Abstract
In an era where the search for innovative drug leads faces challenges, our study pivots towards exploring the untapped potential of plant-derived compounds, focusing on the period of 2021 to 2022. We assess the classes of compounds these new structures belong to; the plants and families these compounds belong to; and the degree of novelty of the compound compared with already-known structures. The review was conducted following the Preferred Reporting Items for Systematics Reviews and Meta-Analyses (PRISMA) statement checklist for the guided reporting of systematic reviews. A total of 464 articles were selected for the new compounds of natural origin survey. We included 117 complete articles in this review and reported approximately 109 new structures elucidated during the years 2021 and 2022. Many of the compounds showed small structural variations in relation to already-known molecules. For some, however, this small modification was decisive for the biological activity reported, demonstrating the importance of descriptive phytochemical studies.
1. Introduction
Plants have always been used as a source of ingredients for developing drugs, cosmetics and several types of products [1]. The study of active plant components has given rise to or inspired the development of numerous medicines, present in our daily life [2]. One reason for this is the vast chemical diversity of structures derived from plant secondary metabolism, which has several functions for plant survival, such as acting as a defense mechanism against predators, attracting insects for pollination, and other factors that contribute to the plant’s resistance [1]. Since they are substances that can act on biological receptors, humans can benefit from them in the development of bioactive products of pharmaceutical interest.
The small molecules from secondary metabolism, also called “natural products” (NPs) [3], can originate from a variety of sources, including plants, animals, and microorganisms. They significantly contribute to the discovery of new molecular entities (NMEs) [4], accounting for about a quarter of drugs approved by the FDA.
Despite plants’ substantial contribution to NME discovery, several studies report a decline in their contribution to new compounds [3,4,5]. This decrease may stem from various factors, such as the high costs of research and investments, particularly in the identification and isolation of molecules and the re-isolation of known and abundant molecules, among other reasons.
The application of high-throughput screening (HTS) and metabolomics helps accelerate the discovery of new bioactive molecules from plants, though reporting NMEs remains a challenge [6,7]. Most newly reported compounds are minor variations of known ones.
Given the reduced contribution of plant-derived natural products to new chemical structure discovery, this study’s goal was to compile articles from 2021 to 2022, assessing (1) the classes of compounds these new structures belong to; (2) the plants and families these compounds are derived from; and (3) the degree of novelty of the compounds compared to known structures.
This review was conducted following the Preferred Reporting Items for Systematics Reviews and Meta-Analyses (PRISMA) statement checklist for a guided reporting of systematic reviews [8]. The search strategy for study identification involved the following steps.
Step 1—We systematically retrieved the articles from the SciFinder database using automation tools to first filter the criteria established for the review as shown in Figure 1. Keywords included “natural products”, “isolation”, and “new compounds”. The selected records were required to have been published between 1 January 2021 and 31 December 2022. We excluded case reports, letters, other reviews, books, patent articles, meeting abstracts, and non-English articles.
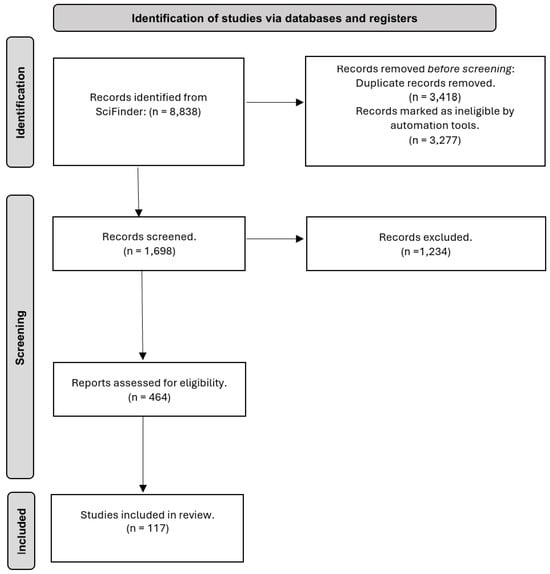
Figure 1.
Flow diagram for identifying, selecting, and including studies on new compounds.
Step 2—Data including article titles, abstracts, author names, and DOIs were downloaded in original xlm. files directly from the database. In this document, the filters used in the first step were downloaded. The tabulated titles were divided by publication year, and two different researchers conducted a preliminary screening based on the titles and abstracts.
Step 3—Articles screened in step two were fully downloaded and coded for a complete read to assess eligibility criteria.
For this review, the articles were selected using the following criteria: 1. Original articles published between the years 2021 and 2022, reporting new compounds; and 2. The new compounds must be derived from plant species, except aquatic plants.
The articles were placed in folders separated by year of publication and coded numerically for organization. In an Excel document, a systematized tabulation was made with relevant information about each article, such as the code article, the plant species classification, plant parts used for the extract, the chemical class, and the name of the new compound, to proceed with the descriptive analysis.
Frequency distributions of observations were carried out in R software 3.6.0 focusing on 1. The five botanical families with the highest number of publications between 2021 and 2022; 2. The five main classes of compounds with the highest number of isolated and elucidated compounds; and 3. The five main genera with the highest study rate, and their species.
2. Included Studies
Figure 1 represents the systematic review study selection flow chart. The 464 articles were organized according to the botanical family under study, genus and species, class of compounds elucidated, plant organ from which the compounds were extracted, and journal of publication. Out of the 464 articles, 117 studies were discussed in this review, exemplifying the novelty of the structures founded.
Although the discovery of new compounds is limited to new chemical skeletons within known classes, the frequent appearance of structural diversity within some classes can contribute to new studies focused on the biological activity of newly reported molecules.
3. Plant Families
Regarding the plant families, we found 122 different families from a total of 464 articles and identified 436 different species. The most frequently occurring family was Asteraceae with an absolute frequency of 40 and a relative frequency of 8.5% of the total of 464, followed by Lamiaceae (27 absolute frequency and 5.7% relative frequency) and Fabaceae (26 absolute frequency and 5.5% relative frequency). Other families also had significant frequencies in the study, such as Ranunculaceae (18 absolute frequency and 3.8% relative frequency), Solanaceae (16 absolute frequency and 3.4% relative frequency), Rutaceae (16 absolute frequency and 3.4% relative frequency), Euphorbiaceae, Rubiaceae, and Apocynaceae, as shown in Table 1.

Table 1.
Most abundant botanical families’ absolute and relative frequency.
The most frequent classes of compounds in the Asteraceae were terpenes with 27 reports out of the 40 articles, followed by phenols with 3 and alkaloids with only 2. In the Fabaceae family, flavonoids appeared in 13 of the 26 articles, followed by alkaloids with 5 and terpenes with 4 appearances. In the Lamiaceae family, terpenes were present in 18 of the 27 articles, followed by phenols and alkaloids with 3 and 2 appearances, respectively.
It is remarkable that the families with the highest number of species also appear as sources of new structures, such as the Asteraceae family, which has an estimated 24,700 species; Fabaceae, with 19,500 species; Rubiaceae, with 13,620, Lamiaceae, with 7530 species; Euphorbiaceae, with 6252 species; and Apocynaceae, with 5100 species [9].
One family, however, draws special attention because, despite having a considerable number of species, it does not appear in our list—the family Orchidaceae. With an estimated 28,000 species, this monocot family is considered the most evolved within the Asparagales order by some authors. This is also the case for the Cyperaceae (5500 species), Myrtaceae (5950 species), Melastomataceae (5115 species), and Poaceae (12,000) families. Although this assessment is just a snapshot, it is worth considering whether these families have already been exhausted by studies, whether they hardly provide any new structures, or whether more studies are needed in view of the potential they offer.
4. Plant Genera
The top five botanical genera with the highest number of published articles between 2021 and 2022 were Hypericum, Piper, Garcinia, Artemisia, and Thalictrum genera (Table 2). One hundred and fifty-three new compounds were isolated, the majority belonging to the terpene class. We report the main botanical species with new molecules elucidated from each genus, and the results found in biological studies using the new compounds.

Table 2.
The five main botanical genera with the highest number of publications between the years 2021 and 2022.
Piper
A member of the Piperaceae family, the genus Piper is the most important of the family, with approximately 2000 species distributed throughout the temperate regions of both the southern and northern hemispheres. The phytochemical investigation of the genus species has led to numerous important scientific studies on the isolation of new bioactive compounds, placing the genus in the spotlight of research to this day.
Polyketides (Kavalactones), alkaloids (aristolactams), polyphenols (lignoids, chromones), simple phenols (phenylpropanoids, prenylated benzoic acids), and terpenes (monoterpenes, sesquiterpenes) are the main secondary compounds produced by plants of the genus Piper, which give it economic and medicinal importance. The NPs have antifungal, insecticidal, bactericidal, antitumor, trypanocidal, antiparasitic, antimicrobial, antiprotozoal, antinociceptive, and antioxidant activity, already proven in biological studies [10,11,12,13,14].
Between 2021 and 2022, a total of 42 new compounds were isolated from five different species of the genus: Piper betle L. [15,16,17], Piper longum L. [18], Piper puberulum Seem. [19,20], and Piper wallichii (Miq.) Hand.-Mazz [21].
Seventeen new compounds were isolated from the leaves of the species Piper betle L. The new structures were described in three different studies, where two new phenolic skeletons, one sesquionelignan and fourteen neolignans, were elucidated.
The new phenolic compounds showed antioxidant potential and significant cytotoxic activity against human oral cancer cell lines [15]. The sesquioneolignan and four neolignans elucidated in a study published in 2021 demonstrated anti-inflammatory activity against nitric oxide (NO) production in murine macrophages activated by lipopolysaccharide [16]. Another 10 new neolignans were isolated through a synergistic antibacterial screen conducted to elucidate the structures and absolute configurations of the metabolites, based on spectroscopic data, single crystal X-rays, diffraction analysis, and experimental ECD. The study revealed potent synergistic activity on the part of the compounds in antibacterial assays against the antibiotic-resistant strain Staphylococcus aureus [17].
In the fruit extracts of the species Piper logum L., three new amide alkaloids, a piperic ester, and two new natural compounds were isolated and evaluated for their biological and cytotoxic activity. Molecular docking simulations were carried out to identify the interaction and binding mechanisms of these active metabolites with proteins related to inflammation and cancer [18].
In Piper puberulum Seem., three new tyramine-type alkamides, three new natural products and five new N-acylated/formylated aporphine alkamides with different proportions of rotational isomers have shown potential inhibitory effects against lipopolysaccharide-induced NO release in microglial cells [19,20].
The last two new compounds found in studies of the Piper genus through the review were extracted from the stems and leaves of Piper wallichiiv (Miq.) Hand. -Mazz. A new dioxoaporfin alkaloid skeleton showed inhibitory activity against the pathogenic bacteria Bacillus cereus, B. subtilis, and Staphylococcus aureus. In addition to this compound, an aryl alkaloid has also been reported [21].
Hypericum
Hypericum is the genus with the second-highest number of newly isolated compounds reviewed in this study. This is the largest genus in the Hypericaceae family, comprising around 500 plant species worldwide. Hypericum species are found in a wide variety of habitats; in the tropics, they are usually confined to high elevations, and their greatest profusion is found in temperate and subtropical regions [22,23]. Among the compounds of greatest pharmacological importance produced by the genus species, the polycyclic polyprenylated acylphloroglucinols and naphthodianthrons (PAPPs) have received a great deal of attention for their promising antidepressant activity, for the treatment of mild to moderate depression [24].
Hypericum perforatum L. is the most chemically and pharmacologically relevant Hypericum species, due to its wide variety of different secondary metabolites. This species has at least ten classes of biologically active compounds and is widely used to treat diseases in folk medicine, with confirmed nephro-protective, antioxidant, antifungal, anxiolytic, antiviral, and healing effects. The study compiled by this review describes the absolute configuration of new terpenoid-based bicyclic dihydropyran enantiomers, isolated from the aerial parts of Hypericum perforatum L. The study observed that the new compound promoted glucose consumption and exhibited a moderate promotion of glucose uptake activity in hepatocytes, suggesting a potential hypoglycemic activity effect [25].
Thirteen new molecular skeletons were elucidated from the Hypericum forrestii (Chitt.) N. Robson. Three polyprenylated acylphloroglucinol meroterpenoids were isolated from the extract of the aerial parts of the plant, with potent inhibitory effects on protein tyrosine phosphatase [26], and ten polyprenylated polycyclic acylphloroglucinols were isolated from the fruit with potential effects against nonalcoholic steatohepatitis [27].
Four new prenylated phloroglucinol isolated from the species Hypericum erectum Thunb. have the potential to increase the effects of various anticancer drugs [28].
The study carried out using the extract of Hypericum longistylum Oliv. isolated a new Lupane-type triterpenoid with immunosuppressive activity [29], and in species of Hypericum japonicum Thunb., three new phloroglucinol showed important biological activity with potential ferroptosis activity [30].
Artemisia
This genus comprises approximately 200 species of economic, medicinal, and food importance. Among the classes of compounds known to be produced by species of this genus are terpenes, phenylpropanoids, flavonoids, triterpenes, and sesquiterpene lactones.
Artemisia rupestris L. was one of the species detected in this review. New thiophene derivatives and a new sesquiterpene were isolated and showed inhibitory activity against neuraminidase enzymes involved in the release of newly synthesized virions by cells infected by the influenza virus [31].
The Artemisia scoparia Waldst. & Kit. is an herb used in natural medicine to treat jaundice in neonates and ear problems. In the survey of studies on new compounds, two new isomers of diprenylated coumaric acid were isolated, and biological tests showed a beneficial ability to modulate adipogenesis in studies with cells [32].
Two new compounds named Integrin A and Integrin B were isolated from the supercritical fluid extract of the aerial parts of the species A. integrifolia L. [33]. The species is characterized by the presence of phenylpropanoids, acetylenes, terpenoids, and fatty acids and is an herb of traditional use with a reported antihyperlipidemic effect [34].
Artemisia atrovirens Hand.-Mazz. is a perennial herb distributed mainly in central and western China and Thailand. Studies focusing on the investigation of new sesquiterpenoids have elucidated a total of 24 compounds, sixteen new guaiane-type sesquiterpenoids [35] and eight new sesquiterpenoid dimers [36]. The evaluation of the biological activity of the guaiane-type compounds showed cytotoxic activity against two hepatoma cell lines [35]. The study with sesquiterpenoid dimers evaluated the anti-inflammatory effect of the new compounds on microglial cells [36].
Searching for sesquiterpenoid compounds, 14 new eudesmane sesquiterpenoids were isolated from the whole plant Artemisia hedinii. Eudesmane sesquiterpenoids have already been elucidated in numerous medicinal plants, especially species from the Asteraceae family. Due to their important biological antibacterial, anti-inflammatory, cytotoxic, and immunostimulant activities, eudesmanes are an important class mainly reported in the genus Artemisia. The species A. hedinii is endemic to the Qinghai and Gnasu Provinces of mainland China. Among its known therapeutic effects, the herb is used to treat inflammation, olecystitis, jaundice, dysentery, chronic gastritis, diabetes, ovarian cancer, positional vertigo, herpes zoster, and exudative erythema multiforme. The study published in 2021 demonstrated the consistent anti-inflammatory effects of the isolated and elucidated metabolites [37].
Garcinia
The genus Garcinia is the most predominant of the family Clusiaceae, comprising 450 species. Several Garcinia species are widely used as traditional or folk medicines, and their medicinal applications are supported by the presence of chemical constituents, mainly varieties of xanthones, flavonoids, and benzophenones. These natural compounds exhibited biological activity effects, including antimicrobial, antifungal, antioxidant, and antimalarial [38]. Most studies reporting new molecular species discovered new compounds derived from the xanthones class and polyprenylated structures.
A total of 23 new compounds were isolated and reported from 5 different species of Garcinia sp. From the leaves of Garcinia xipshuanbannaensis, four new prenylated xanthone analogues were elucidated and evaluated for their cytotoxicity toward human cancer cells [38]. In Garcinia oligantha, four new caged-polyprenylated xanthonoids, a rare class of natural products, and two new simple xanthones were isolated [39]. One new xanthone was isolated from Garcinia nobilis Engl. leaf extract [40]. From the flower and twig extracts of Garcinia mckeaniana, a new biphenyl and one new simple xanthone were reported [41], and in studies using the fruit extract of Garcinia cambogia, ten new polyisoprenylated benzophenone derivatives were isolated and tested for their biological activity [42].
The special interest in natural xanthones produced by the Garcinia genus is due to the bioactive biological variety of the class. Most parts of the studies seen in this review reported the excellent cytotoxic potential and antiproliferative activity in tumor cells of the new molecules.
Thalictrum
A member of the Ranunculaceae family, the genus Thalictrum comprises about 200 species. Approximately 67 species are recorded in the flora of China, and many of them have been used as traditional medicine for the treatment of many diseases, including influenza, gastroenteritis, cancer, dysentery, measles, and conjunctivitis. The most common class produced by the plants of the Thalictrum genus is alkaloids [43].
Alkaloids represent an important broad spectrum of biological activities such as anticancer, antiparasites, antiplatelet aggregation, and antivirus activity. Phytochemical investigations of the Thalictrum genus have focused on elucidating their structures and preliminarily evaluating their bioactivity potential.
In the new compounds review, four studies reported new molecular structures derived from the alkaloids class, totaling nine new molecules, including two new chromeno[3,2-c] pyridine alkaloids from Thalictrum scabrifolium, three new anti-rotavirus quinoline alkaloids from the whole plant of Thalictrum glandulossissimun, two new chromeno[3,2-c] pyridine derivates from Thalictrum finetii, and two new anti-tobacco mosaic virus alkaloids from Thalictrum microgynum [43,44,45,46,47].
5. Compounds Classes
The compound classes with the highest frequency and variety in different botanical species identified in this study were terpenes, alkaloids, phenols, and flavonoids (Figure 2).
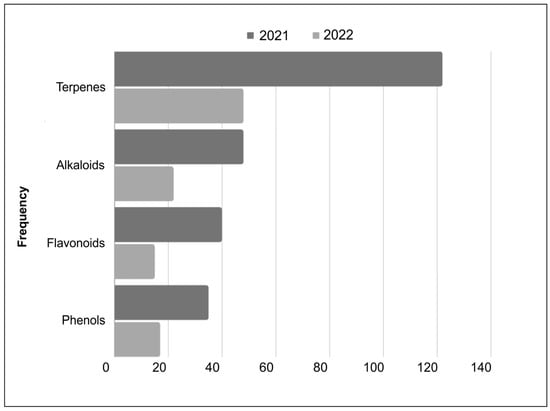
Figure 2.
Distribution of compound classes, with higher number of new structures elucidated between 2021 and 2022.
Terpenes dominated the ranking of new structures, with 192 new compounds elucidated from 184 different botanical species in 188 studies between 2021 and 2022. In second place are alkaloids, with approximately 70 new molecular skeletons described in 69 different botanical species. The flavonoids class had 55 new molecules elucidated, in a variety of 53 botanical species. New phenolic structures were elucidated from 54 different botanical species, with 52 new molecules reported between 2021 and 2022.
We also included the classes of coumarins and anthraquinones in our compounds class section, which even with a relatively smaller number of new compounds elucidated, are among the most important studies on new natural structures.
5.1. Terpenes
Terpenes are composed of simple hydrocarbons formed by isoprenic units from the mevalonate pathway. The classification is established by the number of isoprenic units present in the molecule, with hemiterpenes characterized by the presence of only one isoprenic unit (C5), monoterpenes by two units (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), and tetraterpenes (C40).
Three new polyprenylated acylphloroglucinol meroterpenoids were obtained from the aerial parts of Hypericum forrestii (Chitt.) N. Robson (Hypericaceae), called hyperiforins A–C [26]. Hyperiforins A (1) and C (2) (Figure 3) showed potent inhibitory action on the enzyme tyrosine phosphatase 1B.
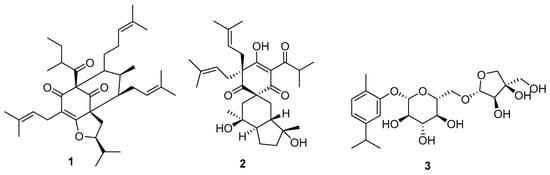
Figure 3.
Hyperiforins A (1) and C (2), and a glycosidic monoterpene (3).
New glycosidic monoterpene compounds (3) (with glucopyranosyl and apiofuranosyl groups) derived from Carvacrol were obtained from the roots of Lilium dauricum Ker Gawl. (Liliaceae). It is interesting to note that, despite the small structural difference (exchange of the position of the sugars and methyl), a different potency in the α-glucosidase inhibitory action was found [48].
Iridoids are undoubtedly the class of monoterpenes with the greatest potential for providing new structures [49,50,51,52]. Cornusdiridoids (4–5) (Figure 4) with unusual cornuside–morroniside secoiridoid dimers and small stereochemical variations were obtained from the fruits of Cornus officinalis. Their small differences consisted in stereochemical and sugar positions. They were tested for antidiabetic activity, but only the already-known compounds were active [53]. Chlorine-containing iridoid glycosides were reported from Plantago maxima Juss. ex Jacq (Plantaginaceae) (6) [54], and Valeriana jatamansi Jones (7) (Caprifoliaceae) [55], as well as secoiridoids (8–10) [50,52,56].
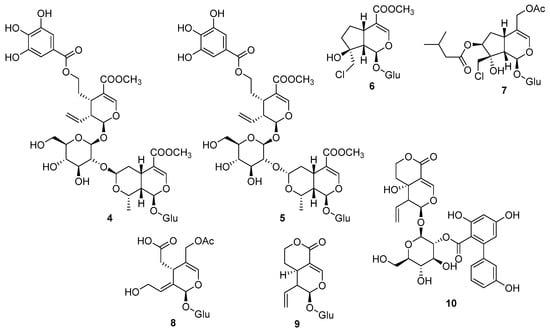
Figure 4.
Chemical structures of Cornusdiridoids (4–5), chlorine-containing iridoid glycosides (6–7), and secoiridoids (8–10).
Considering sesquiterpenes, there was a wide variety of subclasses making up the new structures identified. A dimeric guaiane-type skeleton (11) was identified in the leaves of Xylopia vielana Pierre (Annonaceae) together with different analogs [57] (Figure 5). Compound 12 was obtained from the leaves of Ammoides atlantica (Coss & Durieu) H. Wolff (Apiaceae), together with 15 new compounds [58]. The guaianolide lactone 13 was obtained from Chrysanthemum indicum L. (Asteraceae), a traditional herbal medicine in South Korea [59]. It showed inhibitory effects on lipopolysaccharide (LPS)-induced nitric oxide production in RAW 264.7 cells. The guaianolide 14 was obtained from the plant Ambrosia artemisiifolia L. (Asteraceae), an invasive plant with known allelopathic effects [60].
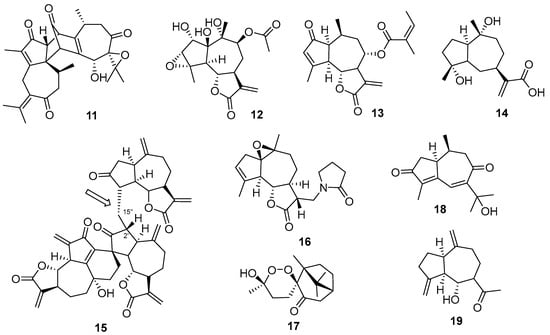
Figure 5.
New sequiterpenes chemical structures.
An unusual guaianolide trimer (15) was found in Ainsliaea fragrans Champ. (Compositae), a plant with medicinal use as an antibacterial and anti-inflammatory effect [61] (Figure 5). The third sesquiterpene unit is attached to C2′, providing a rare linkage (C2′-C15″). Cytotoxicity results showed this compound being active against five cancer cell lines with IC50 values of 0.4–8.3 μM. Compound 16 is notable for the presence of a γ-lactam group [35], while 17 has an unusual 1,4-peroxy hemiacetal xanthanolide skeleton [62]. Other minor structural modifications can be found in the reported sesquiterpenes 18 and 19 [63].
Eudesmane-type sesquiterpenoids are another important subclass of sesquiterpenoids with several examples of new structures (20–26) [37,58,64,65,66,67,68] (Figure 6). Compounds 25 and 26 have a rare peroxide-substituted group and a γ-lactam ring. The first was isolated from Sonchus arvensis L. (Asteraceae) and showed phytotoxic activity. Compound 26 was obtained from Sarcandra glabra (Thunb.) Nakai (Chloranthaceae) together with five other new eudesmane-type sesquiterpenoids.
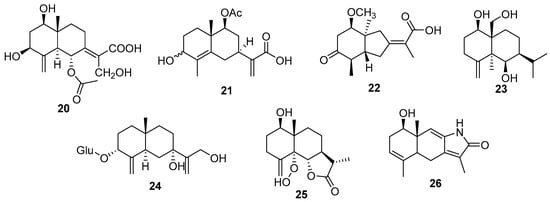
Figure 6.
New Eudesmane-type sesquiterpenoids chemical structures.
Tian and co-workers isolated 5,5-spiroketal sesquiterpenes (27) (Figure 7) from the roots of Angelica pubescens, which possessed inhibitory activity against nitric oxide (NO) production induced by lipopolysaccharide (LPS) in RAW264.7 macrophage cells [69]. Dihydro-β-agarofuran-type sesquiterpenoids were identified from the stems of Celastrus monospermus Roxb. (Celastraceae) with a significant inhibition of osteoclastogenesis [70]. Celasmondin C (28) possessed an unusual nicotinoyloxy group attached to C-1.
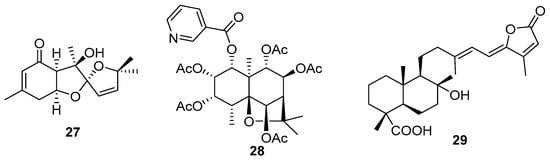
Figure 7.
New sesquiterpenes (27–28) and sesterterpenoid (29) chemical structures.
Germacrenolide-type compounds were found in Carpesium lipskyi Winkl. (Asteraceae) [71], Polydora serratuloides (DC.) H. Rob (Asteraceae) [72], Asteriscus graveolens (Forsk) Less. (Asteraceae) [73], and Carpesium divaricatum Sieb.et Zucc (Compositae) [74].
Sesterterpenoids are relatively rare in nature. Mirzania and co-workers isolated six new (29) from Salvia mirzayanii Rech. f. and Esfand [75] (Figure 7).
Regarding diterpenes, a new ent-kaurane diterpenoid compound named daturoside A (30) (Figure 8) was obtained from the pericarp of Datura metel L. from the Solanaceae family [76]. The authors indicate anti-inflammatory action through the production of nitric oxide (NO) induced by lipopolysaccharide (LPS). The compound hupehenoside A (31) was obtained from Inula hupehensis (Y. Ling) Y. Ling and has a carbon bond in the glycosidic structure and a carboxylic acid in the cyclic structure [77]. Wang et al. (2022) also identified another 15 new compounds with a similar structure, with slight variations in the hydroxyls of the sugars (mostly acetylation substitutions). The authors evaluated the neuro-anti-inflammatory activity, and only one of the compounds showed inhibition of NO production.
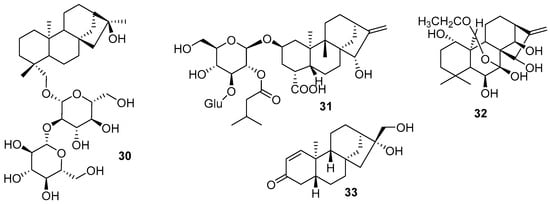
Figure 8.
New diterpenes chemical structures.
Other diterpenes with ent-kaurane skeletons were reported by Wei et al. (2022) [78] and Xin et al. (2022) [79]. Molecule 32 (Figure 8) has more hydroxyls in its structure than the already-known molecule phyllanthone A. It was isolated from the ethanolic extract of the aerial parts of Rabdosia rubescens (Hemsl.) Hara (Lamiaceae). The study by Xin et al. (2022) also reported two other ent-kaurane diterpenes from the roots and stems of Phyllanthus acidus (L.) Skeels (Phyllanthaceae) with cytotoxic activity against some cancer cell lines and moderate anti-inflammatory action (33).
In their study on Euphorbia dracunculoides Lam. from the Euphorbiaceae family, Yan et al. (2022) [80] reported 15,16,17-trinorabietane aromatic diterpenoid and an unusual 17-norabietane diterpenoid. They showed significant antiproliferative activity in four cancer cell lines, above all by inhibiting the proliferation of K562 cells.
Abietane diterpenoids are characterized by having a tricyclic chain and 20 carbons. Six new compounds were reported by Liu et al. (2022) [81], which were obtained from the heartwood of Juniperus formosana Hayata (Cupressaceae). Among the compounds obtained were four cadnene sesquiterpenoids Junipertriol (34) (Figure 9), an abietane diterpenoid (35), and a β-naphthol1-(6-hydroxy-4-methylnaphthalen-2-yl) ethan-1-one derivative (36). Junipertriol (34) demonstrated significant NO inhibitory potential, and all the new compounds showed anti-inflammatory effects, as indicated by measured expressions of IL-1β, IL-6, and TNF-α following LPS stimulation in RAW264.7 cells.
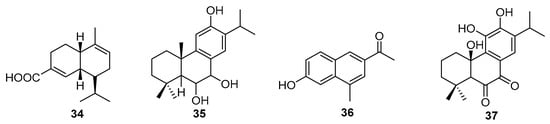
Figure 9.
New abietane diterpenoids chemical structures.
Another reported abietane diterpene, named mutabilol (37) (Figure 9), was obtained from the leaves of Plectranthus mutabilis Codd (Lamiaceae) [82]. Its chemical structure shows two carbonyls in its non-aromatic cyclic structure.
Pimarane (38 and 39) and abietane (40) diterpenoids were obtained from the aerial parts of Blumea balsamifera (L.) DC (Asteraceae) [83] (Figure 10). The compounds showed anti-inflammatory action by inhibiting TNF-α induced by LPS. Among the pimarane-type structures, blusamiferoid E has a hydroxyl in the cyclic structure and an extra unsaturation, while blusamiferoid A has a carboxylic acid.
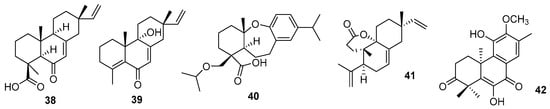
Figure 10.
New pimarane (38,39), abietane (40), 3,4-seco pimarane diterpene and 15,16-dinor-ent-pimarane diterpenes.
Li et al. (2022) reported three new 3,4-seco pimarane diterpene compounds from the leaves and twigs of Isodon flavidus (Hand.-Mazz.) H. Hara (Lamiaceae) [84]. The structure shows a δ-lactone ring and the carbon–carbon double bond of the isopropyl group is associated with antiviral activity. Among the compounds discovered, fladin C (41) (Figure 10) was the only one to show inhibition of Ebola virus replication.
A new 15,16-dinor-ent-pimarane diterpene was obtained from Croton yunnanensis W.W. Sm. (Euphorbiaceae) and was named Crotonyunnan E (42) [85] (Figure 10). In addition to this compound, four other 19-cleodane diterpenes (Crotonyunnan A–D) were also obtained [85]. Crotonyunnan E (42) showed selective cytotoxicity against three tumor cell lines, SMMC-7721 (human hepatoma cells), HL-60 (pre-myelocytic leukemia), and A-549 (lung cancer cells).
Labdane diterpenes are characterized by a bicyclic structure, with decalin as the core. From the leaves of Stevia rebaudiana (Bertoni) Bertoni, Kang et al. (2022) [86] reported a new labdane diterpene compound (6-O-acetyl-(12R)-epiblumdane) (43) (Figure 11). Studies of this compound showed stimulation of insulin secretion in INS-1 β-pancreatic cells from rats.
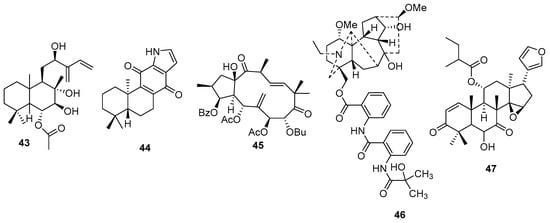
Figure 11.
New labdane (43), indolic (44), C-19-diterpene alkaloid (46) and limonoid (47) diterpenes chemical structures.
An unusual indolic diterpene with a C-17 norcassane structure was obtained from the roots of Euphorbia fischeriana Steud. (Euphorbiaceae) [87], along with another ent-atisane-type diterpene compound. The unusual compound was named Euphkanoid H (44) (Figure 11) and according to the author, it is the first example of an indolic diterpene with a C-17 norcassane structure in nature. In addition, the compound showed biological activity by inhibiting the proliferation of HEL cells, which could be effective in the development of drugs for leukemia.
A jatrophane diterpene was obtained from Euphorbia glomerular (Prokh.) Prokh. (Euphorbiaceae) and was named euphoglophane V (45) (Figure 11); other jatrophane- and ingenane-type diterpenes have also been reported [88]. According to the authors, the compound has an isobutanoyloxyl group on the C-8 carbon and this new molecule showed high efficiency in reversing resistance to multiple drugs, promoting the accumulation of Rh123 and DOX in drug-resistant cells, as well as inhibiting the transport function of P-glycoprotein. This action is of interest in therapies against cancer and fungal infections.
New C-19-diterpene alkaloids of the aconitine type were obtained from the aerial parts of Aconitum apetalum (Huth) B. Fedtsch. (Ranunculaceae) and were given the names apetalrines A–E [89]. The authors’ research indicates that apetalrine B (46) (Figure 11) has neuroprotective action by inhibiting the production of reactive oxygen species (ROS) in SH-SY5Y cells, thereby inhibiting H2O2-induced cell apoptosis.
A new limonoid (47) (Figure 11) was obtained from the leaves and twigs of Walsura yunanensis C.Y.Wu (Meliaceae) and showed cytotoxic action on four cancer cell lines, including A549, HepG2, HCT116 p21KO, and CNE-2 [90]. The α,β-unsaturated ketone and portions of the A and B rings are essential for cytotoxic activity, according to the authors.
A new saponin triterpenoid (48) (Figure 12) was isolated from the roots of Gardenia ternifolia Schumach. & (Rubiaceae) and showed antimicrobial activity against Salmonnella typhi [91]. The compound named ternifoliaoside A (48) has a saponin triterpene nucleus and two glucopyranosidic bonds.
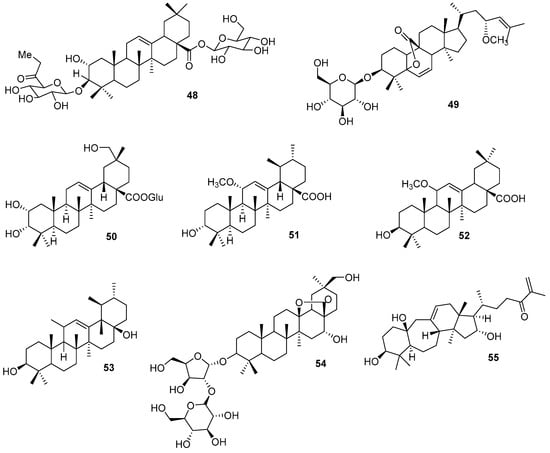
Figure 12.
New triterpenes chemical structures.
Two new triterpenoid glycoside compounds were isolated from the fruits of Momordica charantia L. (Cucurbitaceae) and were named Momordicoside Y and Z [92]. Momordicoside Y (49) (Figure 12), along with other known compounds, were evaluated for their antidiabetic potential, showing inhibitory activity on hepatic gluconeogenesis.
Seven new triterpenes were obtained from the aerial parts of Elsholtzia penduliflora W. W. Smith (Lamiaceae) [93]. Two of the new compounds, named penduloside C (50) (Figure 12) and G (51), showed significant inhibitory activity against tumor cells.
Hu et al. (2022) [94] reported new triterpenoids obtained from the leaves of Alstonia scholaris (L.) R. Br and were named alstolarnoid (A–D). These compounds—alstolarnoid A (52) and D (53)—showed a reduction in uric acid levels in vitro and in vivo.
Three new oleanane-type saponin triterpene compounds with a 13, 28 epoxy bridge were obtained from the roots of Ardisia crispa (Thunb.) A. DC. and were named ardisiacrispin D–F [95]. These compounds showed cytotoxic action against three cancer cell lines (HeLa, HepG2, and U87 MG) in vitro. According to the authors, these new compounds are the first examples of a monosaccharide linked directly to the C3 aglycone of saponin triterpenes in this plant genus (ardisiacrispin D—54) (Figure 12).
New triterpenic compounds were isolated from Lepidozia reptans (L.) Dumort. and named lepidozin A–J [96]. Lepidozin G (55) (Figure 12) is a 9,10-dry cycloartane that contains a carbonyl portion in an α,β-unsaturated portion in its structure that may be related to the inhibition of cancer cell lines by inducing the death of PC-3 cells by mitochondria-related apoptosis.
Among the terpenes, the sesqui- and diterpene subclasses have the greatest potential to present new structures, both due to the presence of atypical bonds between the carbons of the rings for the formation of extra rings, and the presence of some nitrogen derivatives. The possibility of skeleton types within sesqui- and diterpenes provides an even greater chance of finding new derivatives. In the other subclasses of terpenes, mostly minor modifications of functional groups and sugars are found.
5.2. Alkaloids
Piperidine alkaloids show various types of biological activity. Statistics considering the scaffolds of FDA-approved drugs show a widespread presence of the piperidine core [97]. Wu et al. (2022) [98] report eight new 2,6-disubstituted piperidin-3-ol alkaloids (56) (Figure 13) possessing angiogenesis-inhibitory activity from the plant Microcos paniculata L. (Tiliaceae). Structural variations were observed in the number of hydroxyl or carbonyl groups in the carbon chain. Other piperidine alkaloids [99] with small stereochemical variations and the presence of -CH3 e -OH attached to the piperidinic ring have been reported from the plant Alocasia macrorrhiza (L.) Schott (Araceae) (57) (Figure 13). These isolated alkaloids were screened for antiproliferative activity through an MTT assay against HepG2, AGS, and MCF-7 tumor cells.
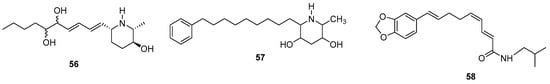
Figure 13.
New piperidine alkaloids chemical structures.
New amide alkaloids were isolated from the fruits of Piper longum L. (Piperaceae) and given the names piperlongumamides D–F [18]. The structure of piperlongumamide E (58) (Figure 13) is similar to the known compound retrofractamide A, differing in the stereochemistry of one of the double bonds. According to the authors, piperlongumamide E (58) showed inhibitory activity against NO production.
Diterpene alkaloids are found mainly in the genera Aconitum, Delphinium, and Spiraea in the Ranunculaceae family. Usually, they have a complex heterocycle scaffold (59) (Figure 14) and a variable number of carbons (C18-, C19-, C20-). Three new C20-diterpenoid alkaloids from Aconitum kusnezoffii Reichb, named napellines, were evaluated in vitro for their proliferative activities against A549, HL-60, MCF-7, Bel-7402, BGC-823, and RAW264.7 cells, but only the already-known compounds showed activity [100]. A similar new structure was found by Wang and co-workers (2021) [101] in the plant Aconitum carmichaelii Debx.
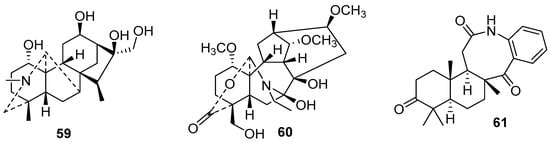
Figure 14.
New diterpene alkaloids chemical structures.
A rare C20-type diterpene alkaloid was isolated from the plant Delphinium gyalanum C. Marquand & Airy Shaw [102]. This compound possesses a hemiacetal ring linking C-2 to C-19 and showed a cardiotonic effect by isolated frog’s heart perfusion (60) (Figure 14). Other activities were reported for diterpene alkaloids, such as protection against cardiomyocytes H2O2-induced injuries [103] and anti-inflammatory effects against NO production [104]. Kemgni et al. (2021) [105] isolated sesquiterpenes alkaloids with an unusual eight-membered lactam ring (61) (Figure 14). They were obtained from the leaves of a Cameroonian medicinal plant Greenwayodendron oliveri (Engl.) Verdc and showed antimicrobial activity.
Steroidal alkaloids have been reported from the plants Veratrum grandiflorum (Maxim. ex Miq.) O.Loes. (Melianthiaceae) [106,107] with anti-inflammatory and cytotoxic activities. Two new pregnane alkaloid derivatives were obtained from Pachysandra terminalis Sieb. et Zucc. (Buxaceae).
Several alkaloids with minor structural modifications of already-known alkaloids have been reported. Wu et al. (2022) [108] report new aglain derivatives (62) (Figure 15) isolated from Aglaia odorata Lour. However, the structural differences from the already-reported aglain derivatives were the absence of methoxy groups. These compounds exhibited cytotoxic activities on human leukemia cells (HELs) and human breast cancer cells with IC50 values in the range of 0.03–8.40 μM.
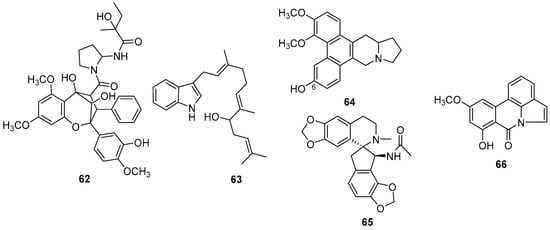
Figure 15.
New aglain (62), indole (63), indolizidine (64), isoquinoline (65) and pyrrolofenanthridone (66) alkaloids.
A new farnesylindole alkaloid was obtained from the flowers of Anomianthus dulcis (Dunal) J. Sinclair (Annonaceae) and it was named (R)-3-(8′-hydroxyfarnesyl)-indole (63) (Figure 15) [109]. The structure of the new compound is very similar to a known compound 3-(R)-3-(8′-hydroxyfarnesyl)-indole, differing in the presence of a hydroxyl in the new compound. According to the authors, the new compound showed significant cytotoxic activity against KB cell lines.
A new phenanthroindolizidine alkaloid was obtained from the leaves of Cryptocarya densiflora Blume (Lauraceae) and it was named (R)-13aα-densiindolizidine (64) (Figure 15) [110]. According to the authors, the new compound exhibited binding interactions with crucial amino acid residues in the active sites of severe acute respiratory syndrome coronavirus MPro (SARS-CoV-MPro). The main difference is the presence of a hydroxyl group at C-6 in the new compound, instead of a methoxyl group.
Six new isoquinoline alkaloids were obtained from the whole plant of Hypecoum erectum L. (Papaveraceae) [111]. NMR data indicated that the new compound hyperectumine B (65) has a similar structure to dihydrofumariline; however, the new compound has an acetamido group in place of the hydroxyl.
A new pyrrolofenanthridone alkaloid was obtained from the stem, root, and bulb of Crinum amabile Donn (Amaryllidaceae) and named Amabiloid A (66) (Figure 15) [112]. According to the author, the new compound showed low inhibition against acetylcholinesterase. The structure of the new compound is similar to that of pratorimine, with the difference being the position of the hydroxyl in the aromatic ring.
5.3. Phenols
Most phenols come from the shikimate pathway and have a benzene ring in their structure containing one or more hydroxyls as substituent groups. The phenol group is present in a variety of compounds and is related to defense mechanisms in plants. Herein, we classify phenols as those structures that contain the chemical group and do not fit into other chemical classes.
A polycyclic phenol with α-glucosidase inhibitory action was obtained from the leaves of Spermacoce latifolia Aubl. (Rubiaceae), named 3,8,10-trihydroxy-4,9-dimethoxy-6H-benzo[c]chromen-6-one (67) (Figure 16) [113]. Considering the structural innovation, it has two methoxyls on the C-4 and C-9 carbons, differing from the known compound 3,4,8,9,10-pentahydroxydibenzo[b,d]pyran-6-one, which has two hydroxyls on these same carbons.
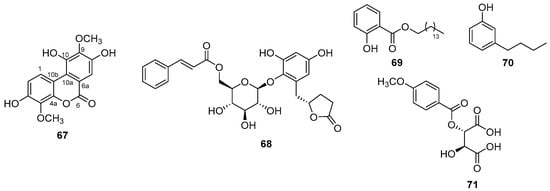
Figure 16.
New phenols compounds chemical structures.
Two new compounds were obtained from the leaves of Ardisia crenata Sims (Primulaceae) and were named ardisicreolides A-B (68) (Figure 16) [114]. The new compounds are similar to the known compound myrsinoside A, differing in the presence of the cinnamoyl and pentadactyl lactone rings and the configuration of the double bond of the cinnamoyl group. The two novel compounds showed NO inhibitory action, as well as a reduced release of the tumor necrosis factor (TNF-α), interleukin 1-β (IL-1 β), interleukin 1–4 (IL-4), and interleukin 1–10 (IL-10) in RAW264.7 macrophage cells induced by lipopolysaccharide (LPS).
Two new phenolic derivative compounds were isolated from the leaves of Piper betle L. (Piperaceae) and named 1-n-decanoyl hydroxy-benzoic acid/1-n-decanoyl phenol (69) and 3-butylphenol (70) (Figure 16) [15]. According to the authors, both compounds showed cytotoxic activities against two oral cancer cell lines (SCC-40 and SCC-29B) and antioxidant activity by scavenging 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radicals. Compound M1 has an aliphatic chain, giving the molecule greater hydrophobicity, while compound H2 has an ester group and a long carbon chain. These carbon chains may be important for biological activity.
A new phenolic acid was obtained from the Zanthoxylum nitidum (Roxb.) DC. plant (Rutaceae) and named nitomentosin (71) (Figure 16) [115]. The structure of the new compound is similar to that of the known compound O-p-anisoyl-D-tartaric acid, with the methoxy group replaced by hydroxyl and one more carboxylic acid than the known molecule.
Among the selected examples of new phenols, most are small modifications of molecules already reported in the literature. The low level of structural innovation is also due to the small size characteristic of this class of molecules.
5.4. Flavonoids
New flavonoids were obtained from the roots and rhizomes of Notopterygium incisum C.T. Ting ex H.T. Chang (Apiaceae) and were named notoflavinols A (72) (Figure 17) and B, notophenitols A–E and (2R)-5,4′-dihydroxy-7-O-[(E)-3,7-dimethyl-2,6-octadienyl]flavanone (73) [116]. According to the author, the carbonyl group on carbon 4, the side chain on carbon 7, and the oxygen on carbon 5 may be related to the anti-inflammatory action by inhibiting nitric oxide (NO).
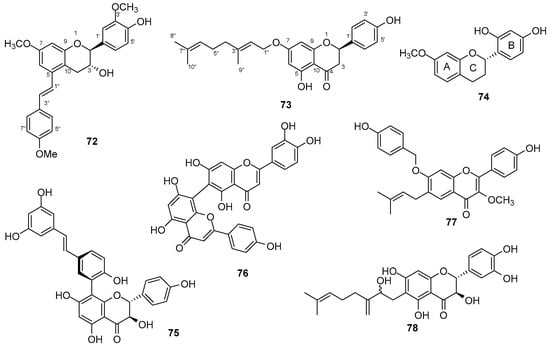
Figure 17.
New flavonoids chemical structures.
Four new catechins were obtained from the aerial parts of Dianella ensifolia (L.) Redouté (Asphodelaceae) [117]. The 2(S)-20,40-dihydroxy-7-methoxyflavan (74) (Figure 17) is similar to a known compound 2(S)-3,‘4’-dihydroxy-7-methoxyflavan, differing only in the position of the hydroxyl in the B ring.
Eleven new flavonostilbenes were obtained from the stem of Rhamnoneuron balansae (Drake) Gilg (Thymelaeaceae) and named rhamnoneuronal D–N [118]. According to the authors, the compound rhamnoneuronal D (75) (Figure 17) was shown to be a potential anti-aging agent in in vitro results for sirtuin 1 (SIRT1).
A new biflavonoid was isolated from the leaves of Schinus polygama (Cav.) Cabrera (Anacardiaceae) and named luteolin-(6→8″)-apigenin (76) (Figure 17), showing anti-inflammatory activity through its membrane-stabilizing effect on erythrocytes [119]. The new compound is similar to agathisflavone, differing in the presence of luteolin in the new compound instead of apigenin.
A new flavonoid called 4′-hydroxy-7-O-(4-hydroxybenzyl)-3-methoxy-6-prenylflavone (77) (Figure 17) was isolated from the leaves of Apocynum venetum L. (Apocynaceae) [120]. According to the author, the new compound exhibited moderate inhibitory action on NO production.
Ten new flavonoid derivatives were obtained from the fruits of Paulownia tomentosa (Thunb.) Steud. (Paulowniaceae) [121]. According to the author, among the compounds, Paulodiplacol A (78) (Figure 17) showed better anti-inflammatory activity by decreasing the action of NF-κβ after the addition of LPS. The new compound is similar to a known compound called paulodiplacone A, differing in the presence of an extra hydroxyl at the C-ring.
Two new C-benzylated chalcones were obtained from the twigs and leaves of Caesalpinia digyna Rottler (Fabaceae) and were identified as 2′,4′-dihydroxy-3′-(2- hydroxylbenzyl) chalcone and 2′,4′-dihydroxy-5′-(2-hydroxybenzyl) chalcone (79) [122]. Both compounds showed cytotoxicity against SMMC-7721, A-549, and MDA-MB-231 cell lines. Compound 79 (Figure 18) is similar to a compound known as 2′,4′-Dihydroxy-3′-(2-hydroxybenzyl)-6′-methoxychalcone, with one change of methoxyl to hydroxyl in the new compound.
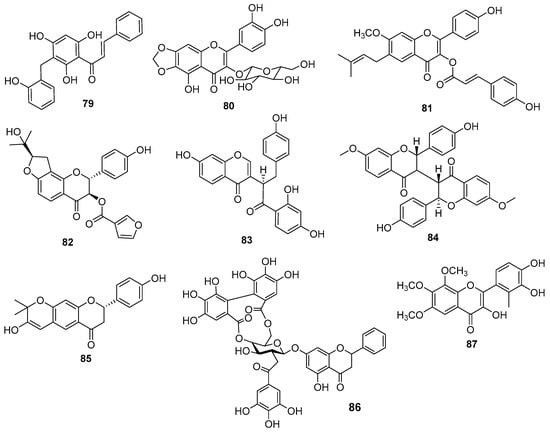
Figure 18.
New flavonoids chemical structures.
A new flavonoid was obtained from the aerial parts of Polygonum tinctorium Aiton (Polygonaceae) and was identified as 3,5,3′,4′-tetrahydroxy-6,7-methylendioxyflavone-3-O-β-d-glucopyranoside (80) (Figure 18) [123].
Two new compounds were isolated from the whole plant of Centella asiatica (L.) Urb. (Apiaceae) and were identified as 4′-hydroxyl-7-methoxyl-6-prenyl-3-O-trans-p-coumaroyl-flavonol (81) and (2R,3R,2′′S)-3-furanoyl-brosimacutin E (82) (Figure 18) [124]. According to the authors, both molecules exhibited high cytotoxic activity in HepG2 and SGC-7901 cells.
A new isoflavonoid was obtained from the bark and roots of Ochna kirkii Oliv. (Ochnaceae) and named kirkinone A (83) (Figure 18) [125]. The structural difference with the well-known compound lophirone A is one aromatic ring less in the new compound. The authors also reported a new biflavonoid kirkinone B (84) (Figure 18), similar to a known compound 4,4′,7-tri-o-methylisocampylospermone A, the difference being the presence of methoxyls instead of hydroxyls.
A new chromenoflavanone was obtained from the fruits of Cullen corylifolium (L.) Medik. (Ericaceae) and named corylifol H (85) [126]. The new compound showed dose-dependent inhibition of NO in LPS-activated RAW 264.7 macrophages. The structure of the new molecule is similar to that of an already-known compound 7,8-dihydro-8-(4-hydroxyphenyl)-2,2-dimethyl-2H,6Hbenzo [1,2-b:5,4-b’]dipyran-6-one, the difference being the presence of an extra hydroxyl.
New flavonoids were obtained from the aerial parts of Penthorum chinense Pursh (Penthoraceae) and named penthorumside A–C [127]. The structure of the compound penthorumside B (86) (Figure 18) is similar to the known compound pinocembrin-7-O-[3″-O-galloyl-4″,6″- hexahydroxydiphenoyl]-b-glucose, differing in the position of the galloyl group.
Polyoxygenated flavonoids were obtained from the aerial parts of Blumea eriantha DC (Asteraceae) and determined as a 3,3′,4′-trihydroxy-6,7,8-trimethoxy flavone (87) (Figure 18) with antiproliferative activity in NCI-H23 cell lines [128].
Flavonoids are notably the compounds with the fewest structural innovations. The new compounds mainly change from the known ones by the location of groups, the exchange of hydroxyls for methoxyls (and vice versa), or the presence of a sugar different from the one already found.
5.5. Coumarins
Coumarins are recurring compounds in various plants, but they can also be found in fungi and bacteria. Their chemical structure consists of an organic heterocyclic, fused to a benzo-α-pyrone ring, divided into subclasses such as furanocoumarins, phenylcoumarins, and isocoumarins, among others.
A new prenylated coumarin ester was obtained from the leaves, fruits, and twigs of Glycosmis ovoidea Pierre (Rutaceae) and identified as 1-(7-methoxy-2-oxo-2H-chromen-8-yl)-3-methyl-1-oxobut-2-en-2-yl (S)-2-methylbutanoate (88) (Figure 19) [129].
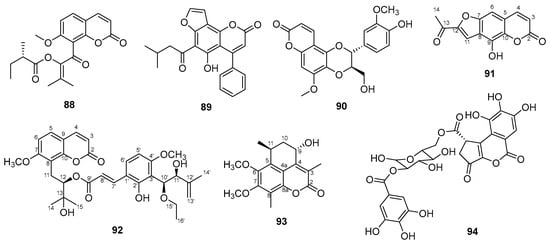
Figure 19.
New coumarins chemical structures.
A new coumarin was obtained from the roots of Calophyllum pisiferum Planch. & Triana (Calophyllaceae) and was named calopisifuran (89) (Figure 19) [130]. According to the authors, the new compound showed significant cytotoxicity against the MDA-MB-231 cell line.
New coumarolignans were obtained from the roots of Waltheria indica L. (Malvaceae) and were named walthindicins A-F [131]. Among the new compounds, walthindicin A (90) (Figure 19) exhibited the greatest inhibition of reactive oxygen species (ROS) and showed dose-dependent inhibition of the NF-κβ transcription factor in human embryonic kidney 293 cells (Luc-HEK-293).
Coumarin derivatives similar to xanthumol were obtained from the whole plant of Spermacoce latifolia Aubl. (Rubiaceae) and identified as 2-acetyl-4-hydroxy-6H-furo [2,3-g]chromen-6-one (91) (Figure 19) and 2-(1′,2′- dihydroxypropan-2′-yl)-4-hydroxy-6H-furo [2,3-g]chromen-6-one [132]. The authors also reported in vitro antimicrobial action of the new compounds against Staphyloccocus aureus, Bacillus subtilis, and B. cereus.
A new coumarin was isolated from the leaves and twigs of Murraya exotica L. (Rutaceae) and identified as 5-demethoxy-10′-ethoxyexotimarin F (92) (Figure 19) [133]. The new compound showed inhibitory activity against the enzyme monoamine oxidase B (MAO-B). The structure of the new compound resembles the known compound 10′-ethoxyexotimarin F, with the difference being the absence of the methoxy group at C-5.
A new coumarin derivative was obtained from the stem of Ulmus elongata L.K. Fu & C.S. Ding (Ulmaceae) and named ulmuselactone A (93) (Figure 19) [134]. The structure of the compound is similar to a known coumarin, except for the presence of methoxyls at C-6 and C-7 in the novel compound instead of hydroxyls.
A new isocoumarin was isolated from the bark of Fraxinus chinensis subsp. rhynchophylla (Hance) A.E. Murray (Oleaceae) and named fraxicoumarin (94) (Figure 19) [135]. The new compound showed LPS-induced NO inhibitory activity in RAW 264.7 cells.
5.6. Antraquinones
Anthraquinones are natural products composed of two aromatic rings, together with two carbonyls, forming a flat aromatic structure. New anthraquinones were isolated from Hedyotis diffusa Willd. (Rubiaceae) and named diffusaquinone A–G [136]. According to the authors, diffusaquinone A (95) (Figure 20) showed the greatest anti-inflammatory activity by inhibiting the generation of superoxide anion and elastase. The structure of the new compound has a rare 2-isopropyldihydrofuran portion.
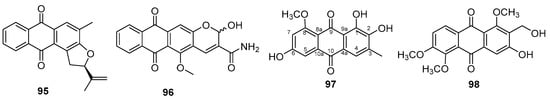
Figure 20.
New anthraquinones chemical structures.
Two new quinones were isolated from the aerial parts of Morinda umbellata L. (Rubiaceae) similar to those already obtained [137]. The structure of umbellata V (96) (Figure 20) resembles that of umbellata S, differing in the presence of hydroxyl instead of ethoxyl. The same had happened with the anthraquinones isolated from the roots of Ventilago denticulata Willd. (Rhamnaceae) [138] (97) (Figure 20).
Two new anthraquinones were obtained from the roots of Prismatomeris filamentosa Craib (Rubiaceae) and named filaments B and C [139]. The structure of filament B (98) (Figure 20) is similar to the known compound 2-hydroxymethylknoxiavaledin, the difference being the replacement of hydroxy groups on carbons 1 and 5 with methoxy groups. The new compounds exhibited moderate antibacterial activity against a range of Gram-positive and Gram-negative bacteria such as B. subtilis, B. cereus, S. aureus, E. coli, P. aeruginosa, and S. sonnei.
6. Conclusions
Through the compilation of articles, we had a total of 464 articles in the years 2021 and 2022 that presented new structures from plants. Among the total number of articles, 122 families and 436 species were represented, with various classes of compounds.
The Asteraceae family provided the largest number of new structures, indeed by the high number of species present. Families with many species that are not among the new structures reported are those that deserve attention, given the potential for new structures they can provide.
Many of the compounds showed small structural variations in relation to already-known molecules. For some, however, this small modification was decisive for the biological activity reported, demonstrating the importance of descriptive phytochemical studies.
Author Contributions
Conceptualization, E.d.S.O., C.N.K. and D.P.D.; methodology, E.d.S.O. and C.N.K.; formal analysis, E.d.S.O., C.N.K., M.T.H., V.F.G. and D.P.D.; investigation, E.d.S.O., C.N.K., M.T.H. and V.F.G.; data curation, E.d.S.O., C.N.K. and D.P.D.; writing—original draft preparation, E.d.S.O., C.N.K. and D.P.D.; writing—review and editing, E.d.S.O., C.N.K., M.T.H., V.F.G. and D.P.D.; supervision, D.P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESP, grant number 2022/08191-9.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Pye, C.R.; Bertin, M.J.; Lokey, R.S.; Gerwick, W.H.; Linington, R.G. Retrospective Analysis of Natural Products Provides Insights for Future Discovery Trends. Proc. Natl. Acad. Sci. USA 2017, 114, 5601–5606. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An Analysis of FDA-Approved Drugs: Natural Products and Their Derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the Decline in Pharmaceutical R&D Efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Demarque, D.P.; Dusi, R.G.; de Sousa, F.D.M.; Grossi, S.M.; Silvério, M.R.S.; Lopes, N.P.; Espindola, L.S. Mass Spectrometry-Based Metabolomics Approach in the Isolation of Bioactive Natural Products. Sci. Rep. 2020, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Oliveira, F.A.D.S.; Passarini, G.M.; Medeiros, D.S.S.D.; Santos, A.P.D.A.; Fialho, S.N.; Gouveia, A.D.J.; Latorre, M.; Freitag, E.M.; Medeiros, P.S.D.M.D.; Teles, C.B.G.; et al. Antiplasmodial and Antileishmanial Activities of Compounds from Piper Tuberculatum Jacq Fruits. Rev. Soc. Bras. Med. Trop. 2018, 51, 382–386. [Google Scholar] [CrossRef]
- Oliveira, E.R.; Menini Neto, L. Levantamento Etnobotânico de Plantas Medicinais Utilizadas Pelos Moradores Do Povoado de Manejo, Lima Duarte-MG. Rev. Bras. Plantas Med. 2012, 14, 311–320. [Google Scholar] [CrossRef]
- Sunila, E.S.; Kuttan, G. Piper Longum Inhibits VEGF and Proinflammatory Cytokines and Tumor-Induced Angiogenesis in C57BL/6 Mice. Int. Immunopharmacol. 2006, 6, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Wirotesangthong, M.; Inagaki, N.; Tanaka, H.; Thanakijcharoenpath, W.; Nagai, H. Inhibitory Effects of Piper Betle on Production of Allergic Mediators by Bone Marrow-Derived Mast Cells and Lung Epithelial Cells. Int. Immunopharmacol. 2008, 8, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.G.; Rebelo, R.A.; Dalmarco, E.M.; Guedes, A.; De Gasper, A.L.; Cruz, A.B.; Schmit, A.P.; Cruz, R.C.B.; Steindel, M.; Nunes, R.K. Composição Química e Avaliação Da Atividade Antimicrobiana Do Óleo Essencial Das Folhas de Piper Malacophyllum (C. Presl.) C. DC. Quim. Nova 2012, 35, 477–481. [Google Scholar] [CrossRef]
- Atiya, A.; Salim, M.A.; Sinha, B.N.; Ranjan Lal, U. Two New Anticancer Phenolic Derivatives from Leaves of Piper Betle Linn. Nat. Prod. Res. 2021, 35, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- San, T.T.; Wang, Y.H.; Hu, D.B.; Yang, J.; Zhang, D.D.; Xia, M.Y.; Yang, X.F.; Yang, Y.P. A New Sesquineolignan and Four New Neolignans Isolated from the Leaves of Piper Betle, a Traditional Medicinal Plant in Myanmar. Bioorg. Med. Chem. Lett. 2021, 31, 127682. [Google Scholar] [CrossRef]
- Xiao, C.Y.; Sun, Z.L.; Huang, J.; Li, R.S.; He, J.M.; Gibbons, S.; Ju, D.W.; Mu, Q. Neolignans from Piper Betle Have Synergistic Activity against Antibiotic-Resistant Staphylococcus aureus. J. Org. Chem. 2021, 86, 11072–11085. [Google Scholar] [CrossRef] [PubMed]
- Viet Phong, N.; Thi Nguyet Anh, D.; Yeong Chae, H.; Young Yang, S.; Jeong Kwon, M.; Sun Min, B.; Ah Kim, J. Anti-Inflammatory Activity and Cytotoxicity against Ovarian Cancer Cell Lines by Amide Alkaloids and Piperic Esters Isolated from Piper Longum Fruits: In Vitro Assessments and Molecular Docking Simulation. Bioorg. Chem. 2022, 128, 106072. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.K.; Su, B.J.; Wang, Y.Q.; Wang, H.S.; Liao, H.B.; Liang, D. New Tyramine- and Aporphine-Type Alkamides with NO Release Inhibitory Activities from Piper puberulum. J. Nat. Prod. 2021, 84, 1316–1325. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Wang, Y.Q.; Su, B.J.; Wang, H.S.; Liao, H.B.; Liang, D. New Enantiomeric Lignans and New Meroterpenoids with Nitric Oxide Release Inhibitory Activity from Piper puberulum. Bioorg. Chem. 2022, 119, 105522. [Google Scholar] [CrossRef]
- Nongmai, C.; Kanokmedhakul, K.; Promgool, T.; Paluka, J.; Suwanphakdee, C.; Kanokmedhakul, S. Chemical Constituents and Antibacterial Activity from the Stems and Leaves of Piper wallichii. J. Asian Nat. Prod. Res. 2022, 24, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, A.S.; Aldasoro, J.J.; Sanmartín, I. Bayesian Inference of Phylogeny, Morphology and Range Evolution Reveals a Complex Evolutionary History in St. John’s Wort (Hypericum). Mol. Phylogenet. Evol. 2013, 67, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Nürk, N.M.; Scheriau, C.; Madriñán, S. Explosive Radiation in High Andean Hypericum-Rates of Diversification among New World Lineages. Front. Genet. 2013, 4, 175. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.S.; Moraes, D.C.; De Freitas, G.B.L.; Almeida, D.J. Botanical, Chemical, Pharmacological and Therapeutic Aspects of Hypericum perforatum L. Rev. Bras. Plantas Med. 2014, 16, 593–606. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Xu, Q.; Shi, Z.; Xiang, M.; Li, H.; Wang, Y.; Qi, C.; Zhang, Y. (±)-Hyperpyran A: Terpenoid-Based Bicyclic Dihydropyran Enantiomers with Hypoglycemic Activity from Hypericum Perforatum (St. John’s Wort). Fitoterapia 2022, 161, 105221. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.F.; Zhang, M.M.; Zhou, Y.B.; Li, J.; Hou, A.J.; Lei, C. Polyprenylated Acylphloroglucinol Meroterpenoids with PTP1B Inhibition from Hypericum Forrestii. Fitoterapia 2021, 153, 104959. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Xu, W.J.; Zhang, M.H.; Zhang, Y.Q.; Li, Y.R.; Zhang, H.; Luo, J.; Kong, L.Y. Diverse Polycyclic Polyprenylated Acylphloroglucinol Congeners with Anti-Nonalcoholic Steatohepatitis Activity FromHypericum Forrestii. J. Nat. Prod. 2021, 84, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Watanabe, T. Isolation and Structure Elucidation of Constituents of Citrus Limon, Isodon Japonicus, and Lansium Domesticum as the Cancer Prevention Agents. Genes Environ. 2020, 42, 17. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Feng, H.; Sun, L.; Shi, Z.; Hu, H.; Duan, Y.; Guo, Y.; Tan, X.; Chen, G.; Qi, C.; et al. Discovery of Immunosuppressive Lupane-Type Triterpenoids from Hypericum Longistylum. Nat. Prod. Res. 2022, 36, 4394–4400. [Google Scholar] [CrossRef]
- Peng, X.; Tan, Q.; Zhou, H.; Xu, J.; Gu, Q. Discovery of Phloroglucinols from Hypericum Japonicum as Ferroptosis Inhibitors. Fitoterapia 2021, 153, 104984. [Google Scholar] [CrossRef]
- Cao, Y.; Zang, Y.; Huang, X.; Cheng, Z. Chemical Constituents from Artemisia Rupestris and Their Neuraminidase Inhibitory Activity. Nat. Prod. Res. 2021, 35, 1775–1782. [Google Scholar] [CrossRef]
- Ribnicky, D.; Beom Kim, S.; Poulev, A.; Wang, Y.; Boudreau, A.; Raskin, I.; Bisson, J.; Ray, G.J.; Chen, S.N.; Richard, A.; et al. Prenylated Coumaric Acids from Artemisia Scoparia Beneficially Modulate Adipogenesis. J. Nat. Prod. 2021, 84, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Bao, W.; Pa, B.; Xu, Y. Two New Compounds from Supercritical Fluid Extract of Artemisia Integrifolia L. Nat. Prod. Res. 2021, 35, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Q.; Bao, W.; Pa, B. Antihyperlipidemic Effect, Identification and Isolation of the Lipophilic Components from Artemisia Integrifolia. Molecules 2019, 24, 725. [Google Scholar] [CrossRef]
- Su, L.H.; Ma, Y.B.; Geng, C.A.; Li, T.Z.; Huang, X.Y.; Hu, J.; Zhang, X.; Tang, S.; Shen, C.; Gao, Z.; et al. Artematrovirenins A–P, Guaiane-Type Sesquiterpenoids with Cytotoxicities against Two Hepatoma Cell Lines from Artemisia Atrovirens. Bioorg. Chem. 2021, 114, 105072. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Li, L.; Zheng, Y.; Gong, Q.; Ke, C.Q.; Yao, S.; Zhang, H.; Tang, C.; Ye, Y. Anti-Inflammatory Sesquiterpenoid Dimers from Artemisia Atrovirens. Fitoterapia 2022, 159, 105199. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Tang, C.; Zhou, S.; Ke, C.Q.; Liu, Y.; Yao, S.; Ai, J.; Ye, Y. Anti-Inflammatory Eudesmane Sesquiterpenoids from Artemisia Hedinii. J. Nat. Prod. 2021, 84, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, Z.; Li, Y.; Wang, H.; Zhang, S.; Reid, A.M.; Lall, N.; Zhang, J.; Wang, C.; Lee, D.; et al. Cytotoxic and Antiangiogenetic Xanthones Inhibiting Tumor Proliferation and Metastasis from Garcinia Xipshuanbannaensis. J. Nat. Prod. 2021, 84, 1515–1523. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, H.; Wang, X.; Zhou, L.; Wang, S.; Shen, T.; Ren, D. Cytotoxic New Caged-Polyprenylated Xanthonoids from Garcinia Oligantha. Fitoterapia 2022, 156, 105092. [Google Scholar] [CrossRef]
- Fouotsa, H.; Dzoyem, J.P.; Lannang, A.M.; Stammler, H.G.; Mbazoa, C.D.; Luhmer, M.; Nkengfack, A.E.; Allémann, É.; Delie, F.; Meyer, F.; et al. Antiproliferative Activity of a New Xanthone Derivative from Leaves of Garcinia Nobilis Engl. Nat. Prod. Res. 2021, 35, 5604–5611. [Google Scholar] [CrossRef]
- Auranwiwat, C.; Limtharakul, T.; Pyne, S.G.; Rattanajak, R.; Kamchonwongpaisan, S. A New Xanthone and a Biphenyl from the Flower and Twig Extracts of Garcinia Mckeaniana. Nat. Prod. Res. 2021, 35, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chen, J.; Feng, L.; Chen, C.; Ye, Y.; Lin, L. Polyisoprenylated Benzophenone Derivatives from Garcinia Cambogia and Their Anti-Inflammatory Activities. Food Funct. 2021, 12, 6432–6441. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.Y.; Zhu, Y.N.; Wu, F.; Mi, Q.L.; Shi, J.Q.; Gao, Q.; Zhu, L.C.; Zhou, T.; Li, J.; Liu, X.; et al. Two New Antibacterial Chromeno[3,2-c]Pyridine Alkaloids from Whole Plants of Thalictrum Scabrifolium. Chem. Nat. Compd. 2022, 58, 506–510. [Google Scholar] [CrossRef]
- Hu, Q.F.; Zhang, L.F.; Liu, M.X.; Cai, B.B.; Li, Y.; Zhou, T.; Li, M.F.; Wang, H.S.; Xu, Y.; Kong, W.S.; et al. Two New Chromeno[3,2-c]Pyridine Derivatives from the Whole Plants of Thalictrum Finetii and Their Antirotavirus Activity. Chem. Nat. Compd. 2022, 58, 511–515. [Google Scholar] [CrossRef]
- Wu, Y.P.; Lin, Z.L.; Zhao, G.K.; Zhou, M.; Yao, H.; Zhang, G.H.; Li, W.; Yang, G.Y.; Li, Y.K.; Hu, Q.F.; et al. Two New Anti-Tobacco Mosaic Virus Alkaloids from the Whole Plants of Thalictrum microgynum. Chem. Nat. Compd. 2022, 58, 699–703. [Google Scholar] [CrossRef]
- Xu, L.; Yang, W.; Hu, J.; Han, C.M.; Li, P.F. Three New Isoquinoline Alkaloids from the Whole Plants of Thalictrum tenue with Cytotoxic Activities. J. Asian Nat. Prod. Res. 2020, 22, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.J.; Jiang, C.Y.; Zou, D.L.; Li, B.J.; Lu, J.C.; Li, D.H.; Lin, B.; Li, Z.L.; Hua, H.M. Baicalensines A and B, Two Isoquinoline Alkaloids from the Roots of Thalictrum baicalense. Org. Lett. 2020, 22, 7439–7442. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, J.; Wang, X.J.; Lu, Y.; Chen, D.F. New Phenolic Glycosides and Lignans from the Roots of Lilium dauricum. Planta Med. 2022, 88, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Yu, M.; Zhu, H.; Luo, Y.; Li, Y.; Li, N.; Zhang, H.; Zhang, J.; Liu, G.; Wei, X.; et al. Two New Iridoid Glycosides from Gardeniae Fructus. Carbohydr. Res. 2021, 501, 108259. [Google Scholar] [CrossRef]
- Yu, J.; Wang, K.; Zhao, H.; Chen, L.; Wang, X. Bioactive Constituents from the Leaves of Lonicera Japonica. Fitoterapia 2022, 162, 105277. [Google Scholar] [CrossRef]
- Sun, S.; Fu, J.; Liu, K.; Dai, M.; Li, Y.; Liu, Y.; Ma, S.; Qu, J. Two New Iridoid Glucosides from the Whole Plant of Patrinia scabiosifolia Link. Molecules 2021, 26, 4201. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Lv, J.; Jiang, N.; Li, Y.; Liu, X.; Zhao, H. Iridoid Compounds from the Aerial Parts of Swertia Mussotii franch. with Cytotoxic Activity. Nat. Prod. Res. 2021, 35, 1544–1549. [Google Scholar] [CrossRef]
- Peng, Z.C.; He, J.; Pan, X.G.; Zhang, J.; Wang, Y.M.; Ye, X.S.; Xia, C.Y.; Lian, W.W.; Yan, Y.; He, X.L.; et al. Secoiridoid Dimers and Their Biogenetic Precursors from the Fruits of Cornus Officinalis with Potential Therapeutic Effects on Type 2 Diabetes. Bioorg. Chem. 2021, 117, 105399. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wang, L.L.; Zhang, Y.F.; Jiang, X.F.; Zhu, X.L.; Pan, K.; Wan, C.X.; Zhou, Z.B. A New Chlorine-Containing Iridoid Glycoside from Plantago Maxima. Nat. Prod. Res. 2021, 35, 1491–1496. [Google Scholar] [CrossRef]
- Tang, J.X.; Quan, L.Q.; Xie, K.; Zhou, Y.; Ye, R.R.; Liu, D.; Li, R.T.; Li, H.M. Jatavaleridoids A-H, Eight New Iridoids from the Roots and Rhizomes of Valeriana Jatamansi Jones. Fitoterapia 2022, 162, 105286. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hao, E.W.; Zhang, M.; Pan, X.L.; Qin, J.F.; Xie, J.L.; Zhou, J.Y.; Hou, X.T.; Deng, J.G. Chemical Constituents from Jasminum Pentaneurum Hand.-Mazz and Their Cytotoxicity against Human Cancer Cell Lines. Nat. Prod. Res. 2021, 35, 921–929. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Zhang, C.; Zhang, Y.L.; Lei, J.L.; Kong, L.Y.; Luo, J.G. Dimeric Guaianes from Leaves of Xylopia Vielana as Snail Inhibitors Identified by High Content Screening. Bioorg. Chem. 2021, 108, 104646. [Google Scholar] [CrossRef]
- Boudermine, S.; Parisi, V.; Lemoui, R.; Boudiar, T.; Chini, M.G.; Franceschelli, S.; Pecoraro, M.; Pascale, M.; Bifulco, G.; Braca, A.; et al. Cytotoxic Sesquiterpenoids from Ammoides Atlantica Aerial Parts. J. Nat. Prod. 2022, 85, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Lee, J.W.; Le, T.P.L.; Han, J.S.; Cho, Y.B.; Kwon, H.; Lee, D.; Lee, M.K.; Hwang, B.Y. Sesquiterpenoids from Chrysanthemum Indicum with Inhibitory Effects on NO Production. J. Nat. Prod. 2021, 84, 562–569. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, H.; He, H.; Qiu, L.; Gao, Q.; Li, Y.; Ding, W. Sesquiterpenoids from the Inflorescence of Ambrosia Artemisiifolia. Molecules 2022, 27, 5915. [Google Scholar] [CrossRef]
- Ding, N.; Wang, J.; Liu, J.; Zhu, Y.; Hou, S.; Zhao, H.; Yang, Y.; Chen, X.; Hu, L.; Wang, X. Cytotoxic Guaianolide Sesquiterpenoids from Ainsliaea Fragrans. J. Nat. Prod. 2021, 84, 2568–2574. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chen, J.; Wang, L.; Qian, F.; Li, G.; Wu, X.; Zhang, L.; Li, Y. Eighteen Structurally Diversified Sesquiterpenes Isolated from Pogostemon Cablin and Their Inhibitory Effects on Nitric Oxide Production. Fitoterapia 2022, 156, 105098. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, J.; Liu, J.; Ni, L.; Du, Y.; Wei, X. Sesquiterpenoids and Diterpenoids from the Flowers of Nicotiana Tabacum L. and Their Antifungal Activity. Rec. Nat. Prod. 2022, 16, 483–487. [Google Scholar] [CrossRef]
- Nhoek, P.; Chae, H.S.; Kim, Y.M.; Pel, P.; Huh, J.; Kim, H.W.; Choi, Y.H.; Lee, K.; Chin, Y.W. Sesquiterpenoids from the Aerial Parts of Salvia Plebeia with Inhibitory Activities on Proprotein Convertase Subtilisin/Kexin Type 9 Expression. J. Nat. Prod. 2021, 84, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Song, M.; Sun, Y.; Yang, F.; Yu, H.; Wu, C.; Sun, Y.; Chang, W.; Ge, D.; Zhang, H. Antifungal and Allelopathic Activities of Sesquiterpenes from Solidago Canadensis. Curr. Org. Chem. 2021, 25, 2676–2682. [Google Scholar] [CrossRef]
- Hanh, T.T.H.; Cham, P.T.; My, N.T.T.; Cuong, N.T.; Dang, N.H.; Quang, T.H.; Huong, T.T.; Cuong, N.X.; Nam, N.H.; Van Minh, C. Sesquiterpenoids from Saussurea Costus. Nat. Prod. Res. 2021, 35, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Ma, L.H.; Li, X.M.; Liu, T.T. Selective Phytotoxic Effects of Sesquiterpenoids from Sonchus Arvensis as a Preliminary Approach for the Biocontrol of Two Problematic Weeds of Wheat. J. Agric. Food Chem. 2022, 70, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Li, Q.R.; Chi, J.; Li, J.X.; Kong, L.Y.; Luo, J. Sesquiterpenoids from the Leaves of Sarcandra Glabra. Chin. J. Nat. Med. 2022, 20, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Cao, L.; Li, Q.; Huang, H.; Xu, W.; Chen, G.; Song, Z.; He, Y.; Yao, X.; Tang, J. Benzannulated 5,5-Spiroketal Sesquiterpenes from the Roots of Angelica Pubescens. Bioorg. Chem. 2021, 107, 104604. [Google Scholar] [CrossRef]
- Ning, R.; Mu, H.; Chen, L.; Wang, T.; Xu, X.; He, S.; Jiang, M.; Zhao, W. First Report on Inhibitory Effect against Osteoclastogenesis of Dihydro-β-Agarofuran-Type Sesquiterpenoids. J. Agric. Food Chem. 2022, 70, 554–566. [Google Scholar] [CrossRef]
- Zhong, W.; Li, M.; Han, S.; Sun, J.; Cao, L.; Mu, Z.; Du, X.; Cui, Y.; Feng, Y.; Zhong, G. Carpelipines C and D, Two Anti-Inflammatory Germacranolides from the Flowers of Carpesium Lipskyi Winkl. (Asteraceae). Chem. Biodivers. 2022, 19, e202200415. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, A.B.; Koorbanally, N.A.; Moodley, B.; Chenia, H.Y. Sesquiterpene Lactones from Polydora Serratuloides and Their Quorum Sensing Inhibitory Activity. Nat. Prod. Res. 2021, 35, 4517–4523. [Google Scholar] [CrossRef] [PubMed]
- Achoub, H.; Mencherini, T.; Esposito, T.; Luca, R.; Aquino, R.; Gazzerro, P.; Zaiter, L.; Benayache, F.; Benayache, S. New Sesquiterpenes from Asteriscus Graveolens. Nat. Prod. Res. 2021, 35, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, H.; Lin, C.; Fu, L.; Zou, Z. New 11-Methoxymethylgermacranolides from the Whole Plant of Carpesium divaricatum. Molecules 2022, 27, 5991. [Google Scholar] [CrossRef] [PubMed]
- Mirzania, F.; Moridi Farimani, M.; Sarrafi, Y.; Nejad Ebrahimi, S.; Troppmair, J.; Kwiatkowski, M.; Stuppner, H.; Alilou, M. New Sesterterpenoids from Salvia Mirzayanii Rech.f. and Esfand. Stereochemical Characterization by Computational Electronic Circular Dichroism. Front. Chem. 2022, 9, 783292. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.D.; Zhou, Y.Q.; Wu, J.T.; Qi, Z.T.; Algradi, A.M.; Pan, J.; Guan, W.; Yang, B.Y.; Kuang, H.X. A New Ent-Kaurane Diterpenoid from the Pericarps of Datura Metel. J. Asian Nat. Prod. Res. 2022, 24, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Yu, M.; Li, H.; Zhang, G.J. Structures and Biological Activities of Polyacylated Ent-Kaurane Diterpenoid Glycosides from the Aerial Parts of Inula Hupehensis. J. Nat. Prod. 2022, 85, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.J.; Zhu, B.; Si, Y.; Guo, T.; Kang, J.; Dai, L. Cytotoxic Ent-Kaurane Diterpenoids from Rabdosia Rubescens. Chem. Biodivers. 2022, 19, e202200497. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Xu, J.; Lv, J.J.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. New Ent-Kaurane and Cleistanthane Diterpenoids with Potential Cytotoxicity from Phyllanthus Acidus (L.) Skeels. Fitoterapia 2022, 157, 105133. [Google Scholar] [CrossRef]
- Yan, X.L.; Zou, M.F.; Chen, B.L.; Yuan, F.Y.; Zhu, Q.F.; Zhang, X.; Lin, Y.; Long, Q.D.; Liu, W.L.; Liao, S.G. Euphorane C, an Unusual C17-Norabietane Diterpenoid from Euphorbia Dracunculoides Induces Cell Cycle Arrest and Apoptosis in Human Leukemia K562 Cells. Arab. J. Chem. 2022, 15, 104203. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; He, F.; Fu, W.; Tang, B.; Bin, Y.; Fang, M.; Wu, Z.; Qiu, Y. Anti-Inflammatory Sesquiterpenoids from the Heartwood of Juniperus Formosana Hayata. Fitoterapia 2022, 157, 105105. [Google Scholar] [CrossRef]
- Ntungwe, E.N.; Stojanov, S.J.; Duarte, N.M.; Candeias, N.R.; Díaz-Lanza, A.M.; Vágvölgyi, M.; Hunyadi, A.; Pešić, M.; Rijo, P. C20- nor-Abietane and Three Abietane Diterpenoids from Plectranthus Mutabilis Leaves as P-Glycoprotein Modulators. ACS Med. Chem. Lett. 2022, 13, 674–680. [Google Scholar] [CrossRef]
- Huang, X.L.; Wang, D.W.; Liu, Y.Q.; Cheng, Y.X. Diterpenoids from Blumea Balsamifera and Their Anti-Inflammatory Activities. Molecules 2022, 27, 2890. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Liang, Z.M.; Zhao, C.L.; Tsang, N.Y.; Li, J.X.; Liu, Y.H.; He, K.; Pan, L.T.; Rong, L.; Zou, J.; et al. 3,4-Seco-Isopimarane Diterpenes from the Twigs and Leaves of Isodon Flavidus. Molecules 2022, 27, 3098. [Google Scholar] [CrossRef]
- Su, X.M.; Liang, Q.; Hu, J.X.; Zhang, X.M.; Jia, R.L.; Xu, W.H. Diterpenoids from the Whole Plants of Croton Yunnanensis and Their Bioactivities. Bioorg. Med. Chem. 2021, 51, 116495. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, D.; Kang, K.S.; Kim, K.H. A New Labdane-Type Diterpene, 6-O-Acetyl-(12R)-Epiblumdane, from Stevia Rebaudiana Leaves with Insulin Secretion Effect. Biomedicines 2022, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Zhu, Q.F.; Zhang, X.; Lin, Y.; Long, Q.D.; Liu, W.L.; Yan, X.L. An Unusual Indole-Diterpenoid with C-17 Norcassane Skeleton from Euphorbia Fischeriana Induces HEL Cell Cycle Arrest and Apoptosis. Fitoterapia 2022, 159, 105195. [Google Scholar] [CrossRef]
- Hasan, A.; Tang, D.; Nijat, D.; Yang, H.; Aisa, H.A. Diterpenoids from Euphorbia Glomerulans with Potential Reversal Activities against P-Glycoprotein-Mediated Multidrug Resistance. Bioorg. Chem. 2021, 117, 105442. [Google Scholar] [CrossRef]
- Wan, L.X.; Zhang, J.F.; Zhen, Y.Q.; Zhang, L.; Li, X.; Gao, F.; Zhou, X.L. Isolation, Structure Elucidation, Semi-Synthesis, and Structural Modification of C19-Diterpenoid Alkaloids from Aconitum apetalum and Their Neuroprotective Activities. J. Nat. Prod. 2021, 84, 1067–1077. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Fei, J.; Dong, X.; Wang, Y.; Yang, S.; Hao, X.; Ding, X.; Zhao, Y. Identification of Limonoids from Walsura Yunnanensis and Evaluation of Their Cytotoxicity against Cancer Cell Lines. Fitoterapia 2022, 157, 105120. [Google Scholar] [CrossRef]
- Bernard, D.; Hassana, Y.; Djaouda, M.; Mathieu, M.; Bouba Romeo, W.; Benoît, K.; Tul Wahab, A. Antibacterial Effects of a New Triterpenoid Saponin from Roots of Gardenia Ternifolia Schumach. & Thonn (Rubiaceae). Results Chem. 2022, 4, 100366. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, Y.; Liu, H.; Zhang, Y.; Wei, Z.; Liu, G.; Tang, X.; Jia, X. Structure Determination, Bitterness Evaluation and Hepatic Gluconeogenesis Inhibitory Activity of Triterpenoids from the Momordica Charantia Fruit. Food Chem. 2022, 372, 131224. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.D.H.; Van Le, T.K.; Do, T.H. Triterpene Glycosides from the Aerial Parts of Elsholtzia Penduliflora W. W. Smith and Their Cytotoxic Activity. Fitoterapia 2022, 162, 105264. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.Y.; Zhao, Y.L.; Ma, D.Y.; Xiang, M.L.; Zhao, L.X.; Luo, X.D. Anti-Hyperuricemic Bioactivity of Alstonia Scholaris and Its Bioactive Triterpenoids in Vivo and in Vitro. J. Ethnopharmacol. 2022, 290, 115049. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Hu, R.; Zhou, Y.; Zhu, W.; Zhou, Y. Cytotoxic 13,28 Epoxy Bridged Oleanane-Type Triterpenoid Saponins from the Roots of Ardisia Crispa. Molecules 2022, 27, 1061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Chu, Z.J.; Zhou, J.C.; Liu, S.G.; Zhang, J.Z.; Qian, L.; Lou, H.X. Cytotoxic Activities of 9,10-Seco-Cycloartane-Type Triterpenoids from the Chinese Liverwort Lepidozia Reptans. J. Nat. Prod. 2021, 84, 3020–3028. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Zhang, W.Y.; Zhong, J.C.; Huang, X.J.; Xu, W.; Chen, M.F.; Weng, S.Q.; Zhang, D.M.; Che, C.T.; Ye, W.C.; et al. Angiogenesis-Inhibitory Piperidine Alkaloids from the Leaves of Microcos Paniculata. J. Nat. Prod. 2022, 85, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, Y.; Wang, R.; Wang, Y.H.; Xu, J.W.; He, X.J. Antiproliferative Piperidine Alkaloids from Giant Taro (Alocasia Macrorrhiza). Chin. J. Nat. Med. 2022, 20, 541–550. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Zhang, X.; Liu, C.; Chen, N.; Naruo, A.; Qin, Z.; Ao, M. New Napelline-Type Diterpenoid Alkaloids from Aconiti Kusnezoffii Roots: Structure Elucidation, Plausible Biogenetic Pathway and Biological Activities. Phytochem. Lett. 2021, 43, 53–59. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhou, Q.M.; Guo, L.; Dai, O.; Meng, C.W.; Miao, L.L.; Liu, J.; Lin, Q.; Peng, C.; Xiong, L. Cardioprotective Effects and Concentration-Response Relationship of Aminoalcohol-Diterpenoid Alkaloids from Aconitum Carmichaelii. Fitoterapia 2021, 149, 104822. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.Z.; Li, X.Y.; Xie, J.; Chen, L.; Gao, F.; Zhou, X.L.; Huang, S. Gyalanunines A and B, Two New C20-Diterpenoid Alkaloids from Delphinium Gyalanum. Tetrahedron Lett. 2022, 108, 154153. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, D.; Chen, Y.; Xu, J.; Xu, Y.; Yue, X.; Jia, J.; Li, H.; Chen, L. Alkaloids of Delphinium Grandiflorum and Their Implication to H2O2-Induced Cardiomyocytes Injury. Bioorg. Med. Chem. 2021, 37, 116113. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yan, Y.; Li, X.; Gong, G.; Wang, W. Three New Diterpenoid Alkaloids from Delphinium Tatsienense. Phytochem. Lett. 2021, 41, 142–146. [Google Scholar] [CrossRef]
- Kemgni, M.F.; Chenda, L.B.N.; Tchamgoue, J.; Kenfack, P.T.; Ngandjui, Y.A.T.; Wouamba, S.C.N.; Tiani, G.L.M.; Green, I.R.; Kouam, S.F. Greenwaylactams A, B and C, the First Group of Sesquiterpene Alkaloids with an Eight-Membered Lactam Ring from Greenwayodendron Oliveri. ChemistrySelect 2021, 6, 1705–1709. [Google Scholar] [CrossRef]
- Xie, T.Z.; Zhao, Y.L.; Wang, H.; Chen, Y.C.; Wei, X.; Wang, Z.J.; He, Y.J.; Zhao, L.X.; Luo, X.D. New Steroidal Alkaloids with Anti-Inflammatory and Analgesic Effects from Veratrum Grandiflorum. J. Ethnopharmacol. 2022, 293, 115290. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.J.; Zhu, P.Y.; Qiao, M.; Gao, W.F.; Li, G.D.; Wang, X.J.; Sheng, J. Two New Steroidal Alkaloids with Cytotoxic Activities from the Roots of Veratrum Grandiflorum Loes. Phytochem. Lett. 2021, 46, 56–60. [Google Scholar] [CrossRef]
- Wu, P.F.; Liu, J.; Li, Y.N.; Ding, R.; Tan, R.; Yang, X.M.; Yu, Y.; Hao, X.J.; Yuan, C.M.; Yi, P. Three New Aglain Derivatives from Aglaia Odorata Lour. and Their Cytotoxic Activities. Chem. Biodivers. 2022, 19, e202101008. [Google Scholar] [CrossRef] [PubMed]
- Promchai, T.; Thaima, T.; Rattanajak, R.; Kamchonwongpaisan, S.; Pyne, S.G.; Limtharakul, T. (R)-3-(8′-Hydroxyfarnesyl)-Indole and Other Chemical Constituents from the Flowers of Anomianthus Dulcis and Their Antimalarial and Cytotoxic Activities. Nat. Prod. Res. 2021, 35, 2476–2481. [Google Scholar] [CrossRef]
- Wan Othman, W.N.N.; Salim, F.; Abdullah, N.N.; Abu Bakar, S.I.; Awang, K.; Jayasinghe, L.; Ismail, N.H. (R)-13aα-Densiindolizidine, A New Phenanthroindolizidine Alkaloid from Cryptocarya Densiflora Blume (Lauraceae) and Molecular Docking against SARS-CoV-2. Nat. Prod. Commun. 2022, 17, 1934578X221114227. [Google Scholar] [CrossRef]
- Yuan, H.L.; Zhao, Y.L.; Qin, X.J.; Liu, Y.P.; Yang, X.W.; Luo, X.D. Diverse Isoquinolines with Anti-Inflammatory and Analgesic Bioactivities from Hypecoum Erectum. J. Ethnopharmacol. 2021, 270, 113811. [Google Scholar] [CrossRef] [PubMed]
- Panthong, K.; Ingkaninan, K. Amabiloid A from Crinum × Amabile Donn Ex Ker Gawl. Nat. Prod. Res. 2021, 35, 3220–3225. [Google Scholar] [CrossRef]
- Liu, S.B.; Zeng, L.; Xu, Q.L.; Chen, Y.L.; Lou, T.; Zhang, S.X.; Tan, J.W. Polycyclic Phenol Derivatives from the Leaves of Spermacoce Latifolia and Their Antibacterial and α-Glucosidase Inhibitory Activity. Molecules 2022, 27, 3334. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Zhou, Y.; Yin, X.; Wei, X.; Zhou, Y. Two New Phenolic Glycosides with Lactone Structural Units from Leaves of Ardisia Crenata Sims with Antibacterial and Anti-Inflammatory Activities. Molecules 2022, 27, 4903. [Google Scholar] [CrossRef]
- Liao, N.; Li, M.S.; Qin, F.; Jiang, J.C.; Luo, R.D.; Chen, X.L.; Zhou, M.M.; Zhu, Y.K.; Wang, H.S. A New Phenolic Acid from Zanthoxylum Nitidum Var. Tomentosum (Rutaceae) and Its Chemotaxonomic Significance. Biochem. Syst. Ecol. 2021, 99, 104351. [Google Scholar] [CrossRef]
- Zheng, X.; Wen, R.; Liu, Y.; Gan, L.; Zhang, Q.; Jiang, Y.; Tu, P. Nitric Oxide Inhibitory Phenolic Constituents Isolated from the Roots and Rhizomes of Notopterygium Incisum. Bioorg. Chem. 2022, 128, 106060. [Google Scholar] [CrossRef]
- Nhung, L.T.H.; Linh, N.T.T.; Cham, B.T.; Thuy, T.T.; Tam, N.T.; Thien, D.D.; Huong, P.T.M.; Tan, V.M.; Tai, B.H.; Hoang Anh, N.T. New Phenolics from Dianella Ensifolia. Nat. Prod. Res. 2021, 35, 3063–3070. [Google Scholar] [CrossRef]
- Cho, H.M.; Zhang, M.; Park, E.J.; Lee, B.W.; Park, Y.J.; Kim, H.W.; Pham, H.T.T.; Chin, Y.W.; Oh, W.K. Flavonostilbenes Isolated from the Stems of Rhamnoneuron Balansae as Potential SIRT1 Activators. J. Nat. Prod. 2022, 85, 70–82. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Singab, A.N.B. A New Antidiabetic and Anti-Inflammatory Biflavonoid from Schinus Polygama (Cav.) Cabrera Leaves. Nat. Prod. Res. 2022, 36, 1182–1190. [Google Scholar] [CrossRef]
- Fu, H.M.; Yin, C.L.; Shen, Z.Y.; Yang, M.H. Flavonoids from the Leaves of Apocynum Venetum and Their Anti-Inflammatory Activity. J. Chem. Res. 2022, 46, 4–7. [Google Scholar] [CrossRef]
- Molčanová, L.; Treml, J.; Brezáni, V.; Maršík, P.; Kurhan, S.; Trávníček, Z.; Uhrin, P.; Šmejkal, K. C-Geranylated Flavonoids from Paulownia Tomentosa Steud. Fruit as Potential Anti-Inflammatory Agents. J. Ethnopharmacol. 2022, 296, 115509. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Bi, D.; Li, F.; Luo, R.; Zhuang, H.; Wang, L. Two New C-Benzylated Chalcones and One New Mimosin-Type Homoisoflavonoid from the Twigs and Leaves of Caesalpinia Digyna. Fitoterapia 2022, 162, 105279. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Jang, J.; Lee, M.K.; Lee, K.Y. Cell Extraction Method Coupled with LC-QTOF MS/MS Analysis for Predicting Neuroprotective Compounds from Polygonum Tinctorium. J. Pharm. Biomed. Anal. 2022, 220, 114988. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Q.; Cao, X.; Meng, Y.; Jiang, G.; Xu, P. Two New Flavonol Derivatives from the Whole Plants of Centella Asiatica and Their Cytotoxic Activities. Phytochem. Lett. 2022, 47, 34–37. [Google Scholar] [CrossRef]
- Kalenga, T.M.; Ndoile, M.M.; Atilaw, Y.; Munissi, J.J.E.; Gilissen, P.J.; Rudenko, A.; Bourgard, C.; Sunnerhagen, P.; Nyandoro, S.S.; Erdelyi, M. Antibacterial and Cytotoxic Biflavonoids from the Root Bark of Ochna Kirkii. Fitoterapia 2021, 151, 104857. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Yu, H.; Zhang, J.; Du, J.; Wang, X.; Wang, Y.; Chai, X. Chemical Constituents from the Fruits of Cullen Corylifolium (L.) Medik. by the Targeted Separation Mode. Nat. Prod. Res. 2021, 35, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.W.; Guo, W.W.; Guo, J.F.; Wang, X.; Chen, X.Q.; Wu, X. Three New Flavonoids from Penthorum Chinense Pursh and Their Docking Studies. Nat. Prod. Res. 2021, 35, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Tambewagh, U.U.; Javir, G.R.; Joshi, K.S.; Rojatkar, S.R. Two New Polyoxygenated Flavonoids from Blumea Eriantha DC, Methanol Extract and Their Anti-Proliferative Activity. Nat. Prod. Res. 2021, 35, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Blanco Carcache, P.J.; Anaya Eugenio, G.D.; Ninh, T.N.; Moore, C.E.; Rivera-Chávez, J.; Ren, Y.; Soejarto, D.D.; Kinghorn, A.D. Cytotoxic Constituents of Glycosmis Ovoidea Collected in Vietnam. Fitoterapia 2022, 162, 105265. [Google Scholar] [CrossRef]
- Chukaew, A.; Chaniad, P. Chemical Constituents from the Roots of Calophyllum Pisiferum Planch. & Triana and Their Cytotoxic and Antioxidant Activities. Rec. Nat. Prod. 2022, 16, 66–73. [Google Scholar] [CrossRef]
- Liu, F.; Mallick, S.; O’donnell, T.J.; Rouzimaimaiti, R.; Luo, Y.; Sun, R.; Wall, M.; Wongwiwatthananukit, S.; Date, A.; Silva, D.K.; et al. Coumarinolignans with Reactive Oxygen Species (ROS) and NF-ΚB Inhibitory Activities from the Roots of Waltheria Indica. Molecules 2022, 27, 3270. [Google Scholar] [CrossRef] [PubMed]
- SShen, H.Y.; Zhang, M.; Ouyang, J.K.; Jia, Y.X.; Luo, Y. Two New Coumarin Derivatives from the Whole Plant of Spermacoce Latifolia. Phytochem. Lett. 2022, 51, 82–85. [Google Scholar] [CrossRef]
- Xia-Hou, Z.R.; Feng, X.F.; Mei, Y.F.; Zhang, Y.Y.; Yang, T.; Pan, J.; Yang, J.H.; Wang, Y.S. 5-Demethoxy-10′-Ethoxyexotimarin F, a New Coumarin with MAO-B Inhibitory Potential from Murraya Exotica L. Molecules 2022, 27, 4950. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wu, S.Y.; Jiang, C.X.; Tong, Y.P.; Zhao, T.; Zhang, B.; Nong, X.H.; Jin, Z.X.; Hu, J.F. A New Coumarin Derivative from the Stems of the Endangered Plant Ulmus Elongata. Nat. Prod. Res. 2021, 35, 3562–3568. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Z.; Kim, C.S.; Jeong, S.K.; Lee, S.; Lee, I.S.; Hong, K.W. Anti-Inflammatory Isocoumarins from the Bark of Fraxinus Chinensis Subsp. Rhynchophylla. Nat. Prod. Res. 2021, 35, 4380–4387. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.Y.; Cheng, K.C.; Kuo, P.C.; Chen, I.T.; Li, Y.C.; Hwang, T.L.; Lam, S.H.; Wu, T.S. Chemical Constituents of Hedyotis Diffusa and Their Anti-Inflammatory Bioactivities. Antioxidants 2022, 11, 335. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Dong, C.; Xie, J.; Lai, S.; Liu, J.; Chen, R.; Kang, J. New Quinones, a Sesquiterpene and Phenol Compounds with Cytotoxic Activity from the Aerial Parts of Morinda Umbellata L. Fitoterapia 2022, 156, 105089. [Google Scholar] [CrossRef] [PubMed]
- Hangsamai, N.; Photai, K.; Mahaamnart, T.; Kanokmedhakul, S.; Kanokmedhakul, K.; Senawong, T.; Pitchuanchom, S.; Nontakitticharoen, M. Four New Anthraquinones with Histone Deacetylase Inhibitory Activity from Ventilago Denticulata Roots. Molecules 2022, 27, 1088. [Google Scholar] [CrossRef]
- Wisetsai, A.; Lekphrom, R.; Schevenels, F.T. New Anthracene and Anthraquinone Metabolites from Prismatomeris Filamentosa and Their Antibacterial Activities. Nat. Prod. Res. 2021, 35, 1582–1589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).