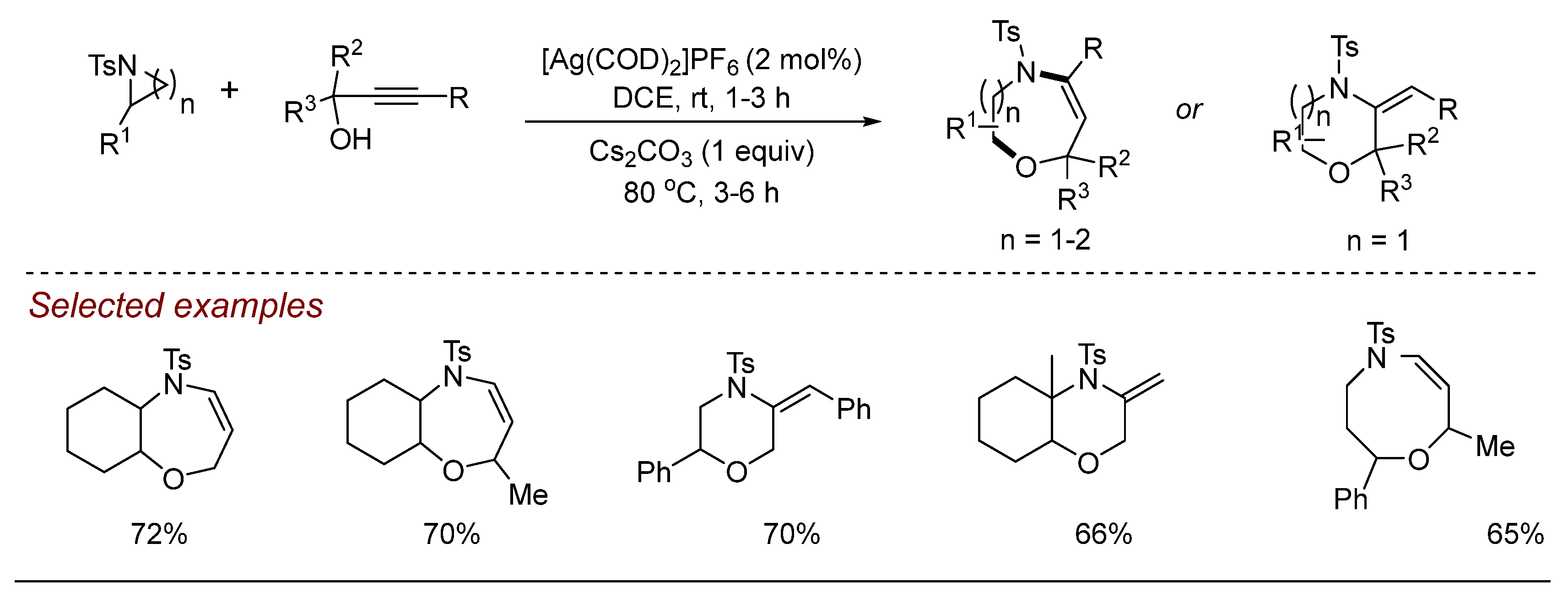
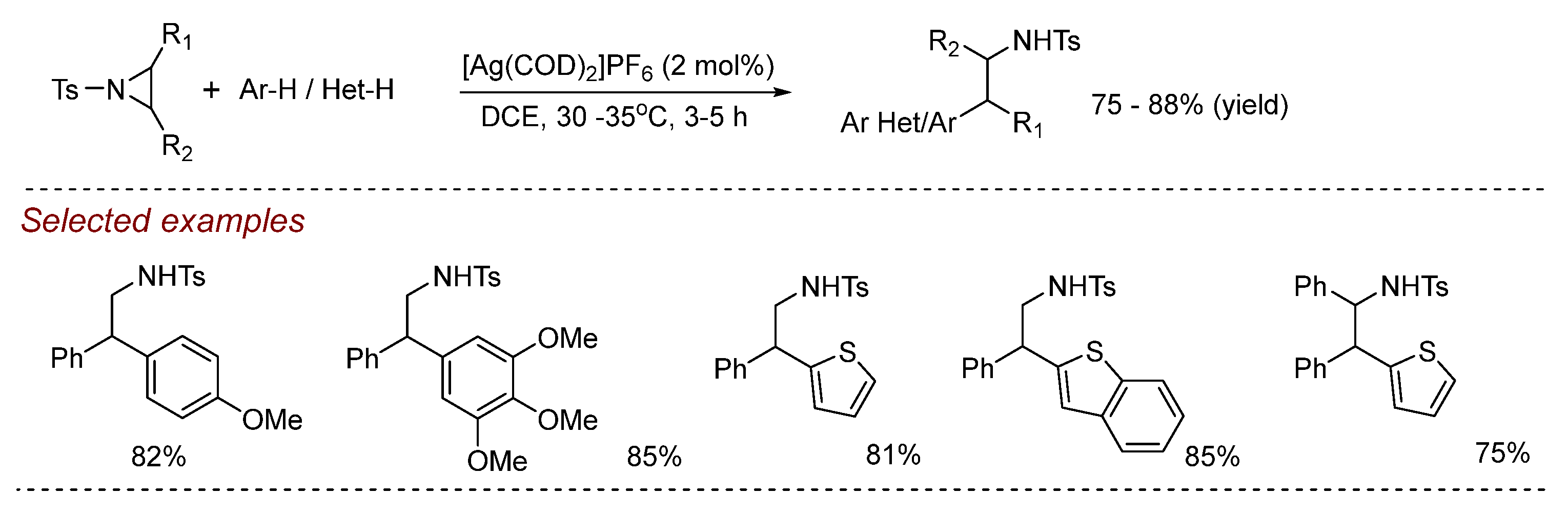
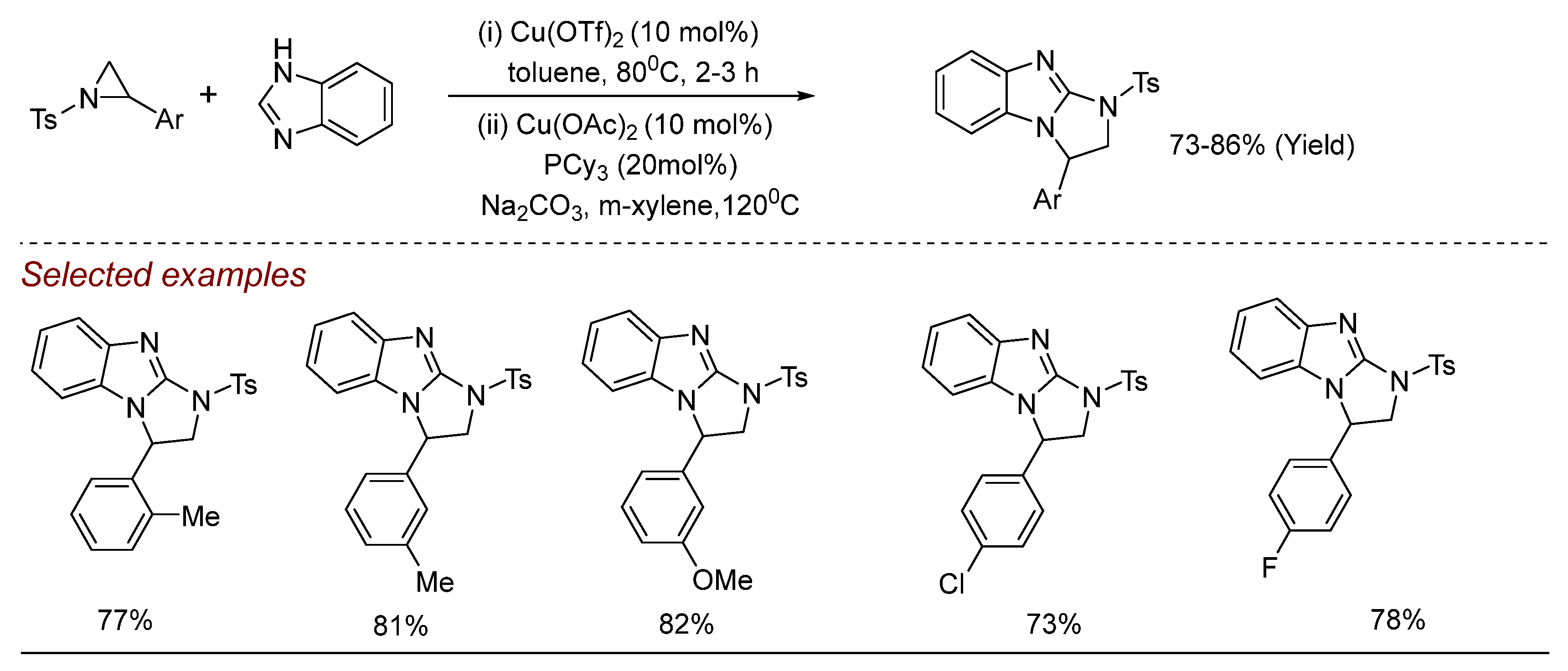
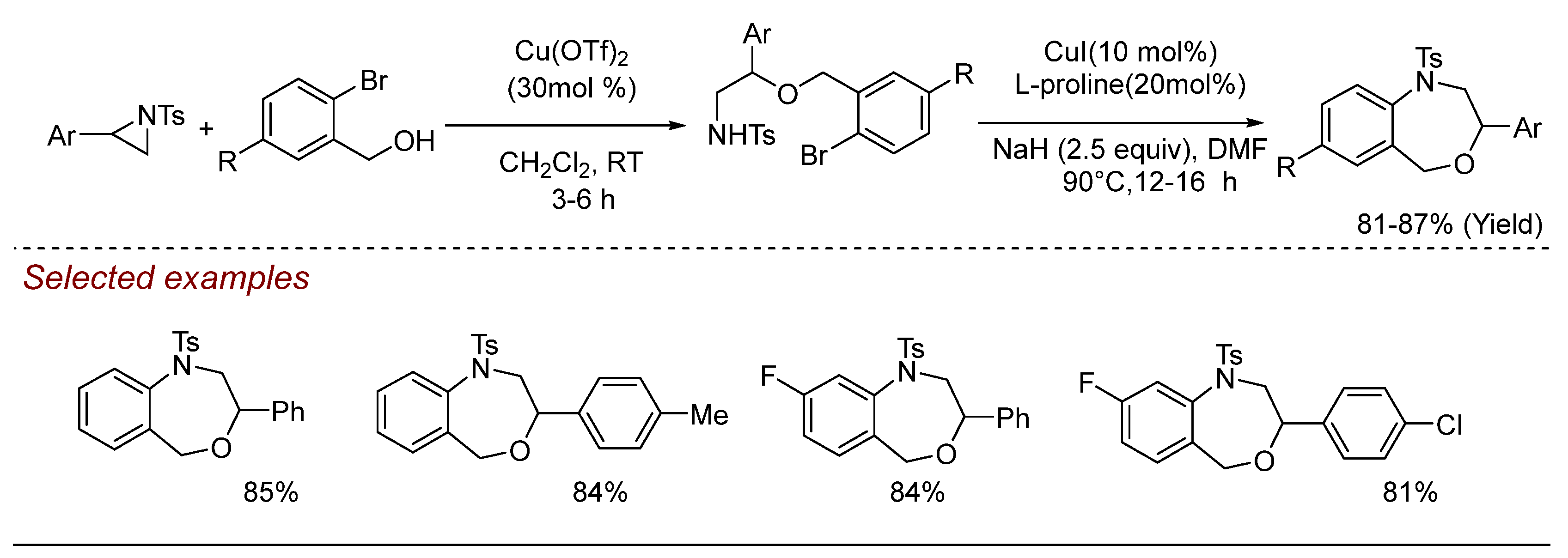
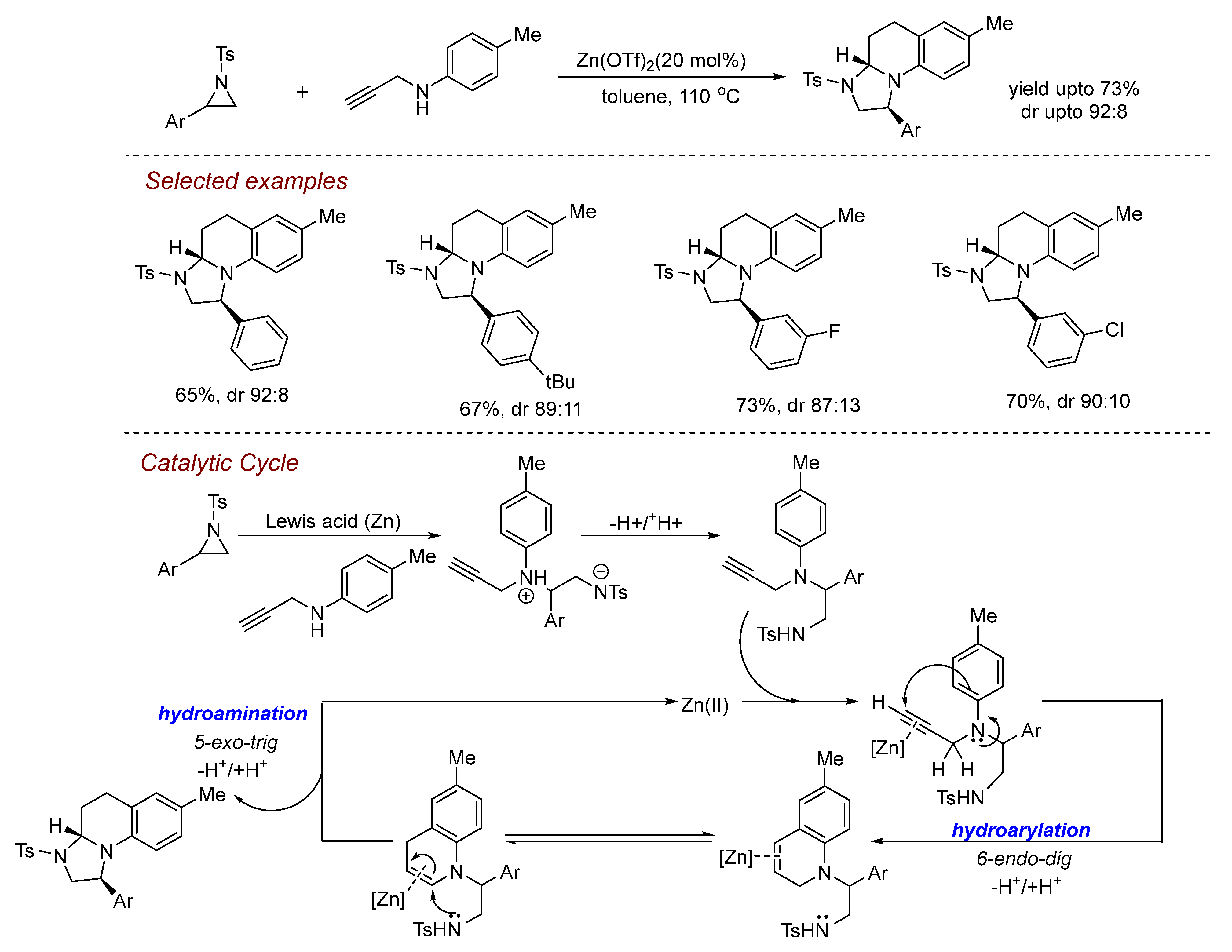
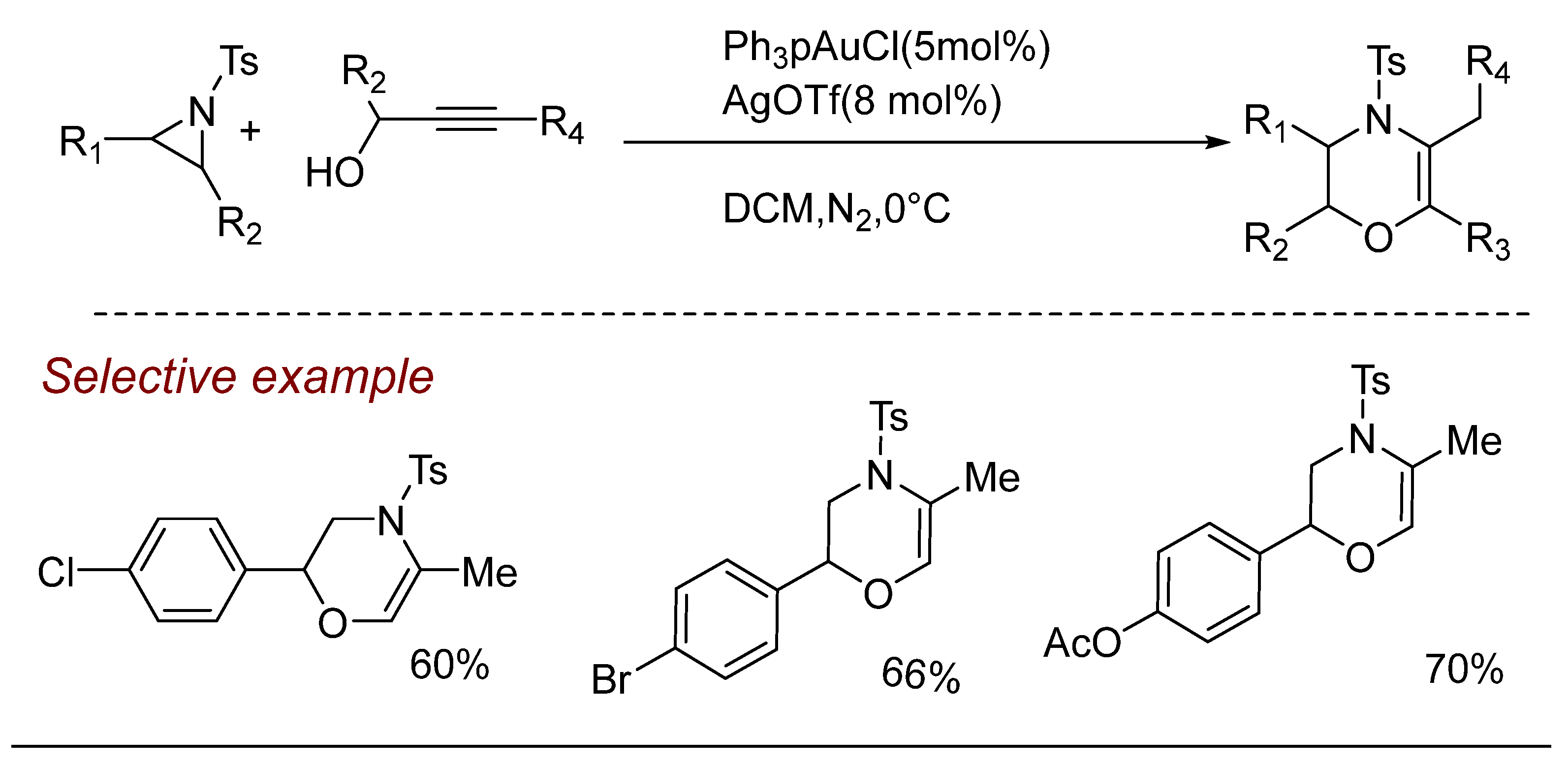
5. Other Transition Metal-Catalyzed Aziridine Ring-Opening Reactions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pineschi, M. Asymmetric Ring-Opening of Epoxides and Aziridines with Carbon Nucleophiles. Eur. J. Org. Chem. 2006, 2006, 4979–4988. [Google Scholar] [CrossRef]
- Dequina, H.J.; Jones, C.L.; Schomaker, J.M. Recent updates and future perspectives in aziridine synthesis and reactivity. Chem 2023, 9, 1658–1701. [Google Scholar] [CrossRef] [PubMed]
- Dank, C.; Ielo, L. Recent advances in the accessibility, synthetic utility, and biological applications of aziridines. Org. Biomol. Chem. 2023, 21, 4553–4573. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S.; Sudheesh, S.; Keroletswe, N. Recent applications of aziridine ring expansion reactions in heterocyclic synthesis. ARKIVOC 2018, 2018, 50–113. [Google Scholar] [CrossRef]
- Hu, X.E. Nucleophilic ring opening of aziridines. Tetrahedron 2004, 60, 2701–2743. [Google Scholar] [CrossRef]
- Wang, G.; Franke, J.; Ngo, C.Q.; Krische, M.J. Diastereo- and Enantioselective Iridium Catalyzed Coupling of Vinyl Aziridines with Alcohols: Site-Selective Modification of Unprotected Diols and Synthesis of Substituted Piperidines. J. Am. Chem. Soc. 2015, 137, 7915–7920. [Google Scholar] [CrossRef]
- Srivastava, N.; Ha, H.-J. Regioselective ring opening of aziridine for synthesizing azaheterocycle. Front. Chem. 2023, 11, 1280633. [Google Scholar] [CrossRef]
- Wu, B.; Gallucci, J.C.; Parquette, J.R.; RajanBabu, T.V. Enantioselective Desymmetrization of meso-Aziridines with TMSN3 or TMSCN Catalyzed by Discrete Yttrium Complexes. Angew. Chem. Int. Ed. 2009, 48, 1126–1129. [Google Scholar] [CrossRef]
- Degennaro, L.; Trinchera, P.; Luisi, R. Recent Advances in the Stereoselective Synthesis of Aziridines. Chem. Rev. 2014, 114, 7881–7929. [Google Scholar] [CrossRef]
- Roxin, A.; Chen, J.; Scully, C.; Rotstein, B.; Yudin, A.K.; Zheng, G. Conformational modulation of in vitro activity of cyclic RGD peptides via aziridine aldehyde-driven macrocyclization chemistry. Bioconjug. Chem. 2012, 23, 1387–1395. [Google Scholar] [CrossRef]
- Dyer, F.B.; Park, C.M.; Joseph, R.; Garner, P. Aziridine-Mediated Ligation and Site-Specific Modification of Unprotected Peptides. J. Am. Chem. Soc. 2011, 133, 20033–20035. [Google Scholar] [CrossRef] [PubMed]
- Ibuka, T. The aza-Payne rearrangement: A synthetically valuable equilibration. Chem. Soc. Rev. 1998, 27, 145–154. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in asymmetric aziridination. Tetrahedron 2010, 66, 1509–1555. [Google Scholar] [CrossRef]
- Botuha, C.; Chemla, F.; Ferreira, F.; Pérez-Luna, A. Aziridines in Natural Product Synthesis. In Heterocycles Nat. Prod. Synthesis; Majumdar, K.C., Chattopadhyay, S.K., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 1–39. [Google Scholar]
- Da Silva, A.R.; dos Santos, D.A.; Paixão, M.W.; Corrêa, A.G. Stereoselective Multicomponent Reactions in the Synthesis or Transformations of Epoxides and Aziridines. Molecules 2019, 24, 630. [Google Scholar] [CrossRef]
- Reddy, G.V.; Rao, G.V.; Sreevani, V.; Iyengar, D.S. An enantioselective synthesis of (1S,2S)-pseudoephedrine. Tetrahedron Lett. 2000, 41, 953–954. [Google Scholar] [CrossRef]
- Mazur, D.M.; Grishina, G.V.; Lebedev, A.T. Molecular recognition of pseudodistamine isomeric precursors trans-3(4)-aminopiperidin-4(3)-ols by EI mass spectrometry. J. Pharm. Biomed. Anal. 2017, 140, 322–326. [Google Scholar] [CrossRef]
- Abzianidze, V.V.; Efimova, K.P.; Poluektova, E.V.; Trishinb, Y.G.; Kuznetsova, V.A. Synthesis of natural phaeosphaeride A and semi-natural phaeosphaeride B derivatives. Mendeleev Commun. 2017, 27, 490–492. [Google Scholar] [CrossRef]
- Kumar, P.; Dubeya, A.; Puranikb, V.G. A general and concise asymmetric synthesis of sphingosine, safingol and phytosphingosines via tethered aminohydroxylation. Org. Biomol. Chem. 2010, 8, 5074–5086. [Google Scholar] [CrossRef]
- Hayashi, Y.; Ogasawara, S. Time Economical Total Synthesis of (−)-Oseltamivir. Org. Lett. 2016, 18, 3426–3429. [Google Scholar] [CrossRef]
- Tranka, T.M.; Grubbs, R.H. The Development of L2X2RuCHR Olefin Metathesis Catalysts: An Organometallic Success Story. Acc. Chem. Res. 2001, 34, 18–29. [Google Scholar] [CrossRef]
- Katsuki, T. Mn-salen catalyst, competitor of enzymes, for asymmetric epoxidation. J. Mol. Catal. A Chem. 1996, 113, 87–107. [Google Scholar] [CrossRef]
- Stanković, S.; D’hooghe, M.; Catak, S.; Eum, H.; Waroquier, M.; Speybroeck, V.V.; Kimpe, N.D.; Ha, H.J. Regioselectivity in the ring opening of non-activated aziridines. Chem. Soc. Rev. 2012, 41, 643–665. [Google Scholar] [CrossRef] [PubMed]
- Coull, W.M.; Davis, F.A. Recent Synthetic Applications of Chiral Aziridines. Synthesis 2000, 10, 1347–1365. [Google Scholar]
- Singh, G.S.; D’hooghe, M.; Kimpe, N.D. Synthesis and Reactivity of C-Heteroatom-Substituted Aziridines. Chem. Rev. 2007, 107, 2080–2135. [Google Scholar] [CrossRef]
- Takeda, Y.; Sameera, W.M.C.; Minakata, S. Palladium-Catalyzed Regioselective and Stereospecific Ring-Opening Cross-Coupling of Aziridines: Experimental and Computational Studies. Acc. Chem. Res. 2020, 53, 1686–1702. [Google Scholar] [CrossRef]
- Mohite, S.B.; Bera, M.; Kumar, V.; Karpoormath, R.; Baba, S.B.; Kumbhar, A.S. O-Benzoylhydroxylamines: A Versatile Electrophilic Aminating Reagent for Transition Metal-Catalyzed C–N Bond-Forming Reactions. Top. Curr. Chem. 2023, 381, 4. [Google Scholar] [CrossRef]
- Mohite, S.B.; Mane, M.V.; Bera, M.; Karpoormath, R. Palladium-Catalyzed Regiodivergent C-H Olefination of Imidazo[1,2a]pyridine Carboxamide and Unactivated Alkenes. Chem. Eur. J. 2023, 29, e202302759. [Google Scholar] [CrossRef]
- Mohite, S.B.; Mirza, Y.K.; Kumar, V.; Partap, S.; Baba, S.B.; Alake, J.; Bera, M.; Karpoormath, R. Palladium-Catalyzed C-H Olefination of Imidazo[1,2a] pyridine Carboxamide in Aqueous Ethanol under Oxygen. Chem. Eur. J. 2024, 30, e202304329. [Google Scholar]
- Mirza, Y.K.; Bera, P.S.; Mohite, S.B.; Pandey, A.K.; Bera, M. Silanes as a versatile hydride source for Ni–H catalysis: A promising tool for π-hydro functionalization. Org. Chem. Front. 2024, 11, 4290–4317. [Google Scholar] [CrossRef]
- Torabi, S.; Jamshidi, M.; Amooshahi, P.; Mehrdadian, M.; Khazalpour, S. Transition metal-catalyzed electrochemical processes for C–C bond formation. New J. Chem. 2020, 44, 15321–15336. [Google Scholar] [CrossRef]
- Huang, K.; Sunwa, W.C.L.; Shi, Z.J. Transition-metal-catalyzed C–C bond formation through the fixation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 2435–2452. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiao, H.; Shu, X.Z.; Wu, L. Zirconium and hafnium catalyzed C–C single bond hydroboration. Nat. Commun. 2024, 15, 1846. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, J.; Hu, R.; Liu, C.; Bartholome, T.A.; Ge, H.; Li, B. Transition-Metal-Catalyzed C−C Bond-Forming Reactions via C−H Activation for the Development of Fluorescent Materials with Practical Value. ACS Catal. 2022, 12, 2796–2820. [Google Scholar] [CrossRef]
- Bera, S.; Hu, X. Nickel-Catalyzed Regioselective Hydroalkylation and Hydroarylation of Alkenyl Boronic Esters. Angew. Chem. Int. Ed. 2019, 58, 13854–13859. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I.; Tzamarioudaki, M.; Li, Z.; Donovan, R.J. Transition Metal-Catalyzed Carbocyclizations in Organic Synthesis. Chem. Rev. 1996, 96, 635–662. [Google Scholar] [CrossRef]
- Negishi, E. Transition Metal-Catalyzed Organometallic Reactions that Have Revolutionized Organic Synthesis. Bull. Chem. Soc. Jpn. 2007, 80, 233–257. [Google Scholar] [CrossRef]
- Omae, I. Transition metal-catalyzed cyclocarbonylation in organic synthesis. Coord. Chem. Rev. 2011, 255, 139–160. [Google Scholar] [CrossRef]
- Vollhardt, K.P.C. Transition-Metal-Catalyzed Acetylene Cyclizations in Organic Synthesis. Acc. Chem. Res. 1977, 10, 1–8. [Google Scholar] [CrossRef]
- Cheng, W.; Shang, R. Transition Metal-Catalyzed Organic Reactions under Visible Light: Recent Developments and Future Perspectives. ACS Catal. 2020, 10, 9170–9196. [Google Scholar] [CrossRef]
- Gandeepan, P.S.; Cheng, C.H. Transition-Metal-Catalyzed p-Bond-Assisted CH Bond Functionalization: An Emerging Trend in Organic Synthesis. Chem. Asian J. 2015, 10, 824–838. [Google Scholar] [CrossRef]
- Nakamura, I.; Yamamoto, Y. Transition Metal-Catalyzed Reactions of Methylenecyclopropanes. Adv. Synth. Catal. 2002, 344, 111–129. [Google Scholar] [CrossRef]
- Wolfe, J.P.; Ney, J.E. A New, Mild Synthesis of N-Sulfonyl Ketimines via the Palladium-Catalyzed Isomerization of Aziridines. Org. Lett. 2003, 5, 4607–4610. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Rachwal, S.; Rachwal, B. Recent Progress in the synthesis of 1,2,3,4-tetrahydroquinolines. Tetrahedron 1996, 52, 15031–15070. [Google Scholar] [CrossRef]
- Ghorai, M.K.; Nanaji, Y.; Yadav, A.K. Ring Opening/C–N Cyclization of Activated Aziridines with Carbon Nucleophiles: Highly Diastereo- and Enantioselective Synthesis of Tetrahydroquinolines. Org. Lett. 2011, 13, 4256–4259. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Lv, M.-F.; Yi, W.-B.; Cai, C. Synthesis of 1,4-Benzoxazepine Derivatives via a Novel Domino Aziridine Ring-Opening and Isocyanide-Insertion Reaction. Adv. Synth. Catal. 2013, 355, 3401–3406. [Google Scholar] [CrossRef]
- Kjellgren, J.; Aydin, J.; Wallner, O.A.; Saltanova, I.V.; Szabó, K.J. Palladium Pincer Complex Catalyzed Cross-Coupling of Vinyl Epoxides and Aziridines with Organoboronic Acids. Chem. Eur. J. 2005, 11, 5260–5268. [Google Scholar] [CrossRef]
- Yin, J.; Mekelburg, T.; Hyland, C. Unusual (Z)-selective palladium(ii)-catalysed addition of aryl boronic acids to vinylaziridines. Org. Biomol. Chem. 2014, 12, 9113–9115. [Google Scholar] [CrossRef]
- Steiman, T.J.; Liu, J.; Mengiste, A.; Doyle, A.G. Synthesis of β-Phenethylamines via Ni/Photoredox Cross Electrophile Coupling of Aliphatic Aziridines and Aryl Iodides. J. Am. Chem. Soc. 2020, 142, 7598–7605. [Google Scholar] [CrossRef]
- Duda, M.L.; Michael, F.E. Palladium-Catalyzed Cross-Coupling of N-Sulfonylaziridines with Boronic Acids. J. Am. Chem. Soc. 2013, 135, 18347–18349. [Google Scholar] [CrossRef]
- Takeda, Y.; Ikeda, Y.; Kuroda, A.; Tanaka, S.; Minakata, S. Pd/NHC-Catalyzed Enantiospecific and Regioselective Suzuki–Miyaura Arylation of 2-Arylaziridines: Synthesis of Enantioenriched 2-Arylphenethylamine Derivatives. J. Am. Chem. Soc. 2014, 136, 8544–8547. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sameera, W.M.C.; Takeda, Y.; Minakata, S. Computational Study on the Mechanism and Origin of the Regioselectivity and Stereospecificity in Pd/SIPr-Catalyzed Ring-Opening Cross-Coupling of 2-Arylaziridines with Arylboronic Acids. ACS Catal. 2019, 9, 4582–4592. [Google Scholar] [CrossRef]
- Takeda, Y.; Matsuno, T.; Sharma, A.K.; Sameera, W.M.C.; Minakata, S. Asymmetric Synthesis of β2-Aryl Amino Acids through Pd-Catalyzed Enantiospecific and Regioselective Ring-Opening Suzuki–Miyaura Arylation of Aziridine-2-carboxylates. Chem. Eur. J. 2019, 25, 10226–10231. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, B.S. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, H.; Ju, C.-W.; Qin, Y.; Xue, X.-S.; Zhao, D. A Ring Expansion Strategy Towards Diverse Azaheterocycles. Nat. Chem. 2021, 13, 1006. [Google Scholar] [CrossRef] [PubMed]
- Hunag, C.-Y.; Doyle, A.B. Nickel-Catalyzed Negishi Alkylations of Styrenyl Aziridines. J. Am. Chem. Soc. 2012, 134, 9541. [Google Scholar] [CrossRef]
- Nielsen, D.K.; Hunag, C.-Y.; Doyle, A.B. Directed Nickel-Catalyzed Negishi Cross Coupling of Alkyl Aziridines. J. Am. Chem. Soc. 2013, 135, 13605–13609. [Google Scholar] [CrossRef]
- Woods, B.P.; Orlandi, M.; Huang, C.-Y.; Sigman, M.S.; Doyle, A.B. Nickel-Catalyzed Enantioselective Reductive Cross-Coupling of Styrenyl Aziridines. J. Am. Chem. Soc. 2017, 139, 5688–5691. [Google Scholar] [CrossRef]
- Jensen, K.L.; Standley, E.A.; Jamison, T.F. Highly Regioselective Nickel-Catalyzed Cross-Coupling of N-Tosylaziridines and Alkylzinc Reagents. J. Am. Chem. Soc. 2014, 136, 11145–11152. [Google Scholar] [CrossRef]
- Fan, P.; Jin, Y.; Liu, J.; Wang, R.; Wang, C. Nickel/Photo-Cocatalyzed Regioselective Ring Opening of N-Tosyl Styrenyl Aziridines with Aldehydes. Org. Lett. 2021, 23, 7364–7369. [Google Scholar] [CrossRef]
- Xu, S.; Hirano, K.; Miura, M. Nickel-Catalyzed Regio- and Stereospecific C–H Coupling of Benzamides with Aziridines. Org. Lett. 2021, 23, 5471–5475. [Google Scholar] [CrossRef]
- Drewniak-Świtalska, M.; Barycza, B.; Rudzińska-Szostak, E.; Morawiak, P.; Berlicki, Ł. Constrained beta-amino acid-containing miniproteins. Org. Biomol. Chem. 2021, 19, 4272–4278. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Janssen-Müller, D.; Zimin, D.P.; Day, C.S.; Yanagi, T.; Elfert, J.; Martin, R. Ni-Catalyzed Carboxylation of Aziridines en Route to β-Amino Acids. J. Am. Chem. Soc. 2021, 143, 4949–4954. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fan, P. Nickel-Catalyzed Cross-Electrophile Ring Opening/gem Difluoroallylation of Aziridines. Org. Lett. 2023, 25, 5756–5761. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.A.; Raj, V.; Saha, S. Indole-fused azepines and analogues as anticancer lead molecules: Privileged findings and future directions. Eur. J. Med. Chem. 2017, 142, 244–265. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-J.; Lin, T.-Y.; Hai-Hong Wu, H.-H.; Zhang, J. Transfer of Chirality in the Rhodium-Catalyzed Intramolecular Formal Hetero-[5 + 2] Cycloaddition of Vinyl Aziridines and Alkynes: Stereoselective Synthesis of Fused Azepine Derivatives. J. Am. Chem. Soc. 2015, 137, 3787–3790. [Google Scholar] [CrossRef]
- Feng, J.-J.; Lin, T.-Y.; Zhu, C.-J.; Wang, H.; Wu, H.-H.; Zhang, J. The Divergent Synthesis of Nitrogen Heterocycles by Rhodium(I) Catalyzed Intermolecular Cycloadditions of Vinyl Aziridines and Alkynes. J. Am. Chem. Soc. 2016, 138, 2178–2181. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Wu, H.-H.; Feng, J.-J.; Zhang, J. Transfer of Chirality in the Rhodium-Catalyzed Chemoselective and Regioselective Allylic Alkylation of Hydroxyarenes with Vinyl Aziridines. Org. Lett. 2017, 19, 2897–2900. [Google Scholar] [CrossRef]
- Suryavanshi, R.H.; Rathore, M.M. Synthesis and biological activities of piperazine derivatives as antimicrobial and antifungal agents. Org. Commun. 2017, 10, 228–238. [Google Scholar] [CrossRef]
- Dequina, J.H.; Eshon, J.; Raskopf, T.W.; Fernandez, I.; Schomaker, M.J. Rh-Catalyzed Aziridine Ring Expansions to Dehydropiperazines. Org. Lett. 2020, 22, 3637–3641. [Google Scholar] [CrossRef]
- Kumar, D.; Aggarwal, N.; Bibi, S.; Chopra, H.; Kumar, V.; Kumar, H.; Marwaha, K.R.; Deep, A.; Alshammari, A.; Alharbi, M.; et al. Synthesis, Anticancer, Antimicrobial and Antioxidant Potential of Novel 4-(Substituted phenyl-1,3,4-oxadiazol/thiadiazol-2-yl) 4-(4-substituted phenyl) Azetidin-2-One Derivatives. Pharmaceuticals 2023, 16, 517. [Google Scholar] [CrossRef]
- Ning, Y.; Chen, H.; Ning, Y.; Zhang, J.; Bi, X. Rhodium-Catalyzed One-Carbon Ring Expansion of Aziridines with Vinyl-N-triftosylhydrazones for the Synthesis of 2-Vinyl Azetidines. Angew.Chem. 2024, 63, 202318072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Vogelsang, E.; Qu, Z.W.; Grimme, S.; Gansauer, A. Titanocene-Catalyzed Radical Opening of N-Acylated Aziridines. Angew. Chem. Int. Ed. 2017, 56, 12654–12657. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yuan, F.R.; Jin, L.W.; Mei, G.J.; Shi, F. Metal-Catalyzed (4+3) Cyclization of Vinyl Aziridines with para-Quinone Methide Derivatives. ACS Catal. 2018, 8, 10234–10240. [Google Scholar] [CrossRef]
- Bera, M.; Roy, S. Silver(I)-Catalyzed Dual Activation of Propargylic Alcohol and Aziridine/Azetidine: Triggering Ring Opening and Endo-Selective Ring-Closing in a Cascade. J. Org. Chem. 2009, 74, 8814–8817. [Google Scholar] [CrossRef]
- Bera, M.; Roy, S. Silver(I)-Diene Complexes as Versatile Catalysts for the C-Arylation of N-Tosylaziridines: Mechanistic Insight from In Situ Diagnostics. J. Org. Chem. 2010, 75, 4402–4412. [Google Scholar] [CrossRef]
- De, P.B.; Pradhan, S.; Punniyamurthy, T. Stereoselective Copper-Catalyzed Cross-Coupling of Aziridines with Benzimidazoles via Nucleophilic Ring Opening and C(sp2)-H Functionalization. J. Org. Chem. 2017, 82, 3183–3191. [Google Scholar] [CrossRef]
- Ghorai, M.K.; Sahoo, A.K.; Bhattacharyya, A. Syntheses of Imidazo-, Oxa-, and Thiazepine Ring Systems via Ring Opening of Aziridines/Cu-Catalyzed C−N/C−C Bond Formation. J. Org. Chem. 2014, 79, 6468–6479. [Google Scholar] [CrossRef]
- Sayyad, M.; Mal, A.; Wani, I.A.; Ghorai, M.K. A Synthetic Route to Chiral Tetrahydropyrroloindoles via Ring Opening of Activated Aziridines with 2-Bromoindoles Followed by Copper-Catalyzed C–N Cyclization. J. Org. Chem. 2016, 81, 6424–6432. [Google Scholar] [CrossRef]
- Pradhan, S.; Chauhan, N.; Shahi, C.K.; Bhattacharyya, A.; Manas, K.; Ghorai, K.M. Stereoselective Synthesis of Hexahydroimidazo[1,2 a]quinolines via SN2 Type Ring-Opening Hydroarylation−Hydroamination Cascade Cyclization of Activated Aziridines with N Propargylanilines. Org. Lett. 2020, 22, 7903–7908. [Google Scholar] [CrossRef]
- Zhang, S.; Shan, C.; Shuai Zhang, S.; Yuan, L.; Wang, J.; Tung, C.-H.; Xing, L.-B.; Xu, Z. Breaking aziridines to construct morpholines with a gold(I)-catalyzed tandem ring-opening and cycloisomerization reaction. Org. Biomol. Chem. 2016, 14, 10973–10980. [Google Scholar] [CrossRef]



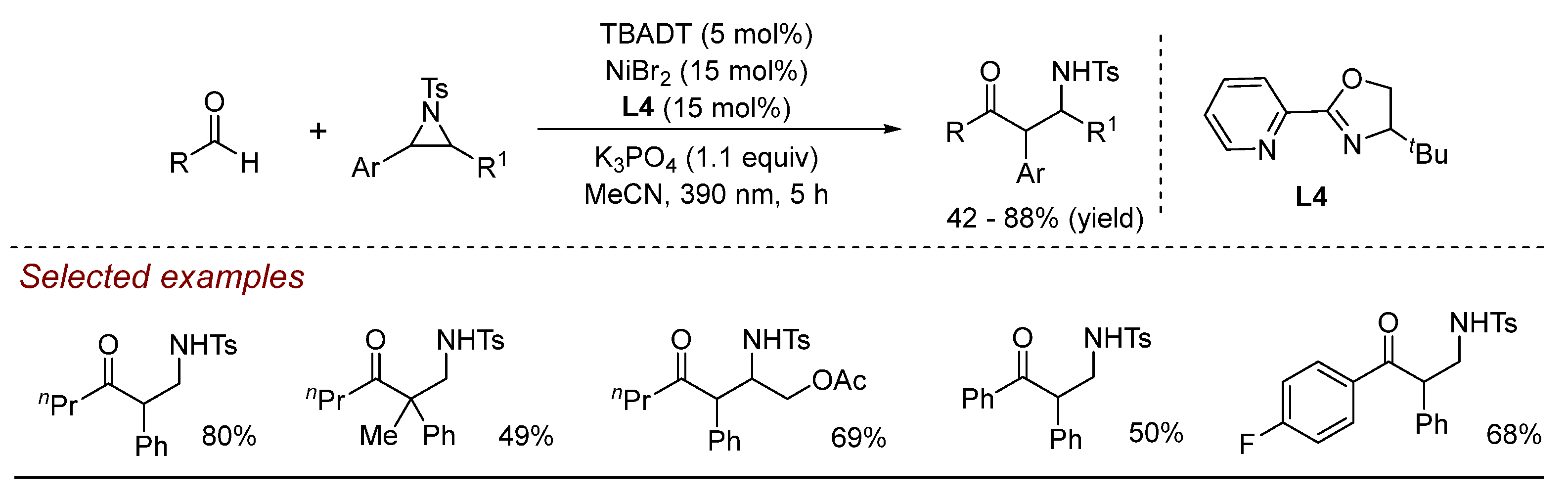
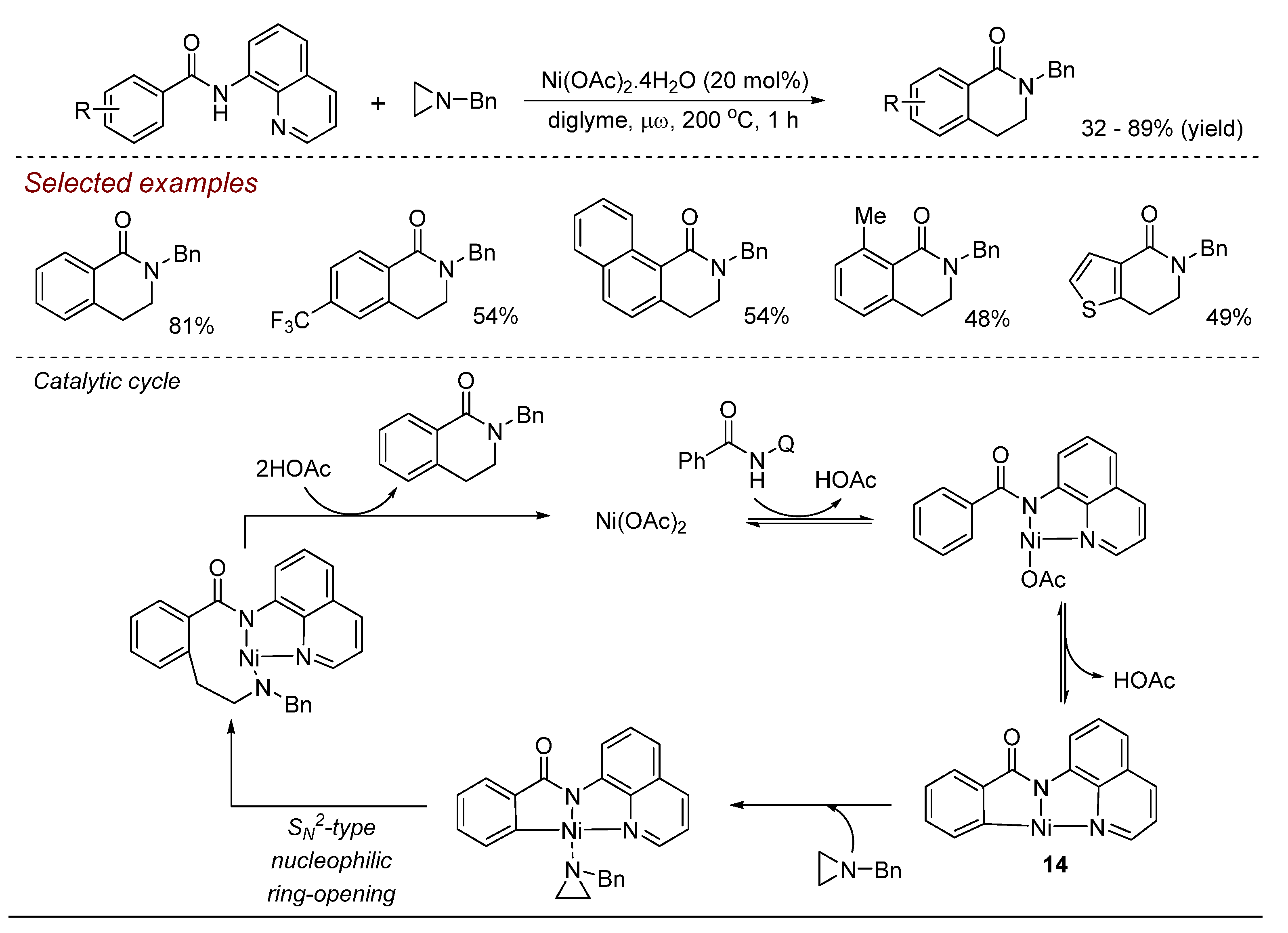
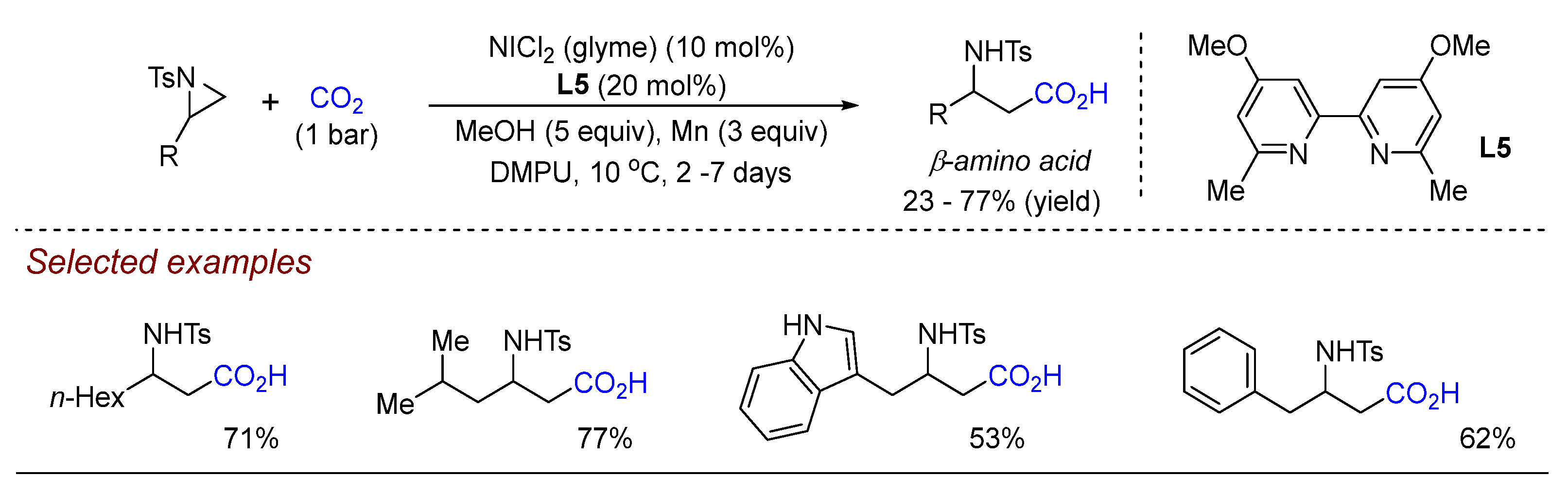
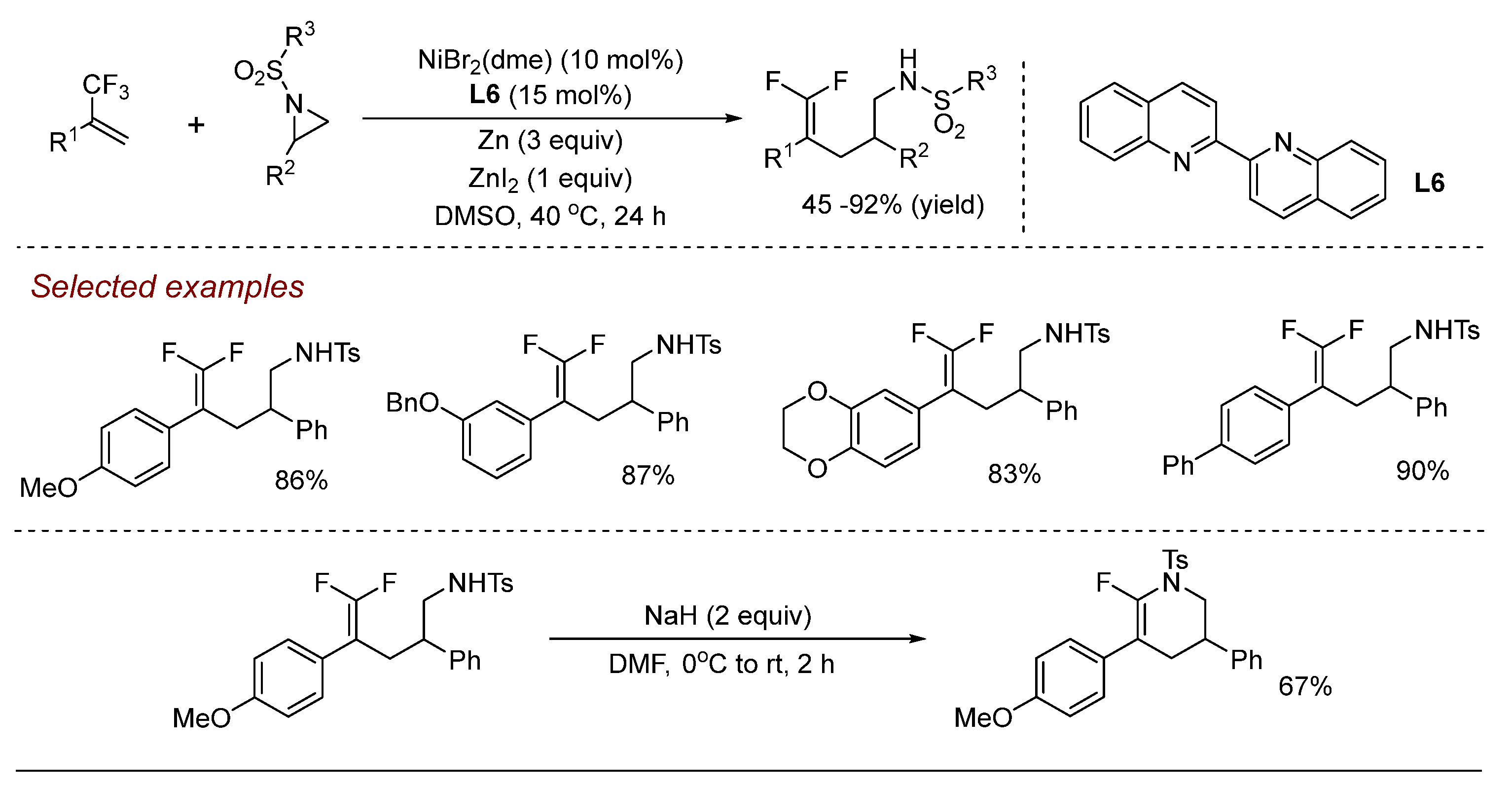


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bera, P.S.; Mirza, Y.K.; Sachdeva, T.; Bera, M. Recent Advances in Transition Metal-Catalyzed Ring-Opening Reaction of Aziridine. Compounds 2024, 4, 626-649. https://doi.org/10.3390/compounds4040038
Bera PS, Mirza YK, Sachdeva T, Bera M. Recent Advances in Transition Metal-Catalyzed Ring-Opening Reaction of Aziridine. Compounds. 2024; 4(4):626-649. https://doi.org/10.3390/compounds4040038
Chicago/Turabian StyleBera, Partha Sarathi, Yafia Kousin Mirza, Tarunika Sachdeva, and Milan Bera. 2024. "Recent Advances in Transition Metal-Catalyzed Ring-Opening Reaction of Aziridine" Compounds 4, no. 4: 626-649. https://doi.org/10.3390/compounds4040038
APA StyleBera, P. S., Mirza, Y. K., Sachdeva, T., & Bera, M. (2024). Recent Advances in Transition Metal-Catalyzed Ring-Opening Reaction of Aziridine. Compounds, 4(4), 626-649. https://doi.org/10.3390/compounds4040038





