Unlocking the Potential of Hydroxycinnamic Acid Bioconjugates: Tailored Derivatives for Biomedical, Cosmetic, and Food Applications
Abstract
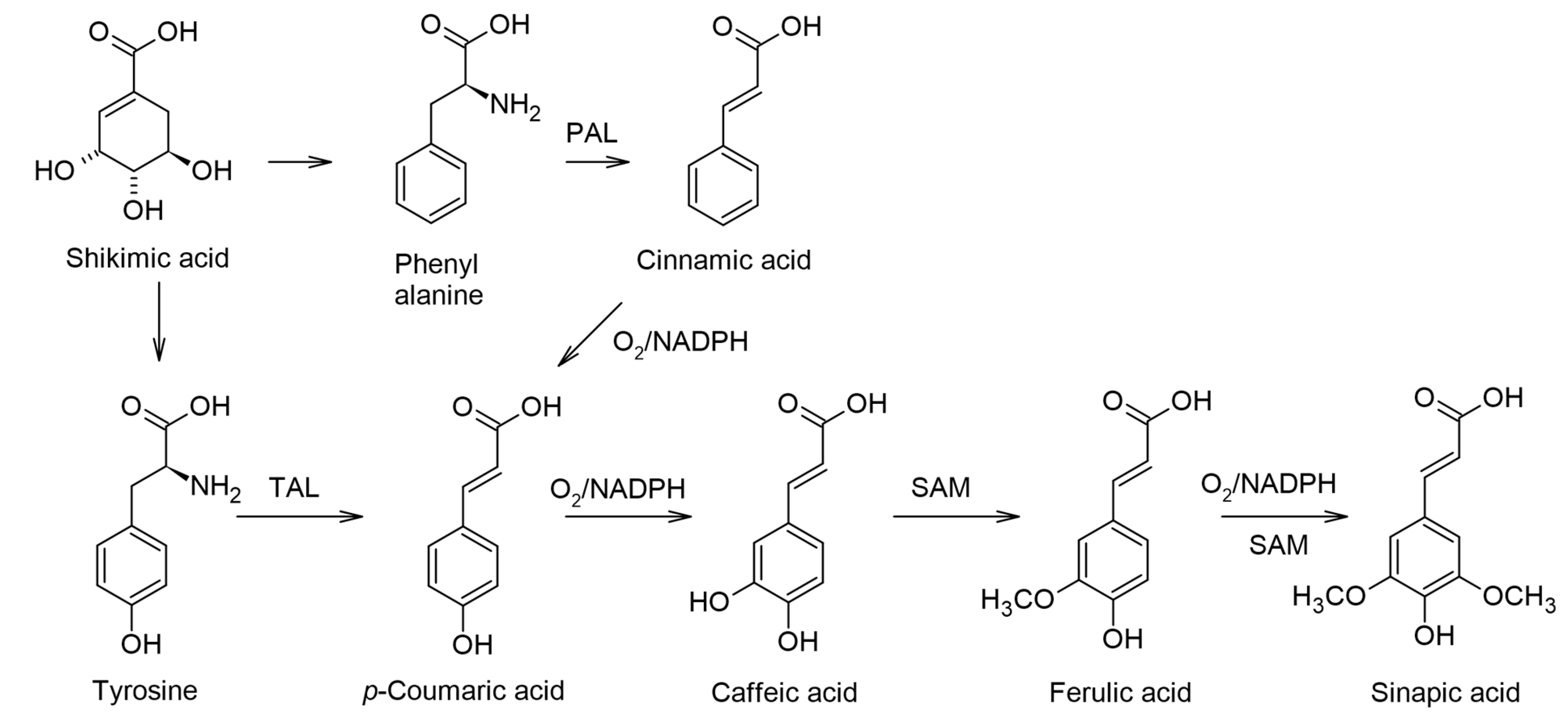
1. Introduction
2. The General Perspectives of Modified HCA Bioconjugates for Various Applications
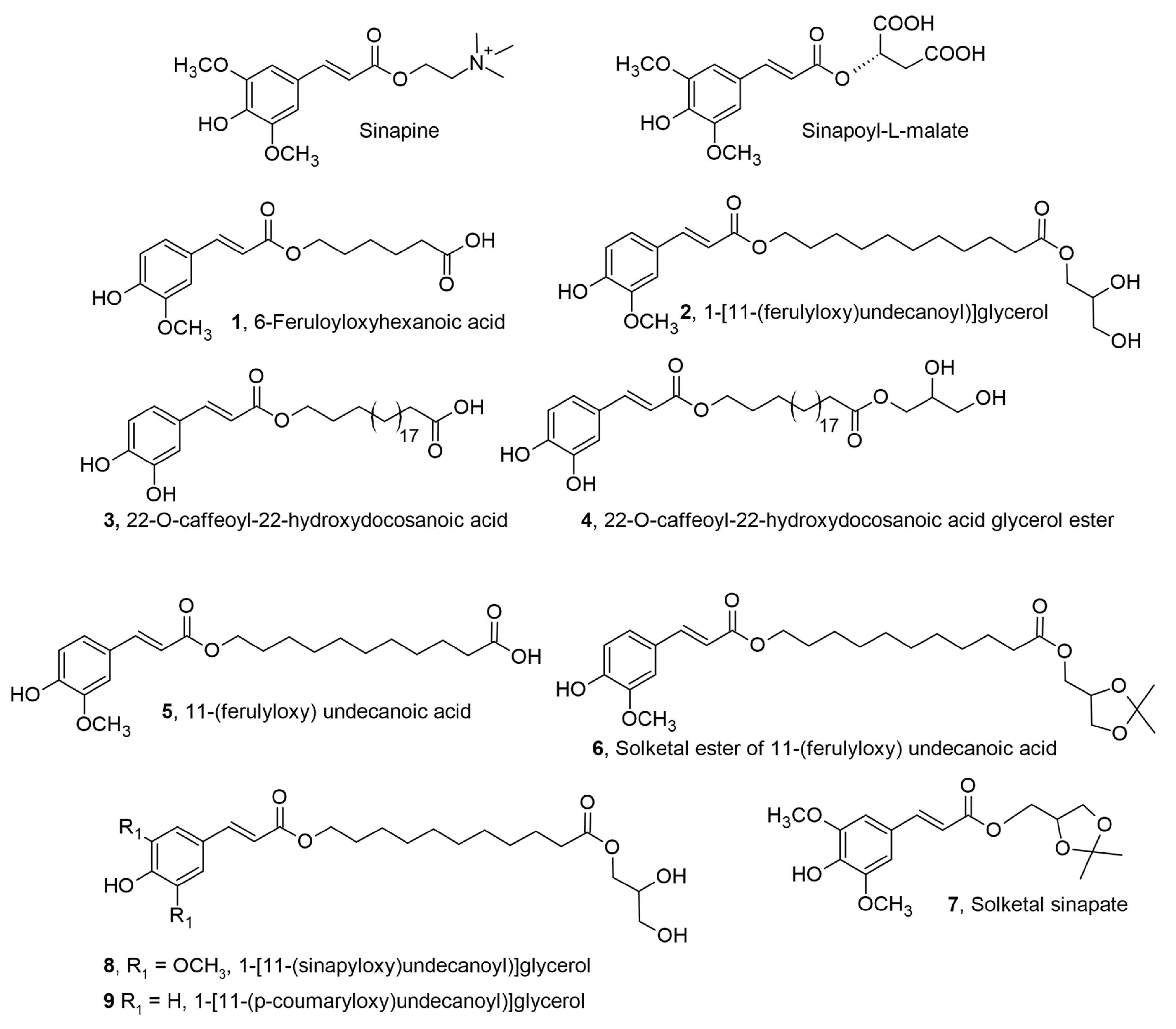
2.1. ω-hydroxycinnamoyloxy Fatty Acids and Derivatives for Biomedical Applications
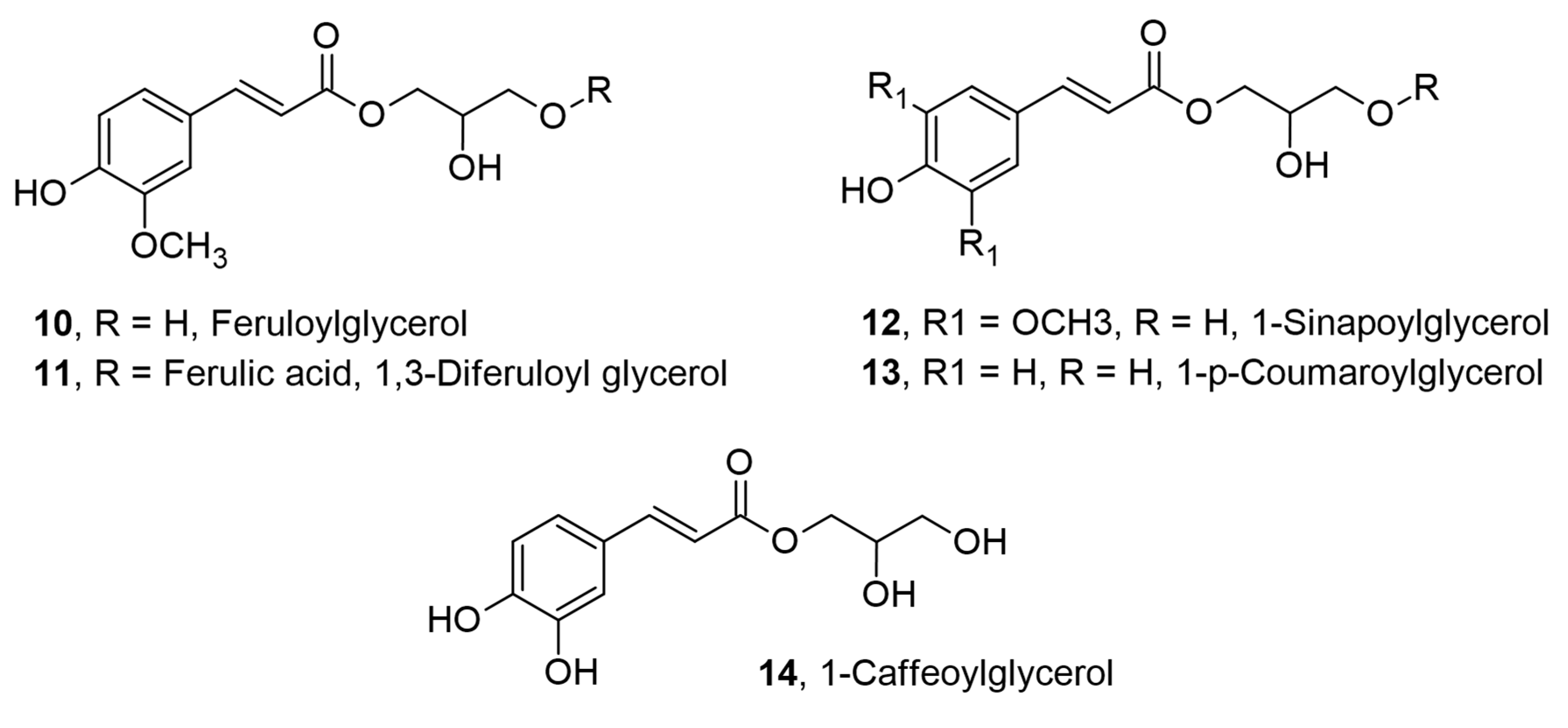
2.2. Monoglyceryl, Diglyceryl, and Glycerol Fatty Acid Esters of Hydroxycinnamic Acids for Cosmetic and Other Applications
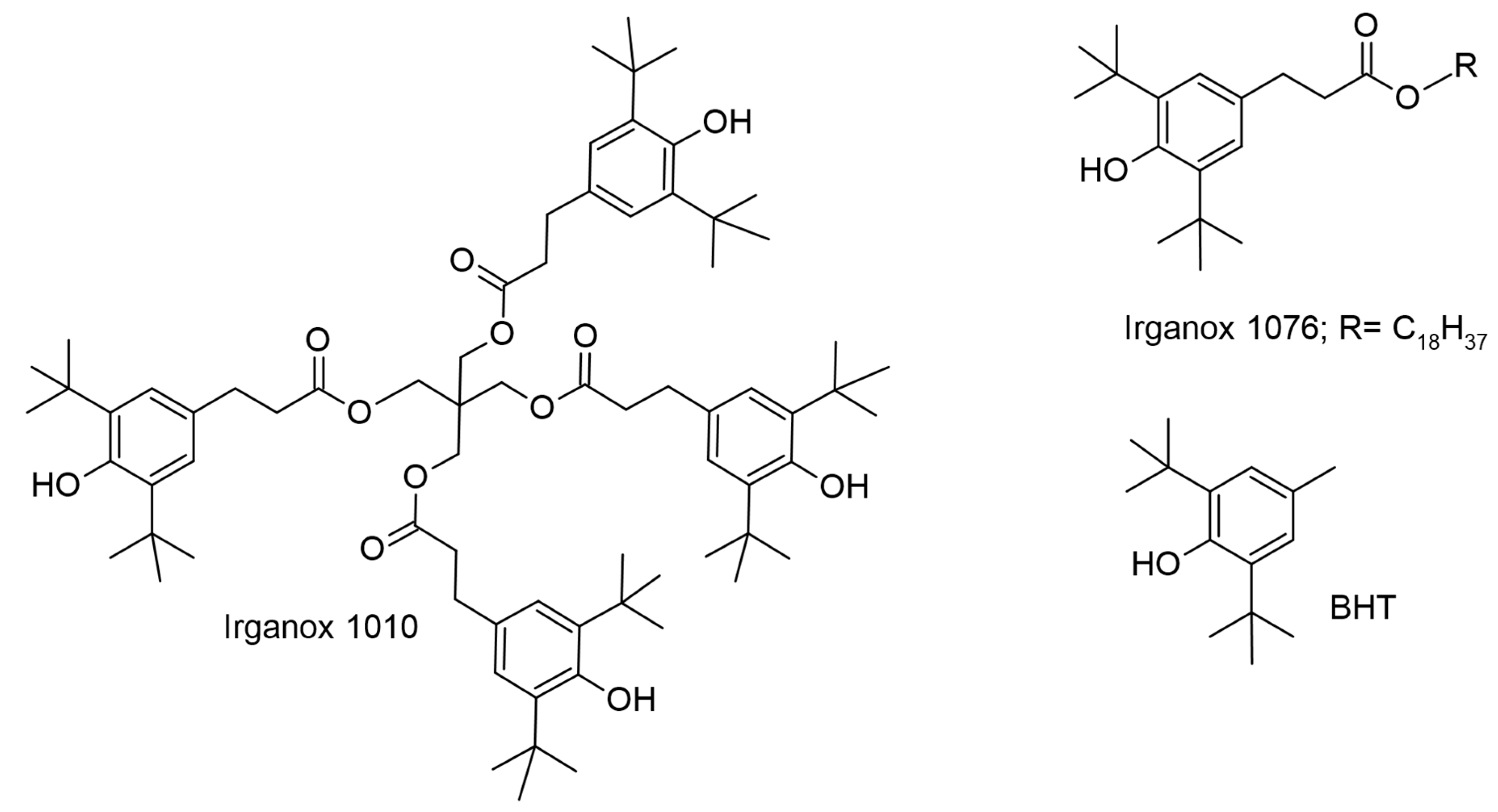
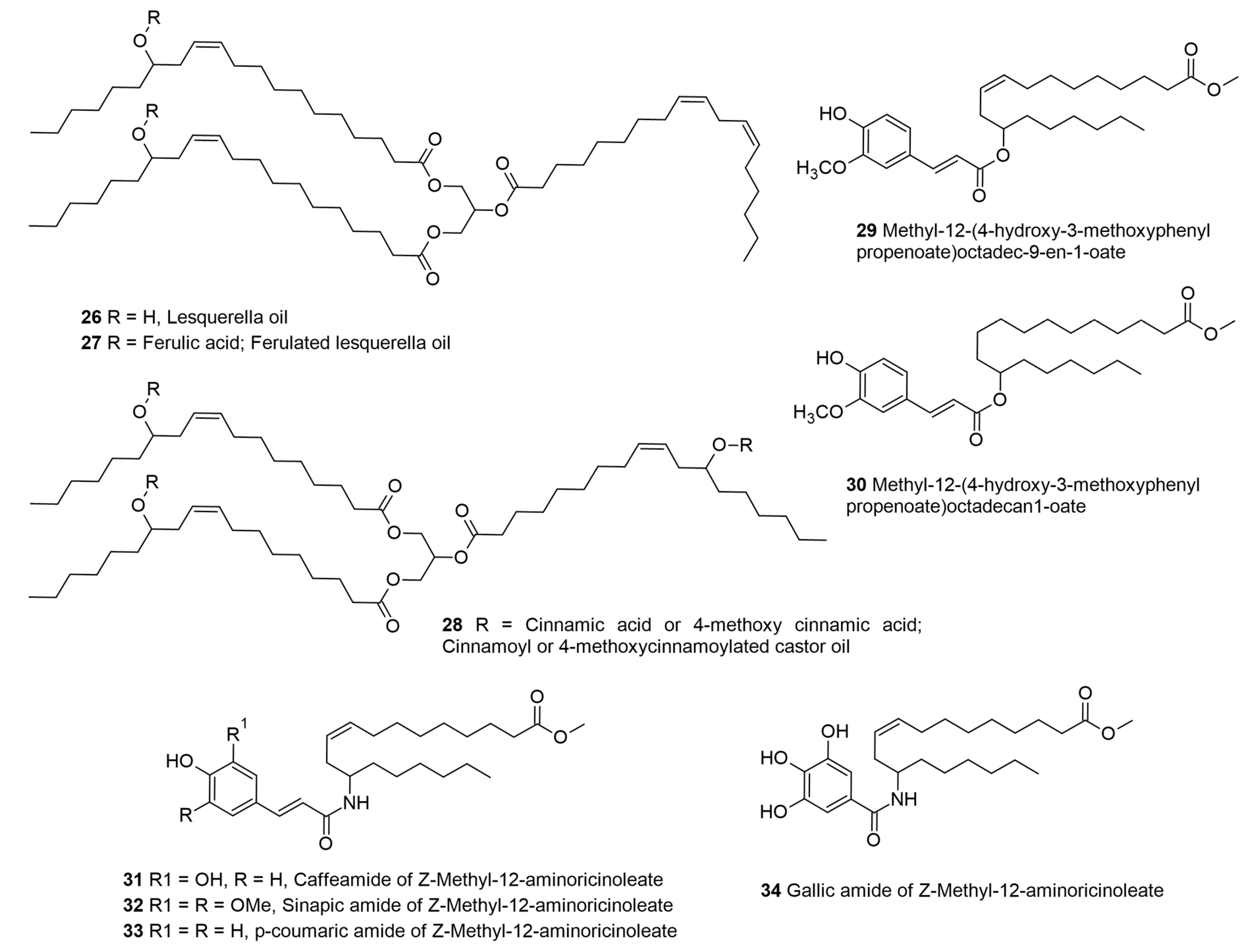
2.3. Lesquerella and Castor-Oil-Based Phenolipids for Cosmetic and Other Applications
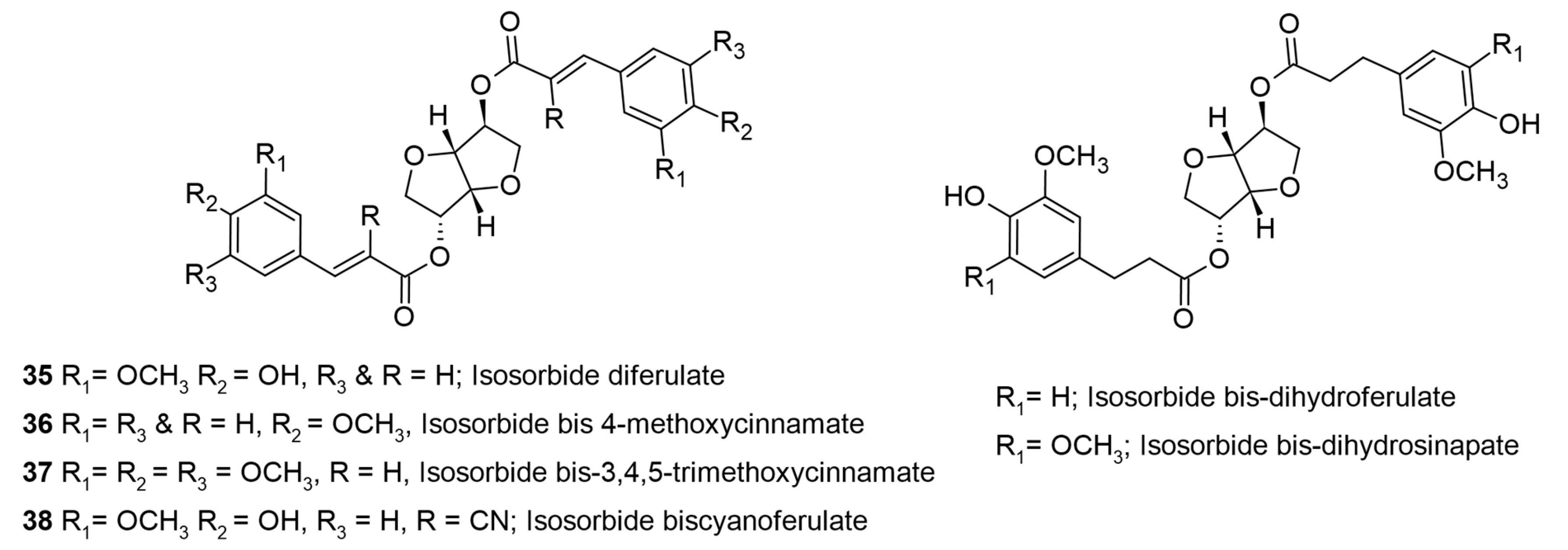
3. HCA-Polysaccharide-Based Polyols for Anti-UV and Cosmetic Applications
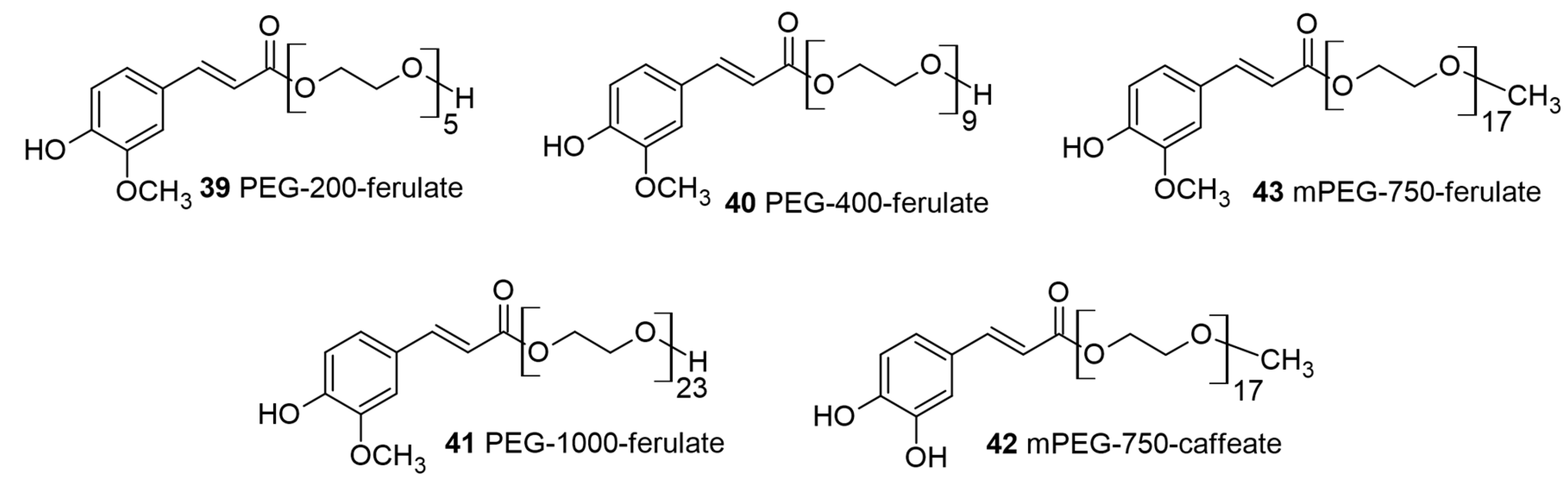
4. Polyethylene-Glycol-Based HCA Conjugates in Food, Blood–Brain Barrier (BBB) Transport, and Skin Applications
5. Encapsulation Approaches and Modification of Biopolymeric Solid Supports for Hydroxycinnamate-Based Bioconjugates
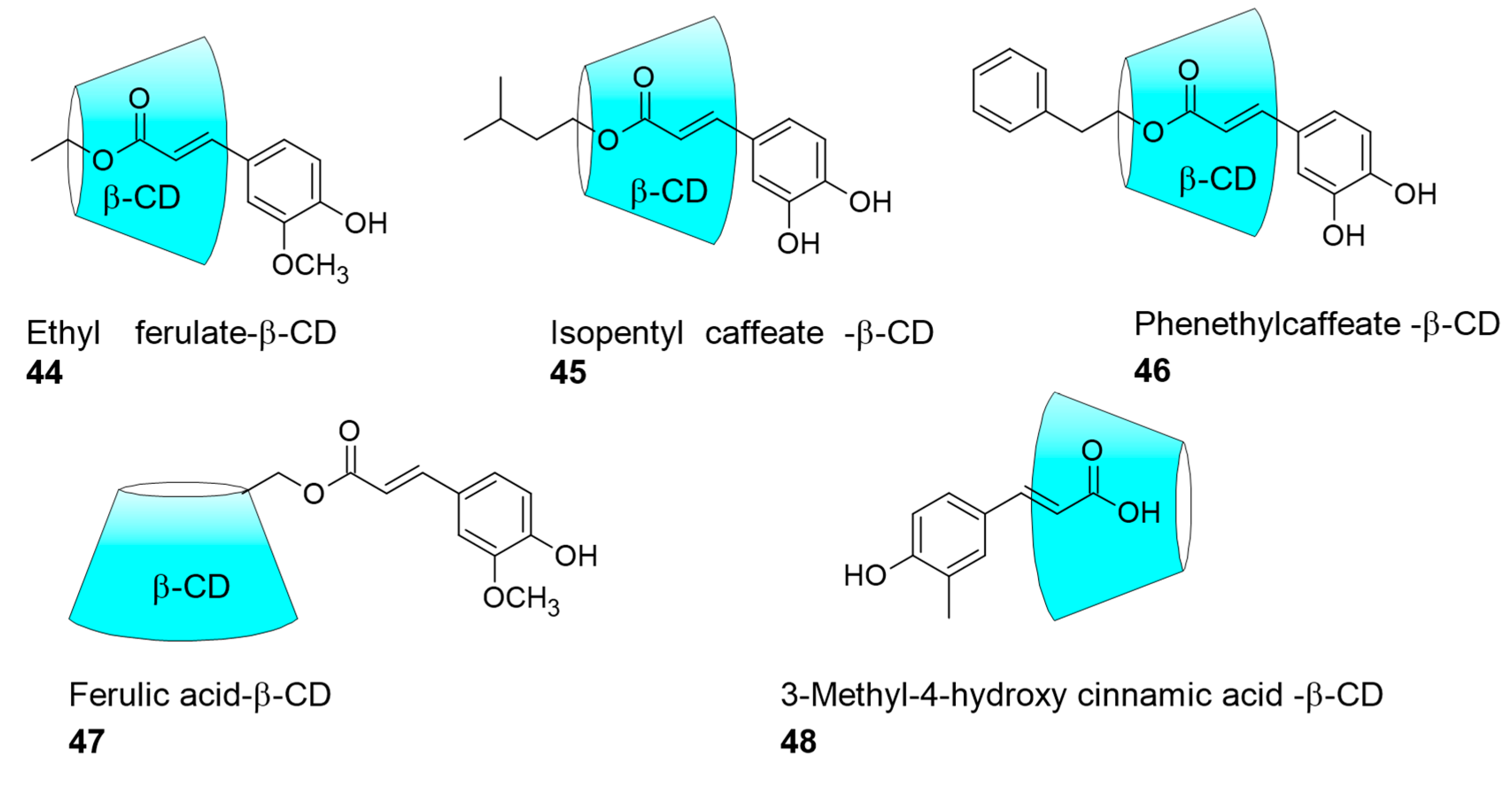
5.1. Hydroxycinnamate–Cyclodextrin-Inclusion Complex for Biological Applications
5.2. Nutraceutical Applications of HCA Polysaccharide Bioconjugates
5.3. Cinnamate-Grafted Cellulose Nanocrystals (CNC) for UV Applications
6. Conclusions and Perspectives
Authorship Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Dewick, P.M. The Shikimate Pathway: Aromatic Amino Acids and Phenylpropanoids. In Medicinal Natural Products; Wiley: Hoboken, NJ, USA, 2009; p. 131. [Google Scholar]
- Van Schijndel, J.; Canalle, L.A.; Molendijk, D.; Meuldijk, J. The green Knoevenagel condensation: Solvent-free condensation of benzaldehydes. Green Chem. Lett. Rev. 2017, 10, 404–411. [Google Scholar] [CrossRef]
- Flourat, A.L.; Combes, J.; Bailly-Maitre-Grand, C.; Magnien, K.; Haudrechy, A.; Renault, J.-H.; Allais, F. Accessing p-Hydroxycinnamic Acids: Chemical Synthesis, Biomass Recovery, or Engineered Microbial Production? ChemSusChem 2021, 14, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, C.; Peru, A.A.M.; Mouterde, L.M.M.; Allais, F. Proline-Mediated Knoevenagel–Doebner Condensation in Ethanol: A Sustainable Access to p-Hydroxycinnamic Acids. ACS Sustain. Chem. Eng. 2019, 7, 9422–9427. [Google Scholar] [CrossRef]
- Domergue, F.; Kosma, D.K. Occurrence and Biosynthesis of Alkyl Hydroxycinnamates in Plant Lipid Barriers. Plants 2017, 6, 25. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Edraki, N.; Kamat, S.P.; Khoshneviszadeh, M.; Kayani, Z.; Mirzaei, H.H.; Miri, R.; Erfani, N.; Nejati, M.; Cavaleiro, J.A.S.; et al. Long Chain Alkyl Esters of Hydroxycinnamic Acids as Promising Anticancer Agents: Selective Induction of Apoptosis in Cancer Cells. J. Agric. Food Chem. 2017, 65, 7228–7239. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Bourlieu, C.; Durand, E.; Lecomte, J.; Villeneuve, P. Lipophilized Antioxidants. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 193–201. [Google Scholar]
- Michels, B.; Zwaka, H.; Bartels, R.; Lushchak, O.; Franke, K.; Endres, T.; Fendt, M.; Song, I.; Bakr, M.; Budragchaa, T.; et al. Memory enhancement by ferulic acid ester across species. Sci. Adv. 2018, 4, eaat6994. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Campos, V.R. Bench to any side-Pharmacology and applications of Natural and Synthetic Alkylated Hydroxy Cinnamates and Cinnamides. SSRN 2024. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Kamat, S.P.; Cavaleiro, J.A.S.; Gaspar, A.; Garrido, J.; Borges, F. Synthesis and antioxidant activity of long chain alkyl hydroxycinnamates. Eur. J. Med. Chem. 2011, 46, 773–777. [Google Scholar] [CrossRef]
- Abiola, T.T.; Auckloo, N.; Woolley, J.M.; Corre, C.; Poigny, S.; Stavros, V.G. Unravelling the Photoprotection Properties of Garden Cress Sprout Extract. Molecules 2021, 26, 7631. [Google Scholar] [CrossRef]
- Nguyen, V.P.T.; Stewart, J.D.; Ioannou, I.; Allais, F. Sinapic Acid and Sinapate Esters in Brassica: Innate Accumulation, Biosynthesis, Accessibility via Chemical Synthesis or Recovery From Biomass, and Biological Activities. Front. Chem. 2021, 9, 664602. [Google Scholar] [CrossRef]
- Rioux, B.; Mouterde, L.M.M.; Alarcan, J.; Abiola, T.T.; Vink, M.J.A.; Woolley, J.M.; Peru, A.A.M.; Mention, M.M.; Brunissen, F.; Berden, G.; et al. An expeditive and green chemo-enzymatic route to diester sinapoyl-l-malate analogues: Sustainable bioinspired and biosourced UV filters and molecular heaters. Chem. Sci. 2023, 14, 13962–13978. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, C.; Mention, M.M.; Fournier, R.; Brunissen, F.; Couvreur, J.; Balaguer, P.; Allais, F. Expeditious and sustainable two-step synthesis of sinapoyl-l-malate and analogues: Towards non-endocrine disruptive bio-based and water-soluble bioactive compounds. Green Chem. 2020, 22, 6510–6518. [Google Scholar] [CrossRef]
- Mention, M.M.; Flourat, A.L.; Peyrot, C.; Allais, F. Biomimetic regioselective and high-yielding Cu(i)-catalyzed dimerization of sinapate esters in green solvent Cyrene™: Towards sustainable antioxidant and anti-UV ingredients. Green Chem. 2020, 22, 2077–2085. [Google Scholar] [CrossRef]
- Michels, B.; Franke, K.; Weiglein, A.; Sultani, H.; Gerber, B.; Wessjohann, L.A. Rewarding compounds identified from the medicinal plant Rhodiola rosea. J. Exp. Biol. 2020, 223, jeb223982. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhou, J.; Wang, X.; Xie, X.; Zhao, Y.; Ni, F.; Huang, W.; Wang, Z.; Xiao, W. A new ferulic acid ester from Rhodiola wallichiana var. cholaensis (Crassulaceae). Nat. Prod. Res. 2018, 32, 77–84. [Google Scholar] [CrossRef]
- Cong, Y.; Guo, J.-G.; Liu, J. Two New Chemical Constituents of Veratrum dahuricum (Turcz.) Loes. f. Helv. Chim. Acta 2013, 96, 345–349. [Google Scholar] [CrossRef]
- Woo, M.H.; Nguyen, D.H.; Zhao, B.T.; Seo, U.M.; Nguyen, T.T.; Jun, D.Y.; Kim, Y.H. Echinochlorins A-C from the grains of Echinochloa utilis (Barnyard Millet) and their anti-inflammatory activity. Planta Medica 2015, 81, PX66. [Google Scholar] [CrossRef]
- Ma, M.; Hussain, M.; Memon, H.; Zhou, W. Structure of pigment compositions and radical scavenging activity of naturally green-colored cotton fiber. Cellulose 2016, 23, 955–963. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Phang, S.J.; Kamaruzaman, N.; Salleh, A.; Zawani, M.; Sanyal, A.; Maarof, M.; Fauzi, M.B. Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review. Antioxidants 2023, 12, 787. [Google Scholar] [CrossRef]
- Sonar, V.P.; Fois, B.; Distinto, S.; Maccioni, E.; Meleddu, R.; Cottiglia, F.; Acquas, E.; Kasture, S.; Floris, C.; Colombo, D.; et al. Ferulic Acid Esters and Withanolides: In Search of Withania somnifera GABAA Receptor Modulators. J. Nat. Prod. 2019, 82, 1250–1257. [Google Scholar] [CrossRef]
- Maccioni, R.; Cottiglia, F.; Maccioni, E.; Talani, G.; Sanna, E.; Bassareo, V.; Kasture, S.B.; Acquas, E. The biologically active compound of Withania somnifera (L.) Dunal, docosanyl ferulate, is endowed with potent anxiolytic properties but devoid of typical benzodiazepine-like side effects. J. Psychopharmacol. 2021, 35, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Johny, J.; Kontham, V.; Veeragoni, D.; Misra, S.; Kaki, S.S. Bioorganic synthesis, characterization and evaluation of a natural phenolic lipid. Biotechnol. Rep. 2019, 24, e00375. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, I.; Faria, R.P.V.; Rodrigues, A.E. Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustain. Chem. 2021, 2, 286–324. [Google Scholar] [CrossRef]
- Peyrot, C.; Mention, M.M.; Brunissen, F.; Allais, F. Sinapic Acid Esters: Octinoxate Substitutes Combining Suitable UV Protection and Antioxidant Activity. Antioxidants 2020, 9, 782. [Google Scholar] [CrossRef]
- Gandhi, B.; Juliya, J.; Dileep, V.; Uma Rajeswari, B.; Misra, S.; Kaki, S.S. Antioxidant and Biological Activities of Novel Structured Monoacylglycerol Derivatives with Phenolic Acids. Eur. J. Lipid Sci. Technol. 2021, 123, 2100055. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef]
- Monhaphol, T.; Albinsson, B.; Wanichwecharungruang, S.P. 2-Ethylhexyl-2,4,5-trimethoxycinnamate and di-(2-ethylhexyl)-2,4,5-trimethoxybenzalmalonate as novel UVA filters†. J. Pharm. Pharmacol. 2010, 59, 279–288. [Google Scholar] [CrossRef]
- Zambrano, D.; Millán, D.; Guevara-Pulido, J. In silico design, synthesis and evaluation of a less toxic octinoxate alternative with suitable photoprotection properties. Eur. J. Pharm. Sci. 2023, 180, 106332. [Google Scholar] [CrossRef]
- Taniguchi, H.; Nomura, E.; Tsuno, T.; Minami, S. Ferulic Acid Ester Antioxidant/UV Absorbent. European Patent EP0681825A2, 15 November 1995. [Google Scholar]
- Zhou, Y.; Lips, A.; Nanavaty, F.S.; Bartolone, J.B. Stabilization of Ferulic Acid in Cosmetic. Compositions. Patent WO 01/07004 A1, 1 February 2001. [Google Scholar]
- Kaeswurm, J.A.H.; Scharinger, A.; Teipel, J.; Buchweitz, M. Absorption Coefficients of Phenolic Structures in Different Solvents Routinely Used for Experiments. Molecules 2021, 26, 4656. [Google Scholar] [CrossRef]
- Ferulan Active. GfN & Selco. Available online: https://www.gfn-selco.de/en/Productsearch/ferulan-active (accessed on 19 August 2022).
- Matsuo, T.; Kobayashi, T.; Kimura, Y.; Tsuchiyama, M.; Oh, T.; Sakamoto, T.; Adachi, S. Synthesis of glyceryl ferulate by immobilized ferulic acid esterase. Biotechnol. Lett. 2008, 30, 2151–2156. [Google Scholar] [CrossRef]
- Kikugawa, M.; Tsutsuki, H.; Ida, T.; Nakajima, H.; Ihara, H.; Sakamoto, T. Water-soluble ferulic acid derivatives improve amyloid-β-induced neuronal cell death and dysmnesia through inhibition of amyloid-β aggregation. Biosci. Biotechnol. Biochem. 2016, 80, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiyama, M.; Sakamoto, T.; Tanimori, S.; Murata, S.; Kawasaki, H. Enzymatic Synthesis of Hydroxycinnamic Acid Glycerol Esters Using Type A Feruloyl Esterase from Aspergillus niger. Biosci. Biotechnol. Biochem. 2007, 71, 2606–2609. [Google Scholar] [CrossRef] [PubMed]
- Compton, D.L.; Laszlo, J.A.; Evans, K.O. Antioxidant properties of feruloyl glycerol derivatives. Ind. Crops Prod. 2012, 36, 217–221. [Google Scholar] [CrossRef]
- Compton, D.L.; Appell, M.; Kenar, J.A.; Evans, K.O. Enzymatic Synthesis and Flash Chromatography Separation of 1,3-Diferuloyl-sn-Glycerol and 1-Feruloyl-sn-Glycerol. Methods Protoc. 2020, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.O.; Compton, D.L.; Laszlo, J.A.; Appell, M. Feruloyl glycerol and 1,3-diferuloyl glycerol antioxidant behavior in phospholipid vesicles. Chem. Phys. Lipids 2016, 195, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Sun, S.; Xu, R. Enhancement of the hydrophilic feruloyl glycerol synthesis using A-35 as a catalyst and its functional characteristics. Food Funct. 2021, 12, 9763–9772. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Xu, Y.; Wu, J.-X.; Zhu, C.-T.; Zhang, D.-Y.; Wu, G.-H.; Wu, F.-A.; Wang, J. Enzymatic Synthesis and Antioxidant Activity of 1-Caffeoylglycerol Prepared from Alkyl Caffeates and Glycerol. J. Am. Oil Chem. Soc. 2018, 95, 149–159. [Google Scholar] [CrossRef]
- Eudes, A.; Mouille, M.; Robinson, D.S.; Benites, V.T.; Wang, G.; Roux, L.; Tsai, Y.-L.; Baidoo, E.E.K.; Chiu, T.-Y.; Heazlewood, J.L.; et al. Exploiting members of the BAHD acyltransferase family to synthesize multiple hydroxycinnamate and benzoate conjugates in yeast. Microb. Cell Factories 2016, 15, 198. [Google Scholar] [CrossRef]
- Guo, X.; Rong, Z.; Ying, X. Calculation of hydrophile–lipophile balance for polyethoxylated surfactants by group contribution method. J. Colloid Interface Sci. 2006, 298, 441–450. [Google Scholar] [CrossRef]
- De Freitas, Z.M.F.; dos Santos, E.P.; da Rocha, J.F.; Dellamora-Ortiz, G.M.; Gonçalves, J.C.S. A new sunscreen of the cinnamate class: Synthesis and enzymatic hydrolysis evaluation of glyceryl esters of p-methoxycinnamic acid. Eur. J. Pharm. Sci. 2005, 25, 67–72. [Google Scholar] [CrossRef]
- Compton, D.L.; Laszlo, J.A.; Berhow, M.A. Lipase-catalyzed synthesis of ferulate esters. J. Am. Oil Chem. Soc. 2000, 77, 513–519. [Google Scholar] [CrossRef]
- Laszlo, J.A.; Smith, L.J.; Evans, K.O.; Compton, D.L. Phenol Esterase Activity of Porcine Skin. Eur. J. Pharm. Biopharm. 2015, 89, 175–181. [Google Scholar] [CrossRef]
- Totani, N.; Tateishi, S.; Takimoto, T.; Shinohara, R.; Sasaki, H. Ferulic Acid Esters and Weight-Loss Promoting Effects in Rats. J. Oleo Sci. 2012, 61, 331–336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laszlo, J.A.; Evans, K.O.; Vermillion, K.E.; Appell, M. Feruloyl Dioleoylglycerol Antioxidant Capacity in Phospholipid Vesicles. J. Agric. Food Chem. 2010, 58, 5842–5850. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, P.; Bourlieu-Lacanal, C.; Durand, E.; Lecomte, J.; McClements, D.J.; Decker, E.A. Lipid oxidation in emulsions and bulk oils: A review of the importance of micelles. Crit. Rev. Food Sci. Nutr. 2023, 63, 4687–4727. [Google Scholar] [CrossRef] [PubMed]
- Hollande, L.; Domenek, S.; Allais, F. Chemo-Enzymatic Synthesis of Renewable Sterically-Hindered Phenolic Antioxidants with Tunable Polarity from Lignocellulose and Vegetal Oil Components. Int. J. Mol. Sci. 2018, 19, 3358. [Google Scholar] [CrossRef]
- Compton, D.L.; Laszlo, A.; Isbell, T.A. Cinnamoyl esters of lesquerella and castor oil: Novel sunscreen active ingredients. J. Am. Oil Chem. Soc. 2004, 81, 945–951. [Google Scholar] [CrossRef]
- Compton, D.L.; Laszlo, J.A.; Evans, K.O. Phenylpropanoid esters of lesquerella and castor oil. Ind. Crops Prod. 2015, 63, 9–16. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, S.; Bi, Y. Solvent-free enzymatic synthesis of feruloylated structured lipids by the transesterification of ethyl ferulate with castor oil. Food Chem. 2014, 158, 292–295. [Google Scholar] [CrossRef]
- Sun, S.; Chen, X.; Jiang, C. Enhanced synthesis of feruloylated acylglycerols by the lipase-catalyzed transesterification of glyceryl monoferulate with different acyl donors using ionic liquids as reaction solvents. J. Biotechnol. 2018, 280, 31–37. [Google Scholar] [CrossRef]
- Sun, S.; Wang, P.; Zhu, S. Enzymatic incorporation of caffeoyl into castor oil to prepare the novel castor oil-based caffeoyl structured lipids. J. Biotechnol. 2017, 249, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.K.; Ravinder, T.; Kanjilal, S. Synthesis and evaluation of antioxidant and antifungal activities of novel ricinoleate-based lipoconjugates of phenolic acids. Food Chem. 2012, 134, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Yarra, M.; Kaki, S.S.; Prasad, R.B.N.; Mallampalli, K.S.L.; Yedla, P.; Chityala, G.K. Synthesis of novel (Z)-methyl-12-aminooctadec-9-enoate-based phenolipids as potential antioxidants and chemotherapeutic agents. Eur. J. Lipid Sci. Technol. 2016, 118, 622–630. [Google Scholar] [CrossRef]
- Bonnin, I.; Mereau, R.; Tassaing, T.; De Oliveira Vigier, K. One-pot synthesis of isosorbide from cellulose or lignocellulosic biomass: A challenge? Beilstein J. Org. Chem. 2020, 16, 1713–1721. [Google Scholar] [CrossRef]
- Saxon, D.J.; Luke, A.M.; Sajjad, H.; Tolman, W.B.; Reineke, T.M. Next-generation polymers: Isosorbide as a renewable alternative. Prog. Polym. Sci. 2020, 101, 101196. [Google Scholar] [CrossRef]
- East, A.; Zhang, Y.; Jaffe, M. Ultraviolet Absorber for Cosmetics and Polymeric. Materials. Patent WO 2007/098022 A2, 30 August 2007. [Google Scholar]
- Feng, X. Synthesis of Corn-Derived Carbohydrate Derivatives as Effective Multifunctional Sunscreen. Master’s Thesis, New Jersey Institute of Technology, Newark, NJ, USA, 2008. [Google Scholar]
- Reano, A.F.; Chérubin, J.; Peru, A.M.M.; Wang, Q.; Clément, T.; Domenek, S.; Allais, F. Structure–Activity Relationships and Structural Design Optimization of a Series of p-Hydroxycinnamic Acids-Based Bis- and Trisphenols as Novel Sustainable Antiradical/Antioxidant Additives. ACS Sustain. Chem. Eng. 2015, 3, 3486–3496. [Google Scholar] [CrossRef]
- Nicks, F.; Richel, A.; Dubrowski, T.; Wathelet, B.; Wathelet, J.-P.; Blecker, C.; Paquot, M. Effect of new synthetic PEGylated ferulic acids in comparison with ferulic acid and commercial surfactants on the properties of wheat flour dough and bread. J. Sci. Food Agric. 2013, 93, 2415–2420. [Google Scholar] [CrossRef]
- Fernandes, C.; Pinto, M.; Martins, C.; Gomes, M.J.; Sarmento, B.; Oliveira, P.J.; Remião, F.; Borges, F. Development of a PEGylated-Based Platform for Efficient Delivery of Dietary Antioxidants Across the Blood–Brain Barrier. Bioconjugate Chem. 2018, 29, 1677–1689. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef]
- Duceac, I.A.; Coseri, S. Biopolymers and their derivatives: Key components of advanced biomedical technologies. Biotechnol. Adv. 2022, 61, 108056. [Google Scholar] [CrossRef]
- EMA. Background Review for Cyclodextrins Used as Excipients; EMA/CHMP/333892/2013; EMA: London, UK, 2014. [Google Scholar]
- Cunha, F.V.M.; do Nascimento Caldas Trindade, G.; da Silva Azevedo, P.S.; Coêlho, A.G.; Braz, E.M.; Pereira de Sousa Neto, B.; de Rezende, D.C.; de Sousa, D.P.; de Assis Oliveira, F.; Nunes, L.C.C. Ethyl ferulate/β-cyclodextrin inclusion complex inhibits edema formation. Mater. Sci. Eng. C 2020, 115, 111057. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Barreto, N.S.; Oliveira, S.S.D.; Santos, A.L.; Branquinha, M.H.; Sousa, D.P.D.; Castro, M.; Andrade, L.N.; Pereira, M.M.; Silva, C.F.D.; et al. β-Cyclodextrin/Isopentyl Caffeate Inclusion Complex: Synthesis, Characterization and Antileishmanial Activity. Molecules 2020, 25, 4181. [Google Scholar] [CrossRef] [PubMed]
- Garrido, E.M.P.J.; Cerqueira, A.S.; Chavarria, D.; Silva, T.; Borges, F.; Garrido, J.M.P.J. Microencapsulation of caffeic acid phenethyl ester and caffeic acid phenethyl amide by inclusion in hydroxypropyl-β-cyclodextrin. Food Chem. 2018, 254, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, Z.; Shen, H.; Zheng, J.; Zhang, G. Comparison of structures, physicochemical properties and in vitro bioactivity between ferulic acid-β-cyclodextrin conjugate and the corresponding inclusion complex. Food Res. Int. 2019, 125, 108619. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Cai, Y.; Yuan, C.; Chen, S.; Ding, T.; Liu, D.; Hu, Y. Ferulic acid-β-cyclodextrin inclusion complexes: Application on the preservation of hairtail (Trichiurus lepturus). Int. J. Food Prop. 2020, 23, 282–296. [Google Scholar] [CrossRef]
- Ikeda, N.; Inoue, Y.; Ogata, Y.; Murata, I.; Meiyan, X.; Takayama, J.; Sakamoto, T.; Okazaki, M.; Kanamoto, I. Improvement of the Solubility and Evaluation of the Physical Properties of an Inclusion Complex Formed by a New Ferulic Acid Derivative and γ-Cyclodextrin. ACS Omega 2020, 5, 12073–12080. [Google Scholar] [CrossRef]
- Kenar, J.A.; Compton, D.L.; Little, J.A.; Peterson, S.C. Formation of inclusion complexes between high amylose starch and octadecyl ferulate via steam jet cooking. Carbohydr. Polym. 2016, 140, 246–252. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Li, M.; Chen, G.; Ma, Y.; Wang, Y.; Chen, J. Construction of ferulic acid modified porous starch esters for improving the antioxidant capacity. RSC Adv. 2022, 12, 4253–4262. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Gao, X.; Chen, G.; Wang, Z. Probing the structure-antioxidant activity relationships of four cinnamic acids porous starch esters. Carbohydr. Polym. 2021, 256, 117428. [Google Scholar] [CrossRef]
- Ou, S.; Yang, A.L.A. A study on synthesis of starch ferulate and its biological properties. Food Chem. 2001, 74, 91–95. [Google Scholar] [CrossRef]
- Kaur, J.; Mehta, V.; Kaur, G. Preparation, development and characterization of Leucaena leucocephala galactomannan (LLG) conjugated sinapic acid: A potential colon targeted prodrug. Int. J. Biol. Macromol. 2021, 178, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.M.; Lee, H.; Jayabalan, R.; Suh, J.-W. Ferulic acid grafted self-assembled fructo-oligosaccharide micro particle for targeted delivery to colon. Carbohydr. Polym. 2020, 247, 116550. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhong, Y.; Chen, Q.; Wu, L.; Ji, S.; Yang, B.; Zhang, Y.; Shen, J.; Lu, B. Modulating the digestibility of cassava starch by esterification with phenolic acids. Food Hydrocoll. 2022, 127, 107432. [Google Scholar] [CrossRef]
- Mendoza, D.J.; Mouterde, L.M.M.; Browne, C.; Raghuwanshi, V.S.; Simon, G.P.; Garnier, G.; Allais, F. Grafting Nature-Inspired and Bio-Based Phenolic Esters onto Cellulose Nanocrystals Gives Biomaterials with Photostable Anti-UV Properties. ChemSusChem 2020, 13, 6552–6561. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.J.; Maliha, M.; Raghuwanshi, V.S.; Browne, C.; Mouterde, L.M.M.; Simon, G.P.; Allais, F.; Garnier, G. Diethyl sinapate-grafted cellulose nanocrystals as nature-inspired UV filters in cosmetic formulations. Mater. Today Bio 2021, 12, 100126. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.J.; Browne, C.; Raghuwanshi, V.S.; Mouterde, L.M.M.; Simon, G.P.; Allais, F.; Garnier, G. Phenolic Ester-Decorated Cellulose Nanocrystals as UV-Absorbing Nanoreinforcements in Polyvinyl Alcohol Films. ACS Sustain. Chem. Eng. 2021, 9, 6427–6437. [Google Scholar] [CrossRef]


| Hydroxycinnamic Acid | Conjugation or Encapsulation | Property | Application | Reference |
|---|---|---|---|---|
| Ferulic acid | ω-hydroxyl fatty acid | Antioxidant | Protective effects against H2O2-induced myocardial cell injury in cultured H9c2 cells | [11] |
| Caffeic acid | ω-hydroxyl fatty acid | Antioxidant | Biomedical/antioxidant based wound-healing patches | [12,13] |
| Sinapic acid; p-coumaric acid | Mono(ω-hydroxyl fatty acyl) glycerol | Antioxidant, Antimicrobial | Cosmetic ingredients | [14] |
| Ferulic acid | Glycerol | Antioxidant, UV filter | Cosmetics and neuroprotection | [15,16] |
| Sinapic acid; p-coumaric acid | Glycerol | Antioxidant; UV filter; solubility | Cosmetic ingredient | [17] |
| Caffeic acid | Glycerol | Antioxidant; UV filter | Cosmetic ingredient | [18] |
| p-methoxy cinnamic acid; Ferulic acid | Mono-acyl and Di-acyl glycerol | UV filter, emollient, antioxidant | Cosmetic ingredient | [19,20,21] |
| p-methoxy cinnamic acid; | Lesquerella and castor oils | UV filter; emollient | Cosmetic ingredient | [22,23] |
| Ferulic acid | Isosorbide | UV filter | Cosmetic ingredient | [24] |
| Ferulic acid; caffeic acid | Polyethylene glycol | Antioxidant | Food additive; Neuroprotection | [25,26] |
| Ferulic acid esters; Caffeic acid ester | β-cyclodextrin encapsulation | Solubility | anti-inflammatory activity; anti-Leishmanial | [27,28] |
| Ferulic acid ester; Ferulic acid; Sinapic acid | Amylose/porous starch encapsulation | Antioxidant | Modified starches; targeted colon delivery | [29,30,31,32,33] |
| Diethyl ferulate; diethyl sinapate | Cellulose nanofibers | Antioxidant, UV filter | Cosmetic ingredient | [34,35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes, J.C.J.M.D.S.; Campos, V.R. Unlocking the Potential of Hydroxycinnamic Acid Bioconjugates: Tailored Derivatives for Biomedical, Cosmetic, and Food Applications. Compounds 2024, 4, 604-625. https://doi.org/10.3390/compounds4040037
Menezes JCJMDS, Campos VR. Unlocking the Potential of Hydroxycinnamic Acid Bioconjugates: Tailored Derivatives for Biomedical, Cosmetic, and Food Applications. Compounds. 2024; 4(4):604-625. https://doi.org/10.3390/compounds4040037
Chicago/Turabian StyleMenezes, José C. J. M. D. S., and Vinícius R. Campos. 2024. "Unlocking the Potential of Hydroxycinnamic Acid Bioconjugates: Tailored Derivatives for Biomedical, Cosmetic, and Food Applications" Compounds 4, no. 4: 604-625. https://doi.org/10.3390/compounds4040037
APA StyleMenezes, J. C. J. M. D. S., & Campos, V. R. (2024). Unlocking the Potential of Hydroxycinnamic Acid Bioconjugates: Tailored Derivatives for Biomedical, Cosmetic, and Food Applications. Compounds, 4(4), 604-625. https://doi.org/10.3390/compounds4040037







